Abstract
Purpose
The aim of this study was to investigate the changes in oxidative stress and antioxidants in lung tissue under different tidal volume ventilation conditions.
Methods
Forty-eight male Wistar rats were randomized into four groups, namely, group C, the control group, which was not ventilated, and groups C1, C2 and C3, the treatment groups, which were ventilated for 2 h with tidal volumes of 8, 30 and 42 ml/kg, respectively. The right middle lobe was assayed for malondialdehyde (MDA), the right posterior lobe was assayed using Western blotting for Nrf2, GCLm and SrX1 and the left lobe was assayed for Nrf2, GCLm and SrX1 mRNA.
Results
The MDA levels were increased in the three treatment groups, with MDA levels highest in group C3 and lowest in group C1 (C3 > C2 > C1) (all P < 0.05). The mRNA expression of Nrf2, GCLm and SrX1 was highest in group C3 and lowest in group 1 (C3 > C2 > C1) (all P < 0.05). No significant difference was observed between group C1 and group C (P > 0.05). A Western blot analysis showed that Nrf2, GCLm and SrX1 expression was highest in group C3 and lowest in group C1 (C3 > C2 > C1) (all P < 0.05). No significant difference was observed between group C1 and group C (P > 0.05).
Conclusions
Oxidative stress and antioxidant enzyme levels in the lungs of rats were positively associated with the tidal volumes of mechanical ventilation, suggesting that higher tidal volumes cause more severe oxidative stress and increased antioxidant responses.
Keywords: Mechanical ventilation, Tidal volume, Oxidative stress, Antioxidant, Lung
Introduction
Ventilator-induced lung injury (VILI) is associated with a high rate of mortality and has an important social impact [1]. Mechanical ventilation (MV) is the only known effective method to support patients’ lives during general anesthesia and is the cornerstone therapy for acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). The mechanisms implicated in VILI include high inspiratory volumes (“volutrauma”), mechanical stress-induced inflammation (“biotrauma”) and cyclical airway collapse and reopening [2]. As early as 1988, Dreyfuss et al. [3] reported novel data demonstrating to the critical care community that MV with large tidal volume is injurious, coining the term “volutrauma.” This terminology proved useful as it emphasizes that peak lung volume and tidal volume are better surrogates for lung stress than peak airway pressure, plateau airway pressure or airway pressure swings.
Emerging evidence suggests a role for antioxidant and oxidant imbalance in the exacerbation of lung injury and inflammation caused by various injurious insults, including MV [4–7]. The production of high levels of reactive oxygen species (ROS) generally leads to oxidative stress. ROS act both as a direct cell toxin and as a secondary messenger that modulates intracellular signaling, causing activation of a cascade of inflammation, neutrophil infiltration and cytokine production, while simultaneously causing apoptosis by activating c-JunN-terminal kinase (JNK). The induction of cytoprotective antioxidant enzymes in response to injurious insults or stressful stimuli is critical in the cellular detoxification of ROS and in the maintenance of cellular redox homeostasis. Specific biological defense systems, including catalase, superoxide dismutase and glutathione (GSH), exist in the lung to counteract oxidative stress [8]. These enzymes are regulated by the transcription factor nuclear factor (erythroid-derived 2)-like (Nrf2) and a number of speed-limited synthetic enzymes. Nrf2 regulates cellular redox status via the antioxidant response element, and genetic disruption of this transcription factor enhances the susceptibility of various experimental murine models to pro-oxidant-induced lung diseases. These effects are primarily due to decreased levels of the basal and inducible expression of several critical antioxidant enzymes, including the glutamate-cysteine ligase catalytic subunit (GCLc), glutamate cysteine ligase modifier subunit (GCLm) and sulfiredoxin-1 (Srx1) [9].
Recent in vitro studies have shown that a cyclic stretch similar to that associated with conventional high tidal volume MV generates ROS and a redox imbalance in lung epithelial and endothelial cells. However, the exact relevance of redox imbalance to the onset of MV-induced lung injury in vivo remains unclear. Based on these observations, we hypothesized that redox imbalance caused by conventional MV might relate to the tidal volume, which in turn might promote and/or perpetuate the pathogenesis of VILI. To test our hypothesis, we evaluated lung oxidant stress and antioxidant response changes under different tidal volumes during MV.
Methods
Reagents
Forty-eight Wistar rats were fed a normal diet, provided with water ad libitum and housed under controlled conditions (25 ± 2 °C; 12-h light:dark periods). All experimental animal protocols were performed in accordance with guidelines approved by the Animal Care and Use Committee of Zhengzhou University, Henan, China. The experiments were approved by the Committee on Animal Experimentation of Zhengzhou University, Henan, China.
Mechanical ventilation
Exposure to MV was performed as previously described [10]. Briefly, 8-week-old male rats were anesthetized intraperitoneally with 10 % chloral hydrate (3 ml per kg body weight), and a supplemental anesthetic was provided regularly at approximately 1 ml/kg per hour as maintenance during the experimental period. Rectal temperatures were continuously monitored and maintained at 37 ± 1 °C by a recirculating heating blanket (ALC-HTP; Alcott Biotech Co. Ltd., Shanghai, China), and the heart rate was monitored via a lead II electrocardiograph. A pulse oximeter (Radical; Masimo, Irvine, CA) was placed on a shaved foreleg. A midline incision of the neck was performed to expose the trachea to facilitate endotracheal intubation with a 16-gauge, 10-cm-long catheter. The right carotid artery was exposed and cannulated for invasive blood pressure monitoring, and a tail vein was cannulated with an intravenous cannula (24G) for fluid infusion and anesthetic administration. The rats were randomly divided into three ventilation (treatment) groups, with 12 rats in each group. The rats in groups C1, C2 and C3 were subjected to MV (Inspira ASV; Harvard Apparatus, Holliston, MA) for 2 h with a tidal volume of 8, 30 or 42 ml/kg, respectively. The tidal volumes and the duration of ventilation were based on the results of pilot experiments which demonstrated that these conditions caused VILI in rats with reproducible alterations in lung mechanics and inflammation. The respiratory rates for each group were selected to achieve normal values for end-tidal carbon dioxide (ETCO2) (BeneView T5; Mindray Medical Int. Ltd., Shenzhen, China) (maintained between 35–45 mm Hg) based on the preliminary experiments. The control group (C) consisted of sham-operated, anesthetized and spontaneously breathing rats. The MV rats were ventilated with room air. Each animal was euthanized by exsanguination under anesthesia, disconnected from the ventilator, and then a thoracotomy was performed. The heart–lung block was removed. The lung was harvested and frozen immediately at −80 °C.
Malondialdehyde measurement
Malondialdehyde (MDA) level is an indicator of increased oxidant stress level. Consequently, MDA measurement has been used as a method to test lipid peroxidation because polyunsaturated fatty acid peroxides generate MDA upon decomposition. The right middle lobe of each harvested lung was assayed for MDA as follows. The frozen tissue samples were homogenized in 200 μl of phosphate buffer containing 4 μl of butylated hydroxytoluene, and the crude homogenate was centrifuged at 4,000 rpm for 10 min at 4 °C. The protein concentration in the supernatant was measured using a bicinchoninic acid (BCA) assay (Pierce Biotechnology Ltd., Rockford, IL). MDA was measured in the supernatant using a commercial kit (NanJing JianCheng Bioengineering Institute, Nanjing, China) and normalized to the protein concentration. The MDA levels in the lung tissue were calculated from the standard curve and expressed as nanomoles per milligram protein.
Western blot analysis
The right posterior lobe tissues of each harvest lung were homogenized, and the protein concentration of the cytosolic fraction of each sample was estimated using the BCA reagent (Pierce) and then stored at −80 °C. Western blot analyses of Nrf2, GCLm and Srx1were performed, as previously described [11]. Briefly, 50 μg of total protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 15 % gels and transferred to polyvinylidene difluoride (PVDF) membranes by semidry blotting. The PVDF membranes were blocked with 2 % nonfat milk. Detection was accomplished by incubation with polyclonal rabbit anti-Nrf2 antibody (1:500), polyclonal goat anti-GCLm antibody (1:200), polyclonal goat anti-Srx1 antibody (1:200) or β-actin (1:1000) followed by incubation with horseradish peroxidase-conjugated secondary antibodies. The blots were developed using an enhanced chemiluminescence (ECL) detection system. The absolute values for each protein were normalized to β-actin expression. All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Reverse transcription-PCR
The left lung tissues of each harvested lung were immediately processed for total RNA isolation using TRIzol reagent (LifeTechnologies, Grand Island, NY). The mRNA levels of genes encoding rat Nrf2, Gclm, Srx1 and β-actin in the lungs of the rats (n = 3–4 per group) were quantified in triplicate using TaqMan gene expression assays according to the supplier’s recommendations. The absolute values for each gene were normalized to β-actin expression.
Statistical analysis
All data from the animal experiments were collected in a double-blind fashion. The data are expressed as the mean ± standard deviation. Analysis of variance was used to compare means of multiple groups. Multiple comparison between the groups was performed using the Dunnet method. Statistical significance was defined as P < 0.05.
Results
Forty-eight Wistar rats were included in the study and divided into one of four groups [1 control (C) and 3 treatment (C1, C2, C3) groups; n =12/group]. All rats survived the experimental procedures.
Systemic and biologic response to MV
Heart rate, systolic blood pressure (SBP) and colonic (body) temperature were maintained relatively constant during the MV protocols (ranges: heart rate 300–420 bpm; SBP 92–130 mmHg; temperature 37–38 °C). Importantly, there were no significant differences among the experimental groups for any of these parameters. The pulse oxygen saturation of blood (SPO2) and CO2 (ETCO2) were also maintained at relatively constant levels during MV. Specifically, SPO2 ranged from 91 to 100 %, whereas ETCO2 ranged from 35 to 45 mmHg. In addition, at the completion of the MV protocols, there were no visual abnormalities of the lungs or thoracic cavity, no evidence of lung infarction and no evidence of infection, indicating that our aseptic surgical technique was successful.
Oxidative stress increased with increasing MV tidal volumes
To determine whether the oxidative stress increased as a function of the MV tidal volume, we measured the level of MDA in the lung tissue homogenates. MDA is a general indicator of lipid peroxidation and is commonly used to measure oxidative stress. Figure 1 shows that all three treatment (MV) groups had higher levels of MDA than control group C (8.18 ± 1.97 nmol/mg protein); this difference between group C and groups C1, C2 and C3 was significant at P < 0.05. MDA levels were highest in group C3 (32.11 ± 5.10 nmol/mg of protein), followed by group C2 (22.00 ± 5.81 nmol/mg of protein) and by group C1 (12.25 ± 3.96 nmol/mg of protein) (all P < 0.05).
Fig. 1.
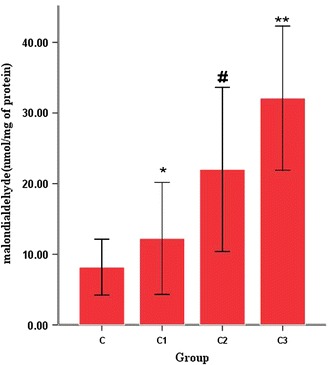
The levels of lung malondialdehyde (MDA) in the four groups of experimental rats. Mechanical ventilation (MV) at three different tidal volumes differentially affected lung oxidative stress responses in the rats. C1, C2, C3 Groups of rats (treatment) ventilated for 2 h with tidal volumes of 8, 30 and 42 ml/kg, respectively, C control group (no MV). The level of oxidative stress in lung tissue was measured MDA level. The MDA level was higher in group C1 than in group C (*P < 0.05), higher in group C2 than in group C1 (# P < 0.05) and higher in group C3 than group C2 (**P < 0.05)
Antioxidant enzymes increased as a function of increased MV tidal volumes
To determine whether the levels of antioxidant enzymes in the lungs of the rats were associated with increased MV tidal volumes, we evaluated the levels of Nrf2, GCLm and SrX1 by reverse transcription-PCR and Western blot analysis. Nrf2, GCLm and SrX1 mRNA levels were upregulated significantly in the lungs of rats subjected to MV with tidal volumes of 30 or 42 ml/kg. The mRNA levels of group C3 were higher than those of group C2, and those of group C2 were higher than those of group C1 (C3 > C2 > C1; all P < 0.05). However, the mRNA levels of Nrf2, GCLm and SrX1 in the rats ventilated at 8 mg/kg were not significantly different from those of the controls (Fig. 2). Western blot analysis of the lung proteins showed that the protein levels of Nrf2, GCLm and Srx1 in the lungs were significantly increased when the rats were subjected to MV with tidal volumes of 30 or 42 ml/kg for 2 h. Moreover, the protein levels of Nrf2, GCLm and Srx1 were higher in group C3 than in group C2 (P < 0.05) and higher in group C2 than in group C1 (C3 > C2 > C1) (all P < 0.05). No significant differences between group C1 and group C were observed (Fig. 2). Specifically, there were no significant differences between the animals receiving MV with the 8 mg/kg tidal volume and the controls for any of the lung antioxidant proteins.
Fig. 2.
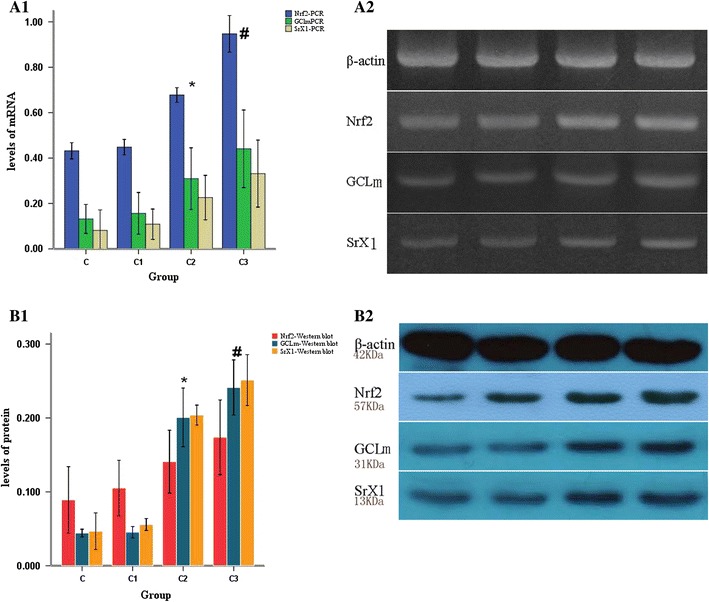
The lung antioxidant response in the ventilated rats. The lung antioxidant response was induced in rats subjected to the different MV tidal volumes. a1, a2 The expression level of the housekeeping gene β-actin was used to normalize expression of the three candidate genes: nuclear factor (erythroid-derived 2)-like (Nrf2), glutamate cysteine ligase modifier subunit (GCLm) and sulfiredoxin-1(Srx1). b1, b2 The level of the housekeeping protein β-actin was used to normalize the levels of the three candidate proteins: Nrf2, GCLm and SrX1. The results are given as the mean ± standard deviation. *P < 0.05. The expression of all three genes and proteins was higher in group C2 than group C1 (P < 0.05) and higher in group C3 than group C2 (# P < 0.05) (C3 > C2 > C1). There were no significant differences between the levels of these biomarkers in group C1 and group C (P > 0.05)
Discussion
Our results demonstrate that the level of oxidative stress, quantified as MDA, in MV groups correlated with the tidal volume. The antioxidant protein levels increased following MV with tidal volumes of 30 and 42 ml/kg. MV is often a necessary treatment for respiratory failure and is a supportive measure in critically ill patients. It is well-known that MV with high volume or pressure may be harmful, causing damage to previously healthy lungs or exacerbating injury in those already damaged. MV with low tidal volume is a lung protective strategy that has been shown to diminish the damage induced by MV in experimental models of ALI/ARDS and to decrease mortality in patients with ALI/ARDS compared with the use of higher tidal volumes. However, some patients develop VILI despite low-volume MV. The mechanisms responsible for the VILI are complex [12], but oxidative stress and inflammation mediator (e.g. interleukin-6) play key roles in the pathogenesis of VILI [10]. In our study, MV tidal volume as low as 8 ml/kg increased MDA levels compared to those of the control group. This result is consistent with those reported previously, which suggested that oxidative stress contributes to VILI [13, 14]. The expression of the antioxidant proteins Nrf2, GCLm and SrX1 in lung tissue of the ventilated rat lungs was also upregulated in parallel with increasing tidal volumes.
MV can provoke oxidative stress and an inflammatory response and subsequently cause VILI [15]. During MV, there are many potential oxidant-producing sources, including leukocytes, parenchymal cells, prooxidant enzymes, as well as the high oxygen concentration in the gases inhaled during MV. Neutrophils release free radicals, which contribute to maintaining the inflammation and inducing a prooxidant state that exceeds the antioxidative defense mechanisms. The tidal volumes used in our study were selected on the basis of earlier studies. We found that the level of MDA in the group subjected to the 8 ml/kg tidal volume (C1) increased by nearly 50 % compared to that of control group, in the 30 ml/kg group (C2), the level of this indicator increased by approximately 80 % compared to that of the 8 ml/kg group and in the 42 ml/kg group (C3) it increased by more than 45 % compared to the 30 ml/kg group. These results demonstrate that oxidative stress increases rapidly as a function of the increased MV tidal volumes.
Nrf2 regulates the expression of several antioxidant genes, including those involved in two major redox systems, namely, the GSH and thioredoxin systems, and has been demonstrated to be a key factor in antioxidant responses [16, 17]. GSH, a major cellular antioxidant, is the central component of the glutathione system. The rate-limiting enzyme in the synthesis of GSH is glutamate cysteine ligase (GCL), formerly called γ-glutamylcysteine synthetase (γ-GCS). GCL is a heterodimer composed of catalytic (GCLc) and regulatory (GCLm) subunits. GCLc possesses all of the catalytic activity and is the site of GSH feedback inhibition. Current evidence suggests that GCLc is responsible for the constitutive synthesis of GSH, while the association of GCLc with GCLm is required to overcome feedback inhibition by GSH when a higher rate of synthesis is required, as may occur during stress [18]. Thus, GCLm is critical for the biosynthesis of GSH [19]. Thioredoxin acts as an antioxidant by controlling cellular redox balance. In this context, thioredoxin promotes cell growth, inhibits apoptosis, modulates inflammation and prevents human immunodeficiency virus replication [20]. Srx1 is associated with the thioredoxin system. Sulfiredoxin catalyzes the reduction of the sulfinic form of peroxiredoxin (Prx) to the sulfenic acid form, thus restoring the functional cysteine residues and activity of Prx enzymes [21, 22]. Srx1 was the first protein identified as a mediator of the removal of a GSH moiety from proteins, a process also known as deglutathionylation [17, 23]. We found that both the gene transcription and protein expression of Nrf2, GClm and SrX1 were upregulated in the rats ventilated with tidal volumes of 30 and 42 ml/kg, respectively. The antioxidant enzyme expression was greater in the MV group subjected to the 42 ml/kg tidal volume than in the group subjected to the 30 ml/kg procedure (C3 > C2), but no significant difference was found between the rats subjected to the 8 ml/kg MV (C1) and the control group ©). These results for upregulation of antioxidant enzymes and oxidative stress differed between group C1 and C. It is possible that the antioxidant response is induced by excessive oxidant stress. Alternatively, the antioxidant capacity may be sufficient to maintain homeostasis under the conditions of the 8 ml/kg tidal volume MV but that greater tidal volumes induce the upregulation of the antioxidant response. Another possibility is that the antioxidant may appear as a cascade response when the tidal volume increased.
Oxidative stress involved in cellular signaling includes the activation of major signaling pathways, including the MAPK, PI3 K/Akt, NFκB, ERK, JNK and p53 pathways. The fundamental question regarding the role of antioxidant processes in the pathogenesis of VILI is whether the process is beneficial or adverse. The answer is clear: “it depends.” Currently, there are no pharmacological treatments that can act synergistically with protective MV strategies to minimize or prevent VILI. The optimal strategy is to maintain the balance of oxidant stress and antioxidants and avoid high tidal volume during the MV unless high volume ventilation is absolutely necessary.
This study had a number of limitations, the high tidal volume in the treatment groups exceeded clinical application. We also did not monitor airway pressure, which may introduce an inaccuracy in all groups. However, we did demonstrate that oxidative stress and antioxidant enzymes in the lungs of rats were related to the MV tidal volume: specifically, the larger the tidal volume, the more severe the oxidative stress and antioxidant response.
Acknowledgments
This work was supported by the Department of Anesthesia, the First Affiliated Hospital of Zhengzhou University, the Department of respiratory medicine, the First Affiliated Hospital of Zhengzhou University, and the Department of Anesthesia, Henan Cancer Hospital, Henan, China. This is an unfunded investigator-originated research report.
Ethical standard
Ethical approval for this study was provided by the Ethical Committee of The First Affiliated Hospital of Zhengzhou University on 16 June 2011.
References
- 1.Ventrice EA, Marti-Sistac O, Gonzalvo R, Villagra A, Lopez-Aguilar J, Blanch L. [Molecular and biophysical mechanisms and modulation of ventilator-induced lung injury]. Mecanismos biofisicos, celulares y modulacion de la lesion pulmonar inducida por la ventilacion mecanica. Medicina intensiva/Sociedad Espanola de Medicina Intensiva y Unidades Coronarias. 2007;31(2):73–82. doi: 10.1016/S0210-5691(07)74779-4. [DOI] [PubMed] [Google Scholar]
- 2.Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L834–L841. doi: 10.1152/ajplung.00069.2005. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137(5):1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 4.Papaiahgari S, Yerrapureddy A, Hassoun PM, Garcia JG, Birukov KG, Reddy SP. EGFR-activated signaling and actin remodeling regulate cyclic stretch-induced NRF2-ARE activation. Am J Respir Cell Mol Biol. 2007;36(3):304–312. doi: 10.1165/rcmb.2006-0131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu P, Murley JS, Grdina DJ, Birukova AA, Birukov KG. Induction of cellular antioxidant defense by amifostine improves ventilator-induced lung injury. Crit Care Med. 2011;39(12):2711–2721. doi: 10.1097/CCM.0b013e3182284a5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis RC, Bloch KD, Ichinose F, Zapol WM. Protective and detrimental effects of sodium sulfide and hydrogen sulfide in murine ventilator-induced lung injury. Anesthesiology. 2011;115(5):1012–1021. doi: 10.1097/ALN.0b013e31823306cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An LLC, Qin XB, Liu QH, Liu Y, Yu SY. Protective effects of hemin in an experimental model of ventilator-induced lung injury. Eur J Pharmacol. 2011;661(1–3):102–108. doi: 10.1016/j.ejphar.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Kosower NS, Kosower EM. The glutathione status of cells. Int Rev Cytol. 1978;54:109–160. doi: 10.1016/S0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJY, Shin BS, Kim H, Song H, Bae SH, Rhee SG, Jeong W. Redox regulation of lipopolysaccharide-mediated sulfiredoxin induction, which depends on both AP-1 and Nrf2. J Biol Chem. 2010;285(45):34419–34428. doi: 10.1074/jbc.M110.126839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papaiahgari S, Yerrapureddy A, Reddy SR, Reddy NM, Dodd OJ, Crow MT, Grigoryev DN, Barnes K, Tuder RM, Yamamoto M, Kensler TW, Biswal S, Mitzner W, Hassoun PM, Reddy SP. Genetic and pharmacologic evidence links oxidative stress to ventilator-induced lung injury in mice. Am J Respir Crit Care Med. 2007;176(12):1222–1235. doi: 10.1164/rccm.200701-060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3(10):e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care. 2005;11(1):82–86. doi: 10.1097/00075198-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Oeckler RA, Hubmayr RD. Ventilator-associated lung injury: a search for better therapeutic targets. Eur Respir J. 2007;30(6):1216–1226. doi: 10.1183/09031936.00104907. [DOI] [PubMed] [Google Scholar]
- 14.Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal. 2009;11(7):1651–1667. doi: 10.1089/ars.2008.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CSKT, Lee S, Tochigi N, Shigemura N, Buchholz BM, Kloke JD, Billiar TR, Toyoda Y, Nakao A. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit Care. 2010;14(6):R234. doi: 10.1186/cc9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8(1–2):76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 17.Singh A, Ling G, Suhasini AN, Zhang P, Yamamoto M, Navas-Acien A, Cosgrove G, Tuder RM, Kensler TW, Watson WH, Biswal S. Nrf2-dependent sulfiredoxin-1 expression protects against cigarette smoke-induced oxidative stress in lungs. Free Radic Biol Med. 2009;46(3):376–386. doi: 10.1016/j.freeradbiomed.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson DA, Levonen A-L, Moellering DR, Arnold EK, Zhang H, Darley-Usmar VM, Forman HJ. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic Biol Med. 2004;37(8):1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Reddy SP, Hassoun PM, Brower R. Redox imbalance and ventilator-induced lung injury. Antioxid Redox Signal. 2007;9(11):2003–2012. doi: 10.1089/ars.2007.1770. [DOI] [PubMed] [Google Scholar]
- 20.Burke-Gaffney A, Callister ME, Nakamura H. Thioredoxin: friend or foe in human disease? Trends Pharmacol Sci. 2005;26(8):398–404. doi: 10.1016/j.tips.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Rhee SG, Jeong W, Chang TS, Woo HA. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney Int Suppl. 2007;106:S3–S8. doi: 10.1038/sj.ki.5002380. [DOI] [PubMed] [Google Scholar]
- 22.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425(6961):980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 23.Findlay VJ, Townsend DM, Morris TE, Fraser JP, He L, Tew KD. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer res. 2006;66(13):6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]


