Abstract
Introduction
Surfactant protein D (SP-D) plays an important role in the innate responses against pathogens and its production is altered in lung disorders.
Methods
We studied the circulating levels of SP-D in 37 patients with acute respiratory distress syndrome due to the A/H1N1 virus infection and in 40 healthy controls. Cox logistic regression models were constructed to explore the association of SP-D levels and risk of death.
Results
Mortality rate after a 28-day was 32.42 %. Significant higher levels of SP-D were detected in A/H1N1 patients with fatal outcome (p < 0.05). After adjusting for confounding variables, levels of SP-D ≥250 ng/mL were associated with increased the risk of death (HR = 8.27, 95 % CI 1.1–64.1, p = 0.043).
Conclusions
Our results revealed that higher circulating levels of SP-D are associated with higher mortality risk in critically ill A/H1N1 patients. SP-D might be a predictive factor of poor outcomes in viral pneumonia.
Keywords: Surfactant protein D (SP-D), A/H1N1 virus, Influenza, ARDS, Mortality, Biomarker
Introduction
Host factors including cardiac, respiratory, and metabolic comorbidities are associated with poorer outcomes of A/H1NI infection and development of acute respiratory distress syndrome (ARDS) [1–3]. Recent studies support the hypothesis that a dysregulated immune activation is a key factor that determines the development of severe pneumonia [4–6].
Surfactant protein D (SP-D) is synthesized by type II pneumocytes, belongs to the collectin family and its main function is to recognize pathogen associated molecular patterns allowing microbial elimination. SP-D participates in neutralization and clearance of influenza viruses given its molecular affinity to viral hemagglutinin [7, 8]. SP-D levels correlates with pro-inflammatory immune responses, mainly when alveolar macrophages interact with the trimeric form trough their CD91 receptor, leading the activation of p38 MAPK signaling pathway to elicit Th1 responses [8]. SP-D production is influenced by diverse lung disorders [9, 10], and its role as a biomarker of lung inflammation has been described [11, 12]. Based on the central role of SP-D in the pulmonary host defense and in the regulation of inflammatory responses and its dysregulation in lung diseases, we hypothesize that circulating levels of SP-D are modified as a result of lung tissue damage in critically ill A/H1N1-infected patients, and that SP-D is a useful biomarker to predict poor outcomes in ARDS patients with A/H1N1 infection.
We aimed to determine if circulating levels of SP-D correlates with the risk of mortality after a 28-day follow-up in critically ill patients with A/H1N1 infection.
Materials and Methods
Patients
A group of 37 patients with ARDS associated to pandemic A/H1N1 virus infection was included. Patients were hospitalized at the intensive care unit (ICU) of the National Institute for Respiratory Diseases (INER) in Mexico City during the A/H1N1 virus outbreak in April 2009. Our Institute is a reference center for emerging respiratory diseases in Mexico. In the outbreak of influenza in April 2009, the Medical and Research staff was early involved in the description of clinical characteristics of patients with severe respiratory illness associated to the novel A/H1N1 virus. The infrastructure of the ICU consists in 15 beds for critical patients and 230–250 patients with different respiratory diseases including COPD, HIV, TB, pneumonia, and influenza-like disease are admitted annually. The overall mortality per year in critically ill patients at the ICU is around 25–32 %.
Diagnosis of A/H1N1 infection was assessed by the presence of influenza-like symptoms, bilateral pulmonary infiltrates, and a positive test RT-PCR for the A/H1N1 virus. The diagnosis of ARDS was based on standard definitions [13].
A group of 40 asymptomatic household contacts, which were in close contact with A/H1N1 patients but did not developed acute respiratory disease, was recruited. Importantly, 76.5 % of the contacts exhibited significant titers of anti-A/H1N1 antibodies (1:16), supporting the fact that they were in contact with the A/H1N1 virus.
The Institutional Review Board of the INER reviewed and approved the study. All subjects or their legally authorized relatives provided written informed consent.
A/H1N1 Virus Detection
Nasopharyngeal samples were obtained and viral RNA was isolated using the viral RNA-mini kit (Qiagen Westburg, Leusden, The Netherlands). Detection of A/H1N1 virus was assessed by real time RT-PCR.
SP-D Levels Determination
Circulating levels of SP-D were measured by ELISA (BioVendor Research Products ELISA kit, Asherville, NC, USA). Briefly, 100 μL of serum or standards were incubated during 1 h with 100 μL of anti-SP-D antibody. After three washes, 100 μL of HRP solution was added and incubated by 1 h. One-hundred microliters of TMB substrate was added to each well and reaction was stopped after 15 min by adding 100 μL of stop solution. Absorbance at 450 nm was measured with a microplate ELISA reader (Bio-Rad Laboratories, Inc., CA, USA).
Anti-A/H1N1 Antibody Titers
The titers of serum anti-A/H1N1 antibodies were measured using the hemagglutination inhibition (HAI) assay. Briefly, serially diluted serum samples (25 mL) were mixed with 25 mL of the A/H1N1 virus strain. The serum/virus dilutions were incubated for 30 min at room temperature. Fifty milliliters of 0.5 % chicken erythrocytes were added and after 30 min, HAI activity was evaluated. Serum antibody titers were established as the reciprocal of the last serum dilution with no hemagglutination activity and titers greater than 1:16 were considered positive.
Statistical Analysis
Demographic characteristics, vital signs, clinical outcomes, treatments, mechanical ventilation and gas exchange parameters, laboratory tests, and APACHE II scores were determined at the admission to the ICU. Continuous variables were compared using the Student’s t test for variables with normal distribution and the Mann–Whitney U test otherwise. Categorical variables were analyzed using χ 2 test. Cox’s multivariate proportional hazard models were constructed to explore the factors associated with risk of death. The independent variables tested in the models were demographic and anthropometric data, lung function, laboratory findings, and comorbid conditions.
We chose the cut-off of 250 ng/mL to determine the two groups for survival analysis. This cut-off was arbitrarily selected after a extensive review of the literature in which we found that in general serum levels of SP-D in healthy volunteers is typically lower than 100 ng/mL. In addition, in certain pathological conditions such as idiopathic pulmonary fibrosis, COPD, and interstitial pneumonia serum mean levels of SP-D are usually higher than 300 ng/mL [14]. The correlation between SP-D levels and BMI was assessed using Spearman’s correlation coefficient.
The significance level was set at two-tailed p < 0.05, and at p < 0.01 in case of multiple comparisons. All analyses were performed using Stata v.10.0 (StataCorp, College Station, TX, USA).
Results
The clinical and demographic characteristics of the patients are presented in Table 1.
Table 1.
Demographic characteristics, clinical features, and serum SP-D values of the study population at study entry
| Variable | All patients, n = 37 | Non-survivors, n = 12 | Survivors, n = 25 | p a Value |
|---|---|---|---|---|
| Age, years (IQR25–75) | 40 (29–52) | 43.5 (28.5–48.5) | 39 (29–52) | 0.90 |
| Male, n (%) | 23 (62) | 9 (75) | 14 (56) | 0.35 |
| Body mass index (IQR25–75) | 29.3 (27.1–33.3) | 31.1 (27.2–35.2) | 28.9 (26.9–32.3) | 0.45 |
| Smokers, n (%) | 21 (57) | 8 (67) | 13 (52) | 0.68 |
| Symptom onset to admission (IQR25–75) | 7 (2–21) | 5.5 (3–13) | 7 (2–21) | 0.58 |
| Dyspnea onset to discharge or death, days (IQR25–75) | 16 (1–38) | 11 (6–22) | 20 (1–38) | 0.08 |
| Body temperature, °C (IQR25–75) | 37.1 (36.5–38) | 36.8 (36.2–38.2) | 37.1 (36.8–37.8) | 0.73 |
| Pulse rate, bpm (IQR25–75) | 100 (95–112) | 104 (92–113) | 100 (95–100) | 0.9 |
| Pulse-oximetry, % (IQR25–75) | 76 (69–82) | 71 (68–81) | 78 (70–85) | 0.37 |
| PaO2/FiO2 (IQR25–75) | 190.4 (161.9–248.1) | 186.6 (161.9–214.7) | 202.8 (174.7–253.8) | 0.51 |
| SP-D, ng/mL (IQR25–75) | 434.5 (105–776) | 630 (439–931) | 172 (56–476) | 0.02 |
| APACHE II, score (IQR25–75) | 9 (7–12) | 9 (7–12) | 9.5 (7–12.5) | 0.65 |
| Diabetes mellitus, n (%) | 5 (13.5) | 0 (0) | 5 (20) | 0.16 |
a Comparisons between non-survivors and survivors were performed with Student’s t test for continuous variables with normal distribution and the Mann–Whitney U test otherwise. Categorical variables were analyzed using χ 2 test. Data are shown as medians (IQR 25–75) and percentages
The median age of the studied A/H1N1 09 patients and asymptomatic controls was similar. Twenty-three of the patients were male (62 %) and 57 % (21/37) were current or former smokers. No significant differences were detected in the body mass index (BMI). Eighty-nine percent of the patient’s required invasive mechanical ventilation due to respiratory failure defined as PaO2 value <60 mmHg (normal value at the altitude of Mexico City: 70 ± 3 mmHg) despite FiO2 > 60 % or an increased breathing effort with accessory respiratory muscle usage. The mortality rate after a 28-day follow-up was 32.42 % (12 of 37 patients).
The main signs and symptoms at the start of the illness included fever, myalgias, cough, and headaches, while chest pain, dyspnea, and cyanosis occurred usually after the third day. All patients were treated with oseltamivir (150 mg/day) during the period of required mechanical ventilation. In addition, all patients received ceftriaxone and clarithromycin from the time of admission, and treatment was sustained, while the patients were on mechanical ventilation, but was upgraded in case of fever, if leukocytosis developed again, if new infiltrates on chest X-ray appeared, or if antibiotic resistance was documented in samples from bronchial aspirates. Blood, urine, and bronchial secretion cultures performed at the time of hospitalization in the ICU revealed neither bacterial, fungal, nor mycobacterial secondary infections. Systemic corticosteroids were administered to all patients at a dose of 1 mg/kg/day of methylprednisolone during the mechanical ventilation period. No other clinical or biochemical variable, including the APACHE II score showed significant difference among these subgroups.
Fatal Outcome in Critically Ill A/H1N1 Patients is Associated to Increased Levels of Circulating SP-D
As illustrated in Table 1 and Fig. 1, circulating SP-D values were significantly higher in patients who died (630 ng/mL) when compared to survivors (172 ng/mL, p < 0.02) and to healthy controls (49.5 ng/mL, p < 0.0001). After adjusting for confounding variables including age, gender, and PaO2/FiO2, we found that a serum SP-D concentration ≥250 ng/mL significantly increased the risk of death in the study population (HR = 8.27, 95 % CI 1.1–64.1, p = 0.043). In addition, Kaplan–Meier’s survival estimates were calculated for the referred value. Patients with SP-D concentrations <250 ng/mL showed a 28-day survival estimate of 0.91 (95 % CI 0.51–0.98) meanwhile this probability was 0.38 (95 % CI 0.16–0.61) for the ≥250 ng/mL group, (p < 0.021), Fig. 2. Significant correlations between SP-D levels with BMI were not detected.
Fig. 1.
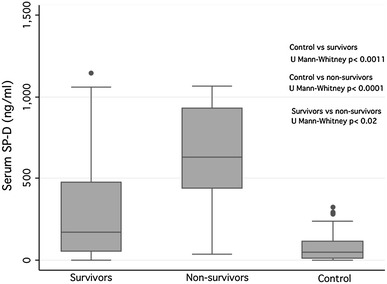
Circulating levels of SP-D in patients with ARDS-A/H1N1 09 infection with fatal outcome, survivors, and controls. A marked increase in the circulating levels of SP-D was observed patients with A/H1N1 09 infection with fatal outcome (non-survivors) when compared to A/H1N1 patients that become recovered (survivors) and asymptomatic controls. Results are shown as medians and interquartile range (IQR25–75). Comparisons among groups were analyzed using the Mann–Whitney U test and p values <0.05 were considered statistically significant
Fig. 2.
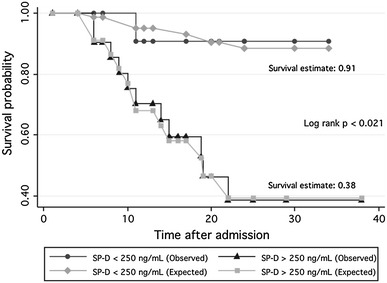
Kaplan–Meier’s survival estimates of all patients with ARDS and A/H1N1 09 infection according to the SP-D circulating levels. Patients with SP-D concentrations <250 ng/mL showed a 28-day survival estimate of 0.91 (95 % CI 0.51–0.98), whereas survival estimate in patients with levels ≥250 ng/mL was 0.38 (95 % CI 0.16–0.61) (p < 0.021)
Discussion
Among its many effects, SP-D has been shown to be a strong chemoattractant for both monocytes and neutrophils with important effects in the pathogenesis of lung disorders [8–10]. In this study, we analyzed the circulating levels of SP-D in a group of patients with severe disease associated to the A/H1N1 virus with and without fatal outcome. The most striking findings were that the patients with fatal outcome displayed a marked increase in the circulating levels of SP-D. After adjustment by age, gender, comorbidities, and APACHE II score, we found that a serum SP-D concentration >250 ng/mL could predict an increased risk of death at 28 days in patients with pneumonia associated to the A/H1N1 virus infection.
SP-D is mainly secreted into the alveoli, thus a rise in serum concentrations may reflect protein translocation caused by the abnormal increase in alveolar-capillary membrane permeability, possibly due to loss of its structural integrity [12, 15]. Serum SP-D has been proposed as a biomarker for acute and chronic respiratory diseases including COPD and IPF in diverse clinical studies [16–18]. These studies support the hypothesis of the potential deleterious effect of the overproduction of SP-D which was directly associated with worse clinical outcomes in lung diseases. Nishida and coworkers [19] reported a non-significant correlation of SP-D and KL-6/MUC1 mucin levels with the clinical progression or poorer outcomes in pediatric A/H1N1 patients. These differences might be explained because pediatric patients had a milder disease and did not require mechanical ventilation, whereas more than 80 % of our patients required mechanical ventilation at the ICU. Interestingly, other studies have described that low levels of SP-D expression in lungs are associated with fatal influenza by A/H1N1 strain [20]. Our findings indicate that serum SP-D levels might be useful to estimate the risk of poorer outcomes in critical patients with severe lung disease associated to A/H1N1.
It is well documented that SP-D is a glycoprotein involved in the regulation of innate immunity and antiviral responses in the lung [9–11]. This protein often translocates to the systemic circulation in clinical conditions in which lung permeability is increased. Different studies have demonstrated that SP-A and SP-D are important biomarkers of IPF where high levels of these surfactant proteins are predictors of mortality [21]. In addition, evidence in large cohorts also supports its importance as a prognostic marker of clinical exacerbations and in COPD progression [18]. Recent reports suggest that the analysis of circulating levels of SP-D is useful as a diagnostic tool in severe sepsis patients with ARDS [22] and to evaluate the progression of lung injury in critically ill patients with mechanical ventilation in whom circulating levels of this protein positively correlates with the lung injury score as a parameter to measure the pathophysiological features of ARDS [23] In this scenario, clinical and experimental studies have demonstrated that the increased plasma SP-D levels could reflect acute alveolar damage and type II cell hyperplasia [24, 25]. Thus, SP-D level appears to be a reliable indicator of alterations in alveolar epithelial permeability because is more hydrophilic than other surfactant proteins such as SP-A that possibly results in a higher capacity to enter to the circulation.
SP-D plays a critical role in the regulation of macrophages, neutrophils, and fibrocytes infiltrates promoting the production of pro-inflammatory and pro-fibrotic cytokines including TGF-β and PDGF-AA [26]. In viral lung infections, SP-D may participate in the recruitment of macrophages contributing to a dysregulated inflammatory response in A/H1N1 critically ill patients. Non-regulated inflammatory responses may exert a deleterious effect in the lung tissue resulting in the development of severe A/H1N1 disease. We and other investigators have reported higher levels of the pro-inflammatory mediators including IL-6, CXCL8, CCL2, and CCL5 in serum and bronchoalveolar lavage from A/H1N1-infected patients with severe pneumonia than those in either the household contacts or healthy controls [4, 27, 28].
Corticosteroid treatment may help to reduce circulating and lung levels of pro-inflammatory mediators. However, recent reports demonstrate that early use of corticosteroid therapy does not reduce mortality of critically ill patients with influenza A/H1N1 virus infection [29, 30]. In contrast, the corticosteroid treatment significantly decreases lung injury and mortality in patients with severe A/H1N1 infection [31] and septic shock [32]. In our study, a dose of 1 mg/kg/day of methylprednisolone was administered to ICU admitted patients during the mechanical ventilation during the outbreak in April 2009. As we mentioned in the results section, a mortality rate of 32.4 % was observed in our patients after 28 days, whereas 54 % of mortality was observed in A/H1N1 patients that received similar doses of steroids after 90 days of follow-up [30]. It is possible that the use of corticosteroid therapy may result in increased susceptibility of secondary bacterial infections due to the pronounced reduction of protective immune mediators [33].
This study has some limitations, including the relatively small number of patients that were studied. Sample size was restricted by the study’s focus on critically ill patients during a short duration outbreak in 2009. Moreover, we choose not to include those patients with influenza-like illness likely associated with A/H1N1 infection but without viral corroboration. A second limitation is our inability to include a replication cohort of patients with ARDS associated to other respiratory viruses different from A/H1N1 strain and third, the lack of experimental assays to understand the possible role of SP-D in the pathogenesis of severe A/H1N1 infection.
In summary, our results indicate that higher circulating levels of SP-D are associated with higher mortality risk in patients with pneumonia due to the A/H1N1 virus. This protein is a potential prognostic biomarker of poorer outcomes of viral pneumonia. Further work is necessary to identify the mechanisms that determine the possible pathogenic effect of high levels of SP-D in infected lung with A/H1N1.
Acknowledgments
The authors thanks to the patients for their participation in this study. This study was supported by a Grant No. 127002 of the “Fondo Sectorial de Investigación en Salud y Seguridad Social” (FOSISSS) from the National Council of Science and Technology of Mexico (CONACYT).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Joaquín Zúñiga and Ivette Buendía-Roldán contributed equally.
Contributor Information
Joaquín Zúñiga, Phone: (5255) 548717 00, Email: joazu@yahoo.com.
Ivette Buendía-Roldán, Phone: 548717 00, Email: ivettebu@yahoo.com.mx.
References
- 1.Meunier I, Pillet S, Simonsen JN, Von Messling V. Influenza pathogenesis: lessons earned from animal studies with H5N1, H1N1 Spanish, and pandemic H1N1 2009 influenza. Crit Care Med. 2010;38:e21–e29. doi: 10.1097/CCM.0b013e3181c8b4d5. [DOI] [PubMed] [Google Scholar]
- 2.Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1), in humans in the United States, 2005–2009. N Engl J Med. 2009;360:2616–2625. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Padilla R, De la Rosa-Zamboni D, Ponce de León S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 4.Bermejo-Martin JF, Martin-Loeches I, Rello J, et al. Host adaptive immunity deficiency in severe pandemic influenza. Crit Care. 2010;14:R167. doi: 10.1186/cc9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee N, Wong CK, Chan PK, et al. Cytokine response patterns in severe pandemic 2009 H1N1 and seasonal influenza among hospitalized adults. PLoS One. 2011;6:e26050. doi: 10.1371/journal.pone.0026050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bautista E, Arcos M, Jimenez-Alvarez L, et al. Angiogenic and inflammatory markers in acute respiratory distress syndrome and renal injury associated to A/H1N1 virus infection. Exp Mol Pathol. 2013;94:486–492. doi: 10.1016/j.yexmp.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Crouch E. Surfactant protein D and pulmonary host defense. Respir Res. 2000;1:93–108. doi: 10.1186/rr19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartshorn K, White M, Tecle T, Sorensen G, Holmskov U, Crouch E. Viral aggregating and opsonizing activity in collectin trimers. Am J Physiol Lung Cell Mol Physiol. 2010;298:L79–L88. doi: 10.1152/ajplung.00223.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo R, Nizet V. Innate barriers against infection and associated disorders. Drug Discov Today. 2008;5:145–152. doi: 10.1016/j.ddmec.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastva A, Wright J, Williams K. Immunomodulatory roles of surfactant proteins A and D. Implications in lung disease. Proc Am Thor Soc. 2007;4:252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene K, Wright J, Steinberg K. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160:1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 12.Winkler C, Atochina-Vasserman E, Holz O, et al. Comprehensive characterisation of pulmonary and serum surfactant protein D in COPD. Respir Res. 2011;12:29. doi: 10.1186/1465-9921-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard GR, Artigas A, Brigham KL, et al. The American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trials coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 14.Honda Y, Kuroki Y, Matsuura E, et al. Pulmonary surfactant protein D in sera and bronchoalveolar lavage fluids. Am J Respir Crit Care Med. 1995;152:1860–1866. doi: 10.1164/ajrccm.152.6.8520747. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Ikegami M, Korfhagen T. Neither SP-A nor NH2-terminal domains of SP-A can substitute for SP-D in regulation of alveolar homeostasis. Am J Physiol Lung Cell Mol Physiol. 2006;291:L181–L190. doi: 10.1152/ajplung.00015.2006. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi H, Kuroki Y, Tanaka H, et al. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am J Respir Crit Care Med. 2000;162:258–263. doi: 10.1164/ajrccm.162.1.9903014. [DOI] [PubMed] [Google Scholar]
- 17.Greene K, King T, Jr, Juroki Y, et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:439–446. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 18.Sin D, Leung R, Gan W, Man S. Circulating surfactant protein D as a potential lung-specific biomarker of health outcomes in COPD: a pilot study. BMC Pulm Med. 2007;7:13. doi: 10.1186/1471-2466-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishida S, Fukazawa R, Imai T, et al. Serum KL-6 and surfactant protein D in children with 2009 pandemic influenza infection. Pediatr Int. 2011;53:910–914. doi: 10.1111/j.1442-200X.2011.03398.x. [DOI] [PubMed] [Google Scholar]
- 20.Boonarkart CH, Suptawiwat O, Uiprasertkul M, et al. A reduced expression of surfactant protein D in the lungs of fatal influenza H1N1 cases in 2009. Acta Virol. 2012;56:253–255. doi: 10.4149/av_2012_03_253. [DOI] [PubMed] [Google Scholar]
- 21.Barlo NP, van Moorsel CH, Ruven HJ, Zanen P, van den Bosch JM, Grutters JC. Surfactant protein-D predicts survival in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vascul Diffus Lung Dis. 2009;26:155–161. [PubMed] [Google Scholar]
- 22.Ware LB, Koyama T, Zhao Z, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17:R253. doi: 10.1186/cc13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Determann RM, Royakkers AA, Haitsma JJ, et al. Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm Med. 2010;10:6. doi: 10.1186/1471-2466-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan T, Nielsen LD, Allen MJ, et al. Serum SP-D is a marker of lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2002;282:L824–L832. doi: 10.1152/ajpcell.00388.2001. [DOI] [PubMed] [Google Scholar]
- 25.Eisner MD, Parsons P, Matthay MA, et al. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58:983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aono Y, Ledford JG, Mukherjee S, et al. Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am J Respir Crit Care Med. 2012;185:525–536. doi: 10.1164/rccm.201103-0561OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zúñiga J, Torres M, Romo J, et al. Inflammatory profiles in severe pneumonia associated with the pandemic influenza A/H1N1 virus isolated in Mexico City. Autoimmunity. 2011;44:562–570. doi: 10.3109/08916934.2011.592885. [DOI] [PubMed] [Google Scholar]
- 28.Ramírez-Martínez G, Cruz-Lagunas A, Jiménez-Alvarez L, et al. Seasonal and pandemic influenza H1N1 viruses induce differential expression of SOCS-1 and RIG-I genes and cytokine/chemokine production in macrophages. Cytokine. 2013;62:151–159. doi: 10.1016/j.cyto.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SH, Hong SB, Yun SC, et al. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. Am J Respir Crit Care Med. 2011;183:1207–1214. doi: 10.1164/rccm.201101-0110OC. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Loeches I, Lisboa T, Rhodes A, et al. Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med. 2011;37:272–283. doi: 10.1007/s00134-010-2078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quispe-Laime AM, Bracco JD, Barberio PA, et al. H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med. 2010;36:33–41. doi: 10.1007/s00134-009-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 33.Li XW, Jiang RM, Guo JZ. Glucocorticoid in the treatment of severe acute respiratory syndrome patients: a preliminary report. Chin J Intern Med. 2003;42:378–381. [PubMed] [Google Scholar]


