Abstract
Human parvovirus B19 (PVB19) is linked to variety of diseases, including erythema infectiosum, transient aplastic crisis, fetal hydrops, cardiomyopathy and, recently, hepatitis and arthritis. Persistence of PVB19 in asymptomatic individuals has been reported in skin, synovium, myocardium and bone marrow. A higher level of PVB19 DNA has been observed in various tissues from cases of disease than in controls. Simultaneously, equal detection of PVB19 DNA has been shown in both cases and controls. Thus, it has become fundamental to study PVB19 DNA persistence in tissues that are unaffected by disease. This will help to better understand PVB19 DNA persistence in symptomatic and asymptomatic individuals and its possible pathogenic role in various diseases. A total of 70 adult autopsies were included and divided into seropositive (SP) and seronegative (SN) groups based on PVB19 IgG. Nested PCR for PVB19 DNA was carried out in myocardium, liver, kidney, and bone marrow. Of the 70 patients, 60 % belonged to the SP group and 40 % to the SN group. Seropositivity ranged from 50 % in the 12 to 20 year old group to 66.7 % in the 61 to 80 year old group. The viral genome was detected in 34.3 % of myocardium, 20 % of bone marrow, 10 % of kidney and 8.6 % of liver samples. There was no significant difference in the persistence rates between the SP and SN groups. The persistence of PVB19 DNA in various tissues ranged from 8.3 % to 36 % in the SP group and 10 % to 30 % in the SN group. The persistence of PVB19 DNA in all the tissues was low, and PVB19 serostatus had no influence on the persistence of PVB19 DNA.
Keywords: Myocarditis, Asymptomatic Individual, Proliferative Glomerulonephritis, Fulminant Liver Failure, Acute Viral Myocarditis
Introduction
Human parvovirus PVB19 (PVB19) is a small, non-enveloped, single-stranded DNA virus [6]. It belongs to the family Parvoviridae, genus Erythroparvovirus, and is the only known parvovirus to infect humans [26]. The virus targets the erythroid progenitors in the bone marrow by binding to the globoside GB4, also known as blood group P antigen. Intense viremia lasts for a few days, followed by production of IgM antibodies, initially followed by IgG antibodies [17]. PVB19 infection is common in childhood, and its seroprevalence has been reported to be 2-21 % in children (1-5 years), 30-40 % in adolescents (15 years), and 40-60 % in young adults (20-40 years), reaching a maximum in the elderly, with a prevalence of over 90 % [31].
The common PVB19-related diseases include erythema infectiosum, arthropathy and hydrops fetalis [12]. PVB19 is also known to be involved in diseases such as myocarditis, cardiomyopathy and rheumatoid arthritis. It has emerged as one of the most commonly encountered viral pathogens of dilated cardiomyopathy, which appears to be a late sequela of acute viral myocarditis [7, 11]. Recently PVB 19 has been linked to hepatitis [28], proliferative glomerulonephritis, and dermatological diseases such as gloves and socks syndrome [31]. According to recent studies, the association of PVB19 with various diseases has been shown by the presence of viral DNA in the affected tissues. However, because PVB19 is a ubiquitous virus, its genome has been reported to be present in some control samples as well [5, 23]. Considering this, the question arises whether myocardial persistence of the PVB19 genome is merely an epiphenomenon or an indication of a latent persistence of the virus, which can then be activated in some individuals by still unknown factors. Recent PCR-based studies have shown that PVB19 DNA may persist in the bone marrow of individuals with serologically documented past PVB19 infection without leading to overt manifestations. Several reports have suggested a pathogenic role for PVB19 in the development of acute hepatitis [9, 10, 13, 30] and fulminant liver failure of unknown pathology [19]. However, whether PVB19 is a pathogenic agent of fulminant liver failure and non-A-E hepatitis or a risk factor accelerating liver dysfunction due to other agents, or alternatively, a bystander with no influence on liver pathology, is still unresolved [29]. Several investigators have attempted to characterize the potential association between PVB19 infection and glomerular disease [18, 27], although PVB19 DNA has also been detected in seemingly normal control kidneys. Therefore, detection of viral DNA in tissues does not necessarily indicate active viral infection.
There are two theories regarding PVB19 infection and the persistence of its viral genome. On one hand, there are studies that have implicated PVB19 as the cause of a range of diseases by demonstrating a higher viral DNA detection rate in cases of disease than in controls. Another group of studies has found an equal, if not a higher, detection rate in controls when compared to disease cases and thus have contradicted previous results. It is therefore of fundamental importance to obtain information about PVB19 DNA persistence in asymptomatic and symptomatic individuals in order to understand its possible pathogenic role.
The present study was designed to investigate the persistence of parvoviral genome in myocardial tissue, kidney, liver and bone marrow in PVB19-seropositive cases at autopsy, excluding cases of myocarditis and cardiomyopathy, and to determine the correlation between seropositivity and persistence of PVB19 genomic DNA. This would throw light on the possible role of detection of parvoviral genomes in diseases associated with PVB19.
Materials and methods
Study population
A total of 70 consecutive adult autopsy cases were included in the study group, excluding cases of cardiomyopathy and myocarditis. The heart blood was collected during autopsy, and serum was separated and stored at −20 °C for PVB19 antibody testing. The study protocol was approved by Institute Ethics Committee (Ref. No. MS/909/MD/7884).
Based on the result of the parvovirus IgG antibody test, the cases were divided in two groups: cases that were PVB19 IgG positive were classified as the seropositive group (SP), whereas cases that were PVB19 IgG negative were classified as the seronegative (SN) group.
For PCR analysis, fresh tissue samples from both groups, including myocardium (n = 35), liver (n = 35), bone marrow (n = 20), and kidneys (n = 20), were collected and stored at −70 °C until testing for PVB19 DNA.
Detection of parvovirus B19 IgG
Parvovirus B19 IgG was detected in serum samples employing a commercially available enzyme-linked immunosorbent assay kit (Novatec, GmBH) following the manufacturer’s instruction.
Detection of PVB19 DNA by PCR
All of the tissues of the SP and SN groups were subjected to detection of parvoviral DNA. The DNA was extracted from tissues using a commercially available viral DNA extraction kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. The extracted DNA was then subjected to nested PCR targeting the NS1 gene as described previously, using the following primers [3, 16]: forward, 5′GACGCACAGAAAGAGAGTAACCAA3′ (from nucleotides 231 to 254); reverse, 5′CCAACCATCTGCTCCAGTAAACAT3′ (from nucleotides 709 to 732). The reaction mixture contained 1X buffer, 0.2 mM dNTP, 1.5 mM MgCl2, 0.5 µM each primer and 1U Taq polymerase (Fermentas, USA). The thermal profile included initial denaturation at 94 °C for 6 min, followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 30 s.
Positive control: A parvovirus B19 clone (a kind gift from Prof. Thomas Tolfvenstam, Karolinska Institute, Sweden) was used as positive control.
Statistical analysis
The PVB19 DNA positivity in different tissues was compared between the SP and SN groups by chi square (χ2) test and ANOVA. The correlation between age, sex and PVB19 seropositivity was established using the Spearman correlation coefficient. A P-value of <0.05 was taken as significant. The results were statistically evaluated using SPSS software version 16.0.
Results
The ages of the individuals in the 70 cases ranged from 12 to 75 years, with a mean of 39 years. There were 46 (65.7 %) males and 24 (34.3 %) females, with a male:female ratio of 1.9:1. The most common cause of death was acute or chronic liver disease in 22 patients (17.1 %), followed by acute respiratory distress syndrome (ARDS) and pneumonia in 10 patients (14.3 %) and septic shock in 15 patients (7.1 %). There were only two patients each with a diagnosis of chronic kidney disease and disseminated fungal infection. In the remaining 19 patients (27 %), terminal ischemia and pneumonia was the cause of death.
ELISA
Of these 70 cases, 42 (60 %) were PVB19 IgG seropositive (SP group) and 28 (40 %) were PVB19 IgG seronegative (SN group). The age distribution of cases in the SP and SN groups is shown in Fig. 1. Most of the patients were below 50 years old (52/70; 74.3 %) and only 18 (25.7 %) patients were above 50 years old.
Fig. 1.
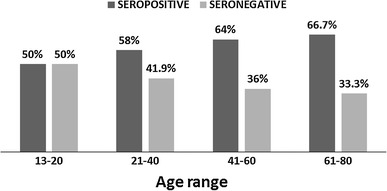
Age distribution of patients in the seropositive and seronegative groups
The seropositivity rate in males was 65.2 % and 50 % in females. The seropositivity showed an increasing trend with increasing age, ranging from 50 % in younger patients (12-20 years) to a maximum of 66.7 % in the 61 to 80 year old group. This indicated increased exposure to PVB19 infection with increasing age. There was no significant correlation between age or sex and seropositivity (p > 0.05).
PCR analysis
The results of tests for detection of PVB19 DNA in various tissues in the SP and SN groups are summarized in Table 1. PVB19 DNA was detected in a total of 12 of 35 (34.3 %) and 3 of 35 (8.6 %) of myocardial and liver tissues, respectively. Of these, 9 of 25 (36 %) myocardial and 2 of 25 (8.3 %) liver tissues belonged to the SP group. Of the remaining 10 samples each of myocardial and liver tissues, 3 of 10 (30 %) and 1 of 10 (10 %), respectively, belonged to the SN group. The difference between the two groups was not statistically significant (p > 0.05) in either myocardial or liver tissue or with regard to the underlying disease or immune status.
Table 1.
Parvovirus B19 DNA positivity in SP and SN cases
| Tissue | Total number of tissues | Total positive samples (%) | Seropositive group (SP) | Seronegative group (SN) | ||
|---|---|---|---|---|---|---|
| Number of samples tested | Positivity (%) | Number of samples tested | Positivity (%) | |||
| Myocardium | 35 | 12 (34.28) | 25 | 9 (36) | 10 | 3 (30) |
| Liver | 35 | 3 (8.57) | 25 | 2 (8.0) | 10 | 1 (10) |
| Bone marrow | 20 | 4 (20) | 10 | 1 (10) | 10 | 3 (30) |
| Kidney | 20 | 2 (10) | 10 | 1 (10) | 10 | 1 (10) |
Of the 20 bone marrow samples studied, PVB19 DNA was detected in 4/20 (20 %), of which one belonged to the SP group and three to the SN group. Of these, three cases had signs indicating secondary immunodeficiency, either due to chemotherapy or leukemia.
In the kidney tissues, there was one sample each positive for PVB19 DNA in the SP (1/10; 10 %) and the SN group (1/10; 10 %). Neither of these patients had clinical renal symptoms or signs.
In the SP group, the persistence of PVB19 DNA was highest in the myocardium (36 %; 9/25) followed by bone marrow (10 %; 1/10), kidney (10 %; 1/10), and liver tissues (8.0 %; 2/25), with a total persistence of 18.6 % (13/70) in all of the samples.
The PVB19 DNA positivity in various tissues in the SP and SN groups was not found to be statistically significant (p > 0.05).
Discussion
PVB19 virus infection has been implicated in a wide spectrum of diseases, including myocarditis, cardiomyopathy and rheumatoid arthritis. It has emerged as an important viral pathogen in dilated cardiomyopathy, which is a late sequela of acute viral myocarditis [11]. Recently, PVB19 has also been linked to hepatitis, proliferative glomerulonephritis, and various dermatological diseases [31]. Most studies have suggested a causal link between PVB19 infection and the disease, based on the viral DNA detection by PCR or in situ hybridization. Other studies have demonstrated viral persistence in different tissues such as bone marrow, skin, synovium, liver, brain, and myocardium in immunocompetent, symptomatic and non-symptomatic individuals [4, 11, 25]. However, data on the serostatus of these patients are scarce and sufficient control populations were rarely included in any of these studies.
We studied the persistence of PVB19 DNA in heart, bone marrow, liver and kidney tissues at autopsy of patients who died of causes other than myocarditis or cardiomyopathy. We also analyzed the sera of these patients for a correlation with serostatus, as there are limited data on the seroprevalence of PVB19. We found seropositivity of 60 % (42/70) for PVB19 IgG antibody. This falls in the range of seroprevalence rates of various population-based studies done in India as well as across the globe [1, 22]. The seroprevalence/positivity rate of PVB19 IgG antibody in adults ranged from a minimum of 37.3 % seen in Malaysia [20] to a maximum of 72 % reported in Germany [22]. However, a study by Abraham et al. from South India found a seroprevalence rate of 70 % among 226 patients [1]. The relatively low seropositivity in our study may be attributed to the small sample size (n = 70) as compared to the previous studies. The variation in the seropositivity status in our study and the previous studies from different geographical regions may be due to the usage of different commercial ELISA kits for PVB19 IgG detection. We used a enzyme-linked immunosorbent assay kit (Novatec, GmBH), which utilizes parvovirus B19 recombinant antigen for detecting virus-specific IgG, whereas PVB19 IgG ELISA kits used in previous studies have employed VP1-VP2 or NS1 epitopes to determine seropositivity [1, 20, 22]. Thus, the different PVB19 antigens used in the ELISA kits and their sensitivity and specificity might be responsible for differences in seropositivity rates in different studies.
We observed that the degree of persistence of PVB19 DNA in the myocardium of asymptomatic individuals was not significant. Also there was no significant relationship between PVB19 DNA persistence and age, sex, underlying disease, or PVB19 serostatus. There is only one autopsy-based study [23] that showed a significant difference in the persistence of PVB19 DNA between SP and SN cases. In that study, the investigators found high persistence of PVB19 DNA in SP cases (46/48; 96 %) compared to SN cases (0/21). We carried out nested PCR in 25 cases from the SP group and 10 from the SN group in myocardial tissue. Of these 35 cases, PVB19 DNA was detected in 9 (36 %) of the SP group and in 3 (30 %) of the SN group, which was much lower than what has been reported previously. The difference between the SP and SN groups was not statistically significant. This could be due to a variation in the sample size and epidemiological distribution of the virus. Similar to the previous studies, we amplified the NS1 region of PVB19, which is a conserved region and hence widely used for PVB19 genome detection [3, 6, 9, 19]. Limited genetic variation has been reported in PVB19 strains, and therefore some strains may not be detected by commercial PCR assays [5].
The persistence of PVB19 DNA reported in the literature ranges from 15 % to 85 % in cases of disease and 0 % to 40 % in controls. There are a few studies [2, 14, 15, 21] in which control groups were included to study the persistence of PVB19 DNA in patients with normal LV systolic function and arterial hypertension. The persistence of myocardial PVB19 DNA varied from 7 % to 40 % and 57 %, respectively, in various studies [2, 14, 21]. In comparison, we found 34.3 % PVB19 DNA persistence in our study, which was higher than in other studies and comparable to the persistence in healthy controls i.e. 0-40 %. The difference in persistence of the PVB19 genome in the myocardium could be due to the varying sensitivity limit of the PCR, influenced by the different primers used or due to differences in PVB19 persistence in different populations. In addition, the methods used for detection were also different, with varying range of sensitivity and specificity, and the serostatus of the patients or controls was also not studied. This could be a significant pitfall for studying viral persistence, as PVB19 has been shown to cause persistent infection even in asymptomatic individuals.
There are several reports and case series of persistent PVB19 infection in the bone marrow of immunodeficient patients. The persistence of PVB19 DNA in bone marrow of asymptomatic patients has ranged from 0 %-57 % to 10 %-20 % in different studies. We found 20 % (4/20) PVB19 DNA persistence in bone marrow samples, including one (10 %) in the SP group and three (30 %) in the SN group. This is in accordance with most of the studies done in asymptomatic and immunocompetent patients. We also observed that three of the four patients who were positive for the PVB19 genome in the bone marrow had an underlying disorder capable of causing secondary immunodeficiency. The immunodeficiency in these patients was further established by the fact that these patients were SN, indicating a lack of humoral immunity. Thus, it is suggested that PVB19 DNA does persist in bone marrow of immunodeficient patients, but persistence in immunocompetent individuals is not seen. However, this cannot be confirmed due to the small sample size.
Recently, a possible role of parvovirus B19 in the development of acute hepatitis [8] and fulminant liver failure of otherwise unknown etiology has been described, with a possible negative effect of PVB19 co-infection in cases of chronic hepatitis B and C [24]. The persistence of PVB19 DNA has been reported to be 27 % to 68 % in cases of acute hepatitis, fulminant hepatic failure, and end-stage liver disease in these studies. However, studies regarding the persistence of PVB19 in liver of asymptomatic individuals are limited. An autopsy-based study by Eis-Hubinger et al. found a high level of PVB19 persistence in liver samples from asymptomatic patients, with a persistence rate of 23.5 % (4/17) [8]. Similarly, a study by Schneider et al. showed a higher level of PVB19 persistence in liver of healthy adults (59/87; 67 %) [24]. We found a low persistence of PVB19 DNA (3/35; 8.6 %) in liver tissue samples, which is in contrast to previous observations. This again could be due to the epidemiological difference in PVB19 infection between the different populations and also needs to be confirmed in a larger study.
Our findings showed that the persistence of PVB19 in kidney samples was low. PVB19 DNA was detected in only two out of 20 samples, one from the SP group and one from the SN group. However, Moudgil et al. have reported parvovirus B19 DNA in 25.9 % of normal renal biopsy samples [18].
In addition, we found the simultaneous presence of PVB19 DNA in liver samples, which may be indicative of a recent PVB19 infection rather than viral persistence. Also, in another two cases there was simultaneous presence of PVB19 DNA in myocardium and bone marrow. This simultaneous presence of PVB19 DNA in more than one tissue in a single case is quite rare and is strongly against the hypothesis of high persistence of PVB19 DNA in asymptomatic individuals. Since there was no simultaneous presence of PVB19 DNA in any tissues samples from the SP group, this statement becomes untrue. Besides, PVB19 IgM antibody assays were not done in our study to test for acute infection. Thus, there is a chance that, in a few cases, the presence of viral DNA was due to recent viral infection rather than the persistence of the viral genome in the respective tissues.
In conclusion, our results showed increasing seropositivity of PVB19 IgG antibody with advancing age, indicting increased exposure to the virus with age.
The overall persistence of PVB19 DNA in asymptomatic individuals ranged from 8.0 % to 36 % in the seropositive group and 10 % to 30 % in the seronegative group. This difference was not significant, and the PVB19 serostatus did not influence the persistence of PVB19 DNA in various tissues. We also suggest that the presence of PVB19 DNA in tissues of patients has to be correlated with the clinical symptoms of the patients before they receive any treatment.
A study with a larger number of samples needs to be carried out to elucidate the role of PVB19 in various diseases.
Conflict of interest
The authors declare that they have no competing interests.
Contributor Information
Uma Nahar Saikia, Email: umasaikia@gmail.com.
Baijayantimala Mishra, Email: bm_mishra@hotmail.com.
References
- 1.Abraham M, Rudraraju R, Kannangai R, George K, Cherian T, Daniel D, Ramalingam S, Sridharan G. A pilot study on the seroprevalence of parvovirus PVB19 infection. Indian J Med Res. 2002;115:139–143. [PubMed] [Google Scholar]
- 2.Andersson B, Lindberg E, Lindeqvist JA, Nilsson F, Magnusson Y, Karason K. Parvovirus is a common finding in diseased and healthy myocardium. Eur Heart J. 2008;29:148–152. doi: 10.1093/eurheartj/ehn189. [DOI] [Google Scholar]
- 3.Bal A, Mishra B, Singh N, Das A, Jindal SK. Fulminant parvovirus B19 associated with haemophagocytic lympho-histiocytosis in an immunocompetent adult. APMIS. 2009;117:773–777. doi: 10.1111/j.1600-0463.2009.02528.x. [DOI] [PubMed] [Google Scholar]
- 4.Cassinotti P, Bas S, Siegl G, Vischer TL. Association between human parvovirus PVB19 infection and arthritis. Ann Rheum Dis. 1995;54:498–500. doi: 10.1136/ard.54.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen BJ, Gandhi J, Clewley JP. Genetic variants of parvovirus B19 identified in the United Kingdom: implications for diagnostic testing. J Clin Virol. 2006;36:152–155. doi: 10.1016/j.jcv.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Corcicolli F, Zackerzewska K, Rinieri A, Fanci R. Tissue persistence of parvovirus PVB19 genotypes in asymptomatic patients. J Med Virol. 2008;80:2005–2011. doi: 10.1002/jmv.21289. [DOI] [PubMed] [Google Scholar]
- 7.Cossart YE, Field AM, Cant B, Widdows D. Parvovirus like particles in human sera. Lancet. 1975;1:72–73. doi: 10.1016/S0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- 8.Dina J, Villedieu F, Labombarda F, Freymuth F, de la Gastine G, Jokic M, Vabret A. Childhood myocarditis and parvovirus B19 genotypes. J Clin Virol. 2010;50:61–64. doi: 10.1016/j.jcv.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Eis-Hubinger AM, Reber U, Abdul-Nour T, Glatzel U, Lauschke H, Putz U. Evidence for persistence of parvovirus PVB19 DNA in livers of adults. J Med Virol. 2001;65:395–401. doi: 10.1002/jmv.2047. [DOI] [PubMed] [Google Scholar]
- 10.Hillingso JG, Jensen IP, Tom-Petersen L. Parvovirus PVB19 and acute hepatitis in adults. Lancet. 1998;351:355–356. doi: 10.1016/S0140-6736(05)60609-5. [DOI] [PubMed] [Google Scholar]
- 11.Ho JK, Tha SP, Coupland R, Dalal BI, Bowie WR, Sreenivasan GM, Krajden M, Yoshida EM. Parvovirus PVB19 in an immunocompetent adult patient with acute liver failure: an underdiagnosed cause of acute non-A-E viral hepatitis. Can J Gastroenterol. 2005;19:161–162. doi: 10.1155/2005/853947. [DOI] [PubMed] [Google Scholar]
- 12.Kuhl U, Paushiinger M, Seebeg B, Lassner D, Noutsias M, Poller W, Schultheiss HP. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 13.Landolsi H, Yacoubi MT, Bouslama M. Detection of human Parvovirus B19 in non-immune hydrops fetalis using immunohistochemistry and nested PCR in formalin-fixed and paraffin-embedded placenta and fetal tissues. Pathologie Biologie. 2009;57:e1–e7. doi: 10.1016/j.patbio.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Langnas AN, Markin RS, Cattral MS, Naides SJ. Parvovirus PVB19 as a possible causative agent of fulminant liver failure and associated aplastic anemia. Hepatology. 1995;22:1661–1665. [PubMed] [Google Scholar]
- 15.Lotze U, Egerer R, Tresselt C, Gluck B, Dannberg G, Stelzner A, Figulla HR. Frequent detection of parvovirus PVB19 genome in the myocardium of adult patients with idiopathic DCM. Med Microbiol Immunol. 2004;193:75–82. doi: 10.1007/s00430-003-0211-0. [DOI] [PubMed] [Google Scholar]
- 16.Lotze U, Egerer R, Gluck B, Zell R, Sigusch H, Erhardt C. Low level of myocardial parvovirus PVB19 persistence is a frequent finding in patients with heart disease but related to ongoing myocardial injury. J Med Virol. 2010;82:1449–1457. doi: 10.1002/jmv.21821. [DOI] [PubMed] [Google Scholar]
- 17.Mishra B, Malhotra P, Ratho RK, Singh MP, Varma S, Varma N. Human Parvovirus B 19 in patients with aplastic anemia. Am J Haematol. 2005;79:166–167. doi: 10.1002/ajh.20347. [DOI] [PubMed] [Google Scholar]
- 18.Modrow S, Dorsch S. Antibody responses in parvovirus PVB19 infected patients. Pathol Biol. 2002;50:326–331. doi: 10.1016/S0369-8114(02)00302-4. [DOI] [PubMed] [Google Scholar]
- 19.Moudgil A, Nast CC, Bagga A, Wei L, Nurmamet A, Cohen AH, Jordan SC, Toyoda M. Association of parvovirus PVB19 infection with idiopathic collapsing glomerulopathy. Kidney Int. 2001;59:2126–2133. doi: 10.1046/j.1523-1755.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 20.Naides SJ, Karetnyi YV, Cooling LL, Mark RS, Langnas AN. Human parvovirus PVB19 infection and hepatitis. Lancet. 1996;347:1563–1564. doi: 10.1016/S0140-6736(96)90720-5. [DOI] [PubMed] [Google Scholar]
- 21.Ooi L, Hooi S, Chua BH, Lam SK, Chua KB. Seroprevalence of human parvovirus PVB19 infection in an urban population in Malaysia. Med J Malaysia. 2005;57:97–103. [PubMed] [Google Scholar]
- 22.Pankuweit S, Lamparter S, Schoppet M, Maisch B. Parvovirus PVB19 genome in EMB specimen. Circulation. 2004;109:179–182. doi: 10.1161/01.CIR.0000124881.00415.59. [DOI] [PubMed] [Google Scholar]
- 23.Rohrer C, Gartner B, Sauerbrei A, Böhm S, Hottenträger B, Raab U, Thierfelder W, Wutzler P, Modrow S. Seroprevalence of parvovirus PVB19 in the German population. Epidemiol Infect. 2008;136:1564–1575. doi: 10.1017/S0950268807009958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenk T, Enders M, Pollak S, Hahn R, Huzley D. High prevalence of human parvovirus PVB19 DNA in myocardial autopsy samples from subjects without myocarditis or DCM. J Clin Microbiol. 2009;47:106–110. doi: 10.1128/JCM.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider B, Hone A, Tolba RH, Fischer HP, Blümel J, Eis-Hübinger AM. Simultaneous persistence of multiple genome variants of human parvovirus PVB19. J Gen Virol. 2008;89:164–176. doi: 10.1099/vir.0.83053-0. [DOI] [PubMed] [Google Scholar]
- 26.Soderlund M, Von Essen R, Haapasaari J, Kiistala U. Persistence of parvovirus PVB19 DNA in synovial membranes of young patients with and without chronic arthropathy. Lancet. 1997;349:1063–1065. doi: 10.1016/S0140-6736(96)09110-6. [DOI] [PubMed] [Google Scholar]
- 27.Takahasi T, Ozawa K, Takahasi K, Asano S, Takatu F. Susceptibility of human erythropoietic cells to PVB19 parvovirus in vitro increases with differentiation. Blood. 1990;75:603–610. [PubMed] [Google Scholar]
- 28.Tanawattanacharoen S, Falk RJ, Jennette JC, Kopp JB. Parvovirus PVB19 DNA in kidney tissue of patients with focal segmental glomerulosclerosis. Am J Kidney Dis. 2000;35:1166–1174. doi: 10.1016/S0272-6386(00)70055-2. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Heim A, Schlaphoff V. Intra hepatic long time persistence Parvovirus B 19 and its role in chronic viral hepatitis. J Med Virol. 2009;81:2079–2088. doi: 10.1002/jmv.21638. [DOI] [PubMed] [Google Scholar]
- 30.Wong S, Young NS, Brown KE. Prevalence of parvovirus PVB19 in liver tissue: no association with fulminant hepatitis or hepatitis- associated aplastic anemia. J Infect Dis. 2003;187:1581–1586. doi: 10.1086/374781. [DOI] [PubMed] [Google Scholar]
- 31.Yoto Y, Kudoh T, Haseyama K, Suzuki N, Chiba S. Human parvovirus PVB19 infection associated with acute hepatitis. Lancet. 1996;347:868–869. doi: 10.1016/S0140-6736(96)91348-3. [DOI] [PubMed] [Google Scholar]


