Abstract
Background
Since the early 1990s, three consecutive pediatric acute myeloid leukemia (AML) trials have been performed in Austria (AML-Berlin-Frankfurt-Münster (BFM) 93, AML-BFM 98, and AML-BFM 2004) in close cooperation with the international BFM study center. Herein, we review the pertinent patient characteristics, therapy, and outcome data.
Patients and methods
From January 1993 to April 2013, 249 children and adolescents (193 protocol patients) diagnosed with AML were enrolled in the three BFM studies. Patients were mainly treated in one of five pediatric hematology/oncology centers distributed over Austria.
Results
Many characteristics and outcome parameters were not statistically different between the three trials. Almost similar proportions of patients were stratified into two risk groups: standard risk (SR) (approximately 37 % overall) and high-risk (HR) (61 %). MLL rearrangements were found in 23 % of patients overall as the most frequent genetic aberration subtype. Complete remission (CR) was achieved by 84–95 % of patients. The most important type of event was leukemic relapse (5-year cumulative incidence 40 ± 8 %, 21 ± 5 %, and 39 ± 6 %; p = 0.058), with a trend to a higher rate specifically in SR patients of study AML-BFM 2004 compared with AML-BFM 98. Importantly, the frequency of death from causes other than relapse sequelae declined over the years (AML-BFM 93: 5/42 12 %, AML-BFM 98: 5/57 9 %, and AML-BFM 2004: 5/94 5 %). Altogether, event-free survival at 5 years varied insignificantly (48 ± 8 %, 61 ± 7 %, and 50 ± 6 %; p = 0.406). Nevertheless, survival (pSU) apparently improved from BFM 93 to subsequent studies, both overall (57 ± 8 %, 75 ± 6 %, and 62 ± 6 %; p = 0.046) and regarding the HR group (5-year-probability of survival (pSU) 40 ± 10 %, 66 ± 8 %, and 52 ± 8 %; p = 0.039).
Conclusion
Treatment of pediatric AML in Austria renders survival rates in the range of international best practice. However, unambiguous statistical comparison of treatment periods is eventually hampered by small numbers and inequalities of recruitment. Hence, only internationally collaborative trials will allow developing treatment further to achieve higher cure rates with fewer events.
Keywords: Pediatric AML, Prognosis, Outcome, Austria, BFM, MLL
Introduction
Acute myeloid leukemia (AML) of childhood is a rare and heterogeneous disease. AML accounts for only 20 % of pediatric leukemias and is associated with a worse outcome compared with acute lymphoblastic leukemia [1]. Due to the rarity of the disease, treatment according to standardized guidelines defined in cooperative treatment protocols and within clinical trials is important to increase cure rates. The treatment strategy for pediatric AML in Austria is conducted in close cooperation with the international Berlin-Frankfurt-Münster (BFM) study center in Germany. Since the early 1990s, a total of 249 patients have been registered in Austria in three consecutive clinical trials (AML-BFM 93, 98, and 2004) [2–6]. Each trial was based on the experiences made in the previous BFM studies and on other relevant observations in the field [3, 5, 7]. With further clinical and molecular characterization, subgroups could be identified that either benefitted from intensification of therapy or from reduced dose intensity [3–5]. Improved survival over the years is also due to optimization of supportive therapy that helped to reduce deaths from hemorrhage and infectious complications [1, 6]. In this paper, we present data from the three Austrian AML-BFM trials and discuss outcome and management of patients.
Patients and methods
The inclusion criteria for the studies were: newly diagnosed AML (diagnosis confirmed by the reference laboratory in Vienna), age between 0 and 18 years, and signed informed consent by parents or legal guardians. Patients with secondary AML, myelodysplastic syndrome with blasts ≤ 20 %, and patients initially treated with non-AML-BFM chemotherapy were registered, but excluded from further analysis. Some details of the recruitment modalities changed within the observation period: patients with Down syndrome, isolated myelosarcoma, and biphenoytpic leukemia have been accepted as protocol patients in the later trials.
The initial diagnosis of AML and assignment to subtypes was made according to the FAB and World Health Organization (WHO) classification [8, 9]. Initial smears were centrally reviewed at the St. Anna Children’s Hospital in Vienna and, in addition, by an annual expert meeting at the University Children’s Hospital of Hannover, Germany. M0 and M7 subtypes always required immunological confirmation. Bone marrow (BM) aspirates from days 15 and 28, after first induction were reviewed centrally for early response assessment. Conventional cytogenetics, fluorescence in situ hybridization (FISH), and polymerase-chain reaction (PCR) screenings were conducted centrally at the St. Anna Children’s Hospital/Children’s Cancer Research Institute (CCRI).
Data of Austrian patients, including clinical, laboratory, toxicity, and outcome data, were collected in the trial office in Vienna (CCRI) and entered into a central database.
Two risk groups were defined: Standard Risk (SR) and High-Risk (HR) [6]. Since trial AML-BFM 93, stratification into the SR-group was based on morphologic findings (FAB M1/M2 with Auer rods, M3, M4Eo) and a good early response (day 15 BM < 5 % blasts). In study BFM 98, genetic findings were added as SR criteria: positivity for t(8;21) or AML1/ETO, and inv16 or CBFB/MYH11—both lesions usually overlap with Auer rod-positive FAB M1/M2- and M4Eo-morphology. In study 2004, AML in patients with Down’s syndrome was added to the SR-group, whereas evidence of FLT3-ITD was considered a HR criterion (except for FAB M3).
The treatment regimens of the three trials are shown schematically in Fig. 1 [6].
Fig. 1.
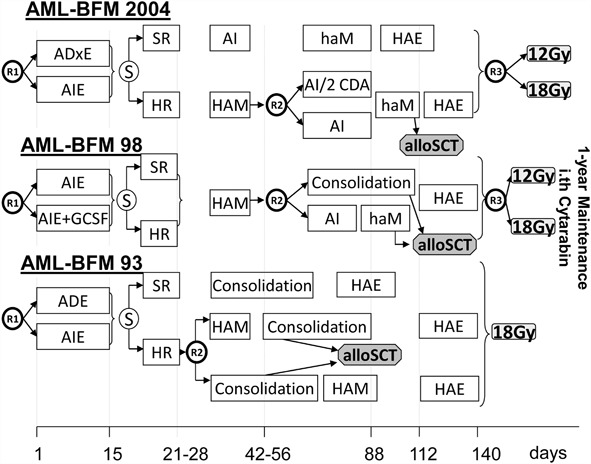
Treatment regimens of study acute myeloid leukemia-Berlin–Frankfurt–Münster 93, 98, and 2004
AML-BFM 93: In the first induction course, the application of daunorubicin was randomized against idarubicin [Ara-C/Daunorubicin/Etoposide (ADE) vs. Ara-C/Idarubicin/Etoposide (AIE)]: Idarubicin was expected to have a higher clinical benefit by showing a faster cellular uptake and increased retention and lower susceptibility to multidrug resistance. The aim of the second randomization was to evaluate the impact of early high-dose Ara-C/Mitoxantrone (HAM) vs. late HAM on outcome of HR patients. The accrual period was from January 1993 to September 1998; 68 patients were registered, of which 42 were accepted as protocol patients.
AML-BFM 98: The prophylactic administration of granulocyte colony-stimulating factor (G-CSF) was evaluated in the first randomization. The second randomization compared two new short consolidation cycles versus the biphasic 6-week consolidation. For standard risk patients (except Down and M3 patients), the therapy was intensified by addition of the second induction (HAM). The question if cranial irradiation could be reduced from 18 to 12 Gy without higher risk of relapse was asked in a third randomization. In total, 82 patients (57 protocol patients) were registered between October 1998 and June 2004.
AML-BFM 2004 (Eudract-Nr. 2006-004710-41): The aim of this study was to evaluate in two randomizations if the prognosis of childhood AML could be further improved by therapy intensification. Liposomal daunorubicin was implemented in first induction in a theoretically higher dosage than idarubicin (first randomization), and 2-CDA was evaluated as intensification in consolidation therapy for HR patients (second randomization). As no advantage could be demonstrated for second induction with HAM in the SR group in AML-BFM 98, administration of this course was abdicated for SR patients. However, further analysis showed a worse outcome for patients with t(8;21) not receiving HAM, leading to an amendment in 2010 [10]. The third randomization of cranial irradiation (12 vs. 18 Gy—started in AML-BFM 98) was continued. A total of 99 patients were recruited (94 protocol patients) between July 2004 and April 2013.
Response definitions: Complete remission (CR) was defined as ≤ 5 % leukemic blasts in the BM with signs of normal hematopoiesis and with clear signs of regeneration of normal blood cells in the peripheral blood (platelets > 80 × 109/l without transfusions, neutrophils > 1.0 × 109/l), and no leukemic cells in the peripheral blood or anywhere else. Definition of nonresponse (NR): all patients not achieving CR or remission with partial regeneration (CRp) and surviving the first 6 weeks of treatment were classified as NR. Definition of early death (ED): death before or within the first 6 weeks of treatment.
Toxicity was assessed according to the Common Terminology Criteria for Adverse Events v3.0 (CTCAE), available online at: http://ctep.cancer.gov/forms/.
Event-free survival (EFS) was calculated from the date of diagnosis to the last follow-up or first event (failure to achieve remission, resistant leukemia, relapse, secondary malignancy, or death of any cause). Patients who did not attain a CR were considered failures at time zero. Survival (SU) was calculated from the date of diagnosis to death of any cause or last follow-up.
Stem cell transplantations (SCT) were performed in the three pediatric transplant centers in Austria (Vienna, Graz, and Innsbruck). While in AML-BFM 93 and 98 all HR patients had an indication for allogeneic SCT if a human leukocyte antigen (HLA)-matched family donor was available, criteria were changed during the course of AML-BFM 2004 after interim analyses could not demonstrate a significant advantage of SCT. From 2006, only patients with primary refractory AML or relapse had an indication for SCT. Autologous SCT was not indicated at all.
Supportive care was stipulated to include prophylaxis against Pneumocystis jirovecci with cotrimoxazole/trimethoprim, as well as gut decontamination and systemic (antifungal) prophylaxis according to institutional practice.
Statistical methods
Mantel–Haenszel Chi-square test was used to compare groups for categorical variables, and Wilcoxon’s rank-sum test (Kruskal–Wallis test for more than two populations) for continuous variables. Survival probabilities were estimated using the Kaplan–Meier method with standard errors according to Greenwood [11, 12]. For overall survival (OS), deaths from any cause were considered an event. NR/aplasia, relapse, progression, secondary malignancy (SM), or death were considered an event. Groups were compared using the log-rank test. Cumulative incidences of events were calculated by the method of Kalbfleisch and Prentice [13], and compared using the Gray test [14].
Results
From January 1993 to April 2013, 249 children and adolescents diagnosed with AML were enrolled in three consecutive BFM studies in Austria (AML-BFM 93, AML-BFM 98 and AML-BFM 2004). Figure 2a shows the annual recruitment of pediatric AML patients in Austria since 1993. Annual recruitment stabilized in the last decade, and the percentage of protocol patients significantly increased over the three study periods from 62 % in AML-BFM 93 up to 94 % in AML-BFM 2004 because of expanded and specified inclusion criteria as discussed above (p < 0.001). Figure 2b demonstrates the distribution of registered patients in the recruiting centers in Austria. The vast majority of patients were treated in one of the five pediatric hematology/oncology centers in Vienna, Graz, Innsbruck, Salzburg, and Linz.
Fig. 2.
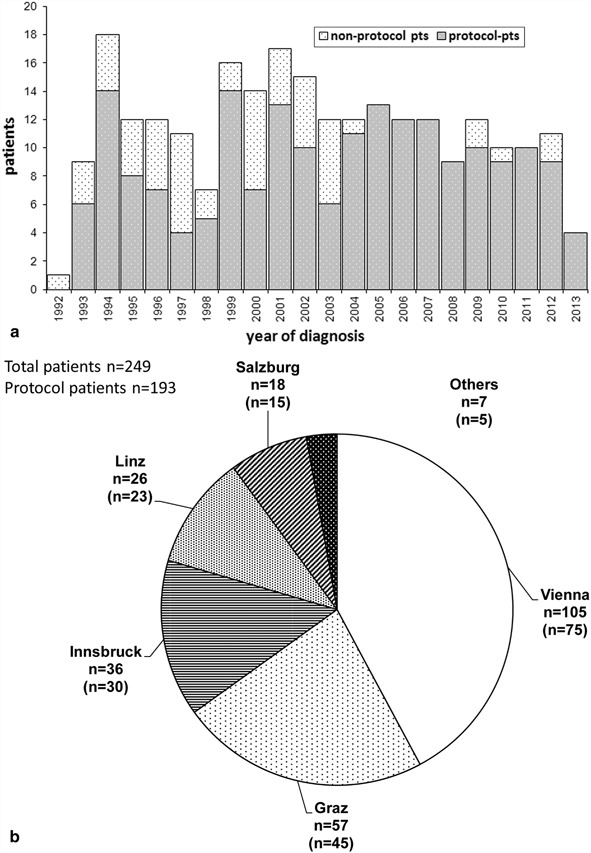
Annual patient recruitment a and recruiting centers in Austria b acute myeloid leukemia-Berlin–Frankfurt–Münster 93, 98, and 2004. Protocol patients in brackets
Patient characteristics (Table 1):
Table 1.
Patient characteristics
| Total | AML-BFM 2004 | AML-BFM 98 | AML-BFM 93 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | p-value | |
| Registered patients | 249 | 99 | 82 | 68 | |||||
| Protocol patients | 193 | 78 | 94 | 95 | 57 | 70 | 42 | 63 | < 0.0001 |
| Age | |||||||||
| Median (Q1–Q3) | 9.5 | (2.5–13.4) | 8.6 | (2.2–14.6) | 9.9 | (3.0–12.8) | 9.5 | (2.9–12.2) | 0.675 |
| < 1 year | 15 | 8 | 7 | 7 | 3 | 5 | 5 | 12 | 0.862 |
| 1≤ 10 years | 88 | 45 | 45 | 48 | 27 | 47 | 16 | 37 | |
| ≥ 10 years | 91 | 47 | 42 | 45 | 27 | 47 | 22 | 51 | |
| Gender | |||||||||
| Males | 96 | 50 | 46 | 49 | 27 | 47 | 23 | 55 | 0.605 |
| Females | 97 | 50 | 48 | 51 | 30 | 53 | 19 | 45 | |
| Leukocytes (× 109/l) | |||||||||
| < 10 | 93 | 48 | 47 | 50 | 27 | 47 | 19 | 45 | 0.552 |
| 10–< 100 | 71 | 37 | 36 | 38 | 17 | 30 | 18 | 43 | |
| ≥ 100 | 29 | 15 | 11 | 12 | 13 | 23 | 5 | 12 | |
| CNS leukemia | 11 | 6 | 8 | 7 | 2 | 4 | 1 | 2 | 0.129 |
| FAB types | |||||||||
| M0 | 12 | 6 | 5 | 5 | 3 | 5 | 4 | 10 | 0.508 |
| M1 | 19 | 10 | 11 | 12 | 7 | 12 | 1 | 2 | |
| M2 | 53 | 27 | 27 | 29 | 13 | 23 | 13 | 31 | |
| M3 | 21 | 11 | 12 | 13 | 5 | 9 | 4 | 10 | |
| M4 | 24 | 12 | 5 | 5 | 10 | 18 | 9 | 21 | |
| M4Eo | 18 | 9 | 8 | 9 | 8 | 14 | 2 | 5 | |
| M5 | 24 | 12 | 13 | 14 | 5 | 9 | 6 | 14 | |
| M6 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | |
| M7 | 19 | 10 | 10 | 11 | 6 | 11 | 3 | 7 | |
| Other | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Not classified | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Genetic aberrations | |||||||||
| t(8;21) | 20 | 10 | 9 | 10 | 5 | 9 | 6 | 14 | 0.606 |
| t(15;17) | 19 | 10 | 10 | 11 | 5 | 9 | 4 | 10 | |
| inv(16) | 18 | 9 | 7 | 7 | 8 | 14 | 3 | 7 | |
| MLL | 45 | 23 | 19 | 20 | 13 | 23 | 13 | 30 | |
| Normal | 26 | 13 | 12 | 13 | 9 | 16 | 5 | 12 | |
| Other | 52 | 27 | 29 | 30 | 13 | 23 | 10 | 23 | |
| Down | 11 | 6 | 7 | 7 | 4 | 7 | n.a. | n.a. | |
| Not classified | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | |
| Risk group | |||||||||
| SR | 72 | 37 | 36 | 38 | 22 | 39 | 14 | 33 | 0.735 |
| HR | 117 | 61 | 58 | 62 | 32 | 56 | 27 | 64 | |
| Not classified | 4 | 2 | 0 | 0 | 3 | 5 | 1 | 2 | |
| Median follow-up | |||||||||
| In years (Q1–Q3) | 6.7 | (4.0–9.7) | 4.0 | (1.8–6.0) | 9.0 | (7.9–10.5) | 11.6 | (9.0–15.0) | |
AML acute myeloid leukemia, CNS central nervous system, SR standard risk, HR high-risk
The 193 protocol patients were followed in median for 6.7 years. Many patient characteristics at presentation were stable over the three study periods: Median age at diagnosis (9.5 years), age distribution, gender ratio (balanced), and proportion of patients with hyperleukocytosis (15 % of patients had ≥ 100 × 109/l leukocytes). Diagnosis of central nervous system (CNS) involvement seemed to increase by trend (2–7 %). The distribution of FAB types as well as the occurrence of classical genetic aberrations was rather similar throughout all studies. Overall, 29 % of patients carried favorable genetic abnormalities (t(8;21), t(15;17), inv(16)). All patients in these three trials were also investigated (retrospectively for the earlier period) for MLL rearrangements as part of a large international project [15]. This investigation led to the identification of quite similar numbers across all trials in the range of 23 % (Table 1). Altogether, 37 % of patients were treated in the SR arm and 61 % in the HR arm. Interestingly, risk group distributions did not vary significantly despite some changes of criteria: The extension of HR-criteria in AML-BFM 2004 by FLT3-ITD in non-FAB M3 had just a minor impact due to small case numbers. Of 13 patients positive for FLT3-ITD overall, two were HR by initial criteria, four were shifted to HR by a poor early response, two remained SR because of FAB M3-phenotype, and only five patients were shifted from SR to HR just because of the FLT3-ITD (three of the latter are in continued first CR following HR chemotherapy, whereas two relapsed).
Response and outcome (Table 2):
Table 2.
Results of different study periods
| AML-BFM 2004 | AML-BFM 98 | AML-BFM 93 | |||||
|---|---|---|---|---|---|---|---|
| n/% | %/SE | n/% | %/SE | n/% | %/SE | p-value | |
| Protocol patients | 94 | 57 | 42 | ||||
| Blasts day 15 > 5 % | 20 | 22 | 13 | 24 | 8 | 20 | |
| Response (first CR) | |||||||
| Early death | 0 | 0 | 2 | 4 | 1 | 2 | 0.868 |
| Nonresponse | 8 | 9 | 6 | 10 | 1 | 2 | |
| CR achieved | 86 | 91 | 49 | 84 | 40 | 95 | |
| 5-year cumulative incidence of the first event | |||||||
| ED/Aplasia/NR | 9 | 3 | 14 | 5 | 5 | 3 | 0.274 |
| Relapses | 39 | 6 | 21 | 5 | 40 | 8 | 0.058 |
| Second malignancy | 1 | 1 | 2 | 2 | 0 | 0 | 0.702 |
| Death in CCR | 1 | 1 | 2 | 2 | 7 | 4 | 0.138 |
| Freedom of all events | 50 | 6 | 61 | 7 | 48 | 8 | 0.406 |
| 5-year cumulative incidence of the first relapse | 41 | 6 | 28 | 6 | 40 | 8 | 0.322 |
| 5-year cumulative incidence of second malignancy | 1 | 1 | 2 | 2 | 0 | 0 | 0.698 |
| 5-year-pSU | 62 | 6 | 75 | 6 | 57 | 8 | 0.046 |
| 5-year-pEFS | 50 | 6 | 61 | 7 | 48 | 8 | 0.406 |
| 5-year-pSU standard risk | 81 | 9 | 91 | 6 | 86 | 9 | 0.625 |
| 5-year-pEFS standard risk | 67 | 10 | 81 | 9 | 79 | 11 | 0.538 |
| 5-year-pSU high-risk | 52 | 8 | 66 | 8 | 40 | 10 | 0.039 |
| 5-year-pEFS high-risk | 41 | 7 | 50 | 9 | 30 | 9 | 0.456 |
AML acute myeloid leukemia, CCR continued complete remission, CR complete remission, ED early death, NR nonresponse, EFS event-free survival, pSU probability of survival, pEFS probability of event-free survival, SU survival, SE standard error
Data on early response on day 15 were available for 183 protocol patients. The distribution of cases with a poor early response (≥ 5 % blasts in BM on day 15) was very similar among the three study periods (8 of 40 patients in AML-BFM 93, 20 %; 13 patients of 54 in AML-BFM 98, 24 %; and 20 of 89 in AML-BFM 2004, 22 %). Overall, 8 of these 41 patients never acquired remission by chemotherapy alone (three alive after SCT), whereas 33 patients achieved CR by subsequent HR treatment. Of the latter, 19 remained in CR (8 FAB M3; further 8 by chemotherapy only; 3 after SCT in CR1), 1 died in CR1, and the other 13 patients suffered from a relapse (alive only one patient after SCT). In total, around 42 % of non-FAB M3 patients with a poor early response achieved a stable CR with their first line of treatment (BFM 93: 0/5; BFM 98: 6/11, 55 %; BFM 2004: 8/17, 47 %).
The overall rate of achieved CR was around 90 % and stable over the three study periods. NR rate was 10 % in AML-BFM 98 and 9 % in AML-BFM 2004. In AML-BFM 93, the NR rate was only 2 %; however, this difference has to be regarded with caution, as a considerable number of patients was excluded from the study for various reasons at that time.
The 5-year cumulative incidence of first relapse was not significantly different over the three study periods (93: 40 ± 8 %, 98: 28 ± 6 %, and 2004: 41 ± 6 %, p = 0.322, Fig. 3). Results for overall and event-free survival for the whole group and split by risk group are demonstrated in Fig. 4a, b. Regarding 5-year-probability of survival (pSU) in general and for HR-patients, a difference between trials could be demonstrated (p = 0.046 and 0.039). Outcome was better in AML-BFM 98 and 2004 compared with AML-BFM 93. As expected, 5-year-probability of event-free survival (pEFS) and 5-year-pSU were higher in SR compared with HR groups, respectively (Fig. 4c–f).
Fig. 3.
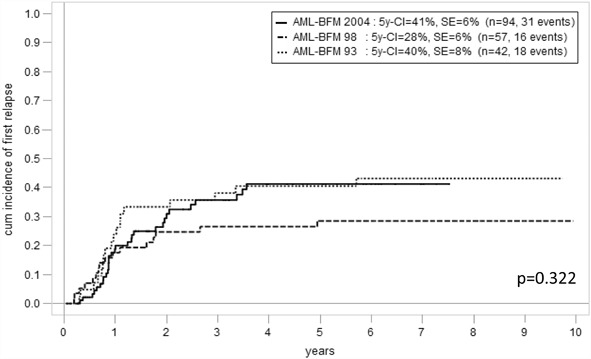
5-year cumulative incidence of first relapse in the three studies
Fig. 4.
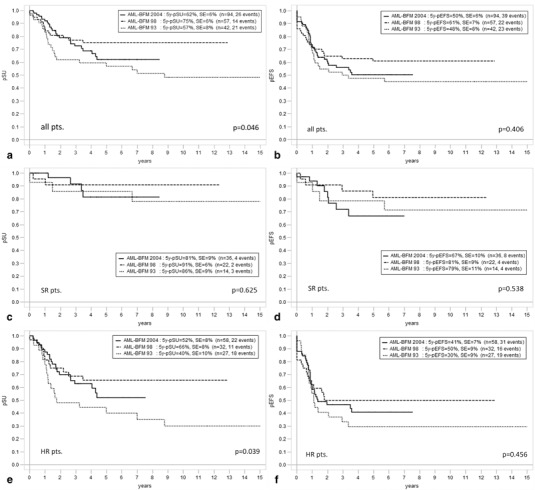
Overall survival (5-year-pSU) and event-free survival (5-year-pEFS) of studies acute myeloid leukemia-Berlin–Frankfurt–Münster 93, 98, and 2004 among all protocol patients (a, b), standard risk patients (c, d), and high-risk patients (e, f)
When comparing results from AML-BFM 98 and 2004, outcome seemed to worsen with the latter study with a higher cumulative incidence of relapse (28 ± 6 vs. 41 ± 6 %), lower 5-year-pEFS (61 ± 7 vs. 50 ± 6 %), and lower 5-year-pSU (75 ± 6 vs. 62 ± 6 %, Figs. 3, 4a, b). This difference is particularly remarkable in the SR group (5-year-pEFS 81 ± 9 vs. 67 ± 10 %, Fig. 4d). While the mere proportional relapse rate in the AML-BFM 2004 HR group so far seemed to be stable compared with AML-BFM 98 (24/58, 41 vs. 14/32 %, 44 %), already 7 of 36 patients in the AML-BFM 2004 SR group relapsed mostly around 2 years of observation (i.e., 19 %; AML-BFM-98 2/22, 9 %). Notably, cumulative incidence measures of relapse indicate a more significant difference in relapses between study AML-BFM 98 and 2004 than mere proportional comparisons because several events occurred quite late in the more recent study (around 4 years of observation, so rather close to the median follow-up time).
Out of the 193 protocol patients of the three studies, 61 died (31.6 % overall). Relapse was the main cause of eventual death throughout (46/193, i.e., 24 % of all patients; 46/61, i.e., 75 % of all deaths). ED was rare overall (n = 3; 1.6 %), but we know of six additional patients who died due to fulminant disease and related complications (mostly hemorrhage) before consent for study procedures could have been acquired and thus were not registered to the trials (FAB M2 n = 1, M3 n = 2, M4/5 n = 3). The rare incidences of death in NR (n = 7; 3.6 %) were mainly caused by SCT-associated toxic, infectious, or immunological problems. Reasons for death in CR (n = 5; 2.6 %) were mainly infectious, including fungal, bacterial, and specifically viral complications, including two cases of fatal Epstein–Barr virus (EBV)-induced hemophagocytosis syndromes. In total, the frequency of death from causes other than relapse sequelae declined over the years (BFM 93: 5/42 12 %, BFM 98: 5/57 9 %, and BFM 2004: 5/94 5 %). Importantly, the rate of secondary malignancy until last follow-up was very low (n = 2 cases overall).
As expected, outcome depended on AML subtypes. For example, of the 21 protocol patients with FAB M3 (11 % overall in the three trials), all patients achieved CR, only 3 patients relapsed, and only 1 eventually died. All but one patient with a complex aberrant karyotype without the typical PML/RARA fusion were treated in the SR group. The three relapsing patients underwent either autologous (n = 2) or allogeneic SCT. While two of them are now in stable second CR, only the patient with a rare STAT5B/RARA fusion died after suffering from an isolated testicular relapse, NR to arsenic trioxide (ATO) and all-trans retinoic acid (ATRA), and a subsequent systemic relapse [16]. Comparatively, the 45 protocol patients with MLL rearrangements fared worse, however, with clear differences found according to partner-genes involved. Events occurred most frequently in MLL/AF6 (all five patients relapsed after HR treatment) and MLL/AF10 (6 relapses among 11 cases). The most frequent fusion, MLL/AF9 (n = 12), had a considerably better outcome (only three relapses and one ED). Other types were too infrequent to draw prognostic conclusions: MLL/ELL (n = 3; one relapse, one ED), MLL/AF1Q (n = 3; one SM), MLL/ENL (n = 2; one relapse), singular others (n = 9; four alive in first CR).
Nonhematological grade 3/4 toxicity:
Data have been extensively collected in study AML-BFM 2004 (Table 3). Overall, grade 3/4 toxicity was relatively infrequent (occurring in less than one-third of patients throughout all items as well as all treatment courses). It consisted mainly of gastrointestinal/hepatic toxicity (elevated transaminases, stomatitis, nausea, and diarrhea) and infectious complications. Severe stomatitis occurred only after anthracycline dose-intensive induction and HAM courses (13 and 10 %, respectively). The rate of severe infectious complications remained stable throughout all intensive chemotherapy courses (22–31 %) and was lower during maintenance therapy (15 %). Renal, neurological, and specifically cardiac toxicity of higher grade were extremely rare.
Table 3.
Toxicity in acute myeloid leukemia-Berlin–Frankfurt–Münster 2004
| Induction | HAM (HR group) | AI | haM | HAE | Maintenance | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade3/4 | Grade3/4 | Grade3/4 | Grade3/4 | Grade3/4 | Grade3/4 | |||||||||||||
| n | % | Total | n | % | Total | n | % | Total | n | % | Total | n | % | Total | n | % | Total | |
| General condition | 12 | 16 | 77 | 9 | 18 | 49 | 9 | 13 | 71 | 5 | 7 | 67 | 5 | 8 | 60 | 2 | 4 | 56 |
| Nausea | 14 | 18 | 76 | 4 | 8 | 49 | 9 | 13 | 71 | 5 | 8 | 65 | 9 | 15 | 60 | 2 | 4 | 56 |
| Diarrhea | 13 | 17 | 77 | 2 | 4 | 49 | 8 | 11 | 71 | 2 | 3 | 66 | 2 | 3 | 60 | 0 | 0 | 56 |
| Stomatitis | 10 | 13 | 77 | 5 | 10 | 49 | 1 | 1 | 71 | 0 | 0 | 65 | 0 | 0 | 60 | 1 | 2 | 56 |
| S-GOT/S-GPT | 13 | 17 | 77 | 9 | 18 | 49 | 23 | 33 | 70 | 8 | 12 | 66 | 13 | 22 | 59 | 6 | 11 | 55 |
| Creatinine | 1 | 1 | 77 | 0 | 0 | 49 | 0 | 0 | 71 | 1 | 1 | 67 | 0 | 0 | 58 | 0 | 0 | 55 |
| Infection | 17 | 22 | 77 | 15 | 31 | 49 | 21 | 30 | 70 | 19 | 29 | 66 | 17 | 30 | 57 | 8 | 15 | 55 |
| Cardiac function | 0 | 0 | 77 | 1 | 2 | 49 | 1 | 1 | 71 | 0 | 0 | 65 | 1 | 2 | 60 | 1 | 2 | 56 |
| Neurotoxicity | 2 | 3 | 77 | 1 | 2 | 49 | 2 | 3 | 71 | 0 | 0 | 66 | 0 | 0 | 59 | 2 | 4 | 56 |
AI Ara-C/Idarubicin, HAE high-dose Ara-C/Etoposide, HR high-risk, S-GOT serum glutamic oxaloacetic transaminase, S-GPT serum glutamic-pyruvic transaminase
Stem cell transplantation:
Despite the fact that indications for SCT changed in 2006 (see above), similar proportions of patients of the last two studies were transplanted (AML-BFM 98: 21/57, 37 %; AML-BFM 2004 37/94, 39 %). However, in study AML-BFM 98, more patients underwent SCT during the first line of therapy (12/21, 57 %) as opposed to AML-BFM 2004 (14/37, 38 %). Altogether, 14 patients were transplanted in first CR (mostly because of unfavorable genetics) of which 9 remained in CR after allogeneic SCT (3/7 in AML-BFM 98; 6/7 in AML-BFM 2004). Of 12 patients with NR transplanted in studies 98 and 2004, only 4 are alive after SCT (four died of treatment-related toxicity, one of NR, three after another relapse). Among the 31 patients transplanted for a first relapse, 4 received autologous peripheral SCT (two for an isolated CNS relapse, one for testicular relapse, one for BM relapse of FAB M3; three are alive in CR, including both CNS relapses). The other 27 patients underwent allogeneic SCT for their relapse, of which 11 died of SCT-related complications (8 in the more recent period), 6 of a further relapse, and 10 are alive (37 %; i.e., 7/20 of study AML-BFM 2004).
Discussion
While pediatric AML was an almost incurable disease before 1970, the prognosis could be gradually improved through 40 years of subsequent AML-BFM trials [6]. Intensification of therapy led to better early response and survival up to the range of 60–70 % nowadays, and subgroups could be identified that have excellent cure rates up to 80–90 % (AML in Down’s syndrome; FAB M2 with t(8;21), M3 with t(15;17), and M4Eo with inv(16)) [4–7, 10, 17]. Treatment of AML in children and adolescents up to the age of 18 years in Austria has been conducted since 1993 in close cooperation with the German BFM study group, and data of Austrian patients were always included into the larger data set of the three international trials conducted since then [3, 5, 7, 10, 17]. This is now the first independent report summarizing characteristics and outcome of only the Austrian patients.
Importantly, over the years, the diagnostic work-up has improved and recruitment criteria were specified and broadened to achieve a more realistic picture of the variable characteristics as well as the general outcome of children with AML. For example, patients with Down’s syndrome were included in the case of AML, but not with the transient form of myeloproliferation occurring below the age of 6 months, as were cases of isolated myelosarcoma and mixed phenotype acute leukemia with dominant myeloid features. In the last study period (AML-BFM 2004), a stable recruitment of approximately 10–12 cases annually was reached and most of those (95 %) could be accepted as fulfilling protocol criteria. Hence, results from the different study periods are not completely comparable as the composition of the cohorts changed over time. In addition, as recruited numbers are anyway small, single cases and their distinct characteristics have a greater influence on overall outcome. Thus, we expect a considerable statistical variability in our data so that more detailed analyses are only possible in the context of large international trials.
Cure rates of children and adolescents with AML in Austria are at the forefront of what is achievable nowadays: 68.4 % of all protocol patients since 1993 are alive (AML-BFM 93: 50 %; AML-BFM 98: 74 %; AML-BFM 2004 73 %). Importantly, results improved significantly in trials AML-BFM 98 and 2004 as compared with the 93 study—mostly due to fewer death events beyond 1 year of follow-up in the HR group. To some extent, this reflects not only improvements in therapy including front-line intensifications, but also consistent relapse treatment since the late 1990s and improved SCT procedures [18, 19]. Surprisingly, in comparison with AML-BFM 98, outcome seemed to worsen slightly in AML-BFM 2004 when looking at time-related probability measures, but not so obviously when looking at mere proportions of protocol patients (98 vs. 2004: relapsing 30 vs. 33 %; deceased 26 vs. 27 %). The lower EF and overall survival probability as well as the higher incidence of relapses in study AML-BFM 2004 is mainly caused by a higher relapse rate in the SR group (more events around 2 years of follow-up) and a few late occurring events among HR patients (around 4 years, which is close to the median follow-up of the trial, thus creating significant statistical weight despite low numbers). In detail, 7 of 36 SR-patients of trial AML-BFM 2004 relapsed so far. Five of these would nowadays receive more therapy upfront or not be included into the SR group according to more recent BFM practice and international consensus (one with AML1/ETO; one with STAT5B/RARA, three fulfilling previous SR-criteria only by morphology—Auer rod positive—but not by genetics) [1, 10]. Cases with AML1/ETO fusion have been recognized to fare worse by not receiving HAM in study 2004 that intended only four instead of five cycles for SR patients [10]. Patients with FAB M3 and STAT5B/RARA fusion have recently been demonstrated to be resistant to both ATRA and ATO, and to carry a very poor prognosis with conventional therapy [16]. Generally speaking, of the six PML noninvolving variant rearrangements of RARA identified to date to occur with a low frequency, specifically those involving the PLZF and STAT5B as fusion partners confer therapy resistance and need special management probably even including SCT in first CR [16, 20]. According to the recent pediatric AML expert consensus, mere evidence of Auer rods has no longer been considered an independent criterion for inclusion into a SR stratum in the future [1]. Hence, our relapse case observations among formerly considered “SR” patients demonstrate the increase of knowledge over the study period as well as the fact that the course of single patients can have a considerable statistical influence in small trials. Outcome differences between the small Austrian cohorts and the much larger German patient series, therefore, need to be interpreted with caution: results in the Austrian BFM 98 patients seemed to be better compared with the entire AML-BFM 98 cohort (5-year-pSU 75 ± 6 vs. 65 ± 2 %, 5-year-pEFS 61 ± 7 vs. 51 ± 2 %), whereas this relation inverted in study 2004 (5-year-pSU 62 ± 6 vs. 74 ± 2 %, 5-year-pEFS 50 ± 6 vs. 55 ± 2 %) [17].
One cornerstone of good management of pediatric myeloid leukemia is avoiding nonleukemic deaths and specifically nonrelapse deaths. Major factors of success in this striving are (i) immediate diagnostics and treatment in dedicated and experienced centers only (i.e., limited to five major centers in Austria serving nearly all cases out of a population of approximately 8 million people) and (ii) supportive care aiming at reducing typical comorbidities and fatalities. In fact, the total percentage of study patients who died for any other reasons besides relapse declined over the three studies from 11 % in study 93 to 5 % in trial 2004. In AML-BFM 93 and 98, the respective numbers were comparable with those published for the whole BFM cohort (12 and 9 %, respectively) [21]. Death before start of treatment (n = 6) and early death (n = 3) were almost exclusively caused either by cerebral hemorrhage due to coagulation disturbances as in FAB M3 subtype (n = 2) or to hyperleukocytosis (n = 4; three cases M4/M5), or due to leukostasis with associated acute respiratory distress syndrome (ARDS) that is typical for FAB M4/M5 subtypes (n = 2). This was also seen in the whole BFM cohort and other international pediatric AML studies [21–23]. A high degree of experienced alertness to initial risk of complications, immediate treatment commencement including external cell reduction modalities (e.g., by exchange transfusion) as needed, and specific therapies like ATRA pretreatment in FAB M3 all translate into a reduced death toll [24]. Nevertheless, it needs to be stated that deaths before study consent and treatment initiation are usually not registered in the database of studies that fall under laws and regulations related to the conduct of clinical trials on medicinal products. Hence, registration also of such patients and of their presentation (and of other patients not eligible for study inclusion) is an overriding aim for a population-based assessment of the welfare of pediatric patients with AML to work on measures to avert fatal outcome.
Death in first CR (n = 5 in total; only one case in study 2004) should be rare with experienced supportive care. Nevertheless, AML treatment is one of the most intense therapy types delivered in pediatric oncology. It is accompanied by long periods of severe neutropenia and immunosuppression with associated invasive bacterial and fungal infections. Related to this, high-grade toxicity in AML-BFM 2004 was particularly caused by infections (between 22 and 31 % following the different chemotherapy courses; Table 3). The usage of anti-infective prophylaxis covering Pneumocystis jirovecci, common molds like Aspergillus species, and gram-positive as well as -negative bacteria has, therefore, been recommended [1, 22, 23, 25, 26]. Currently, oral antifungal prophylaxis with either itraconazole suspension (higher bioavailability than capsules) or voriconazol is probably the most cost-effective praxis in children with AML. However, rare breakthrough infections with more resistant molds like Zygomycetes (which would be amenable to prophylaxis with posaconazol—not licensed for children) need to be considered. A main cause for gram-positive septicemia in neutropenia especially following cycles containing high-dose cytarabin are viridans group streptococci (VGS), which can be associated with significant morbidity and mortality due to pneumonitis and ARDS [25–27]. Antibiotic prophylaxis with intravenous vancomycin (or cephalosporins, but not with oral b-lactam antibiotics) at St. Jude’s Childrens’ Hospital strongly reduced the incidence of VGS sepsis and associated morbidity, whereas oral ciprofloxacin effectively reduced gram-negative infections [26]. In a similar approach, we piloted alternate-day high-dose intravenous teicoplanin (plus oral ciprofloxacin for patients aged > 5 years) and found a similar reduction of VGS septicemia (manuscript in preparation). This prophylaxis can easily be applied also in an out-patient setting. Serious viral infections and associated deaths have been rarely reported in pediatric AML patients [22, 23]; however, we observed two cases of death in CR during maintenance therapy caused by fatal EBV-induced hemophagocytosis syndrome.
Toxicity besides infectious complications was mainly gastrointestinal (Table 3). In contrast to other international AML studies, high-grade cardiotoxicity is very rare, both in the entire BFM cohort and in the Austrian series, and we faced no death caused by cardiac toxicity [22, 28]. To some extent, this may be due to the use of potentially less cardiotoxic anthracyclines [17], as well as to strategic treatment reductions in good risk patients [4, 10, 29]. Treatment reductions and drug targeting of essential pathways of leukemic pathobiology instead of applying conventional, nonselective chemotherapy may further allow improving cure rates and quality of life. As an example, patients with AML FAB M3 and the typical PML/RARA fusion have an excellent prognosis with current ATRA-based chemotherapy already (20/21 of our patients are in continuous CR-2 following SCT after relapse) [29]. More recent observations with combinations of ATO and ATRA suggest that chemotherapy could even be completely avoided in this subtype [30, 31]. Other types of targeted treatment, e.g., using tyrosine-kinase inhibitors tackling aberrant signaling (e.g., by FLT3-ITD), may be options for increasing survival rates in selected cohorts without further increasing classical chemotherapy toxicity [32].
Protracted nonresponse to therapy cycles remains a serious risk of death. Seven of all 15 patients with continued NR died due to toxicities which accumulated during continuous nonregeneration of hematopoiesis and after rescue SCT. Interestingly, not all these cases were contained in the group of patients with a slow early blast reduction at day 15 (> 5 % blasts in BM). Overall, the proportion of patients with poor early response remained at 20–24 % throughout the three treatment periods despite stepwise intensification of induction—similar to the whole BFM cohort [17]. Of these 41 patients in total, only 8 showed continued NR and 33 (80 %) achieved CR with further trial chemotherapy, leading to overall remission rates in the range of 90 %—as in the entire BFM cohort [17]. After excluding 8 FAB M3 patients from analysis who are known for a slow response without impact on outcome (all alive in CR), 15 (45 %) of the remaining 33 eventually acquired a sustained remission (none from trial 93; 8 after HR-chemotherapy only, three after SCT in CR1, three after SCT because of NR, and only one after SCT for relapse—out of 13). In summary, a challenge for the future will be pinpointing patients who need further therapy intensification (e.g., by SCT) already during their first line of treatment because outcome anticipation is otherwise very poor.
While allogeneic SCT for relapsed or refractory AML is an approved concept, the usefulness of SCT in first CR has been discussed controversially [33–36]. Indicating SCT for HR AML by availability of a matched sibling donor—as in study AML-BFM 98—has no proven value overall, but SCT might be beneficial in certain subgroups like those with MLL rearrangements [36]. SCT-related increased morbidity and mortality as compared with conventional intensive chemotherapy needs to be balanced against potentially higher antileukemic efficacy. In that striving for higher cure rates, SCT—in its current forms—is still not a cure-all: despite SCT in first CR, five patients relapsed among 14 transplanted (some of the latter have a very short follow-up time). Nevertheless, our data are in line with the notion that performing SCT in first CR in defined high-risk constellations might allow undergoing SCT under better circumstances and with less toxicity accumulations—in contrast to waiting for a relapse and undergoing further toxic chemotherapies as preparation for eventual SCT in second CR. Importantly, according to our data the proportions of patients eventually transplanted—based on both policies—seem to be similar (37–39 % in study 98 and 2004). Upcoming trials will, therefore, reintegrate the idea of transplanting certain high-risk patients already in first CR based on the nowadays more comprehensive picture of genetic aberrations heralding a very poor outcome upon chemotherapy alone [1]. Among those are MLL translocations specifically involving the quite frequently occurring partner genes AF6 (5/5 relapsed; one survived after SCT) and AF10 (6/11 relapsed; short follow-up in two cases after primary SCT), whereas AF9—the most frequently observed translocation partner in pediatric AML—did not confer such very dismal outcome. Our MLL data are in line with recent large-scale case collections, both regarding the very significant overall incidence (23 % among all study patients—38 % of HR patients) and with respect to partner gene distribution as well as related outcome [37]. Hence, MLL-rearranged pediatric HR-AML is probably the most important genetically defined subcohort needing improved and individualized treatment modalities. Most importantly, both the need for and the potential of individualized management are based on sophisticated genetic reference diagnostics. In line with this, also improved early response assessment with more objective methodology (e.g., multiparameter flow cytometry) might in the future allow us to individualize treatment better [38].
In summary, the Austrian study data are good examples of achievements as well as remaining shortcomings in the management of childhood AML. Our outcome data are at the forefront of what is achievable nowadays. Nevertheless, the growing understanding of the high diversity of pediatric AML underscores the need for very large and multinational studies with sufficient statistical power—even beyond the German–Austrian BFM-cooperation— to develop treatment further and to improve outcome overall and in even smaller, well-circumscribed subgroups.
Acknowledgments
The authors thank all involved clinicians, data managers, nurses, and technicians of the participating hospitals for their most valuable cooperation. The important contribution of the reference diagnostic teams (R. Kornmüller, U. Stalze, E. Neidhart, S. Juhasz; M. König, G. Pass, and co-workers; M. Neßlböck, H. Daxberger, and co-workers; A. Schumich—all at St. Anna/CCRI; as well as W. Pickl and co-workers at the Institute of Immunology, Medical University of Vienna) needs to be emphasized. We also wish to thank R. Marschalek and C. Meyer from DCAL/Klinikum d. Goethe Universität Frankfurt for their valuable cooperation. Last but not least, we wish to thank our German colleagues in the strive against childhood AML, U. Creutzig, D. Reinhardt, M. Zimmermann, N. von Neuhoff, and all co-workers at the BFM study center for their close alliance and continued support.
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
For the AML BFM-Austria study group. Heidrun Boztug and Nora Mühlegger contributed equally.
Contributor Information
Heidrun Boztug, Email: heidrun.boztug@stanna.at.
Michael Dworzak, Email: michael.dworzak@stanna.at.
References
- 1.Creutzig U, van den Heuvel-Eibrink MM, Gibson B. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120(16):3187–205. doi: 10.1182/blood-2012-03-362608. [DOI] [PubMed] [Google Scholar]
- 2.Creutzig U, Berthold F, Boos J. Improved treatment results in children with AML: results of study AML-BFM 93. Klin Padiatr. 2001;213(4):175–85. doi: 10.1055/s-2001-16849. [DOI] [PubMed] [Google Scholar]
- 3.Creutzig U, Zimmermann M, Lehrnbecher T. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24(27):4499–506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- 4.Creutzig U, Reinhardt D, Diekamp S. AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity. Leukemia. 2005;19(8):1355–60. doi: 10.1038/sj.leu.2403814. [DOI] [PubMed] [Google Scholar]
- 5.Creutzig U, Ritter J, Zimmermann M. Improved treatment results in high-risk pediatric acute myeloid leukemia patients after intensification with high-dose cytarabine and mitoxantrone: results of study acute myeloid leukemia-Berlin-Frankfurt-Munster 93. J Clin Oncol. 2001;19(10):2705–13. doi: 10.1200/JCO.2001.19.10.2705. [DOI] [PubMed] [Google Scholar]
- 6.Creutzig U, Zimmermann M, Dworzak MN. Development of a curative treatment within the AML-BFM studies. Klin Padiatr. 2013;225(1):79–86. doi: 10.1055/s-0033-1337968. [DOI] [PubMed] [Google Scholar]
- 7.Creutzig U, Ritter J, Zimmermann M. Idarubicin improves blast cell clearance during induction therapy in children with AML: results of study AML-BFM 93. AML-BFM study group. Leukemia. 2001;15(3):348–54. doi: 10.1038/sj.leu.2402046. [DOI] [PubMed] [Google Scholar]
- 8.Creutzig U, Ritter J, Ludwig WD. [Classification of AML by morphologic, immunologic and cytogenetic criteria. Review with reference to subtypes in the AML-BFM-87 study] Klin Padiatr. 1993;205(4):272–80. doi: 10.1055/s-2007-1025237. [DOI] [PubMed] [Google Scholar]
- 9.Vardiman JW, Thiele J, Arber DA. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 10.Creutzig U, Zimmermann M, Bourquin JP. Second induction with high-dose cytarabine and mitoxantrone: different impact on pediatric AML patients with t(8;21) and with inv(16) Blood. 2011;118(20):5409–15. doi: 10.1182/blood-2011-07-364661. [DOI] [PubMed] [Google Scholar]
- 11.Klein JP, Rizzo JD, Zhang MJ. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant. 2001;28(10):909–15. doi: 10.1038/sj.bmt.1703260. [DOI] [PubMed] [Google Scholar]
- 12.Klein JP, Rizzo JD, Zhang MJ. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: Regression modeling. Bone Marrow Transplant. 2001;28(11):1001–11. doi: 10.1038/sj.bmt.1703271. [DOI] [PubMed] [Google Scholar]
- 13.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Hoboken: John Wiley & Sons, Inc.; 2002.
- 14.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–54. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 15.Balgobind BV, Raimondi SC, Harbott J. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114(12):2489–96. doi: 10.1182/blood-2009-04-215152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strehl S, Konig M, Boztug H. All-trans retinoic acid and arsenic trioxide resistance of acute promyelocytic leukemia with the variant STAT5B-RARA fusion gene. Leukemia. 2013;27(7):1606–10. doi: 10.1038/leu.2012.371. [DOI] [PubMed] [Google Scholar]
- 17.Creutzig U, Zimmermann M, Bourquin JP. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from study AML-BFM 2004. Blood. 2013;122(1):37–43. doi: 10.1182/blood-2013-02-484097. [DOI] [PubMed] [Google Scholar]
- 18.Sander A, Zimmermann M, Dworzak M. Consequent and intensified relapse therapy improved survival in pediatric AML: results of relapse treatment in 379 patients of three consecutive AML-BFM trials. Leukemia. 2010;24(8):1422–8. doi: 10.1038/leu.2010.127. [DOI] [PubMed] [Google Scholar]
- 19.Kaspers GJ, Zimmermann M, Reinhardt D. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013;31(5):599–607. doi: 10.1200/JCO.2012.43.7384. [DOI] [PubMed] [Google Scholar]
- 20.Zelent A, Guidez F, Melnick A. Translocations of the RARalpha gene in acute promyelocytic leukemia. Oncogene. 2001;20(49):7186–203. doi: 10.1038/sj.onc.1204766. [DOI] [PubMed] [Google Scholar]
- 21.Creutzig U, Zimmermann M, Reinhardt D. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22(21):4384–93. doi: 10.1200/JCO.2004.01.191. [DOI] [PubMed] [Google Scholar]
- 22.Riley LC, Hann IM, Wheatley K. Treatment-related deaths during induction and first remission of acute myeloid leukaemia in children treated on the Tenth Medical Research Council acute myeloid leukaemia trial (MRC AML10). The MCR Childhood Leukaemia Working Party. Br J Haematol. 1999;106(2):436–44. doi: 10.1046/j.1365-2141.1999.01550.x. [DOI] [PubMed] [Google Scholar]
- 23.Molgaard-Hansen L, Mottonen M, Glosli H. Early and treatment-related deaths in childhood acute myeloid leukaemia in the Nordic countries: 1984–2003. Br J Haematol. 2010;151(5):447–59. doi: 10.1111/j.1365-2141.2010.08389.x. [DOI] [PubMed] [Google Scholar]
- 24.Mann G, Reinhardt D, Ritter J. Treatment with all-trans retinoic acid in acute promyelocytic leukemia reduces early deaths in children. Ann Hematol. 2001;80(7):417–22. doi: 10.1007/s002770100304. [DOI] [PubMed] [Google Scholar]
- 25.Lehrnbecher T, Varwig D, Kaiser J. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia. 2004;18(1):72–7. doi: 10.1038/sj.leu.2403188. [DOI] [PubMed] [Google Scholar]
- 26.Kurt B, Flynn P, Shenep JL. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer. 2008;113(2):376–82. doi: 10.1002/cncr.23563. [DOI] [PubMed] [Google Scholar]
- 27.Marron A, Carratala J, Gonzalez-Barca E. Serious complications of bacteremia caused by Viridans streptococci in neutropenic patients with cancer. Clin Infect Dis. 2000;31(5):1126–30. doi: 10.1086/317460. [DOI] [PubMed] [Google Scholar]
- 28.Creutzig U, Diekamp S, Zimmermann M. Longitudinal evaluation of early and late anthracycline cardiotoxicity in children with AML. Pediatr Blood Cancer. 2007;48(7):651–62. doi: 10.1002/pbc.21105. [DOI] [PubMed] [Google Scholar]
- 29.Creutzig U, Zimmermann M, Dworzak M. Favourable outcome of patients with childhood acute promyelocytic leukaemia after treatment with reduced cumulative anthracycline doses. Br J Haematol. 2010;149(3):399–409. doi: 10.1111/j.1365-2141.2010.08107.x. [DOI] [PubMed] [Google Scholar]
- 30.Müller E, Seidel MG, Lackner H. Acute promyelocytic leukemia complicated by massive intracerebral hemorrhage: safety and efficacy of replacing conventional chemotherapy with arsenic trioxide in an adolescent. Klin Padiatr. 2013;225(3):172–3. doi: 10.1055/s-0033-1334898. [DOI] [PubMed] [Google Scholar]
- 31.Lo-Coco F, Avvisati G, Vignetti M. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–21. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 32.Inaba H, Rubnitz JE, Coustan-Smith E. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. 2011;29(24):3293–300. doi: 10.1200/JCO.2011.34.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niewerth D, Creutzig U, Bierings MB. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116(13):2205–14. doi: 10.1182/blood-2010-01-261800. [DOI] [PubMed] [Google Scholar]
- 34.Woods WG, Neudorf S, Gold S. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97(1):56–62. doi: 10.1182/blood.V97.1.56. [DOI] [PubMed] [Google Scholar]
- 35.Stevens RF, Hann IM, Wheatley K. Marked improvements in outcome with chemotherapy alone in paediatric acute myeloid leukemia: results of the United Kingdom Medical Research Council’s 10th AML trial. MRC Childhood Leukaemia Working Party. Br J Haematol. 1998;101(1):130–40. doi: 10.1046/j.1365-2141.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 36.Klusmann JH, Reinhardt D, Zimmermann M. The role of matched sibling donor allogeneic stem cell transplantation in pediatric high-risk acute myeloid leukemia: results from the AML-BFM 98 study. Haematologica. 2012;97(1):21–9. doi: 10.3324/haematol.2011.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balgobind BV, Zwaan CM, Pieters R. The heterogeneity of pediatric MLL-rearranged acute myeloid leukemia. Leukemia. 2011;25(8):1239–48. doi: 10.1038/leu.2011.90. [DOI] [PubMed] [Google Scholar]
- 38.Inaba H, Coustan-Smith E, Cao X. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol. 2012;30(29):3625–32. doi: 10.1200/JCO.2011.41.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]


