Abstract
Background
The surgical approaches to the treatment of bleeding esophageal varices in cirrhotic patients have been reduced since the clinical development of endoscopic sclerotherapy, transjugular intrahepatic portosystemic shunt (TIPS), and liver transplantation. However, when acute sclerotherapy fails, and in cases where no further treatment is accessible, emergency surgery may be life saving. In the present study we retrospectively analyzed the results of the modified Sugiura procedure, performed as emergency and semi-elective treatment in the patient with bleeding esophageal varices.
Methods
Ninety patients with cirrhosis and portal hypertension were managed in our department for variceal esophageal bleeding between January 1985 and December 1992. The modified Sugiura procedure was performed in 46 patients on an emergency (25 patients) or semi-elective (21 patients) basis. Liver cirrhosis stage according to Child classification was A in 4 patients, B in 16 patients, and C in 26 patients.
Results
Acute bleeding was controlled in all patients. Postoperative mortality was 23.9% (11 of 46 patients). The mortality rate was 34.6% in Child class C patients (9 of 26 patients), and 12.5% in Child class B patients (2 of 16 patients). Twenty-four patients had long-term follow-up extending from 14 months to 22 years (mean 83.1 months). Ten of 24 patients (41.6%) did not develop rebleeding for 5–22 years (mean 10.3 years). Overall 5-year survival in these 24 patients was 62.5%.
Conclusions
The modified Sugiura procedure remains an effective rescue therapy for patients with bleeding esophageal varices when alternative treatments fail or are not indicated. Moreover, it can be a life-saving procedure in patients with anatomy unsuitable for shunt surgery or for patients treated in nonspecialized centers where surgical expertise for a shunt operation is not available.
Keywords: Portal Hypertension, Transjugular Intrahepatic Portosystemic Shunt, Esophageal Varix, Portal Vein Thrombosis, Gastric Varix
Introduction
The role of surgery in portal hypertension, particularly in the acute management of active variceal bleeding has decreased since the advent of alternative therapies. Widespread use of invasive endoscopy and TIPS (transjugular intrahepatic portosystemic shunt) placement has put forward a different therapeutic approach to the complications of portal hypertension [1, 2]. Once hemostasis is obtained—with endoscopic sclerotherapy, banding, or TIPS placement—the patient with portal hypertension and history of bleeding esophageal varices may undergo portosystemic shunt procedures, on an elective basis, or liver transplantation in certain cases of liver cirrhosis.
Despite the satisfactory results obtained with emergency endoscopic procedures [3], failure of hemostasis ranges from 10 to 20%, and the mortality rate following a second unsuccessful endoscopic sclerotherapy is approximately 60% without further intervention [4]. In specialized centers the use of TIPS achieves control of bleeding in 90% of these cases, rendering it a useful method in patients who are immediate candidates for liver transplantation, the only therapy that significantly prolongs long-term survival in patients with cirrhosis [2].
However, about 20–30% of patients with refractory variceal bleeding are Child A class and may not require transplantation for many years, and there are cases where TIPS either cannot be performed or is contraindicated [1, 5]. Surgical intervention may thus be necessary, as the only means of controlling bleeding in these patients.
Regarding the choice of surgery, the modified Sugiura procedure (transabdominal gastroesophageal devascularization and esophageal transection) is among the most favored emergency procedures. The low rates of encephalopathy and variceal rebleeding recommend the procedure, although controversies exist regarding its long-term results [6, 7]. The Sugiura procedure is indicated in patients who are not candidates for liver transplantation or TIPS placement, and in whom endoscopic hemostasis has not been achieved, or when portosystemic shunt placement is not feasible. Thus it is best suited to patients with portal hypertension and liver failure falling into categories Child class A and B, or those with extensive thrombosis of the portal, splenic, and superior mesenteric veins [4].
From 1985 to 1992, our department was a referral center for the management of variceal esophageal bleeding. It is noteworthy that during this period endoscopic sclerotherapy in our department was performed with unsatisfactory success rates, and the TIPS procedure was unavailable. Our liver transplantation program was being developed. Surgical management of these patients therefore consisted of placement of a portosystemic shunt or the modified Sugiura procedure.
Our preliminary results with the Sugiura procedure were published early and updated later on [8, 9]. In the present study we present the management of these patients and the further long-term follow up results of the transabdominal modified Sugiura procedure.
Materials and methods
Between January 1985 and December 1992, 90 (63 men and 27 women) patients with cirrhosis and portal hypertension were managed in our department for variceal esophageal bleeding. Mean age was 49 years (range 26–73 years). All patients were initially treated with continuous intravenous infusion of vasopressin (Pitressin) at a rate of 0.1–0.4 U/min, or i.v. somatostatin (Sandostatin) at a rate of 50 μg/h. As soon as the patient was resuscitated, upper gastrointestinal (GI) tract endoscopy was performed in order to establish the diagnosis and to rule out any other possible source of upper GI bleeding. In cases of variceal bleeding, endoscopic emergency sclerotherapy of the esophageal varices followed. Balloon tamponade with a Sengstaken-Blakemore tube was mostly performed in patients with bleeding associated with fundal varices.
Among these 90 patients, 60 underwent surgical treatment for variceal bleeding in an emergency or semi-elective setting. Emergency surgery during active bleeding of varices was carried out in 26 patients. In this subset of patients the modified Sugiura procedure was performed in 25 cases, while 1 patient underwent a conventional thoracoabdominal Sugiura procedure performed in one stage. The remaining 34 patients with initially controlled bleeding underwent a semi-elective surgical procedure. In these patients a full preoperative work-up was performed in order to determine the cause of portal hypertension, including needle biopsy of the liver, abdominal computed tomography, angiography of the celiac axis, or splenoportography. Indications for a surgical intervention in these patients were recurrence of bleeding after two attempts of endoscopic sclerotherapy; the presence of symptomatic hypersplenism; patient noncompliance with regular follow-up or medical treatment, and patient inability to undergo regular surveillance. In this group of patients the surgical procedures performed were modified Sugiura in 22 patients; mesocaval shunt (Drapanas) in 5 patients, and distal splenorenal shunt (Warren) in 8 patients. The Sugiura procedure was performed in those with significant co-morbidities or mild encephalopathy, nonpatency of the portal or splenic veins, previous splenectomy, or the presence of severe hypersplenism requiring splenectomy. All surgical procedures were performed by the same surgeon (D.V.) (Fig. 1).
Fig. 1.
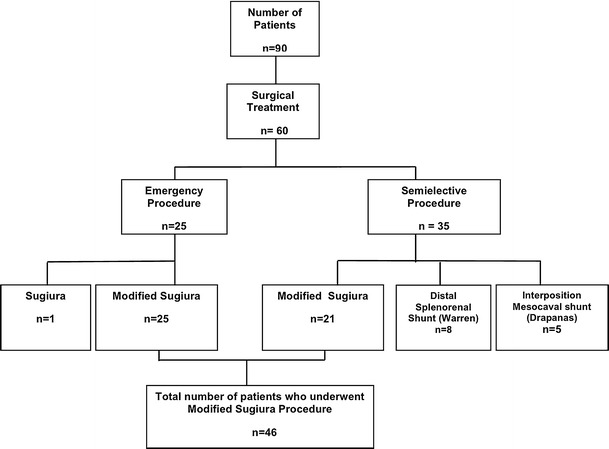
Flow diagram of the management of patients with esophageal bleeding
The modified Sugiura procedure was thus performed in 46 of the 60 patients who underwent surgical treatment on an emergency basis (25 patients) or on a semi-elective basis (21 patients). Mean age of those 46 patients was 49 ± 14 years (range 26–73 years); 31 were men (67%) and 15 were women (33%). Portal hypertension was due to liver cirrhosis in all patients. The cause of cirrhosis was post-viral hepatitis in 31 patients, alcoholic hepatitis in 10, cryptogenic in 3, and autoimmune hepatitis in 2 patients (Table 1). Liver cirrhosis stage according to Child classification was A in 4 patients, B in 16 patients, and C in 26 patients.
Table 1.
Characteristics of the 46 patients who underwent the modified Sugiura procedure
| Gender | |
| Male | 31 (67%) |
| Female | 15 (33%) |
| Age, years (range) | 49 ± 14 (26–73) |
| Cause of portal hypertension | |
| Viral cirrhosis | 31 (67.4%) |
| Hepatitis B virus | 24 |
| Hepatitis C virus | 7 |
| Alcoholic cirrhosis | 10 (21.7%) |
| Cryptogenic cirrhosis | 3 (6.6%) |
| Autoimmune hepatitis | 2 (4.3%) |
The modified Sugiura procedure consisted of complete devascularization of the lesser curvature and the proximal area of the greater curvature of the stomach and transhiatal devascularization of the lower esophagus, 6–10 cm from the gastroesophageal junction. In this step we performed dissection of the short gastric and left gastric vessels, left gastroepiploic vein, coronary vein, as well as the periesophageal collaterals. An EEA (end-to-end anastomosis) stapling device, size 25–28 mm (Autosuture, Covidien, Mansfield, MA), was then introduced into the lower esophagus through an anterior gastrotomy approximately 3 cm from the gastroesophageal junction. An end-to-end anastomosis of the esophagus was performed 2–3 cm above the gastroesophageal junction, allowing simultaneous complete transection of the esophagus. After removal of the stapler device, the lower esophagus was reassessed to confirm the integrity of the anastomosis. In cases of severe hypersplenism or splenic vein thrombosis, or for technical reasons—i.e., enlarged spleen—splenectomy was performed. In emergency cases, liver biopsies were taken during the procedure for histological diagnosis of the liver disease.
After the patient’s discharge from the hospital, a follow-up visit was scheduled every 3 months during the first year, every 6 months for the next 5 years, and once every year thereafter. All patients were scheduled for upper GI endoscopy 6 months after surgery, or sooner, depending on their clinical status.
Results
Perioperative and 30-day postoperative period
The mean operative time was 3.3 h (range 2.5–5.5 h), and the mean intraoperative amount of blood transfused was 3.3 units of PRBC (range 0–12 U). Six patients who underwent the procedure semi-electively did not receive blood transfusions. The mean operative time in the emergency setting was greater than in the semi-elective group (3.6 vs. 2.9 h), as well as the mean amount of packed red blood cells (PRBC) transfused (4.2 vs. 2.3 U). Gastric varices were found in 12 patients (26%) and were oversewn with under running silk suture through gastrotomy of the anterior wall of the stomach. Splenectomy was carried out in 31 patients (67%). Pyloroplasty was performed in 37 patients (80%) in cases where both vagal nerves were sectioned during esophageal devascularization or in patients with gastric ulcer (Table 2).
Table 2.
Intraoperative characteristics of the modified Sugiura procedure
| Total | Emergency | Semielective | |
|---|---|---|---|
| (n = 46) | (n = 25) | (n = 21) | |
| Duration of surgery (h) | 3.4 (2.5–5.5) | 3.6 (3–5.5) | 2.9 (2.5–4.5) |
| Transfused PRBC units/patient | 3.3 (0–12) | 4.2 (0–12) | 2.3 (0–7) |
| Splenectomy | 31 (65%) | 22 | 9 |
| Pyloroplasty | 38 (80%) | 26 | 12 |
| Gastric varices | 12 (25%) | 4 | 8 |
| Mean ICU stay (days) | 8 (2–38) | ||
| Mean hospital stay (days) | 25 (10–56) |
Postoperatively, acute bleeding was controlled in all patients. The mean stay in the surgical intensive care unit (SICU) was 8 days (range 2–38 days). Mean hospital stay was 26 days (range 10–56 days). Early rebleeding developed in seven patients on postoperative day 16 ± 5. In three patients endoscopy revealed grade II residual esophageal varices above the esophageal anastomosis, which were managed with sclerotherapy. One patient with bleeding originating from gastric varices was successfully managed with sclerotherapy. In another case, bleeding was found to be coming from the stapler anastomotic site. In two patients where bleeding presented as melena, endoscopy revealed esophagitis and portal gastropathy, and those conditions were medically managed with H2 blockers or proton pump inhibitors.
Esophageal anastomotic leak occurred in five patients (10.8%), and leak from the pyloroplasty site occurred in one patient. All patients were managed initially with total parenteral nutrition and a computed tomography (CT)-guided percutaneous drainage was performed in three cases. One patient with esophageal leak underwent an exploratory laparotomy and surgical drainage of a subdiaphragmatic collection on the 15th postoperative day. The patient died on the 45th postoperative day after developing sepsis and acute respiratory distress syndrome (ARDS).
Seven patients (six of them Child class C and one Child class B) developed progressive liver failure. Mild hepatic encephalopathy occurred in three of them. One patient presented with portal vein thrombosis and was treated with oral anticoagulants for 4 months. Ascites that was controlled with diuretics appeared in 12 patients (Child class B and C). In two patients with refractory ascites, a LeVeen shunt was placed. Hepatorenal syndrome was diagnosed in three patients, and two patients who developed acute renal failure were treated with hemodialysis. One patient (Child class C) presented with portal vein thrombosis preoperatively and died in the early postoperative period disseminated coagulopathy (DIC). Other complications included respiratory infection—pneumonia in seven patients and pleural effusion in nine patients.
Perioperative and 30-day postoperative mortality was overall 23.9% (11 of 46 patients). The mortality rate was greater (36%) in the subset of patients who had undergone an emergency procedure (9 of 25 patients). Child class C patients who underwent an emergency procedure had a mortality rate of 53%. In the semi-elective group, the mortality rate was 9.5% (2 of 21 patients). Mortality according to Child classification was 34.6% in Child class C (9 of 26 patients), and 12.5% (2 of 16 patients) in Child class B. The cause of death was liver failure in seven patients, coagulopathy (DIC) in one, respiratory failure—acute respiratory distress syndrome (ARDS) in one, and hepatorenal failure in two (Table 3).
Table 3.
Child stage classification and perioperative mortality
| Child stage classification | Emergency procedure (n) | Emergency procedure deaths | Semielective Procedure (n) | Semielective procedures deaths | Total (n) | Total deaths |
|---|---|---|---|---|---|---|
| A | 2 | 0 (0%) | 2 | 0 (0%) | 4 (9%) | 0 (0%) |
| B | 8 | 1 (12.5%) | 8 | 1 (12.5%) | 16 (35%) | 2 (12.5%) |
| C | 15 | 8 (53%) | 11 | 1 (9%) | 26 (56%) | 9 (34.6%) |
| Total | 25 | 9 (36%) | 21 | 2 (9.5%) | 46 | 11 (23.9%) |
Long term follow-up
Surveillance endoscopies performed 6 months after the procedure in the 35 patients who survived revealed mild to moderate anastomotic stricture in 7 patients, grade I or II residual esophageal varices in 8, portal gastropathy in 12, and gastric varices in 3 patients. Four patients with esophageal stricture developed dysphagia and underwent sequential dilatation of the stricture.
During the follow-up period, 11 patients (Child class A: 3, class B: 7 and class C: 1) were excluded from the long-term results in this study because no data could be retrieved. The remaining 24 patients had a long-term follow up from 14 months to 22 years (mean 83.1 months).
Late recurrence of bleeding presented in 7 of 24 patients during a mean period of 6.1 years (range 3.5–11 years). In four cases (16.6%), the source of bleeding was recurrent varices grade II–III and they were managed with sclerotherapy. In 3 patients bleeding was due to hemorrhagic gastritis and portal gastropathy, and all three patients were managed successfully with H2 blockers and proton pump inhibitors. Progressive liver failure and encephalopathy occurred in 9 Child class C patients during a mean period of 4.8 years (range 3–7 years). One Child class C and one Child class B developed hepatocellular carcinoma (HCC) after 4 and 6 years, respectively (Table 4).
Table 4.
Short- and long-term follow-up results of the 46 patients who underwent the modified Sugiura procedure
| Endoscopic findings (6 months postoperation) (n = 36 patients) | Long-term results (14 months–22 years) (n = 24 patients) |
|---|---|
| Recurrence of varices (first and second degree) (n = 8 patients) | Bleeding esophageal varices (n = 4 patients) |
| Hemorrhagic gastritis-portal gastropathy (n = 12 patients) | Hemorrhagic gastritis-portal gastropathy (n = 3 patients) |
| Gastric fundus varices (n = 3 patients) | Hepatic failure (n = 9 patients) |
| Stenosis of the anastomosis (n = 7 patients) | Occurrence of hepatocellular cancer (n = 2 patients) |
| No rebleeding 5 years postoperatively (n = 10 patients) |
The 5-year survival for these 24 patients was 62.5%: 52.9% for Child class C patients, and 83.3% for Child class B patients (Fig. 2a). The overall survival rate for 46 patients including perioperative mortality was 42.8% (32% for Child class C patients, and 44.4% for Child class B patients) (Fig. 2b). There was no Child class C patient with more than 10 years survival. One Child class B patient had a follow-up of 22 years. The cause of death was liver failure in 12 patients, HCC in 2 patients, and recurrence of bleeding in 4 patients. In 6 patients with over 10-year survival, the cause of death could not be retrieved. Considering as an optimum outcome a minimum 5-year survival without recurrence of bleeding, 10 of 24 patients (41.6%) did not present rebleeding for 5–22 years (mean 10.3 years) (Fig. 2).
Fig. 2.
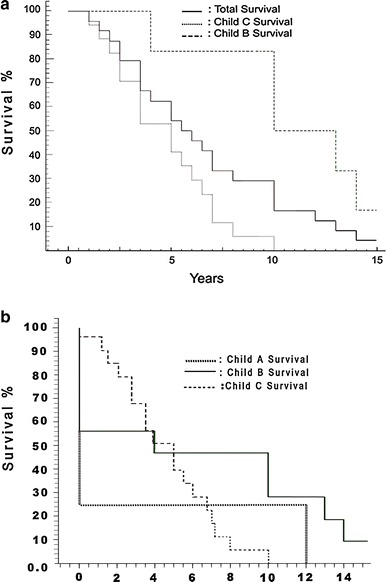
Kaplan–Meier curve showing: a survival of the 24 patients with long-term follow-up (n = 24). The Child class A patient survived for 12 years. b Survival of the 46 patients who underwent the modified Sugiura procedure (n = 46). Intraoperative and in-hospital postoperative deaths are included
Discussion
Variceal bleeding develops in up to one-third of patients with cirrhosis and is associated with an estimated mortality rate of 30–50% during the first episode [4, 10]. Endotherapy (endoscopic band ligation and injection sclerotherapy), combined with pharmacotherapy, remains the first-line treatment and controls bleeding in more than 85% of patients. The early rebleed rate after endoscopic sclerotherapy (EST) and endoscopic variceal band-ligation (EVL) is reported to range from 21 to 52% and from 20 to 33%, respectively [11, 12]. Endoscopic therapy should be considered a failure if hemorrhage cannot be controlled after two sessions [4, 13, 14].
An emergency surgical intervention may be required in patients not scheduled for liver transplantation and when endoscopic and pharmacologic therapies are unsuccessful and TIPS placement is contraindicated, unavailable, or unsuccessful. At present, a surgical shunt procedure is indicated in patients with Child class A cirrhosis, whereas the transjugular intrahepatic portosystemic shunt is indicated in those with Child class B–C cirrhosis scheduled for liver transplantation [4, 15].
As originally described, the nonshunting Sugiura operation includes extensive paraesophagogastric devascularization, esophageal transection, splenectomy, vagotomy, and pyloroplasty, while maintaining the plexus of collaterals that connects the left gastric vein to the azygous system. Separate thoracic and abdominal approaches were performed in one or two stages. By ensuring preservation of portal venous blood flow to the liver, this procedure has advantages over portosystemic shunts, preserving liver function and deterring development of postoperative hepatic encephalopathy. In a Japanese series, the original Sugiura procedure had a rebleeding rate of less than 10% [16]. Several modifications of the original Sugiura procedure have been reported, including left gastric vein ligation, with different results [7, 17–19]. Likely because of a difference in the proportion of patients with alcoholic cirrhosis, these modifications have not been successfully adopted in the Western countries [4, 6, 20].
Being a one-stage abdominal approach, the modified transabdominal Sugiura operation also consists of a simultaneous transection of the distal esophagus with a circular stapler introduced into the esophageal lumen through a gastric incision. Total esophageal transection to interrupt the transmural and paraesophageal varices was firstly performed by Walker [21]; later, Johnston [22] described the use of a stapler device gun in an abdominal esophageal transection. The major advantage of this procedure is that it can be performed by most surgeons. In addition, a devascularization operation may be the only remaining option for sclerotherapy failure with diffuse splanchnic venous thrombosis. Several series have revealed relatively low operative mortality and rebleeding in the acute setting; however, risk of later haemorrhage from gastric or recurrent esophageal varices may be significant [4, 6, 16, 20, 23, 24].
In our patients, 56% of whom had Child class C disease, hospital mortality was 23.9% in emergency and semi-elective settings overall. Patients with Child class C cirrhosis had an overall perioperative mortality of 34.6%, while in emergency settings mortality was 53%. As described by Orozco et al. [17], this can reach 80% in patients with Child class C cirrhosis, contrasting the reported results of this procedure in patients with well-preserved liver function [6, 25]. Overall in Western series, when the operation was performed as an emergency the reported operative mortality varied between 22 and 100% [7, 18, 26–29].
The reported incidence of recurrent varices ranges from 17 to 37% [30–34]. In case of recurrence of esophageal varices, the incidence of rebleeding varies from 30 to 40% [18, 30, 35, 36]. In our series, 4 of 24 patients with long-term follow-up had recurrence of esophageal varices presenting with bleeding, after a mean period of 6.1 years. Ten patients (41.6%), 7 of whom were Child class C, did not present recurrence of bleeding after a mean period of 10.3 years (range 5–22 years) [9].
The significant cause of morbidity associated with this procedure has been attributed to esophageal leak, the incidence of which is reported to be from 6 to 22% with a leak-related mortality rate of 50–60% [7, 18, 37, 38]. Depending upon the recent or long-term sclerotherapy, these patients can have a friable esophagus, which may be the factor that explains the high risk of anastomotic complications. In the present series, esophageal leak from the transection line occurred in 10.8% of patients and was the cause of death in one patient. Johnson et al. [35] reported an equivalent successful result regarding rebleeding rate, omitting the esophageal transection in two-thirds of patients during the modified Sugiura procedure, with lower morbidity in this subset of patients. In addition, several authors suggest that esophageal transection is not essential for the effective control of variceal hemorrhage, avoiding anastomotic leaks in about 20% and death from these leaks in up to 50–66% of all patients [20, 39, 40]. The postoperative encephalopathy rate was 6.5% indicating that devascularization is not associated with a high risk of encephalopathy, which can be as high as 30% in portosystemic shunt procedures and 15% in selective distal splenorenal shunt [31, 41].
The major disadvantage of emergency surgery is that many patients who bleed from portal hypertension are at high operative risk because of their underlying liver disease with poor hepatic reserve. In the emergency settings in Child class C patients, even the use of TIPS is associated with probability of survival rate of 60% at 1 year and less than 50% at 2 years [1, 3, 42, 43]. When TIPS was used as a rescue treatment after sclerotherapy failure, early rebleeding occurred in 16–30% of the patients, and the early mortality amounted to 17–60%, depending on whether the patients received a shunt [3, 44, 45]. Considering that in our series Child class C patients who could tolerate surgery had an overall 5-year survival of 52.9%, the modified Sugiura procedure constitutes a reasonable therapeutic option.
In countries where there is a limited supply of donor organs, liver transplantation is rarely an alternative for the acutely bleeding patient. Where liver transplantation has been proposed as the treatment of choice for bleeding esophageal varices in the absence of any contraindications, the outcome has often been unsatisfactory [4, 46]. In a randomized controlled trial, Orloff et al. [46] concluded that bleeding esophageal varices alone should not be considered an indication for liver transplantation, and that liver transplantation is seldom required following control of bleeding by portocaval shunt.
It is also important to note that numerous patients referred for liver transplantation will be disqualified for various reasons (e.g., alcoholism, drug abuse, and patient noncompliance), and few will ultimately receive a transplant [47].
In conclusion, once sclerotherapy has failed in patients with bleeding esophageal varices, there are limited options other than TIPS or emergency surgery as a bridge to liver transplantation. For patients who are not candidates for liver transplantation and who cannot be shunted because of portal vein thrombosis, the modified devascularization Sugiura procedure remains a reasonable surgical option that can be performed in nonspecialized centers.
Acknowledgments
The research is self-funded from our hospital. No potential and real conflicts of interest exist.
References
- 1.Wolff M, Hirner A. Current state of portosystemic shunt surgery. Langenbecks Arch Surg. 2003;388:141–149. doi: 10.1007/s00423-003-0367-5. [DOI] [PubMed] [Google Scholar]
- 2.Bosch J, Berzigotti A, Garcia-Pagan JC, et al. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48(Suppl 1):S68–S92. doi: 10.1016/j.jhep.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Rosemurgy AS, Zervos EE. Management of variceal hemorrhage. Curr Probl Surg. 2003;40:263–343. doi: 10.1016/S0011-3840(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 4.Wright AS, Rikkers LF. Current management of portal hypertension. J Gastrointest Surg. 2005;9:992–1005. doi: 10.1016/j.gassur.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Burroughs AK, Vangeli M. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy: randomized trials for secondary prophylaxis of variceal bleeding: an updated meta-analysis. Scand J Gastroenterol. 2002;37:249–252. doi: 10.1080/003655202317284138. [DOI] [PubMed] [Google Scholar]
- 6.Selzner M, Tuttle-Newhall JE, et al. Current indication of a modified Sugiura procedure in the management of variceal bleeding. J Am Coll Surg. 2001;193:166–173. doi: 10.1016/S1072-7515(01)00937-1. [DOI] [PubMed] [Google Scholar]
- 7.Dagenais M, Langer B, Taylor BR, et al. Experience with radical esophagogastric devascularization procedures (Sugiura) for variceal bleeding outside Japan. World J Surg. 1994;18:222–228. doi: 10.1007/BF00294405. [DOI] [PubMed] [Google Scholar]
- 8.Voros D, Androulakis G, Mallas E, et al. Immediate results of operations for esophageal varices. Hell J Gastroenterol. 1993;6:261–266. [Google Scholar]
- 9.Voros D, Karapanos K, Kyriazi M et al (2007) Long-term outcome of a modified Sugiura procedure for bleeding esophageal varices. ISW 2007 conference abstract ID: 113
- 10.Grace ND. Prevention of initial variceal hemorrhage. Gastroenterol Clin N Am. 1992;21:149–161. [PubMed] [Google Scholar]
- 11.Stanley AJ, Hayes PC. Portal hypertension and variceal haemorrhage. Lancet. 1997;350:1235–1239. doi: 10.1016/S0140-6736(97)06283-1. [DOI] [PubMed] [Google Scholar]
- 12.Burnett DA, Rikkers LF. Nonoperative emergency treatment of variceal hemorrhage. Surg Clin N Am. 1990;70:291–306. doi: 10.1016/s0039-6109(16)45082-6. [DOI] [PubMed] [Google Scholar]
- 13.D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332–354. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 14.de Franchis R, Primignani M. Endoscopic treatments for portal hypertension. Semin Liver Dis. 1999;19:439–455. doi: 10.1055/s-2007-1007131. [DOI] [PubMed] [Google Scholar]
- 15.Henderson JM, Nagle A, Curtas S, et al. Surgical shunts and TIPS for variceal decompression in the 1990 s. Surgery. 2000;128:540–547. doi: 10.1067/msy.2000.108209. [DOI] [PubMed] [Google Scholar]
- 16.Idezuki Y, Kokudo N, Sanjo K, et al. Sugiura procedure for management of variceal bleeding in Japan. World J Surg. 1994;18:216–221. doi: 10.1007/BF00294404. [DOI] [PubMed] [Google Scholar]
- 17.Orozco H, Juarez F, Uribe M, et al. Sugiura procedure outside Japan. The Mexican experience. Am J Surg. 1986;152:539–542. doi: 10.1016/0002-9610(86)90224-2. [DOI] [PubMed] [Google Scholar]
- 18.Gouge TH, Ranson JH. Esophageal transection and paraesophagogastric devascularization for bleeding esophageal varices. Am J Surg. 1986;151:47–54. doi: 10.1016/0002-9610(86)90010-3. [DOI] [PubMed] [Google Scholar]
- 19.Mercado MA, Orozco H, Vasquez M, et al. Comparative study of 2 variants of a modified esophageal transection in the Sugiura–Futagawa operation. Arch Surg. 1998;133:1046–1049. doi: 10.1001/archsurg.133.10.1046. [DOI] [PubMed] [Google Scholar]
- 20.Jin G, Rikkers LF. Transabdominal esophagogastric devascularization as treatment for variceal hemorrhage. Surgery. 1996;120:641–647. doi: 10.1016/S0039-6060(96)80011-0. [DOI] [PubMed] [Google Scholar]
- 21.Walker RM. Esophageal transection for bleeding varices. Surg Gynecol Obstet. 1964;118:323. [PubMed] [Google Scholar]
- 22.Johnston GW. Treatment of bleeding varices by oesophageal transection with the SPTU gun. Ann R Coll Surg Engl. 1977;59:404–408. [PMC free article] [PubMed] [Google Scholar]
- 23.Burroughs AK, Hamilton G, Phillips A, et al. A comparison of sclerotherapy with staple transection of the esophagus for the emergency control of bleeding from esophageal varices. N Engl J Med. 1989;321:857–862. doi: 10.1056/NEJM198909283211303. [DOI] [PubMed] [Google Scholar]
- 24.Soonawalla ZF, Shah SR, Mathur SK. Modified Sugiura procedure. J Am Coll Surg. 2002;194:247. doi: 10.1016/S1072-7515(01)01146-2. [DOI] [PubMed] [Google Scholar]
- 25.Sugiura M, Futagawa S (1984) Esophageal transection with paraesophagogastric devascularizations (the Sugiura procedure) in the treatment of esophageal varices. World J Surg 673–679. doi:10.1007/BF01655762 [DOI] [PubMed]
- 26.Barbot DJ, Rosato EF. Experience with the esophagogastric devascularization procedure. Surgery. 1987;101:685–690. [PubMed] [Google Scholar]
- 27.Weese JL, Starling JR, Yale CE. Control of bleeding esophageal varices by transabdominal esophageal transection, gastric devascularization, and splenectomy. Surg Gastroenterol. 1984;3:31–36. [PubMed] [Google Scholar]
- 28.Ginsberg RJ, Waters PF, Zeldin RA, et al. A modified Sugiura procedure. Ann Thorac Surg. 1982;34:258–264. doi: 10.1016/S0003-4975(10)62494-0. [DOI] [PubMed] [Google Scholar]
- 29.Cello JP, Crass R, Trunkey DD. Endoscopic sclerotherapy versus esophageal transection of Child class C patients with variceal hemorrhage. Comparison with results of portacaval shunt: preliminary report. Surgery. 1982;91:333–338. [PubMed] [Google Scholar]
- 30.Hosking SW, Johnson AG. What happens to esophageal varices after transection and devascularization? Surgery. 1987;101:531–534. [PubMed] [Google Scholar]
- 31.Qazi SA, Khalid K, Hameed AM, et al. Transabdominal gastro-esophageal devascularization and esophageal transection for bleeding esophageal varices after failed injection sclerotherapy: long-term follow-up report. World J Surg. 2006;30:1329–1337. doi: 10.1007/s00268-005-0372-7. [DOI] [PubMed] [Google Scholar]
- 32.Spence RAJ, Johnston GW. Results in 100 consecutive patients with stapled esophageal transaction for varices. Surg Gynecol Obstet. 1985;160:323–329. [PubMed] [Google Scholar]
- 33.Langer BF, Greig PD, Taylor BR. Emergency surgical treatment of variceal hemorrhage. Surg Clin N Am. 1990;70:307–311. doi: 10.1016/s0039-6109(16)45083-8. [DOI] [PubMed] [Google Scholar]
- 34.Koyanagi N, Iso Y, Higashi H, et al. Recurrence of varices after oesophageal transection: intra-operative and postoperative assessment by endoscopy. Br J Surg. 1988;75:9–11. doi: 10.1002/bjs.1800750105. [DOI] [PubMed] [Google Scholar]
- 35.Johnson M, Rajendran S, Balachandar TG, et al. Transabdominal modified devascularization procedure with or without esophageal stapler transaction—an operation adequate for effective control of a variceal bleed. Is esophageal stapler transection necessary? World J Surg. 2006;30:1507–1518. doi: 10.1007/s00268-005-0754-x. [DOI] [PubMed] [Google Scholar]
- 36.Comar KM, Sanyal AJ. Portal hypertensive bleeding. Gastroenterol Clin N Am. 2003;32:1079–1105. doi: 10.1016/S0889-8553(03)00099-2. [DOI] [PubMed] [Google Scholar]
- 37.Idezuki Y, Sanjyo K. Twenty-five-year experiences with esophageal transection for esophageal varices. J Thorac Cardiovasc Surg. 1989;98:876–883. [PubMed] [Google Scholar]
- 38.Mathur SK, Shah SR, Soonawala ZF, et al. Transabdominal extensive oesophagogastric devascularization with gastro-oesophageal stapling in the management of acute variceal bleeding. Br J Surg. 1997;84:413–417. doi: 10.1002/bjs.1800840346. [DOI] [PubMed] [Google Scholar]
- 39.Goyal N, Singhal D, Gupta S, et al. Transabdominal gastroesophageal devascularization without transection for bleeding varices: results and indicators of prognosis. J Gastroenterol Hepatol. 2007;22:47–50. doi: 10.1111/j.1440-1746.2006.04330.x. [DOI] [PubMed] [Google Scholar]
- 40.Chaudhary A, Aranya RC. Devascularization following endoscopic sclerotherapy of oesophageal varices: dangers and difficulties. Br J Surg. 1991;78:1249–1251. doi: 10.1002/bjs.1800781032. [DOI] [PubMed] [Google Scholar]
- 41.Klempnaue J, Schrem H. Review: surgical shunts and encephalopathy. Metab Brain Dis. 2001;16:21–25. doi: 10.1023/A:1011606326934. [DOI] [PubMed] [Google Scholar]
- 42.Chalasani N, Clark WS, Martin LG, et al. Determinants of mortality in patients with advanced cirrhosis after transjugular intrahepatic portosystemic shunting. Gastroenterology. 2000;118:138–144. doi: 10.1016/S0016-5085(00)70422-7. [DOI] [PubMed] [Google Scholar]
- 43.LaBerge JM, Sombers KA, Lake JR, et al. Two-year outcome following transjugular intrahepatic portosystemic shunt for variceal bleeding: results in 90 patients. Gastroenterology. 1995;108:1143–1151. doi: 10.1016/0016-5085(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 44.Ochs A. Transjugular intrahepatic portosystemic shunt. Dig Dis. 2005;23:56–64. doi: 10.1159/000084726. [DOI] [PubMed] [Google Scholar]
- 45.Sanyal AJ, Freedman AM, Luketic VA, et al. Transjugular intrahepatic portosystemic shunts for patients with active variceal hemorrhage unresponsive to sclerotherapy. Gastroenterology. 1996;111:138–146. doi: 10.1053/gast.1996.v111.pm8698192. [DOI] [PubMed] [Google Scholar]
- 46.Orloff MJ, Isenberg JI, Wheeler HO, et al. Liver transplantation in a randomized controlled trial of emergency treatment of acutely bleeding esophageal varices in cirrhosis. Transplant Proc. 2010;42:4101–4108. doi: 10.1016/j.transproceed.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terblanche J. The management of portal hypertension: controversies. J Gastroenterol Hepatol. 2002;17(Suppl):S439–S440. doi: 10.1046/j.1440-1746.17.s4.6.x. [DOI] [PubMed] [Google Scholar]


