Abstract
Background
Split liver transplantation is still discussed controversially. Utilization of split liver grafts has been declining since a change of allocation rules for the second graft abolished incentives for German centres to perform ex situ splits. We therefore analysed our long-term experiences with the first ex situ split liver transplant series worldwide.
Methods
A total of 131 consecutive adult ex situ split liver transplants (01.12.1987–31.12.2010) were analysed retrospectively.
Results
Thirty-day mortality rates and 1- and 3-year patient survival rates were 13, 76.3, and 66.4 %, respectively. One- and three-year graft survival rates were 63.4 and 54.2 %, respectively. The observed 10-year survival rate was 40.6 %. Continuous improvement of survival from era 1 to 3 was observed (each era: 8 years), indicating a learning curve over 24 years of experience. Patient and graft survival were not influenced by different combinations of transplanted segments or types of biliary reconstruction (p > 0.05; Cox regression). Patients transplanted for primary sclerosing cholangitis had better survival (p = 0.021; log-rank), whereas all other indications including acute liver failure (13.6 %), acute and chronic graft failure (9.1 %) had no significant influence on survival (p > 0.05; log-rank). Biliary complications (27.4 %) had no significant influence on patient or graft survival (p > 0.05; log-rank). Hepatic artery thrombosis (13.2 %) had a significant influence on graft survival but not on patient survival (p = 0.002, >0.05, respectively; log-rank).
Conclusions
Split liver transplantation can be used safely and appears to be an underutilized resource that may benefit from liberal allocation of the second graft.
Keywords: Liver Transplantation, Graft Survival, Biliary Complication, Middle Hepatic Vein, Biliary Leakage
Introduction
The first description of a successful split liver transplantation using a left-lateral graft for a paediatric recipient and the remnant extended right graft for an adult recipient was reported by our institution in 1988 [1]. Before the introduction of split liver transplantation, reduced size liver transplantation had been used for paediatric liver transplantation, which resulted in the waste of liver segments 4–8 plus segment 1 [2]. Since 1988, with increasing experience and improvement of the surgical technique, the significant potential of split liver transplantation became apparent, and this modality soon became common practice [3–9]. Therefore, split liver transplantation from deceased donors still has a place in paediatric as well as adult liver transplantation [6–14]. More advanced developments include the full anatomical liver split procedure for transplantation in two smaller adult liver recipients [10–14]. Today, the splitting procedure can be done either in situ during liver procurement in the deceased heart-beating donor or ex situ [7–9]. Both methods are considered to increase the risk for biliary complications [7–9, 14–22]. Biliary complications belong to the most serious morbidities after all types of liver transplantation [18, 19]. Partial liver grafts have a higher incidence of biliary complications as a result of the risks of biliary leakage from the transected liver surface and as a result of the risks of surgical dissection in the hepatic hilum, which may cause injury to the intra- and extrahepatic bile ducts [3–17, 23, 24]. It could be demonstrated that the in situ split of the liver graft in the deceased donor is associated with significantly shorter cold ischemic times compared with the ex situ split procedure [6–9]. Unfortunately, the in situ split approach often is restrained by logistical and technical efforts and lack of expertise as well as financial expenditures, all of which are necessary for its ultimate success [4–11]. In practice, about half of all split liver transplantation procedures in Europe and the United States are done after ex situ split of the liver graft [7–9, 25–28]. Split liver transplantation is still discussed controversially for high-urgency and retransplant cases and some transplant centres still hesitate to use split liver grafts in adults [7–9, 17]. Our goal is to evaluate the long-term results of ex situ split liver transplantation and the influences of complications and their management.
Patients and methods
This is a retrospective, single-centre analysis with ongoing data collection from a university hospital within the Eurotransplant community. Included were all consecutive ex situ split liver transplants performed in adult recipients. Excluded were all combined organ, living-related organ donor, and reduced size liver transplants. We investigated 131 consecutive split liver transplants, including seven acute retransplants (retransplantation within 30 days) and five chronic retransplants in a total of 131 patients [median age 43.8 (range 18–66) years; males n = 52, 39.7 %; females n = 79, 60.3 %]. All transplants were performed between the 01.12.1987 and the 31.12.2010. The posttransplant observational period ended on the 31.12.2012.
Surgical technique
The details of the surgical technique in this series were described previously [1, 17]. The veno/veno bypass was used routinely in all liver transplants until December 1996 and then completely abandoned without any measurable negative effect on outcome. Severe graft congestion and caval outflow complications during the postoperative course were observed in two early cases after reconstruction of the middle hepatic vein. The middle hepatic vein was preserved in all full right grafts and reconstructed in all full left grafts with an autologous venous patch, harvested either from the graft itself or from an iliac vein of the donor (n = 10; segments 1–4 or segments 2–4). The venous outflow was preserved in all cases for segments 5–8 in the presence of large accessory hepatic veins (V6, V7, V8), because right split grafts were always transplanted with the complete vena cava.
Organ preservation
Euro-Collins solution was only used for the first three adult ex situ split liver transplants in era 1 and abandoned completely afterwards.
Immunosuppression
The immunosuppressant regimen changed over time. Cyclosporine, tacrolimus, mycophenolate mofetil, and sirolimus were introduced very early in our centre due to participation in the respective phase I and II clinical trials. Immunosuppression was the same as for whole organ transplantation.
Statistical analysis
Kaplan–Meier analysis, log-rank tests, Cox regression, logistic regression, Mann–Whitney U tests, and the Chi square tests were used where appropriate. For all statistical tests a p < 0.05 was defined as significant. The PASW statistics software version 20.0 (IBM, Somers, NY) was used for statistical analysis.
Results
Patient and graft survival
Mean observed patient survival was 5.7 years [median 5.6 (range 0–19.0) years], and the mean observed graft survival was 4.7 years [median 3.6 (range 0–16.6) years]. The 30-day mortality rate and the 1- and 3-year patient survival rates were 13, 76.3, and 66.4 %, respectively. The 1- and 3-year graft survival rates were 63.4 and 54.2 %, respectively. Thirty patients were retransplanted (retransplant rate 22.9 %). Sixty-four adult ex situ split liver transplants were performed before 31.12.2001 and resulted in an observed long-term patient survival of more than 10 years for 26 patients (observed 10-year survival rate: 40.6 %; mean: 12.9 years; median: 12.6 years; range 10–19 years). Patient and graft survival have improved continuously from era to era (each era: 8 years; p = 0.054, 0.097, respectively; Kaplan–Meier analysis, log-rank test; Figs. 1, 2).
Fig. 1.
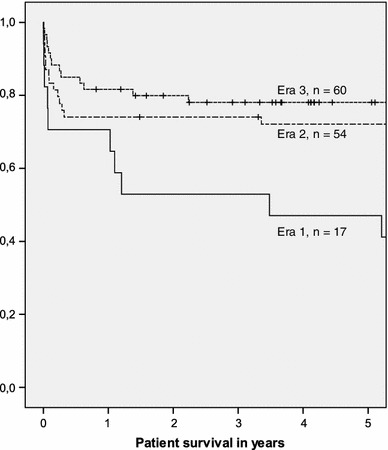
Influence of the era on patient survival (p = 0.054; Kaplan–Meier analysis, log-rank test) (era 1 = 01.01.1987–31.12.1994, era 2 = 01.01.1995–31.12.2002, era 3 = 01.01.2003–31.12.2010). It is interesting to note that the survival has improved continuously from era to era with a growing number of performed transplants
Fig. 2.
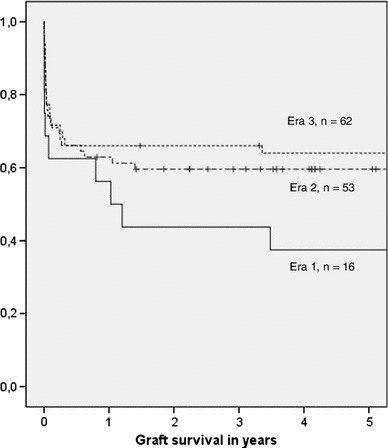
Influence of the era on graft survival (p = 0.097; Kaplan–Meier analysis, log-rank test) (era 1 = 01.01.1987–31.12.1994, era 2 = 01.01.1995–31.12.2002, era 3 = 01.01.2003–31.12.2010). It is interesting to note that graft survival has improved continuously from era to era with a growing number of performed transplants without reaching statistical significance
Influence of the MELD-score
At the time of transplant, the intensive care unit statements of both the donor and recipient were taken into account before transplantation. Retrospective model of end-stage liver disease based on laboratory results (labMELD) was available for 52 of 131 patients (labMELD: mean 14.2, median 12, range 6–40 points). Four of these 52 patients had a labMELD >30 at the time of transplantation with 75 % overall survival versus 79.2 % in patients transplanted with a labMELD <30 (p = 0.867, log-rank).
Influence of the underlying liver disease
Indications for transplantation and causes of death are summarized in Table 1. Patients with acute liver failure as compared to all other indications had no significantly different patient (p = 0.491; Kaplan–Meier, log-rank) or graft survival (p = 0.3; Kaplan–Meier, log-rank). The same was observed for patients with hepatocellular carcinoma, hepatitis B virus-related cirrhosis, and primary or secondary biliary cirrhosis (patient survival p > 0.05, log-rank; graft survival p > 0.05, log-rank). Cases with primary sclerosing cholangitis (PSC) had significantly better patient survival (p = 0.021; Kaplan–Meier, log-rank) compared with all other indications reaching 88 and 80 % patient survival after 1 and 3 years, and 68 and 52 % graft survival after 1 and 3 years, respectively.
Table 1.
Indications for liver transplantation in the study population and the leading causes of death following split liver transplantation
| Indications for split liver transplantation (n = 131 patients) | n | % |
|---|---|---|
| Acute liver failure | 18 | 13.6 |
| Alcoholic cirrhosis | 3 | 2.4 |
| Autoimmune hepatitis | 2 | 1.6 |
| Budd Chiari syndrome | 2 | 1.6 |
| Cryptogenic cirrhosis | 6 | 4.5 |
| Hemangioendothelioma | 1 | 0.8 |
| Metabolic diseases | 3 | 2.4 |
| HBV-related cirrhosis | 11 | 5.3 |
| HCV-related cirrhosis | 7 | 8.3 |
| Hepatocellular carcinoma | 19 | 14.4 |
| Klatskin tumour | 1 | 0.8 |
| Liver adenoma | 3 | 2.4 |
| M. Osler/hemangioma | 1 | 0.8 |
| Neuroendocrine metastases | 2 | 1.6 |
| Primary/secondary biliary cirrhosis | 13 | 9.9 |
| Primary sclerosing cholangitis | 25 | 18.9 |
| Polycystic disease | 2 | 1.6 |
| Retransplantation due to chronic graft failure | 5 | 3.8 |
| Retransplantation due to acute graft failure | 7 | 5.3 |
| Total | 131 | 100 |
| Causes of death (n = 44 fatal long-term outcomes) | n | % |
|---|---|---|
| Cardiovascular event | 3 | 2.3 |
| Cerebral: ischemia | 3 | 2.3 |
| De novo malignancy | 2 | 1.6 |
| Gastrointestinal: bleeding | 1 | 0.8 |
| Gastrointestinal: perforation | 1 | 0.8 |
| Infection: fungal | 2 | 1.6 |
| Infection: sepsis | 11 | 8.3 |
| Intraabdominal bleeding | 3 | 2.3 |
| Liver graft: biliary tract complications | 3 | 2.3 |
| Liver graft: HCV reinfection | 1 | 0.8 |
| Liver graft: PNF | 2 | 1.6 |
| Lung: ARDS | 1 | 0.8 |
| Lung: embolism | 1 | 0.8 |
| Lung: pneumonia | 1 | 0.8 |
| Tumour recurrence | 6 | 4.5 |
| Data not available | 3 | 2.3 |
| Survivors, no death | 87 | 66 |
| Total | 131 | 100 |
HBV hepatitis B, HCV hepatitis C, PNF primary nonfunction, ARDS acute respiratory distress syndrome
Combinations of transplanted segments and types of biliary reconstruction
The combinations of transplanted segments, the types of biliary reconstructions and T-drain usage in this series are summarized in Table 2. It is interesting that the combinations of transplanted segments had no significant influence on patient and graft survival and also not on the later occurrence of biliary complications. The usage of a hepaticojejunostomy (n = 41; 31.3 %) had no statistically significant influence on patient survival (p = 0.26; Kaplan–Meier; log-rank) or graft survival (p = 0.489; Kaplan–Meier; log-rank). The types of biliary reconstruction used (Table 2) had no statistically significant influence on patient survival (p = 0.396; Cox regression analysis), on graft survival (p = 0.138; Cox regression analysis), and the later occurrence of biliary complications (p = 0.251, Chi square). Patient and graft survival were not significantly different for adults who received a full left graft compared with adults who received a full right or an extended right graft (p > 0.05; Cox regression). In nine patients, segment 1 was sacrificed for technical reasons and not retrieved together with segments 4–8.
Table 2.
Transplanted segments, types of biliary reconstruction used, and T-drain usage frequency in this study
| Transplanted segments | n | % |
|---|---|---|
| Not specified | 2 | 1.5 |
| Segments 1–4 | 2 | 1.5 |
| Segments 2–4 | 8 | 6.1 |
| Segments 2+3 | 5 | 3.8 |
| Segments 4–8 | 9 | 6.9 |
| Segments 4–8+1 | 62 | 47.3 |
| Segments 5–8 | 34 | 26 |
| Segments 5–8+1 | 9 | 6.9 |
| Total | 131 | 100 |
| Biliary tract reconstruction | n | % |
|---|---|---|
| Roux-en-Y choledochojejunostomy | 2 | 1.5 |
| End-to-end choledochocholedochostomy | 46 | 35.1 |
| End-to-side choledochocholedochostomy | 2 | 1.5 |
| Side-to-side choledochocholedochostomy | 9 | 6.9 |
| Hepaticocholedochostomy | 9 | 6.9 |
| Hepaticohepaticostomy | 8 | 6.1 |
| Roux-en-Y hepaticojejunostomy | 40 | 30.5 |
| Temporary biliary reconstruction | 8 | 6.1 |
| Secondary biliary reconstruction | 1 | 0.8 |
| Data not available | 6 | 4.6 |
| Total | 131 | 100 |
| T-drain usage | n | % |
|---|---|---|
| Yes | 9 | 6.9 |
| No | 116 | 88.5 |
| Data not available | 6 | 4.5 |
| Total | 131 | 100 |
Biliary complications
Following split-liver transplantation biliary complications occurred in 35 of 128 patients (27.4 %). Late biliary complications were primarily characterised as late anastomotic stenosis alone (n = 4) or combined with early biliary leakage (n = 2) or as progressive ischaemic cholangiopathy (n = 5). Early biliary complications were primarily detected as leakage from the biliary anastomosis (n = 5), biliary leakage from the resection plane (n = 13), biliary leakage from open previously unrecognized central bile ducts (n = 3), or as anastomotic stenosis either alone (n = 1) or combined with biliary leakage (n = 2; Table 3).
Table 3.
Biliary complications, diagnostic methods used to detect them, as well as the time intervals between transplantation and the detection of the complication, the treatment modalities of biliary complications, and the time intervals between liver transplantation and the treatment of biliary complications within the study cohort
| Types of biliary complications (n = 35) | Diagnostic methods used to detect biliary complications | Median days from Tx to diagnosis | Treatment modality for biliary complications | Median days from Tx to treatment of biliary complication |
|---|---|---|---|---|
| Dehiscence of biliary anastomosis (n = 5) |
Intraoperative (n = 2) HBSS (n = 2) ERC (n = 1) |
18 (3–37) |
Reanastomosis of biliary duct (n = 3) ERC with stent (n = 1) Re-LTx (n = 1) |
18 (4–37) |
| Anastomotic stenosis (n = 5) |
Sono (n = 1) ERC/PTCD (n = 4) |
211 (20–522) |
Reanastomosis of biliary duct (n = 1) ERC with stent (n = 3) PTC with stent (n = 1) |
211 (20–522) |
| Biliary leakage from the resection plane (n = 13) |
Intraoperative (n = 6) CT (n = 3) HBSS (n = 2) Sono (n = 1) MRCP (n = 1) |
6 (0–30) |
Suture at the resection plane (n = 8) No specific treatment (n = 2) Interventional drainage (n = 3) |
6 (0–30) |
| Biliary leakage from a central bile duct (n = 3) |
Intraoperative (n = 2) CT (n = 1) |
18 (2–19) |
Suture at the central bile duct followed by reanastomosis of biliary duct (n = 1) Suture at the central bile duct followed by ERC with stent (n = 1) Interventional drainage (CT-guided) followed by Reanastomosis of biliary duct (n = 1) |
Primary treatment 18 (2–19) Secondary treatment 23 (9–40) |
| Progressive ischaemic cholangiopathy (n = 5) |
ERC/PTCD (n = 3) Biopsy (n = 2) |
260 (78–3,436) |
Re-LTx (n = 2) PTC/ERC with stent (n = 2) No specific treatment (n = 1) |
456 (107–3,436) |
| Combined biliary complications: biliary leakage and anastomotic stenosis (n = 4) |
CT (n = 1) HBSS (n = 1) Sono (n = 1) ERC (n = 1) |
Primary diagnosis 15 (6–22) Secondary diagnosis: 1,851 (28–1,869) |
Interventional drainage followed by ERC/PTC with stent (n = 2) Suture at the resection plane followed by reanastomosis of biliary duct (n = 1) Reanastomosis of biliary duct (n = 1) |
Primary treatment 16 (6–22) Secondary treatment 963 (28–1,869) |
Five cases with progressive ischaemic cholangiopathy comprised two cases with ischemic-type biliary lesions (ITBL), two cases with secondary sclerosing cholangitis, and one case with CMV-associated chronic biliary tract destruction
HBSS hepatobiliary sequence scintigraphy, CT computed tomography, ERC endoscopic retrograde cholangiography, PTC percutaneous transhepatic cholangiography, PTCD percutaneous transhepatic cholangio-drainage, MRCP magnetic resonance cholangiopancreatogram
The occurrence of biliary complications had a significant influence on 30-day mortality (p = 0.009, Chi square test) but not on 1- and 3-year patient survival rates (p > 0.05, Chi square test), whereas the individual types of observed biliary complications failed to reach a significant influence on 30-day mortality and 1- and 3-year patient survival (p > 0.05, Chi square test). Central bile duct lesions at the biliary confluence lead to significantly worse long-term patient survival compared with all other types of biliary complications taken together (p = 0.014, log-rank; Table 3). The dissection along the biliary tract should not be close to the wall of the biliary duct so that the fine arterial plexus remains intact and is not damaged. We believe that central bile duct lesions may be the result of extensive dissection in the hepatic hilum, especially in full right and full left splits.
Biliary complications had a significant influence on 1- and 3-year graft survival (p = 0.025 and p = 0.02, respectively, Chi square test), whereas the individual types of observed biliary complications failed to have a statistically significant influence on 1- or 3-year graft survival (p > 0.05, Chi square test; Table 3). The type of biliary complications as well as the chosen treatment modality for biliary complications had no significant influence on patient and graft survival (p > 0.05; Kaplan–Meier, log-rank; Table 4). The majority of patients with biliary complications were treated surgically (57.1 %, n = 20) in all eras, whereas a significant increase in the percentage of interventional treatments for biliary complications was observed from era 1 to era 3 (p = 0.048, Chi square).
Table 4.
Types of biliary complications and their treatment as well as their respective statistical influence on patient and graft survival (Kaplan–Meier analysis with log-rank test)
| Types of biliary complications (n = 35) | No specific treatment (n = 3 in 3 patients) | Interventional treatment (n = 16 in 14 patients) | Surgical treatment (n = 22 in 20 patients) | Influence of the type of biliary complication on patient survival | Influence of the type of biliary complication on graft survival |
|---|---|---|---|---|---|
| Biliary leakage (n = 21) | 2 | 6 | 16 | 0.109 | 0.244 |
| Anastomotic stenosis (n = 5) | – | 4 | 1 | 0.257 | 0.137 |
| Progressive ischaemic cholangiopathy (n = 5) | 1 | 2 | 2 | 0.838 | 0.245 |
| Biliary leakage and anastomotic stenosis (n = 4) | – | 4 | 3 | 0.309 | 0.186 |
| Patients (n) | 3 (9 %) | 14 (40 %) | 20 (57.1 %) | ||
| Influence of the treatment modality on patient survival | 0.935 | 0.776 | 0.284 | n.a. | n.a. |
| Influence of the treatment modality on graft survival | 0.636 | 0.859 | 0.162 | n.a. | n.a. |
Two patients received both interventional and surgical treatment modalities for biliary complications
A large proportion of patients with biliary complications required interventional treatment (40 %, n = 14). A minority of patients with biliary complications, including chronic biliary tract destruction (n = 1) and biliary leakage from the liver resection plane (n = 2), did not receive any specific treatment during follow-up (n = 3; Tables 3, 4). Interestingly, the frequency of biliary complications did not change significantly between the three different eras.
Use of adult ex situ split liver grafts for retransplantation
The 24-year experience with the use of ex situ split liver grafts for retransplantation is summarized in Table 5. A total of 12 retransplants (9.2 %) was performed with ex situ split liver grafts, including seven acute retransplants and five chronic retransplants.
Table 5.
Details of 12 adult split liver retransplants (reLTX) in this series with observed patient and graft survival as well as the indications for the primary liver transplant procedures (LTX) and the retransplant procedures (reLTX) and the time intervals between LTX and reLTX in days
| Recipient sex | Time between LTX and reLTX (days) | Indication for primary LTX | Indication for reLTX | Transplanted segments (reLTX) | Death during the observation period | Patient survival (year) | Graft survival (year) |
|---|---|---|---|---|---|---|---|
| F | 47 | Cryptogenic cirrhosis | Biliary tract complications | 5–8 | Yes | 1.1 | 0.8 |
| F | 37 | PBC | Initial graft non-function | 1–4 | Yes | 9.0 | 0.1 |
| M | 1,159 | HBV HCV-related cirrhosis | Biliary tract complications | 4–8 | Yes | 0.1 | 0.1 |
| F | 10 | PSC | Biliary tract complications | 5–8+1 | Yes | 0.0 | 0.0 |
| F | 7 | HCC | Acute rejection | 5–8+1 | Yes | 0.2 | 0.2 |
| M | 666 | PSC | Chronic graft failure | 4–8+1 | No | 12.1 | 12.1 |
| F | 4,122 | Bylers disease | Chronic graft failure | 4–8+1 | No | 7.5 | 7.5 |
| F | 253 | HBV HDV-related cirrhosis | Biliary tract complications | 5–8+1 | No | 7.2 | 7.2 |
| M | 20 | Budd Chiari syndrome | Hepatic Artery thrombosis | 4–8+1 | No | 7.1 | 7.1 |
| M | 3 | HCC | Hepatic Artery thrombosis | 4–8+1 | Yes | 0.1 | 0.1 |
| M | 5,058 | Caroli syndrome | Chronic graft failure | 5–8 | No | 1.4 | 1.4 |
| M | 2 | Alcoholic cirrhosis | Initial graft nonfunction | 4–8+1 | Yes | 3.5 | 3.5 |
All primary transplants were performed with whole organ grafts
F female, M male, HCC hepatocellular carcinoma, HDV hepatitis D virus, PSC primary sclerosing cholangitis
Hepatic artery thrombosis after transplantation
Hepatic artery thrombosis occurred in a total of 17 of 129 (13.2 %) patients and in 4 of 35 (11.5 %) patients with biliary complications. This difference in frequency did not reach statistical significance (Chi square test: p = 0.654; logistic regression: p = 0.654; Exp(B) = 1.273, 95 % confidence interval 0.443–3.662; Table 6). Patients with biliary leakage demonstrated a lower frequency of hepatic artery thrombosis (2/25, 8 %) compared with the whole cohort (17/129, 13.2 %). Hepatic artery thrombosis was detected in one of eight patients with early or late anastomotic stenosis and in one of five patients with progressive ischemic cholangiopathy during long-term follow-up. The cold ischemic time had a significant influence on the development of hepatic artery thrombosis (p = 0.02; Mann–Whitney U test). This was not the case for the use of arterial interposition grafts (n = 4) as well as the number of intraoperatively transfused units of red blood cells and the number of intraoperatively transfused units fresh-frozen plasma (p > 0.05; Chi square and Mann–Whitney U tests). Hepatic artery thrombosis had a significant influence on graft survival (p = 0.002; Kaplan–Meier, log-rank) but not on patient survival (p > 0.05; Kaplan–Meier, log-rank; Table 6).
Table 6.
Variables, their frequencies in our series, and their statistical influence on the occurrence of biliary complications after split liver transplantation (univariate logistic regression analysis, Chi square test) and on graft and patient survival
| Variables | Influence on biliary complications | Graft survival | Patient survival |
|---|---|---|---|
|
Cold ischemic time (min) Mean 705 min, median 722 min Range 104–1,262 min |
n.s.a | n.s.a | n.s.a |
|
Warm ischemic time (min) Mean 40 min, median 38 min Range 18–112 min |
n.s.a | n.s.a | n.s.a |
| HTK preservation (n = 77) vs. UW preservation (n = 51) | n.s.a | n.s.b | n.s.b |
| Hepatic artery thrombosis yes (n = 17) or no (n = 110) | n.s.a | p = 0.002b | n.s.b |
| Left-lateral graft yes (n = 5) or no (n = 126) | n.s.a | n.s.b | n.s.b |
| Hepaticojejunostomy yes (n = 41) or no (n = 84) | n.s.a | n.s.b | n.s.b |
| Postoperative bleeding complication yes (n = 27) or no (n = 102) | n.s.a | n.s.b | n.s.b |
| Retransplant case yes (n = 12) or no (n = 119) | n.s.a | n.s.a | n.s.a |
|
Units of intraoperatively transfused Red blood cells; mean 7, median 6 range 0–45 |
p = 0.005c Exp(B) = 0.851 (95 % CI 0.761–0.951) |
n.s.a | p = 0.022a |
|
Units of intraoperatively transfused Fresh-frozen plasma; mean 9, median 8 range 0–41 |
p = 0.005c Exp(B) = 0.879 (95 % CI 0.803–0.961) |
n.s.a | n.s.a |
Postoperative portal venous thrombosis did not occur in this series
n.s. not significant, HTK histidine-tryptophan-ketoglutarate organ preservation solution, UW University of Wisconsin organ preservation solution
a1-year survival Chi square test results
bKaplan–Meier analysis with log-rank test results
cLogistic regression analysis
Portal vein thrombosis
Portal vein thrombosis was verified during 8 of 131 split liver transplant procedures leading to significantly worse survival (p = 0.035, log-rank).
Risk factor analysis
Only the number of intraoperatively transfused packs of red blood cells (p = 0.005; Exp(B) = 0.851; 95 % confidence interval 0.761–0.951) and the number of intraoperatively transfused units of fresh-frozen plasma (p = 0.005; Exp(B) = 0.879; 95 % confidence interval 0.803–0.961; logistic regression) demonstrated a statistically significant influence on the occurrence of biliary complications during follow-up after transplantation (Table 6). These results could not be confirmed with the Chi square test. The number of intraoperatively transfused packs of red blood cells had a significant influence on 1-year patient survival (p = 0.022; Chi square), whereas the cold and warm ischemic times did not (Table 6).
Hospital stay and intensive care stay
The absence or presence of biliary complications had no statistically significant influence on the duration of hospital stay (p = 0.059; Kaplan–Meier, log-rank) or the duration of intensive care unit stay (p = 0.893; Kaplan–Meier, log-rank; Fig. 3).
Fig. 3.
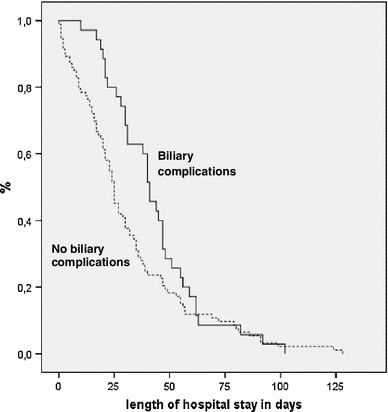
Influence of observed biliary complications after split liver transplantation on hospital stay in days (p = 0.059; Kaplan–Meier analysis, log-rank test)
Discussion
Reports using pooled registry data considered split liver transplantation as an independent predictor of poor patient outcomes for adults and children [29–31], whereas studies from specialised centres demonstrated survival outcomes comparable with whole organ liver transplantation [17, 22, 32–42]. We therefore consider that the long-term results of the first series of adult ex situ split liver transplantation since 1987 will be of interest in times of continued donor organ shortage and resulting deaths on the waiting lists.
Thirty-day mortality in this series appears comparatively high and the overall 1- and 3-year patient survival rates appear comparatively low compared with today’s expectations. As could be demonstrated in Fig. 1, these results were influenced by the era during which the transplants have been performed. In this context, it is very interesting to note that patient survival has improved continuously from era to era. It can be assumed that the development of our series reflects a learning curve since the introduction of split liver transplantation in 1987. The comparatively high retransplant rate (22.9 %) is a result of the very long observational period. Mean graft survival of split liver transplants that were followed by a retransplant procedure during follow-up was 533 (range 1–2,655) days. A significant improvement of graft survival from era 1 to 3 was observed (p = 0.041, log-rank). Taken together, we consider that our results are largely comparable to other recently published series of split liver transplantation from specialised centres within comparable eras [17, 20, 21, 32–41]. It appears noteworthy that this retrospective long-term study is able to demonstrate a mean observed patient survival of 5.7 years (median 5.6 years; range 0–19.0 years) and a mean observed graft survival of 4.7 years (median 3.6 years; range 0–16.6 years) and an observed 10-year survival rate of 40.6 % (mean 12.9 years, median 12.6 years; range 10–19 years) after adult ex situ split liver transplantation. These results underline the long-term value of the concept of adult ex situ split liver transplantation.
We were able to demonstrate earlier with a matched-pair analysis that extended right liver grafts obtained by ex situ split can be used safely for primary and secondary liver transplantation with acceptable biliary morbidity [17]. The possibility that adult ex situ split liver transplantation can be used for retransplantation is important, but as shown in Table 5 long-term results seem quite disappointing. The results of full size liver retransplantation also are disappointing without being significantly better unfortunately.
The present study shows the value of all different segmental combinations in adult ex situ split liver transplantation since its inception. The type of biliary reconstruction, the usage of a hepaticojejunostomy, and the usage of a T-tube all had no significant statistical influence on patient and graft survival and also not on the later occurrence of biliary complications (Table 2). A recently published meta-analysis on the routine use of a T-tube in liver transplantation is in line with our findings [43]. In our experience T tubes can be omitted and are omitted in our current practice of split and full size liver transplantation even though the data presented in our paper does not seem to provide clear evidence that this is the right choice. There is no conclusive evidence available for the influence of different types of biliary tract reconstruction on biliary complications after split liver transplantation or on graft or patient survival due to a complete lack of randomized, controlled trials [15, 17–19, 44]. Interestingly, a recent study including all types of liver transplantation could demonstrate that hepaticojejunostomy was an independent risk factor for the development of hepatic artery thrombosis [45]. We also found a significant influence of hepaticojejunostomy on the development of hepatic artery thrombosis in this series. In our practice, Roux-en-Y biliary reconstruction is limited to cases transplanted for PSC and to cases with destructed central bile ducts (e.g., in retransplant cases) as is the case in full size liver transplantation. The current series contains many patients with primary sclerosing cholangitis because our hepatologists have a research focus and a special clinical interest in this disease.
We believe that it is a progress in surgical technique to abandon the veno/venous bypass. Due to improved surgical skills, the potential advantages of the veno/venous bypass were made redundant.
According to United Network for Organ Sharing (UNOS) data, of the hepatic retransplantations performed between 1996 and 2007, only 8.7 % were done using right or extended right grafts from deceased donors. A small series of five hepatic retransplants using right partial grafts was reported by Gruttadauria et al. [46] in 2009. In our series, the fact whether split liver transplantation was performed as a retransplant or as a primary procedure had no statistically significant influence on patient or graft survival (Table 6). We believe that the results of this study show that adult ex situ split liver transplantation can be used as an option for acute and chronic retransplant cases and can enable long-term survival (Table 5).
Biliary complications are frequently considered the technical “Achilles heel” of liver transplantation because of their high frequency, the need for long-term, repeated treatment, and the potential detrimental effects on graft and patient survival. The incidence of biliary complications in our series (27.4 %) was similar to other published series [15, 47]. The results of this series could not confirm a previous observation of significantly increased mortality caused by biliary complications after adult ex situ split liver transplantation [15].
The results of this study confirm an earlier observation that cold ischemia time is not a major determinant of biliary complications [48]. The use of the histidine-tryptophan-ketoglutarate organ preservation solution (HTK) versus the University of Wisconsin organ preservation solution did not have a significant influence on the occurrence of biliary complications and also not on patient and graft survival (Table 6).
The overall incidence of early and late hepatic artery thrombosis (13.2 %) was lower in our series compared with early reports on paediatric partial liver transplantation (15–25 %) [49] and comparatively higher than other series of all types of liver transplantation (4.9 %) [45] and also compared with more recent reports on paediatric liver transplantation (7.8 %) [50]. Hepatic artery thrombosis had a significant influence on graft survival but not on patient survival. It is interesting to note that the overall incidence of hepatic artery thrombosis after transplantation (n = 17; 13.2 %) was slightly more frequent compared with the incidence of hepatic artery thrombosis (n = 4; 11.8 %) in cases with biliary complications which may be a consequence of early retransplantation after hepatic artery thrombosis before biliary complications could manifest in some cases. It could be demonstrated in an earlier study on hepatic artery thrombosis after all kinds of liver transplantations that cold ischemic time, the use of blood and plasma, and the use of aortic conduits in arterial reconstruction were significantly associated with hepatic artery thrombosis [45]. This association could only be confirmed in this study for cold ischemic time. Although surgical revision used to be the standard treatment for biliary complications after liver transplantation, nonoperative management of biliary complications has more and more become a standard alternative practice over the past two decades in this and other series [47, 51–54]. In this series, surgical and interventional methods were used alone or combined for the treatment of biliary complications, including operative reanastomosis or suture, retransplantation, endoscopic retrograde cholangiography, percutaneous transhepatic cholangiography, endoscopic stent placement, and either computed tomography or ultrasound-guided interventional drainage (Table 3). Interventional treatment versus surgical treatment and the type of biliary complications after adult ex situ split liver transplantation both had no significant influence on patient or graft survival (Table 4).
The type of biliary complications and the chosen treatment modality for biliary complications had no statistically significant influence on patient and graft survival (Table 4). Although these latter statistical results should be interpreted with caution due to the relatively small numbers of patients with different biliary complications and different treatment modalities for these complications it should be noted that they are in line with most published observations on split liver transplantation [4, 6, 7, 9, 14, 16, 17, 20–22, 55].
Case by case evaluation in the present series of cases transplanted after the 01.12.2006 revealed that the survival of patients with a labMELD >30 at the time of split-liver transplantation is not worse as compared to recipients with a labMELD <30. This observation, although based on very small case numbers is in line with a recent study based on the American UNOS data base [33].
Our data is principally in line with the very encouraging results of a recent Italian study which found that split liver transplantation can be successfully performed for two adult recipients including full right and left grafts [56]. A prerequisite for a successful full left and right split liver transplant procedure for two small adults lies in the successful avoidance of a small-for-size liver syndrome. This may be achieved by a split and reconstruction of the middle hepatic vein either for the right lobe graft [56] or like in our series the left lobe graft.
There is a widespread consensus that only liver grafts without extended donor criteria and unquestionable donor organ quality should be considered as potential candidates for the splitting procedure to enable two liver transplants [3–17, 20, 21, 57]. Unfortunately, these organs appear to become rarer and rarer within the Eurotransplant community [58]. Several reports could demonstrate that split liver transplantation has potential equal to that of whole organ liver transplantation not only for adult but also for paediatric recipients [4, 6, 7, 9, 14, 16, 17, 20–22, 39–41, 55]. Merion et al. [29] found that split liver transplantation could provide enough organs to satisfy the entire current demand for paediatric donor livers in the United States and thus provide more aggregate years of life than whole organ transplantation and result in larger numbers of recipients. Encouraging results have been reported from Ghent for adult split liver transplantation with extended right lobe grafts from deceased donors that did not meet the Eurotransplant criteria for optimal donors [59].
It was reported that the quality of donor organs has seen a continuous deterioration in most Eurotransplant countries over the past 10–15 years: 63 % of organs are labelled “sub-optimal” with a donor risk index >1.5 [58].
The graft-to-recipient body weight ratio (GRWR) was not routinely calculated before the splitting procedure in this first series of split liver transplantation. The clinical role of the GRWR in split liver transplantation was only later recognized. Data on the GRWR was available retrospectively for 89 of 131 split liver transplants in this series. The mean GRWR was 1.73 (median 1.67; range 0.85–3.73). It was found previously that a GRWR less than 0.8 does not exclude adult-to-adult right lobe living donor transplantation [57].
Donor livers were selected for the ex situ split procedure on the basis of donor organ availability for paediatric and adult recipients, the clinical urgency of transplantation, and the clinical judgement of donor organ quality. A rationale for exact mathematical benefit calculations for optimal use of available donors is unfortunately not yet available.
During the observational period a total of 297 consecutive ex situ split liver transplants were performed at our centre, 131 adult and 166 paediatric transplants. In 36 of these 131 adult split liver transplants, the other graft was shared with other institutions, and in 95 cases, the other graft also was transplanted at our institution: 7 of these into another adult recipient and the remainder into paediatric recipients. Interestingly, estimated 3- and 10-year survival rates were better for paediatric split liver recipients (78 vs. 72.8 % and 71.8 vs. 56.5 %, Kaplan–Meier) without reaching statistical significance (p = 0.201, log-rank).
At the end of 2005, allocation policies in Germany were changed so that the optional use of the other graft by the same centre was no longer possible. Within the Eurotransplant community, the overall number of transplanted split liver grafts has decreased within recent years. The reasons for this observation may be partially explained by the reported deterioration of donor liver quality and a change in the allocation policy for split liver grafts without a direct incentive for the transplant centre that performs the splitting procedure and no incentive for transplant centres without a paediatric transplant programme.
The increased risk for biliary complications after transplantation of adult ex situ split liver grafts does not render them as irresponsibly unsafe, especially in times of problematic donor organ shortage and resulting deaths on the waiting lists. Biliary complications and their treatment increase morbidity without significantly decreasing patient and graft survival. The low number of full left grafts in this series does not allow definitive conclusions on their influence on the risk of biliary complications or on survival after transplantation.
We are aware that split liver transplantation is limited by some obstacles due to a comparably high rate of biliary and arterial complications, the necessary technical expertise and the requirement of a good graft while a wider use of split liver transplantation would eliminate the shortage of liver grafts for small children. Split liver transplantation is increasingly important in times of decreasing altruistic organ donation in Germany after the recent transplant scandals [60]. Therefore, we believe that split liver transplantation appears to be an underutilized resource that may benefit from a more liberal allocation of the second lobe.
In our experience, the choice of the recipient for split liver transplantation needs to take into account the increased risk of biliary complications with increased posttransplant morbidity, whereas the choice of the graft for an ex situ splitting procedure needs to take into account the absence of macrovesicular steatosis >20 % and a sufficient graft volume with a GRWR that is ideally larger than 0.8. Sufficient technical expertise with split liver transplantation is mandatory for this procedure.
Disclosure
The authors declare that they did not receive any funding for this work. The authors of this manuscript have no conflicts of interest to disclose.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CIT
Cold ischemic time
- CT
Computer tomography
- ERC
Endoscopic retrograde cholangiography
- GRWR
Graft-to-recipient body weight ratio
- HAT
Hepatic artery thrombosis
- HBSS
Hepatobiliary sequence scintigraphy
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- HDV
Hepatitis D virus
- HTK
Histidine–tryptophan–ketoglutarate organ preservation solution
- ICU
Intensive care unit
- labMELD
Model of end-stage liver disease based on laboratory results
- MELD
Model of end-stage liver disease
- MRCP
Magnetic resonance cholangiopancreatogram
- PNF
Primary non-function of the graft
- PSC
Primary sclerosing cholangitis
- PTC
Percutaneous transhepatic cholangiography
- PTCD
Percutaneous transhepatic cholangio-drainage
- UNOS
United Network for Organ Sharing
- UW
University of Wisconsin organ preservation solution
Footnotes
Harald Schrem and Moritz Kleine have contributed equally to this paper.
References
- 1.Pichlmayr R, Ringe B, Gubernatis G, et al. Transplantation of a donor liver to 2 recipients (splitting transplantation)—a new method in the further development of segmental liver transplantation [in German] Langenbecks Arch Chir. 1988;373:127–130. doi: 10.1007/BF01262776. [DOI] [PubMed] [Google Scholar]
- 2.Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;95:367–370. [PubMed] [Google Scholar]
- 3.Busuttil RW, Goss JA. Split liver transplantation. Ann Surg. 1999;229:313–321. doi: 10.1097/00000658-199903000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renz JF, Emond JC, Yersiz H, et al. Split-liver transplantation in the United States: outcomes of a national survey. Ann Surg. 2004;239:172–181. doi: 10.1097/01.sla.0000109150.89438.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirza DF, Achilleos O, Pirenne J, et al. Encouraging results of split-liver transplantation. Br J Surg. 1998;85:494–497. doi: 10.1046/j.1365-2168.1998.00605.x. [DOI] [PubMed] [Google Scholar]
- 6.Yersiz H, Renz JF, Farmer DG, et al. One hundred in situ split-liver transplantations: a single-center experience. Ann Surg. 2003;238:496–505. doi: 10.1097/01.sla.0000089852.29654.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CL, de Villa VH. Split liver transplantation. Asian J Surg. 2002;25(4):285–290. doi: 10.1016/S1015-9584(09)60193-7. [DOI] [PubMed] [Google Scholar]
- 8.Renz JF, Yersiz H, Reichert PR, et al. Split-liver transplantation: a review. Am J Transplant. 2003;3(11):1323–1335. doi: 10.1046/j.1600-6135.2003.00254.x. [DOI] [PubMed] [Google Scholar]
- 9.Emre S, Umman V. Split liver transplantation: an overview. Transplant Proc. 2011;43(3):884–887. doi: 10.1016/j.transproceed.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 10.Rogiers X, Broering D, Topp S, et al. Technical and physiological limits of split liver transplantation in two adults. Acta Chir Belg. 2000;100(6):272–275. [PubMed] [Google Scholar]
- 11.Yersiz H, Renz JF, Hisatake G, et al. Technical and logistical considerations of in situ split-liver transplantation for two adults: part II. Creation of left segment I-IV and right segment V-VIII grafts. Liver Transpl. 2002;8(1):78–81. doi: 10.1053/jlts.2002.31036. [DOI] [PubMed] [Google Scholar]
- 12.Humar A, Khwaja K, Sielaff TD, et al. Technique of split-liver transplant for two adult recipients. Liver Transpl. 2002;8(8):725–729. doi: 10.1053/jlts.2002.34680. [DOI] [PubMed] [Google Scholar]
- 13.Giacomoni A, Lauterio A, Donadon M, et al. Should we still offer split-liver transplantation for two adult recipients? A retrospective study of our experience. Liver Transpl. 2008;14(7):999–1006. doi: 10.1002/lt.21466. [DOI] [PubMed] [Google Scholar]
- 14.Washburn K, Halff G, Mieles L, et al. Split-liver transplantation: results of statewide usage of the right trisegmental graft. Am J Transplant. 2005;5:1652–1659. doi: 10.1111/j.1600-6143.2005.00933.x. [DOI] [PubMed] [Google Scholar]
- 15.Wojcicki M, Silva MA, Jethwa P, et al. Biliary complications following adult right lobe ex vivo split liver transplantation. Liver Transpl. 2006;12:839–844. doi: 10.1002/lt.20729. [DOI] [PubMed] [Google Scholar]
- 16.Corno V, Colledan M, Dezza MC, et al. Extended right split liver graft for primary transplantation in children and adults. Transpl Int. 2006;19:492–499. doi: 10.1111/j.1432-2277.2006.00323.x. [DOI] [PubMed] [Google Scholar]
- 17.Takebe A, Schrem H, Ringe B, et al. Extended right liver grafts obtained by an ex situ split can be used safely for primary and secondary transplantation with acceptable biliary morbidity. Liver Transpl. 2009;15(7):730–737. doi: 10.1002/lt.21745. [DOI] [PubMed] [Google Scholar]
- 18.Greif F, Bronsther OL, Van Thiel DH, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40–45. doi: 10.1097/00000658-199401000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascher A, Neuhaus P. Bile duct complications after liver transplantation. Transpl Int. 2005;18:627–642. doi: 10.1111/j.1432-2277.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 20.Broering DC, Topp S, Schaefer U, et al. Split liver transplantation and risk to the adult recipient: analysis using matched pairs. J Am Coll Surg. 2002;195:648–657. doi: 10.1016/S1072-7515(02)01339-X. [DOI] [PubMed] [Google Scholar]
- 21.Wilms C, Walter J, Kaptein M, et al. Long-term outcome of split liver transplantation using right extended grafts in adulthood: a matched pair analysis. Ann Surg. 2006;244:865–872. doi: 10.1097/01.sla.0000247254.76747.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker NS, Barshes NR, Aloia TA, et al. Analysis of recent pediatric orthotopic liver transplantation outcomes indicates that allograft type is no longer a predictor of survivals. Liver Transpl. 2008;14(8):1125–1132. doi: 10.1002/lt.21491. [DOI] [PubMed] [Google Scholar]
- 23.Kousoulas L, Becker T, Richter N, et al. Living donor liver transplantation: effect of the type of liver graft donation on donor mortality and morbidity. Transpl Int. 2011;24(3):251–258. doi: 10.1111/j.1432-2277.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- 24.Sotiropoulos GC, Radtke A, Molmenti EP, et al. Long-term follow-up after right hepatectomy for adult living donation and attitudes toward the procedure. Ann Surg. 2011;254(5):694–700. doi: 10.1097/SLA.0b013e31823594ae. [DOI] [PubMed] [Google Scholar]
- 25.de Ville de Goyet J. Split liver transplantation in Europe—1988 to 1993. Transplantation. 1995;59:1371–1376. doi: 10.1097/00007890-199505270-00002. [DOI] [PubMed] [Google Scholar]
- 26.Adam R, McMaster P, O’Grady JG, et al. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9:1231–1243. doi: 10.1016/j.lts.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 27.SRTR/OPTN Annual Report 2009 http://www.ustransplant.org. Accessed November 2010
- 28.European Liver Transplant Registry. http://www.eltr.org. Accessed 4 January 2013
- 29.Merion RM, Rush SH, Dykstra DM, et al. Predicted lifetimes for adult and pediatric split liver versus adult whole liver transplant recipients. Am J Transplant. 2004;4(11):1792–1797. doi: 10.1111/j.1600-6143.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- 30.Diamond IR, Fecteau A, Millis JM, et al. Impact of graft type on outcome in pediatric liver transplantation: a report from studies of pediatric liver transplantation (SPLIT) Ann Surg. 2007;246(2):301–310. doi: 10.1097/SLA.0b013e3180caa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adam R, Cailliez V, Majno P, et al. Normalised intrinsic mortality risk in liver transplantation: European Liver Transplant Registry study. Lancet. 2000;356(9230):621–627. doi: 10.1016/S0140-6736(00)02603-9. [DOI] [PubMed] [Google Scholar]
- 32.Vagefi PA, Parekh J, Ascher NL, et al. Outcomes with split liver transplantation in 106 recipients: the University of California, San Francisco, experience from 1993 to 2010. Arch Surg. 2011;146(9):1052–1059. doi: 10.1001/archsurg.2011.218. [DOI] [PubMed] [Google Scholar]
- 33.Nadalin S, Schaffer R, Fruehauf N. Split-liver transplantation in the high-MELD adult patient: are we being too cautious? Transpl Int. 2009;22(7):702–706. doi: 10.1111/j.1432-2277.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 34.Nesher E, Island E, Tryphonopoulos P, et al. Split liver transplantation. Transplant Proc. 2011;43(5):1736–1741. doi: 10.1016/j.transproceed.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 35.Saidi RF, Jabbour N, Li Y, Shah SA, et al. Outcomes in partial liver transplantation: deceased donor split-liver vs. live donor liver transplantation. HPB (Oxford) 2011;13(11):797–801. doi: 10.1111/j.1477-2574.2011.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sepulveda A, Scatton O, Tranchart H, et al. Split liver transplantation using extended right grafts: the natural history of segment 4 and its impact on early postoperative outcomes. Liver Transpl. 2012;18(4):413–422. doi: 10.1002/lt.22479. [DOI] [PubMed] [Google Scholar]
- 37.Viganò L, Laurent A, Tayar C, et al. Outcomes in adult recipients of right-sided liver grafts in split-liver procedures. HPB (Oxford) 2010;12(3):195–203. doi: 10.1111/j.1477-2574.2009.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong JC, Yersiz H, Farmer DG, et al. Long-term outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10-year comparative analysis of 2,988 cases. J Am Coll Surg. 2009;208(5):682–689. doi: 10.1016/j.jamcollsurg.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Cardillo M, De Fazio N, Pedotti P, et al. Split and whole liver transplantation outcomes: a comparative cohort study. Liver Transpl. 2006;12(3):402–410. doi: 10.1002/lt.20720. [DOI] [PubMed] [Google Scholar]
- 40.Humar A, Beissel J, Crotteau S, et al. Whole liver versus split liver versus living donor in the adult recipient: an analysis of outcomes by graft type. Transplantation. 2008;85(10):1420–1424. doi: 10.1097/TP.0b013e31816de1a3. [DOI] [PubMed] [Google Scholar]
- 41.Bonney GK, Aldouri A, Attia M, et al. Outcomes in right liver lobe transplantation: a matched pair analysis. Transpl Int. 2008;21(11):1045–1051. doi: 10.1111/j.1432-2277.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- 42.Hong JC, Yersiz H, Busuttil RW. Where are we today in split liver transplantation? Curr Opin Organ Transplant. 2011;16(3):269–273. doi: 10.1097/MOT.0b013e328346572e. [DOI] [PubMed] [Google Scholar]
- 43.Riediger C, Müller MW, Michalski CW, et al. T-Tube or no T-tube in the reconstruction of the biliary tract during orthotopic liver transplantation: systematic review and meta-analysis. Liver Transpl. 2010;16(6):705–717. doi: 10.1002/lt.22070. [DOI] [PubMed] [Google Scholar]
- 44.Fan ST, Lo CM, Liu CL, et al. Biliary reconstruction and complications of right lobe live donor liver transplantation. Ann Surg. 2002;236(5):676–683. doi: 10.1097/00000658-200211000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva MA, Jambulingam PS, Gunson BK, et al. Hepatic artery thrombosis following orthotopic liver transplantation: a 10-year experience from a single centre in the United Kingdom. Liver Transpl. 2006;12(1):146–151. doi: 10.1002/lt.20566. [DOI] [PubMed] [Google Scholar]
- 46.Gruttadauria S, di Francesco F, Spada M, et al. Different modalities of arterial reconstruction in hepatic retransplantation using right arterial graft. World J Gastroenterol. 2009;15(26):3322–3323. doi: 10.3748/wjg.15.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24(4):379–392. doi: 10.1111/j.1432-2277.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 48.Pirenne J, Van Gelder F, Coosemans W, et al. Type of donor aortic preservation solution and not cold ischemia time is a major determinant of biliary strictures after liver transplantation. Liver Transpl. 2001;7(6):540–545. doi: 10.1053/jlts.2001.24641. [DOI] [PubMed] [Google Scholar]
- 49.Stevens LH, Emond JC, Piper JB, et al. Hepatic artery thrombosis in infants. A comparison of whole livers, reduced-size grafts, and grafts from living-related donors. Transplantation. 1992;53(2):396–399. doi: 10.1097/00007890-199202010-00025. [DOI] [PubMed] [Google Scholar]
- 50.Stringer MD, Marshall MM, Muiesan P, et al. Survival and outcome after hepatic artery thrombosis complicating pediatric liver transplantation. J Pediatr Surg. 2001;36(6):888–891. doi: 10.1053/jpsu.2001.23963. [DOI] [PubMed] [Google Scholar]
- 51.Balderramo D, Sendino O, Burrel M, et al. Risk factors and outcomes of failed endoscopic retrograde cholangiopancreatography in liver transplant recipients with anastomotic biliary strictures: a case-control study. Liver Transpl. 2012;18(4):482–489. doi: 10.1002/lt.23371. [DOI] [PubMed] [Google Scholar]
- 52.Davidson BR, Rai R, Nandy A, et al. Results of choledochojejunostomy in the treatment of biliary complications after liver transplantation in the era of nonsurgical therapies. Liver Transpl. 2000;6(2):201–206. doi: 10.1002/lt.500060215. [DOI] [PubMed] [Google Scholar]
- 53.Righi D, Franchello A, Ricchiuti A, et al. Safety and efficacy of the percutaneous treatment of bile leaks in hepaticojejunostomy or split-liver transplantation without dilatation of the biliary tree. Liver Transpl. 2008;14(5):611–615. doi: 10.1002/lt.21416. [DOI] [PubMed] [Google Scholar]
- 54.Gwon DI, Sung KB, Ko GY, et al. Dual catheter placement technique for treatment of biliary anastomotic strictures after liver transplantation. Liver Transpl. 2011;17(2):159–166. doi: 10.1002/lt.22206. [DOI] [PubMed] [Google Scholar]
- 55.Broering DC, Mueller L, Ganschow R, et al. Is there still a need for living-related liver transplantation in children? Ann Surg. 2001;234:713–721. doi: 10.1097/00000658-200112000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cescon M, Grazi GL, Ravaioli M, et al. Conventional split liver transplantation for two adult recipients: a recent experience in a single European center. Transplantation. 2009;88(9):1117–1122. doi: 10.1097/TP.0b013e3181ba1096. [DOI] [PubMed] [Google Scholar]
- 57.Selzner M, Kashfi A, Cattral MS, et al. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transplant. 2009;15(12):1776–1782. doi: 10.1002/lt.21955. [DOI] [PubMed] [Google Scholar]
- 58.Schlitt HJ, Loss M, Scherer MN, et al. Current developments in liver transplantation in Germany: MELD-based organ allocation and incentives for transplant centres. Z Gastroenterol. 2011;49(1):30–38. doi: 10.1055/s-0029-1245946. [DOI] [PubMed] [Google Scholar]
- 59.Decoster EL, Troisi R, Sainz-Barriga M, et al. Improved results for adult split liver transplantation with extended right lobe grafts: could we enhance its application? Transplant Proc. 2009;41(8):3485–3488. doi: 10.1016/j.transproceed.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Schrem H, Kaltenborn A. Germany: avoid more organ transplant scandals. Nature. 2013;498(7452):37. doi: 10.1038/498037b. [DOI] [PubMed] [Google Scholar]


