Abstract
Background
Flexible bronchoscopy (FB) and bronchoalveolar lavage (BAL) have major roles in the evaluation of parenchymal lung diseases in immunocompromised patients. Given the limited evidence, lack of standardized practice, and variable perception of procedural safety, uncertainty still exists on what constitutes the best approach in critically ill patients with immunocompromised state who present with pulmonary infiltrates in the era of prophylactic antimicrobials and the presence of new diagnostic tests.
Objective
To evaluate the diagnostic yield, safety and impact of FB and BAL on management decisions in immunocompromised critically ill patients admitted to the intensive care unit (ICU).
Methods
A prospective, observational study of 106 non-HIV immunocompromised patients admitted to the intensive care unit with pulmonary infiltrates who underwent FB with BAL.
Results
FB and BAL established the diagnosis in 38 (33%) of cases, and had a positive impact on management in 44 (38.3%) of cases. Escalation of ventilator support was not required in 94 (81.7%) of cases, while 18 (15.7%) required invasive and 3 (2.6%) required non-invasive positive pressure ventilation after the procedure. Three patients (2.6%) died within 24 h of bronchoscopy, and 46 patients (40%) died in ICU. Significant hypoxemia developed in 5% of cases.
Conclusion
FB can be safely performed in immunocompromised critically ill patients in the ICU. The yield can be improved when FB is done prior to initiation of empiric antimicrobials, within 24 h of admission to the ICU, and in patients with focal disease.
Keywords: Bronchoscopy, Bronchoalveolar lavage, Immunocompromised, Critical care, Intensive care
Background
Immunocompromised patients are at high risk of infectious and non-infectious pulmonary complications, which are associated with significant morbidity and mortality [1, 2].
The lungs of immunocompromised patients are common targets of opportunistic infections including viral and fungal organisms [3]. Up to 25% of immunocompromised patients with pulmonary infiltrates have non-infectious pathologies such as drug-induced pulmonary toxicity, pulmonary edema (including acute respiratory distress syndrome, or graft versus host disease), radiation pneumonitis, recurrence of the underlying disease, an immune-mediated pathology such as vasculitis, or idiopathic pathology (e.g., cryptogenic organizing pneumonia) [4]. The distinction among these pathologies based on the clinical and radiologic features can be difficult. Early identification and treatment of the causative pathogen is essential, and is associated with improved survival [5–7].
Flexible bronchoscopy (FB) has been widely used in the evaluation of pulmonary infiltrates in immunocompromised critically ill patients. Studies performed on bronchoalveolar lavage (BAL) can be helpful in identifying the cause of pulmonary infiltrates [8]. The diagnostic yield of FB and BAL in immunocompromised state has varied in different studies [9–24].
Prior studies excluded some of the most critically ill patients (e.g., on mechanical ventilation) or focused on immunocompromised patients with human immunodeficiency virus (HIV) infection and malignancies, while other immunocompromised populations have not been adequately studied. Such patients usually receive aggressive therapy (including empiric broad-spectrum antimicrobials) which has a significant impact on the yield of diagnostic studies. Furthermore, the yield of FB and BAL may have changed in recent years. Lastly, the impact of FB and BAL on management decisions has not been adequately evaluated in prior studies. In our study, we sought to explore the yield, safety and impact of FB on management in this population.
Materials and Methods
Patients
The study was approved by the Mayo Foundation Institutional Review Board (IRB No. 13-004963). This was a prospective observational study, carried out over 1 year (July 2013 through June 2014). Patients considered eligible for the study were: adults ≥ 18 years, critically ill in the intensive care unit, with evidence of pulmonary infiltrates on chest radiographs, and immunocompromised due to one of the following conditions: hematologic malignancy (e.g., leukemia, lymphoma, multiple myeloma, and myelodysplastic syndrome), active use of immunosuppressive medications, use of a chemotherapeutic agent in the last 6 months, receiving long course (20 mg/day prednisone or its equivalent for at least 2 months) or high-dose (60 mg/day prednisone or its equivalent for 2 weeks within 3 months) corticosteroid therapy, or had stem cell or solid organ transplantation. Since HIV infection is uncommon in Olmsted County, patients with HIV infection were excluded. In addition, we excluded patients who denied their medical records to be used for research.
Data Collection
The following data were collected (from medical records) on the first ICU day for each subject: demographics (age, gender, and ethnicity), the reason for ICU admission, code status, smoking history, Acute Physiology and Chronic Health Evaluation (APACHE) III score, and Sequential Organ Failure Assessment (SOFA) score. In addition, we obtained details of underlying cause of the immunocompromised state, active medications including radiologic findings, and laboratory results including complete blood count, coagulation profile, and tests of the BAL panel used for the immunocompromised host at Mayo Clinic in Rochester MN [cell count and differential, cytology, hemosiderin–laden macrophages, gram stain, aerobic bacterial culture, Legionella polymerase chain reaction (PCR), Legionella culture, Nocardia stain, acid fast smear for Mycobacteria, Mycobacterial culture, Aspergillus antigens, fungal smear, fungal culture, adenovirus PCR, influenza A and B PCR, respiratory syncytial virus (RSV) PCR, viral culture, Pneumocystis PCR, and galactomannan]. Active use of antimicrobial prophylaxis was documented. Prophylaxis varied by nature of the immune-compromised state, and included prophylaxis against PCP, cytomegalovirus, herpes simplex virus, varicella-zoster, and fungal pathogens. The critical care physician performing the procedure was not aware of the ongoing study. The clinical presentation, laboratory (serology, microbiological tests, results of BAL) tests, radiographic features on chest imaging, response to therapy, histopathologic and post-mortem findings were used to establish the final diagnosis.
FB and BAL
FB was performed through an endotracheal tube in all patients according to the standard practice at Mayo Clinic. All patients had BAL sampling, but transbronchial biopsy was performed in only eight patients, of which five were on mechanical ventilation before the procedure. BAL samples were taken from the most prominently affected pulmonary segment. Adequate sample for the immunocompromised host panel was defined as recovery of at least 40 ml of BAL fluid following the installation of 100 ml of sterile saline. FB and BAL were performed more than once on few patients during the same hospital stay.
Diagnostic Definitions
Identification of bacterial organisms from BAL or tissue sample along with characteristic clinical features (temperature ≥ 38.3 °C, cough, expectoration of purulent material), and radiologic findings were used to define bacterial pneumonia [25]. The diagnosis of viral pneumonia was made based on the characteristic radiographic features, in the presence of: positive real-time-PCR assay from BAL sample; or identification of virus on tissue culture (or typical viral inclusions) in lung biopsy; or associated viremia. The consensus of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group was used to define proven, probable and possible aspergillosis [26].
Cardiogenic pulmonary edema was diagnosed based on the clinical presentation, in conjunction with cardiac studies such as echocardiography. The diagnosis of alveolar hemorrhage was made when progressive bloody return was encountered on sequential aliquots or > 20% of hemosiderin–laden macrophages identified on BAL cytology. Acute lung rejection was defined by the presence of perivascular and interstitial mononuclear cell infiltrates based on the revised Working Formulation for Classification and Grading of Pulmonary Allograft Rejection. The Spitzer criteria were used to diagnose engraftment syndrome, while other non-infectious complications (e.g., drug-induced toxicity) were diagnosed based on the constellation of characteristic clinical and radiographic features in the absence of infection [27].
Statistical Analysis
All statistical analyses were performed using JMP statistical software and SAS (version 9.0, SAS institute, Cary, NC). Data were summarized as median (interquartile range, IQR) for continuous variables or percentages for categorical variables.
Nine patients received more than one bronchoscopic evaluation during the same hospitalization but on different ICU admission. Therefore, we reported diagnostic yield, impact on management, and complications as percentage per total number of procedures performed. The diagnostic yield of BAL was the percentage of positive microbiologic testing or percentage of cases in which the diagnosis is established based on BAL results. Impact of FB on management was the percentage of cases in whom the therapeutic plan was changed by addition or withdrawal of an antimicrobial or anti-inflammatory agent based on bronchoscopic findings or BAL results. The χ2 test was used to assess the association between categorical variables. Descriptive statistics are also reported, including the frequency and percentage for each category of a categorical variable and the median (IQR) for continuous variables. Univariate and multivariate logistic regression models were also assessed and the odds ratio and confidence intervals (CIs) are reported. A two-sided p value of ≤ 0.05 was considered to be significant.
Results
Patients
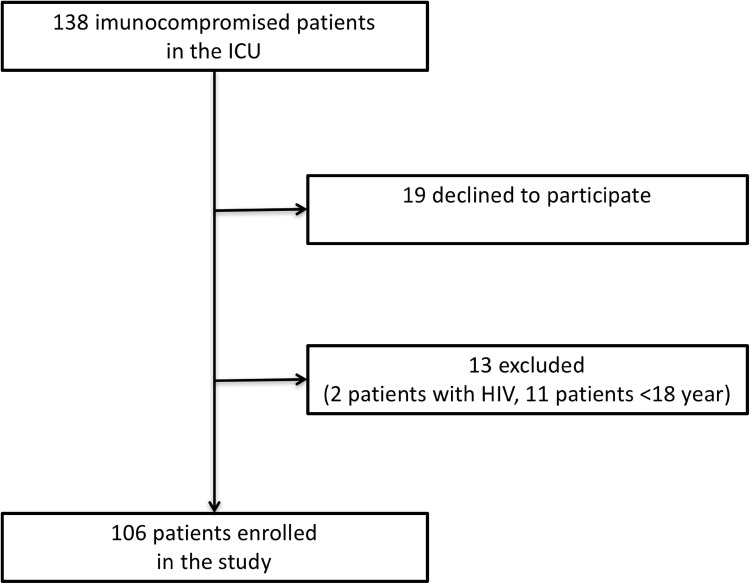
The study included 138 consecutive non-HIV immunocompromised patients who were admitted to the intensive care unit with lung infiltrates, who underwent diagnostic bronchoscopy (BAL with or without transbronchial biopsies). Of those, 106 patients fulfilled the inclusion criteria and had 115 bronchoscopic evaluations (Fig. 1).
Fig. 1.
Consort diagram of screened and included patients
The baseline and demographic features of the study population are summarized in Table 1. The majority of patients were males (62%) and former smokers (64%). Patients were categorized into five groups based on the underlying etiology of the immune-compromised state. Thirty-four patients (32%) had hematologic malignancy, 12 patients (11%) had recent chemotherapy, and 28 patients (26.5%) were immunocompromised due to chronic or high-dose corticosteroids. Twenty-eight patients (26.5%) had received transplantation (10 patients had received allogenic or autologous stem cell transplantation and 18 had received solid organ transplant). The remaining four patients (4%) were receiving active immunosuppressant therapy at the time of admission to the ICU for various indications (connective tissue disease or inflammatory disorder). Acute hypoxemic respiratory failure was the most common cause for ICU admission (64%), followed by severe sepsis or septic shock (19%). The pattern of infiltrates seen on chest imaging (chest X-ray or computed tomography) was divided into: focal (infiltrate confined to one lobe) or diffuse (involving either both lungs, or more than one lobe of one lung). Only 17 patients (16%) had focal pattern.
Table 1.
Baseline characteristics of the patients
| Characteristic | |
|---|---|
| Age, median (IQR) | 61 (54–71) |
| Sex, n (%) | |
| Male | 66 (62) |
| Female | 40 (38) |
| Smoking status, n (%) | |
| Current | 10 (10) |
| Former | 68 (64) |
| Never | 28 (26) |
| Reason for ICU admission, n (%) | |
| Respiratory failure | 68 (64) |
| Severe sepsis/septic shock | 20 (19) |
| Others | 18 (17) |
| Underlying immune-compromised state, n (%) | |
| Hematologic malignancy | 34 (32) |
| Recent chemotherapy within 6 months | 12 (11) |
| Chronic or high-dose corticosteroids | 28 (26.5) |
| Transplantation (stem cell or solid organ) | 28 (26.5) |
| Active immunosuppressive therapy | 4 (4) |
| Disease pattern on chest imaging, n (%) | |
| Diffuse disease | 89 (84) |
| Focal disease | 17 (16) |
| Ventilatory support prior to bronchoscopy, n (%) | |
| None | 30 (28) |
| Non-invasive positive pressure ventilation | 10 (9) |
| Mechanical ventilation | 66 (63) |
| Severity of illness, median (IQR) | |
| APACHE III | 83 (59–106) |
| SOFA | 8 (5–12) |
| PaO2/FiO2 ratio prior to bronchoscopy, median (IQR) | 179 (130–247) |
| Prophylactic antimicrobial prior to bronchoscopy, n (%) | |
| No prophylaxis | 59 (56) |
| Prophylaxis | 47 (44) |
| Empiric antimicrobial prior to bronchoscopy, n (%) | |
| No empiric antimicrobial | 7 (7) |
| Empiric antimicrobial | 99 (93) |
| Time to bronchoscopy (h), median (IQR) | 9.6 (2.8–39.5) |
Non-invasive positive pressure ventilation (NIPPV) was used in 10 patients (9%) prior to bronchoscopy, while 66 patients (63%) were on mechanical ventilation at the time of bronchoscopy.
Forty-seven patients (44%) were receiving appropriate prophylactic antimicrobials. The majority of patients (93%) were treated with empiric antimicrobial agents prior to undergoing FB, while the remaining were believed to have non-infectious pathology and, therefore, did not receive antimicrobials prior to BAL.
Diagnostic Yield
The diagnosis was made by FB in 38 of 115 (33%) bronchoscopic procedures, of which 26 (22.6%) were infectious (some patients had more than one pathogen identified), and 12 (10.4%) were non-infectious. Patients in the corticosteroids group had the highest yield (43.3%) followed by 33.3% in patients with recent chemotherapy. The yield was lowest in the active immunosuppressant group (25%). However, there was no significant difference in the yield based on the etiology of compromised immune status (p = 0.7, Table 2). The ICU mortality in patients with positive BAL was 26% compared to 30% in patients with no diagnosis made by BAL (p = 0.83). Moreover, hospital mortality was not different in patients with positive BAL compared to those with negative BAL (34% and 37% respectively, p = 0.84).
Table 2.
Diagnostic yield (per procedure) by immune status
| Patient groups by immune status (N = 115) | Yield (%) |
|---|---|
| Hematologic malignancies (n = 35) | 31.4 |
| Recent chemotherapy within 6 months (n = 12) | 33.3 |
| Prolonged or high-dose corticosteroids (n = 30) | 43.3 |
| Stem cell or solid transplant (n = 34) | 26.5 |
| Active immunosuppressant (n = 4) | 25 |
Viral Infections
Viral pneumonia was diagnosed in 13 patients. Influenza A was the most common cause (six patients), followed by cytomegalovirus (four patients). Other identified viral infections included: parainfluenza (one patient), RSV (one patient), and metapneumovirus (one patient).
Bacterial Infections
Eight patients had bacterial pneumonia diagnosed by BAL. Staphylococcus aureus was diagnosed in three patients (two methicillin-sensitive S. aureus, one methicillin-resistant S. aureus), while two patients had Stenotrophomonas maltophilia. Other bacterial pneumonias diagnosed by BAL include: Streptococcus pneumoniae (one patient), Serratia marcescens (one patient), and Haemophilus influenza (one patient).
Fungal Infections
The diagnosis of fungal pneumonia was made in 10 patients. Aspergillus fumigatus was the most commonly identified fungus (six patients), followed by Pneumocystis jiroveci ‘PJP’ (three patients). One patient had disseminated infection due to Histoplasma capsulatum. Interestingly, one patient in the corticosteroids group had a negative BAL but was diagnosed to have severe PJP pneumonia based on positive sputum sample.
Alveolar Hemorrhage
Among the non-infectious diagnoses made by BAL, alveolar hemorrhage was the most common (11 cases). Ten of these were diffuse and bilateral, while one patient had focal hemorrhage.
Lung Rejection
Lung rejection was identified in two patients in the transplant group based on bronchoscopy and transbronchial biopsies.
Other non-infectious diagnoses made by bronchoscopic evaluation are: myeloid infiltration (one patient), bronchial obstruction by tumor (one patient), and broncholith (one patient).
Non-invasive Tests
In a subset of cohort, there was one positive test out of 67 for Influenza A on nasopharyngeal swab for viral PCR. Similarly, one was positive for S. pneumoniae antigens (Ag) on urine test for streptococcal, histoplasmosis and legionella Ags. None was positive on serum fungal Ag for 1-3-β-d-Glucan (BDG), Galactomannan, and Cryptococcus Ag. Total of two fungal cultures were positive on sputum culture for Candida lusitaniae and A. fumigatus. Overall, the yield of non-invasive test was very low.
In a univariate analysis (Table 3), the diagnostic yield was significantly higher in patients who did not receive antimicrobials prior to bronchoscopy (85.7% vs. 14.3%, p = 0.002). Focal pattern on chest imaging was associated with a high diagnostic yield compared to diffuse pattern (58.8% vs. 41.2%, p = 0.02). When performed early (within 24 h of admission to the ICU), the yield was higher compared to late bronchoscopy (41% vs 17%, p = 0.02).
Table 3.
Univariate analysis of BAL yield
| Patient characteristics | Positive yield (N = 38) |
Negative yield (N = 77) |
p value |
|---|---|---|---|
| Male gender, n (%) | 23 (60.5) | 45 (58.4) | 1.0 |
| Age, years, median (IQR) | 60 (55–70) | 60 (54.59.9) | 0.75 |
| APACHE III at admission, median (IQR) | 80 (60–98) | 84 (59–107) | 0.43 |
| SOFA day-1, median (IQR) | 8 (4–10) | 9 (6–13) | 0.09 |
| Focal pattern on chest imaging, n (%) | 10 (58.8) | 7 (41.2) | 0.02* |
| No empiric antibiotics prior to bronchoscopy, n (%) | 6 (85.7) | 1 (14.3) | < 0.01* |
| Bronchoscopy within 24 h of admission to the ICU, n (%) | 32 (40.5) | 6 (17) | 0.02* |
*Indicates statistical significance
A multivariate logistic regression model adjusting for focal pattern on chest imaging, prior use of antimicrobials, and time between ICU admission and bronchoscopy (Table 4) revealed a significant difference between focal and diffuse X-ray. Focal X-ray findings were associated with higher yield [odds ratio 3.24 (95% CI 1.08–10.9), p = 0.04]. Absence of empiric antimicrobials prior to bronchoscopy strongly predicted positive yield [odds ratio 12.9 (95% CI 1.9–262), p < 0.01]. Furthermore, early bronchoscopy (performed within 24 h of ICU admission) was associated with significantly higher yield compared to late bronchoscopy [odds ratio 3.2 (95% CI 1.2–9.7), p = 0.02].
Table 4.
Multivariate analysis of BAL yield
| Odds ratio | 95% CI | p value | |
|---|---|---|---|
| Focal pattern on chest imaging | 3.37 | 1.08–10.9 | 0.04* |
| No empiric antimicrobial prior to bronchoscopy | 12.9 | 1.9–262 | < 0.01* |
| Bronchoscopy within 24 h of ICU admission | 3.2 | 1.2–9.7 | 0.02* |
*Indicates statistical significance
Impact of BAL on Management
The overall impact of BAL was positive in 44 cases (38.3%). The impact was most notable in patients receiving corticosteroids therapy (56.7%) and patients with recent chemotherapy (41.7%) and lowest in patients with active immunosuppressive therapy (25%) (Table 5).
Table 5.
Therapeutic impact (per procedure) by immune status
| Patient groups by immune status (N = 115) | Positive impact (%) |
|---|---|
| Hematologic malignancies (n = 35) | 34.3 |
| Recent chemotherapy within 6 months (n = 12) | 41.7 |
| Prolonged or high-dose corticosteroids (n = 30) | 56.7 |
| Stem cell or solid transplant (n = 34) | 26.6 |
| Active immunosuppressant (n = 4) | 25 |
Safety
Invasive mechanical ventilation was required in 18 cases (15.7%) after bronchoscopy, while NIPPV had to be started in three cases (2.6%). Patients spent median (IQR) 5.6 (2.2–11.7) days on mechanical ventilation; and those who required mechanical ventilation had similar ICU and hospital mortality to those who didn’t.
A small pneumothorax developed in one patient (out of eight) who underwent transbronchial biopsies. Three patients (2.6%) died within 24 h of performing the procedure (septic shock in two patients, and withdrawal of care due to futility in one patient), and 46 patients (40%) during the same ICU admission. Significant worsening in hypoxemia (decrease in PaO2/FiO2 ratio by 50% or increase oxygen requirement by 50%) was observed in five cases (4.3%). Other reported complications included new atrial fibrillation (one patient) and non-ST elevation myocardial infarction (one patient).
Discussion
Our study suggests a lower diagnostic yield among critically ill patients with immunocompromised states than previously reported in each group alone. The yield, however, was significantly higher in patients with focal disease, in those who received no antimicrobial prior to the procedure, and in those who underwent FB within 24 h of admission to the ICU.
Early initiation of empiric broad-spectrum antimicrobials in critically ill patients with possible sepsis is a common and appropriate practice. Therapy is typically initiated prior to arrival to the intensive care unit, especially in the immunocompromised. Moreover, the widespread practice of prophylaxis against certain infections (e.g., P. jiroveci) has resulted in reduced incidence of these infections. Therefore, a low yield for infectious etiology can be anticipated.
Non-infectious complications (with few exceptions such as diffuse alveolar hemorrhage) commonly require tissue (rather than BAL) samples for diagnosis, which require more invasive procedures (e.g., transbronchial biopsy). Such procedures are usually avoided in critically ill patients, especially those on mechanical ventilation due to risk of complications. In our study, only eight patients underwent transbronchial biopsies.
The pattern on chest imaging may reflect the nature of the underlying pathology and, therefore, predict the yield. A focal pattern is more likely to be secondary to infectious process (bacterial or fungal), while a bilateral pattern could be infectious (viral) or non-infectious (e.g., ARDS). Despite the low percentage of positive BAL for pathogens, many patients were still considered to have possible pneumonia. Consequently, differentiation between non-infectious and infectious pathologies with negative BAL (e.g., due to prior antimicrobial therapy) can be extremely difficult.
Despite the potential limitations, FB with BAL is still frequently performed to identify the diagnosis. Given the safety of FB with BAL, we suggest that bronchoscopy be performed early (preferably within 24 h of ICU admission), in immunocompromised patients, especially those with focal disease.
In non-HIV patients (similar to our population), the diagnostic yield of FB with BAL was 52–84% in prior studies [9, 10, 28]. Similar yield was observed in immunocompromised patients due to neutropenia, where the diagnosis was made in 49% of patients based on FB and BAL [9]. However, those studies included immunocompromised patients who underwent FB with BAL regardless of their severity of illness. In a recent study by Cracco et al., the diagnostic yield of FB in critically ill non-intubated patients with respiratory failure was 59% [29]. This study included patients regardless of their immune status.
Shannon et al. reported similar improvement in diagnostic yield of FB when the procedure was performed within 24 h of presentation among hematopoietic stem cell transplant recipients [30].
Kottomann et al. also reported a higher yield in immunocompromised patients (56% positive yield), and the yield was significantly higher when performed within the first 3 days of starting antimicrobials. However, the immune deficiency state was not specified and the study was not limited to critically ill patients [31].
Complications of FB are rare (0.5–0.8%) and usually minor [32]. Significant hypoxemia and need for escalation of ventilatory support found in our study are consistent with the recent findings by Cracco et al. [29]. In their study, 35% of critically ill patients with respiratory failure required increase in ventilator support, and 15% required intubation. The presence of immunocompromised state was associated with significant risk of intubation following the procedure. In a randomized multi-center trial by Azoulay and Schlemmer the need for NIPPV and mechanical ventilation following BAL was slightly less (13% and 11% respectively) compared to our study. However, baseline PaO2/FiO2 ratio in their study was higher, reflecting better respiratory status prior to bronchoscopy and BAL. In addition, their study was limited to cancer patients (82% hematologic malignancies) [33].
Our study is limited to a single tertiary center with complex patients. Other limitations include the under-representation of certain immunocompromised patients (i.e., active immune suppressive therapy). Furthermore, HIV patients were not included due to the limited number of HIV patients encountered at our institution. The observational nature of our study further limits the ability to draw firm conclusions about the benefit of bronchoscopy and its effects on outcomes and made it difficult to control for some factors such as standard BAL technique and physician practices. Another major limitation of our study would be the lack of information on non-invasive tests on all patients, thus the interpretation of BAL yield can be difficult.
Conclusion
FB is safe in immunocompromised critically ill patients. The yield of FB is improved when done within 24 h of admission, in patients with focal disease, and in those who have not received empiric antimicrobials.
Author Contributions
MOA, RCC, SGP: performed the literature searches, contributed in the study design, the acquisition, analysis and interpretation of data, and writing of the manuscript. RK: participated in data collection, analysis and interpretation. SK: participated in data collection. MOA, RCC, RK, SK, SGP: revised the manuscript.
Funding
This publication was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study, and the study was approved by the Mayo Foundation Institutional Review Board (IRB No. 13-004963). This article does not contain any studies with animals performed by any of the authors.
References
- 1.Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2004;170:22–48. doi: 10.1164/rccm.200309-1322SO. [DOI] [PubMed] [Google Scholar]
- 2.White P, Bonacum JT, Miller CB. Utility of fiberoptic bronchoscopy in bone marrow transplant patients. Bone Marrow Transplant. 1997;20:681–687. doi: 10.1038/sj.bmt.1700957. [DOI] [PubMed] [Google Scholar]
- 3.Hiorns MP, Screaton NJ, Muller NL. Acute lung disease in the immunocompromised host. Radiol Clin North Am. 2001;39:1137–1151. doi: 10.1016/S0033-8389(05)70335-3. [DOI] [PubMed] [Google Scholar]
- 4.Conces DJ., Jr Pulmonary infections in immunocompromised patients who do not have acquired immunodeficiency syndrome: a systematic approach. J Thorac Imaging. 1998;13(4):234–246. doi: 10.1097/00005382-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Oh YW, Effmann EL, Godwin JD. Pulmonary infections in immunocompromised hosts: the importance of correlating the conventional radiologic appearance with the clinical setting. Radiology. 2000;217(3):647–656. doi: 10.1148/radiology.217.3.r00dc35647. [DOI] [PubMed] [Google Scholar]
- 6.Rañó A, Agustí C, Benito N, Rovira M, Angrill J, Pumarola T, et al. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2002;122:253–261. doi: 10.1378/chest.122.1.253. [DOI] [PubMed] [Google Scholar]
- 7.Dunagan DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of pulmonary infiltrates following bone marrow transplantation. Chest. 1997;111:135–141. doi: 10.1378/chest.111.1.135. [DOI] [PubMed] [Google Scholar]
- 8.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741–1751. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 9.Peikert T, Rana S, Edell E. Safety, diagnostic yield, and therapeutic implications of flexible bronchoscopy in patients with febrile neutropenia and pulmonary infiltrates. Mayo Clin Proc. 2005;80(11):1414–1420. doi: 10.4065/80.11.1414. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein RA, Rohatgi PK, Bergosfsky EH, American Thoracic Society et al. Clinical role of bronchoalveolar lavage in adults with pulmonary disease. Am Rev Respir Dis. 1990;142:481–486. doi: 10.1164/ajrccm/142.2.481. [DOI] [PubMed] [Google Scholar]
- 11.Martin WJ, II, Smith TF, Sanderson DR, et al. Role of bronchoalveolar lavage in the assessment of opportunistic pulmonary infections: utility and complications. Mayo Clin Proc. 1987;62:549–557. doi: 10.1016/S0025-6196(12)62292-7. [DOI] [PubMed] [Google Scholar]
- 12.Stover DE, Zaman MB, Hajdu SI, et al. Bronchoalveolar lavage in the diagnosis of diffuse pulmonary infiltrates in the immunosuppressed host. Ann Intern Med. 1984;101:1–7. doi: 10.7326/0003-4819-101-1-1. [DOI] [PubMed] [Google Scholar]
- 13.Glazer M, Breuer R, Berkman N, et al. Use of fiberoptic bronchoscopy in bone marrow transplantation recipients. Acta Haematol. 1998;99:22–26. doi: 10.1159/000040710. [DOI] [PubMed] [Google Scholar]
- 14.Marra R, Pagano L, Pagliari G, et al. The yield of bronchoal-veolar lavage in the etiological diagnosis of pneumonia in leukemia and lymphoma patients. Eur J Haematol. 1993;51:256–258. doi: 10.1111/j.1600-0609.1993.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 15.Von Eiff M, Zuhlsdorf M, Roos N, et al. Pulmonary infiltrates in patients with hematologic malignancies: clinical usefulness of non-invasive bronchoscopic procedures. Eur J Haematol. 1995;54:157–162. doi: 10.1111/j.1600-0609.1995.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 16.Rano A, Agusti C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax. 2001;56:379387. doi: 10.1136/thorax.56.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson B-M, Dahl H, Wang F-Z, et al. Diagnosis of pulmonary infections in immunocompromised patients by fiberoptic bronchoscopy with bronchoalveolar lavage and serology. Scand J Infect Dis. 1996;28:479–485. doi: 10.3109/00365549609037944. [DOI] [PubMed] [Google Scholar]
- 18.Huaringa AJ, Leyva FJ, Signes-Costa J, et al. Bronchoalveolar lavage in the diagnosis of pulmonary complications of bone marrow transplant patients. Bone Marrow Transplant. 2000;25:975–979. doi: 10.1038/sj.bmt.1702335. [DOI] [PubMed] [Google Scholar]
- 19.Pisani RJ, Wright AJ. Clinical utility of bronchoalveolar lavage in immunocompromised hosts. Mayo Clin Proc. 1992;67:221–227. doi: 10.1016/S0025-6196(12)60096-2. [DOI] [PubMed] [Google Scholar]
- 20.Cordonnier C, Bernaudin J-F, Fleury J, et al. Diagnostic yield of bronchoalveolar lavage in pneumonitis occurring after allogenic bone marrow transplantation. Am Rev Respir Dis. 1985;132:11181123. doi: 10.1164/arrd.1985.132.5.1118. [DOI] [PubMed] [Google Scholar]
- 21.Young JA, Hopkin JM, Cuthbertson WP. Pulmonary infiltrates in immunocompromised patients: diagnosis by cytological examination of bronchoalveolar lavage fluid. J Clin Pathol. 1984;37:390–397. doi: 10.1136/jcp.37.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breuer R, Lossos IS, Lafair JS, Engelhard D. Utility of bronchoalveolar lavage in the assessment of diffuse pulmonary infiltrates in nonAIDS immunocompromised patients. Respir Med. 1990;84(4):313–316. doi: 10.1016/S0954-6111(08)80059-5. [DOI] [PubMed] [Google Scholar]
- 23.Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest. 2004;125(2):712–722. doi: 10.1378/chest.125.2.712. [DOI] [PubMed] [Google Scholar]
- 24.Azoulay E, Schlemmer B. Diagnostic strategy in cancer patients with acute respiratory failure. Intensive Care Med. 2006;32(6):808–822. doi: 10.1007/s00134-006-0129-2. [DOI] [PubMed] [Google Scholar]
- 25.Aguilar-Guisado M, Jiménez-Jambrina M, Espigado I, Rovira M, Martino R, Oriol A, et al. Pneumonia in allogeneic stem cell transplantation recipients: a multicenter prospective study. Clin Transplant. 2011;25:E629–E638. doi: 10.1111/j.1399-0012.2011.01495.x. [DOI] [PubMed] [Google Scholar]
- 26.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–898. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 28.Lucena CM, Torres A, Rovira M, et al. Pulmonary complications in hematopoietic SCT: a prospective study. Bone Marrow Transplant. 2014 doi: 10.1038/bmt.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cracco C, Fartoukh M, Prodanovic H, et al. Safety of performing fiberoptic bronchoscopy in critically ill hypoxemic patients with acute respiratory failure. Intensive Care Med. 2013;39(1):45–52. doi: 10.1007/s00134-012-2687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shannon VR, Andersson BS, Lei X, et al. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:647–665. doi: 10.1038/bmt.2009.203. [DOI] [PubMed] [Google Scholar]
- 31.Kottomann RM, Kelly J, Lyda E, et al. Bronchoscopy with bronchoalveolar lavage: determinants of yield and impact on management in immunosuppressed patients. Thorax. 2011;66(9):823. doi: 10.1136/thx.2010.145540. [DOI] [PubMed] [Google Scholar]
- 32.Pue CA, Pacht ER. Complications of fiberoptic bronchoscopy at a university hospital. Chest. 1995;107:430. doi: 10.1378/chest.107.2.430. [DOI] [PubMed] [Google Scholar]
- 33.Azoulay E, Mokart D, Lambert J, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: a randomized controlled trial. Am J Respir Crit Care Med. 2010;182(8):1038–1046. doi: 10.1164/rccm.201001-0018OC. [DOI] [PubMed] [Google Scholar]