Abstract
Lipopolysaccharide (LPS)-induced autophagy inhibition in lung fibroblasts is closely associated with the activation of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K-Akt-mTOR) pathway. However, the underlying mechanism remains unknown. In this study, we demonstrated that LPS activated the PI3K-Akt-mTOR pathway and inhibited lung fibroblast autophagy by depleting thymocyte differentiation antigen-1 (Thy-1) and upregulating integrin β3 (Itgb3). Challenge of the human lung fibroblast MRC-5 cell line with LPS resulted in significant upregulation of integrin β3, activation of the PI3K-Akt-mTOR pathway and inhibition of autophagy, which could be abolished by integrin β3 silencing by specific shRNA or treatment with the integrin β3 inhibitor cilengitide. Meanwhile, LPS could inhibit Thy-1 expression accompanied with PI3K-Akt-mTOR pathway activation and lung fibroblast autophagy inhibition; these effects could be prevented by Thy-1 overexpression. Meanwhile, Thy-1 downregulation with Thy-1 shRNA could mimic the effects of LPS, inducing the activation of PI3K-Akt-mTOR pathway and inhibiting lung fibroblast autophagy. Furthermore, protein immunoprecipitation analysis demonstrated that LPS reduced the binding of Thy-1 to integrin β3. Thy-1 downregulation, integrin β3 upregulation and autophagy inhibition were also detected in a mouse model of LPS-induced pulmonary fibrosis, which could be prohibited by intratracheal injection of Thy-1 overexpressing adeno-associated virus (AAV) or intraperitoneal injection of the integrin β3 inhibitor cilengitide. In conclusion, this study demonstrated that Thy-1 depletion and integrin β3 upregulation are involved in LPS-induced pulmonary fibrosis, and may serve as potential therapeutic targets for pulmonary fibrosis.
Subject terms: Diseases, Cell biology
This study confirms that the depletion of Thy-1 and upregulation of integrin β3 is an essential mechanism and potential therapeutic target for PI3K-Akt-mTOR pathway-associated inhibition of lung fibroblast autophagy in lipopolysaccharide-induced pulmonary fibrosis.
Introduction
Pulmonary fibrosis is a well-recognized feature of acute respiratory distress syndrome (ARDS) [1–3], and lipopolysaccharide (LPS) is a considerable factor causing sepsis-associated ARDS and pulmonary fibrosis [4–6]. However, the related mechanisms are not fully understood. Previous studies have shown that autophagy inhibition in lung tissues is closely associated with pulmonary fibrosis [7, 8]. In addition, our recent study revealed that LPS could inhibit lung fibroblast autophagy by activating the phosphatidylinositol-3-kinase-protein kinase B-mammalian target of rapamycin (PI3K-Akt-mTOR) pathway [9]. However, the mechanism underlining LPS-induced PI3K-Akt-mTOR pathway activation and lung fibroblast autophagy inhibition remains largely unknown.
Thy-1 is a glycoprotein anchored by glycophosphatidylinositol (GPI) on the surface of fibroblasts [10]. Moderate expression of Thy-1 was detected in lung fibroblasts of normal lung tissues [11]. However, reduced Thy-1 expression was reported to be associated with aberrant proliferation and activation of lung fibroblasts in LPS or bleomycin-induced pulmonary fibrosis [12, 13]. Thy-1 also binds to integrin β3 (Itgb3) and inhibits skin dermal cell proliferation, and its depletion could induce the proliferation of skin dermal cells [14].
Integrins, which serve as a bridge between the extracellular matrix and the intracellular cytoskeleton, transduce a variety of signals from the extracellular matrix that affect cell survival and differentiation [15, 16]. Integrin β3 is the main integrin heterodimer on the surface of lung fibroblasts [17]. Previous studies have shown a protective role for integrin β3 in lung disease, e.g., programmed cell death prevention in lung fibroblasts in response to transforming growth factor-β1 (TGF-β1) stimulation with activation of survival signals during TGF-β1 stimulation [18]. However, little is known regarding the role of integrin β3 in sepsis-induced pulmonary fibrosis. Multiple studies suggested that integrins are involved in the regulation of autophagy by activating its downstream PI3K-Akt signaling pathway, thereby controlling lung fibroblast viability and migration [17, 19]. Therefore, we hypothesized that in LPS-induced pulmonary fibrosis, LPS may activate the PI3K-Akt-mTOR pathway and inhibit lung fibroblast autophagy by downregulating Thy-1 and upregulating integrin β3.
In this study, based on the cellular model of LPS-induced lung fibroblast autophagy inhibition and a mouse model of LPS-induced pulmonary fibrosis, we aimed to assess the role of Thy-1 and integrin β3 in the process of LPS-induced PI3K-Akt-mTOR pathway activation and lung fibroblast autophagy inhibition through genetic or pharmacological interventions.
Material and methods
Ethics statement and animals
Male C57BL/6 mice (8-week-old; 20–25 g) were obtained from Shanghai SLAC Laboratory Animal, China. Animals were housed under controlled temperature (22–24 °C) under a 12 h/12 h light/dark cycle, with free access to food and tap water. All procedures in this study were carried out in accordance with the guidelines for animal care published by the United States’ National Institutes of Health (NIH) for animal care (Guide for the Care and Use of Laboratory Animals, Department of Health and Human Services, NIH Publication No. 86–23, revised 1985). The study was approved by Renji hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China (Permit number: RJ-20170930).
Regents and antibodies
Lipopolysaccharide (Escherichia coli O127:B8) was purchased from Sigma (USA). The integrin β3 inhibitor cilengitide (#S7077) was purchased from Selleckchem (USA). The primary antibodies used in this study were: rabbit anti-Thy-1 (ab225, Abcam, USA), mouse anti- integrin β3 (sc-46655, Santa Cruz, USA), rabbit anti-LC3 I/II (L7453, Sigma, USA), rabbit anti-p62/SQSTM1 (#5114, Cell Signaling Technology, USA), rabbit anti-Akt (#4691, Cell Signaling Technology, USA), rabbit anti-p-Akt (#4060, Cell Signaling Technology, USA), rabbit anti-mTOR (#2983, Cell Signaling Technology, USA), rabbit anti-p-mTOR (#2971, Cell Signaling Technology, USA) and mouse anti-GAPDH (30201ES20, Yeasen, China). Goat anti-mouse (A0216, Beyotime, China) and goat anti-rabbit (A0208, Beyotime, China) secondary antibodies were used as well.
Experimental design and treatment
The human lung fibroblast MRC-5 cell line was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and cultured in Minimum Essential Medium (MEM) (Gibco, Grand island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco), at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
MRC-5 cells in the logarithmic growth phase were seeded into 6-well plates at a density of 2 × 105 cells/mL (2 mL in each well), and stimulated with 1 μg/ml LPS to generate an autophagy inhibition model. Then, treatment with cilengitide (#S7077, Selleck, an integrin β3 inhibitor), integrin β3 knockdown lentivirus (Itgb3-KD), Thy-1 knockdown lentivirus (Thy-1-KD) and Thy-1 overexpression lentivirus (Thy-1-OE) (Genomeditech, Shanghai, China) were used to inhibit or overexpress integrin β3 or Thy-1 at the protein and gene levels. Cells were collected after 6 and 24 h, respectively, for the detection of signaling molecules and autophagy-associated proteins.
Animal experiments were carried out in C57/BL6 mice; 5 mg/kg LPS was intraperitoneally injected for 5 days to establish a pulmonary fibrosis model [20], and the integrin β3 inhibitor cilengitide was intraperitoneally injected to inhibit integrin β3, while Thy-1 overexpression adeno-associated virus (AAV) was intratarsally injected to overexpress Thy-1. Lung tissue samples were collected 30 days after LPS stimulation.
Lentivirus transfection
MRC-5 cells were seeded in a 12-well plate at a density of 5 × 104 cells/well. Subsequently, lentivirus (Shanghai GeneChem, China) and polybrene (5 μg/ml) were added to each well. Transfection efficiency was assessed by green fluorescent protein (GFP) detection, and cells were selected with puromycin (CAS 58–58–2, Santa Cruz, USA) (2 μg/ml). Stable cell lines were established after a 1-week of selection. The expression levels of relevant proteins (Thy-1 and integrin β3) were examined by Western blot.
The primers used were: Thy-1, F-5′-TCGCTCCTGCTAACAGTCT-3′ and R-5′-AGACTGTTAGCAGGAGAGCGA-3′; integrin β3, F-5′-GTGACCTGAAGGAGAATCTGC-3′ and R-5′-CCGGAGTGCAATCCTCTGG-3′.
Thy-1 overexpression by AAV transfection
The mouse Thy-1 was cloned from the PGMThy-1 plasmid by polymerase chain reaction (PCR) using the following primers: forward 5′-CCGGAATTCGCCACCATGAACCCAGCCATCAGCG-3′ and reverse 5′- CCGGGATCCCAGAGAAATGAAGTCCAGGGCTTG-3′. All AAVs are purchased from Genomeditech (Shanghai, China).
AAV expressing vector or Thy-1 was delivered to the mouse lung by intratarsal injection in 50 μl PBS containing 5 × 1010 μg per mouse, and termed vector-AAV and Thy-1-AAV mice, respectively.
Western blot
The protein samples used for western blot were extracted with RIPA lysis buffer (Beyotime Biotechnology, China) supplemented with protease inhibitors (Roche, China). Protein concentrations were determined with the bicinchoninic acid (BCA) assay kit (Thermo scientific, USA). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis, transferred onto polyvinyl fluoride membranes (Merck KGaA, Darmstadt, Germany), and incubated with primary (rabbit anti-Thy-1 (ab225, Abcam, US), mouse anti- integrin β3 (sc-46655, Santa Cruz, USA), rabbit anti-LC3 I/II (L7453, Sigma, USA), rabbit anti-p62/SQSTM1 (#5114, Cell Signaling Technology, USA), rabbit anti-Akt (#4691, Cell Signaling Technology, USA), rabbit anti-p-Akt (#4060, Cell Signaling Technology, USA), rabbit anti-mTOR (#2983, Cell Signaling Technology, USA), rabbit anti-p-mTOR (#2971, Cell Signaling Technology, USA) and mouse anti-GAPDH (30201ES20, Yeasen, China) antibodies, respectively. This was followed by incubation with the appropriate secondary antibodies, including goat anti-mouse (A0216, Beyotime, China) and goat anti-rabbit (A0208, Beyotime, China) antibodies, respectively. Signals were detected using the ECL Plus Western blotting system kit (Beyotime Biotechnology, China); band intensity was measured with the Image LabTM software (Bio-Rad, USA).
Transmission electron microscopy (TEM)
A total of 1 × 105 MRC-5 cells were fixed by treatment with fresh 2.5% glutaraldehyde at 4 °C for at least 4 h, post-fixed with 1% osmium tetroxide for 1 h, dehydrated in an ethanol gradient, embedded, and incubated respectively at 37, 45, and 60 °C for 24 h. Ultrathin sections prepared with an ultra-cut E ultra-microtome (Leica, Wetzlar, Germany) were stained with uranyl acetate and lead citrate, and observed on a TECNAI 10 transmission electron microscopy system from FEI (Hillsboro, USA). The semi-quantification of the results was obtained by counting the numbers of autophagosomes and autolysosomes in at least six cells.
Quantitative real-time PCR (qRT-PCR)
To assess Thy-1 mRNA expression, total RNA was isolated from lung tissue samples with TRIzol regent (Invitrogen) according to the manufacturer’s instructions. Complementary DNA synthesis was performed using Prime Script RT Master Mix (Takara, China), and real-time PCR was carried out on a Light Cycler 480 real-time PCR system (Roche, USA) using iTaq universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). The primers used for real-time PCR were: GAPDH, forward 5′-TGGTGAAGGTCGGTGTGAAC-3′ and reverse 5′-GCTCCTGGAAGATGGTGATGG-3′; Thy-1, forward 5′- CCGGAATTCGCCACCATGAACCCAGCCATCAGCG-3′ and reverse 5′- CCGGGATCCCAGAGAAATGAAGTCCAGGGCTTG -3′.
The 2−ΔΔCt method was employed to assess relative expression levels. GAPDH was used as an endogenous control.
Immunofluorescence
A total of 1 × 105 MRC-5 cells cultured on 12-well chamber slides were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. The slides were then blocked with 10% goat serum at room temperature for 10 min. Subsequently, the samples were incubated with primary antibodies, including rabbit anti-Thy-1 (ab225, Abcam, US) and mouse anti- integrin β3 (sc-46655, Santa Cruz, USA), respectively, at 4 °C overnight, followed by incubation with Alexa Flour 488 or Cy3 secondary antibodies (Beyotime, China) for 1 h at room temperature. The slides were counterstained with DAPI and examined by fluorescence microscopy (Leica, Heidelberg, Germany). Primary antibodies included rabbit anti-Thy-1 from CST and mouse anti-integrin β3 from Santa Cruz.
Co-Immunoprecipitation
Co-immunoprecipitation was performed according to a previous report.16 Briefly, proteins were extracted with cell lysis buffer (KGP701~KGP701–100, KeyGEN BioTECH, China) with 1% PMSF (KGP610, KeyGEN BioTECH, China). A total of 500 μg of protein extract was then incubated with 2 μg of appropriate immunoprecipitation (IP) antibodies, i.e., rabbit anti-Thy-1 (ab225, Abcam, US) or mouse anti- integrin β3 (sc-46655, Santa Cruz, USA) at 4 °C overnight with shaking. Then, 20 μl of protein A/G plus agarose (Thermo) was added, followed by incubation at 4 °C for 3 h with shaking. The pellets were washed five times with cell lysis buffer for 2 min and resuspended in 40 μl of 2 × electrophoresis loading buffer. After boiling for 10 min, 20 μl samples were used for SDS-PAGE and assessed by Western blot as described above.
Flow cytometry
PAC-1 binding to activated integrin β3 was measured by flow cytometry (BD FACSVerseTM, USA) using a modification of the technique previously described [15]. Briefly, 5 × 108 to 1 × 109/ml of MRC-5 cells were incubated for 30 min with FITC mouse anti-human PAC-1 (340507, BD Biosciences, USA) (20 μl) at room temperature. After washing with PBS, the cells were analyzed by flow cytometry; data were analyzed with the Flowjo (version 10) software.
Hematoxylin and eosin (H&E) and Masson’s trichrome staining
Pulmonary tissue samples were fixed by inflation with 4% paraformaldehyde overnight, dehydrated in 70% ethanol and embedded in paraffin wax. Sections of 5 µm thickness were prepared and subjected to H&E and Masson’s trichrome staining, respectively, as previously described [21].
Immunohistochemistry
The levels of α-SMA in the lung were assessed by IHC. Lung tissue sections were deparaffinized and incubated with 1% albumin solution containing 0.1% Triton X-100 at room temperature for 1 h. The slices were then blocked with 3% normal goat serum containing 0.1% Triton X-100 at room temperature for 1 h. Next, the samples were sequentially incubated with rabbit anti-α-SMA primary (1/2000) and goat anti-rabbit IgG secondary (1/1000) antibodies, each for 1 h.
Pulmonary Hydroxyproline (HYP) and collagen measurement
To estimate total collagen deposition, an indicator of pulmonary fibrosis, the hydroxyproline content of the middle lobe of the left lung was measured with a commercially available hydroxyproline detection kit (Nanjing Jiancheng Bioengineering; Nanjing, China) according to the manufacturer’s instructions. The collagen content of the upper lobe of the right lung was measured according to the protocol of a commercial collagen detection kit (Nanjing Jiancheng Bioengineering; Nanjing, China).
Statistical analysis
Data were processed with the Graph Pad PRISM7 software (USA), and presented as mean ± standard deviation (SD). Two-tailed Student’s t test was used to compare two groups. Multiple groups were compared by one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
LPS-induced autophagy inhibition is accompanied with Thy-1 downregulation and integrin β3 upregulation in LPS-induced pulmonary fibrosis in vivo
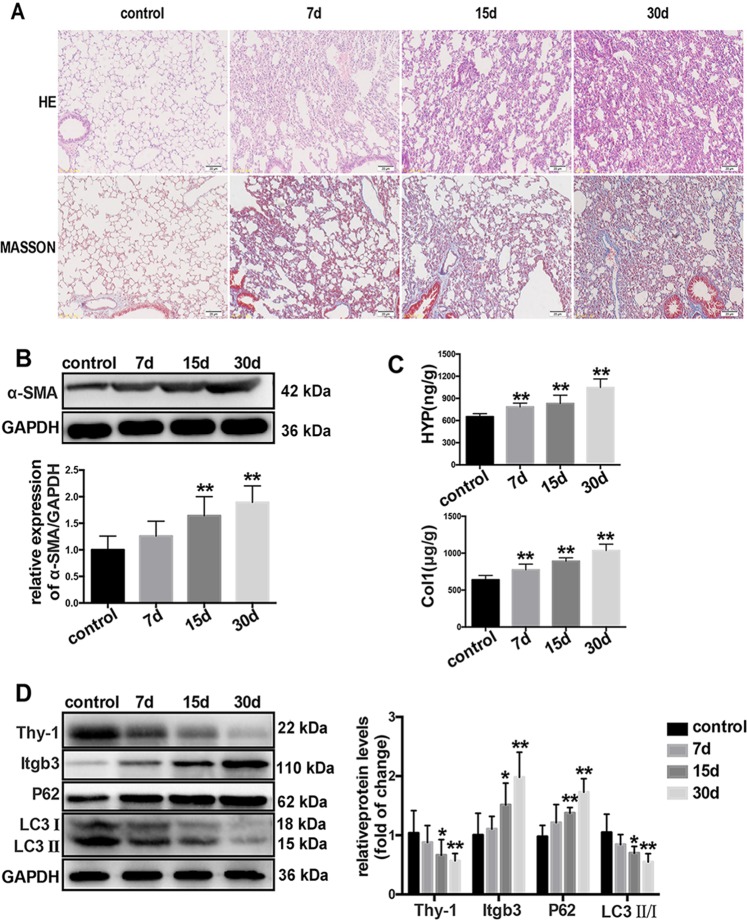
To investigate the time-course of LPS-induced pulmonary fibrosis in vivo, mice were intraperitoneally injected with saline or LPS (5 mg/kg) for 5 consecutive days, and samples were collected at day 7, 15 and 30 after LPS injection, respectively. As shown in Fig. 1a, mild pulmonary fibrosis was detected at 7 and 15 days after LPS treatment, and typical pulmonary fibrosis was observed at 30 days, accompanied with α-SMA upregulation and increased hydroxyproline and collagen contents (Fig. 1b, c).
Fig. 1.
LPS-induced autophagy inhibition is accompanied with Thy-1 downregulation and integrin β3 upregulation in LPS-induced pulmonary fibrosis. The severity of pulmonary fibrosis was determined by hematoxylin-eosin (H&E) staining; collagen deposition was revealed by MASSON staining (magnification, ×200) (a). The expression of α-SMA in lung tissues was also measured by Western blot (b). Collagen deposition was measured by hydroxyproline content and collagen level assessments (c). Representative Western blot images showing expression of Thy-1, integrin β3, LC3 and P62 in the lung tissue (d). Values are mean ± SD (n = 6). *p < 0.05 vs control group; **P < 0.01 vs control group
In addition, LPS also suppressed autophagy by upregulating P62 and decreasing the LC3II-to-LC3 I ratio, accompanied with decreased expression of Thy-1 and increased amounts of integrin β3 in the lung tissue (Fig. 1d).
LPS-induced autophagy inhibition is accompanied with Thy-1 downregulation and integrin β3 upregulation and activation in lung fibroblasts in vitro
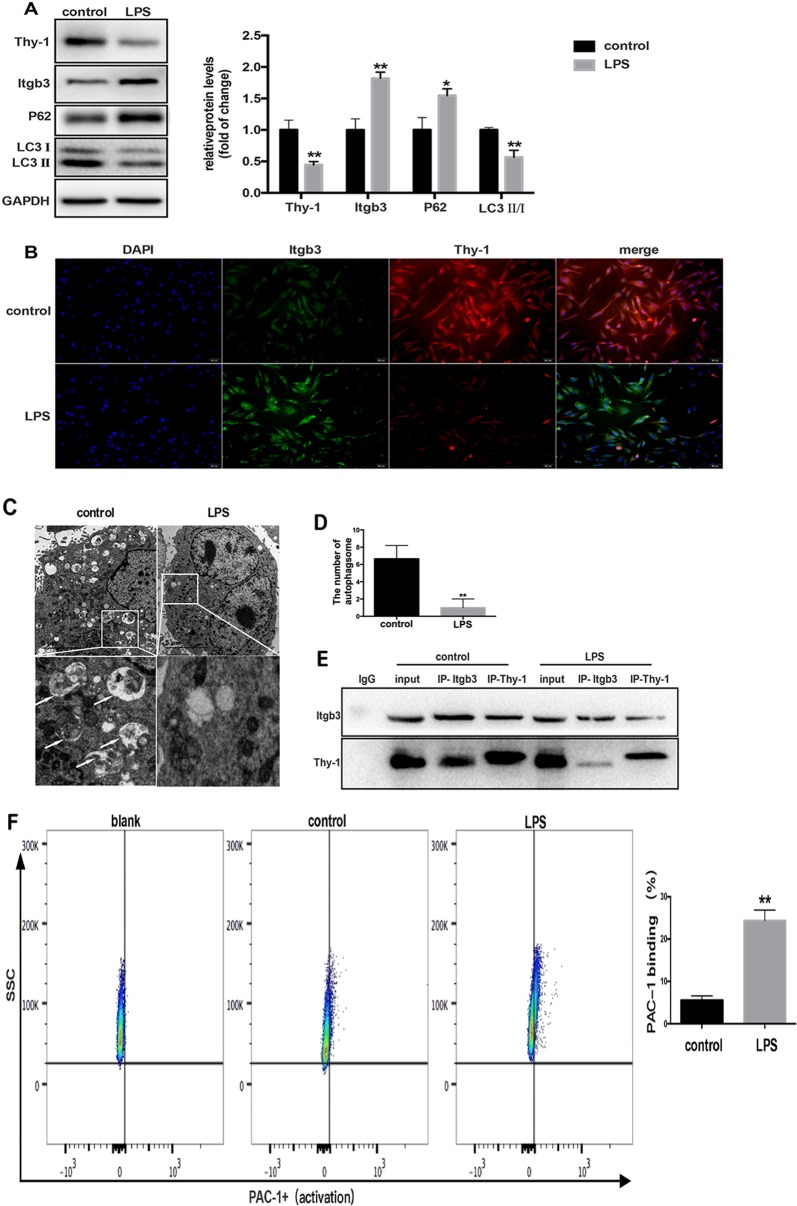
To explore the effects of LPS on autophagy inhibition and Thy-1 and integrin β3 expression levels in lung fibroblasts, MRC-5 cells were challenged by LPS (1 μg/ml) for 6 h. Compared with the control group, LPS challenge significantly inhibited Thy-1 expression and increased integrin β3 amounts in lung fibroblasts (Fig. 2a), which was further confirmed by immunofluorescence (Fig. 2b). Furthermore, autophagy in lung fibroblasts was significantly inhibited after treatment with 1 μg/ml LPS for 24 h, as indicated by increased P62 amounts, decreased LC3 II-to-LC3 I ratios and reduced autophagosome amounts detected by TEM (Fig. 2a, c, d).
Fig. 2.
LPS-induced autophagy inhibition is accompanied with Thy-1 downregulation and integrin β3 upregulation and activation in lung fibroblasts. Western blot was performed to detect Thy-1 and integrin β3 in lung fibroblasts challenged with 1 μg/ml LPS for 6 h, and P62 and LC3 in lung fibroblasts challenged with 1 μg/ml LPS for 24 h (a). Immunofluorescent staining of the nucleus (blue), integrin β3 (green) and Thy-1 (red) in lung fibroblasts cultured in the absence or presence of 1 μg/ml LPS for 6 h (b). Lung fibroblasts were observed by transmission electron microscopy. White arrows indicate autophagosomes (c), the semi-quantification of autophagosomes of per cell were also shown (d). Co-IP was used to detect the interaction between Thy-1 and integrin β3 in MRC-5 cells after LPS challenge. Western blot for co-immunoprecipitates of IgG control, input, Thy-1 and integrin β3 (e). Flow cytometry was used to detect the expression of PAC-1, an integrin β3 activation marker (f).Values are mean ± SD from triplicate experiments. *p < 0.05, **p < 0.01
Next, the interaction between Thy-1 and integrin β3 in MRC-5 cells was assessed by co-immunoprecipitation. After treating lung fibroblasts with LPS, the interaction between Thy-1 and integrin β3 was reduced (Fig. 2e). Then, flow cytometry was used to detect the expression of PAC-1, a marker of integrin β3 activation, in lung fibroblasts. PAC-1 expression was increased dramatically, which indicated integrin β3 activation by LPS in MRC-5 cells (Fig. 2f).
Integrin β3 inhibition precludes LPS-induced PI3K-Akt-mTOR activation and lung fibroblast autophagy inhibition
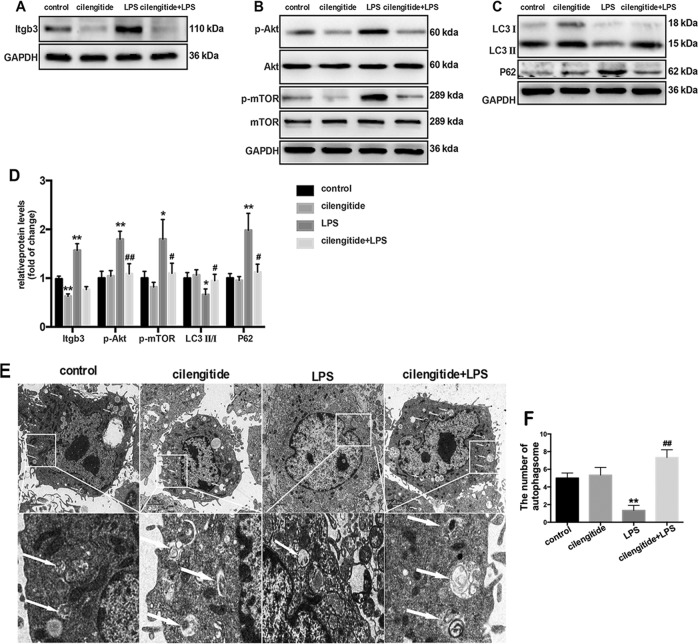
Our previous study showed that LPS activates the PI3K-Akt-mTOR pathway and inhibits lung fibroblast autophagy. To assess whether integrin β3 is essential in LPS-induced PI3K-Akt-mTOR pathway activation and lung fibroblast autophagy inhibition, cilengitide, an inhibitor for integrin β3, was applied. Western blot showed that integrin β3 protein expression in lung fibroblasts was increased significantly 6 h after LPS challenge, and this effect was prohibited by cilengitide treatment (Fig. 3a, d). Meanwhile, activation of the PI3K-Akt-mTOR pathway was also detected by increased amounts of phosphorylated Akt (p-Akt) and phosphorylated mTOR (p-mTOR) 6 h after LPS challenge. However, no differences were observed after cilengitide treatment (Fig. 3b, d). In addition, pretreatment with cilengitide increased the LC3 II-to-LC3 I ratio and decreased p62 levels, suggesting that cilengitide partially avoided LPS-induced autophagy inhibition (Fig. 3c, d). TEM images also showed that autophagosomes reappeared after cilengitide treatment (Fig. 3e, f).
Fig. 3.
Integrin β3 inhibition precludes LPS-induced PI3K-Akt-mTOR activation and lung fibroblast autophagy blockade. Western blot was used to detect the expression of integrin β3 (a), phospho-AKT (p-AKT), total AKT, phospho-mTOR (p-mTOR) and total mTOR (b). Representative images showing protein expression of LC3 I, LC3 II and P62 in MRC-5 cells challenged without or with 1 μg/ml LPS in the absence or presence of the inhibitor cilengitide (2 mM) for 24 h (c). Quantitation of p-AKT/total AKT, p-mTOR/total mTOR, LC3 II/ I, integrin β3 and P62 protein levels normalized to GAPDH (d). Lung fibroblasts were assessed by transmission electron microscopy. White arrows indicate autophagosomes (e), the semi-quantification of autophagosomes of per cell were also shown (f). Values are mean ± SD from triplicate experiments. *p < 0.05 vs control group; **p < 0.01 vs control group; #p < 0.05 vs LPS group; ##p < 0.05 vs. LPS group
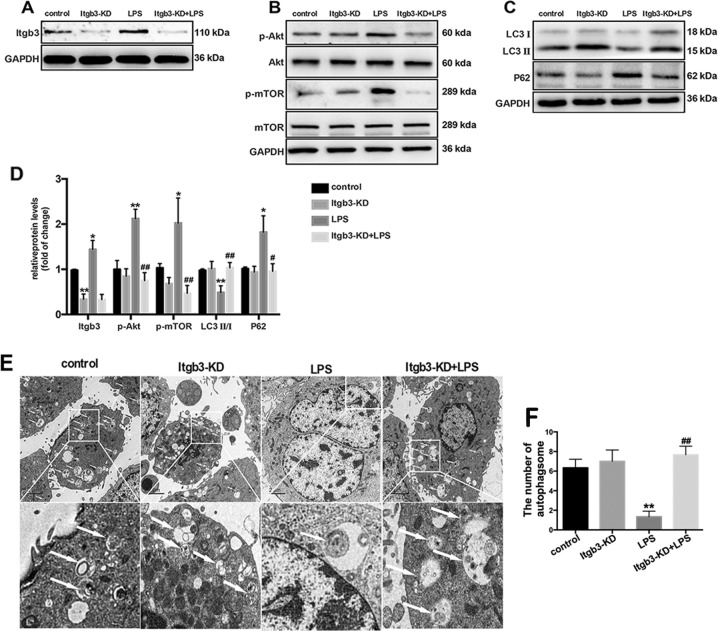
To further assess the role of integrin β3 in the inhibition of LPS-induced lung fibroblast autophagy, integrin β3 was knocked down in lung fibroblasts.
Similar with the effect of the integrin β3 inhibitor cilengitide, Itgb3-KD transfection prohibited LPS-induced integrin β3 expression (Fig. 4a, d), PI3K-Akt-mTOR pathway activation (Fig. 4b, d) and autophagy inhibition in lung fibroblasts (Fig. 4c–f). These findings indicated that LPS activated the PI3K-Akt-mTOR pathway and inhibited lung fibroblast autophagy by upregulating integrin β3.
Fig. 4.
Genetic integrin β3 inhibition prevents LPS-induced PI3K-Akt-mTOR activation and lung fibroblast autophagy inhibition. Western blot was used to detect the expression levels of integrin β3 (a), phospho-AKT (p-AKT), total AKT, phospho-mTOR (p-mTOR) and total mTOR (b) in lung fibroblasts after 6 h of 1 μg/ml LPS challenge after transfection with integrin β3 shRNA or the empty vector. Representative images showing expression of LC3 I, LC3 II and P62 in lung fibroblasts after 24 h of 1 μg/ml LPS challenge after transfection with integrin β3 shRNA or the empty vector (c). Quantification of p-AKT/total AKT, p-mTOR/total mTOR, LC3 II/ I, integrin β3and P62 protein levels normalized to GAPDH (d). Lung fibroblasts were observed by transmission electron microscopy. White arrows indicate autophagosomes (e), the semi-quantification of autophagosomes of per cell were also shown (f). Values are mean ± SD from triplicate experiments. *p < 0.05 vs. control group; **p < 0.01 vs. control group; #p < 0.05 vs. LPS group; ##p < 0.05 vs. LPS group
Thy-1 downregulation induces PI3K-Akt-mTOR activation and lung fibroblast autophagy inhibition
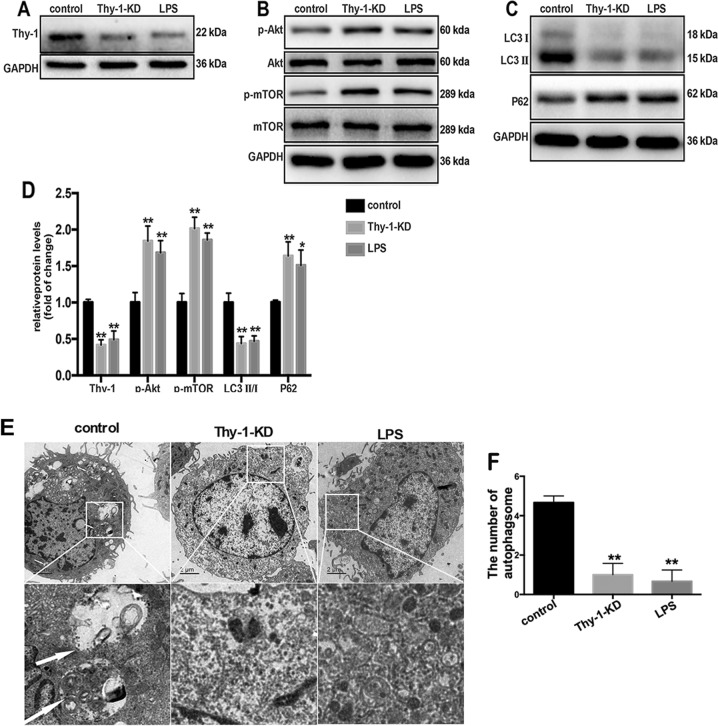
To determine the effect of Thy-1 depletion on autophagy inhibition in lung fibroblasts, Thy-1 was knocked down in lung fibroblasts by transfection of Thy-1 shRNA. Thy-1 expression in lung fibroblasts was reduced significantly 6 h after LPS challenge or Thy-1 shRNA transfection (Fig. 5a, d). Thy-1 downregulation mimicked the effects of LPS, inducing PI3K-Akt-mTOR pathway activation and inhibiting lung fibroblast autophagy, as demonstrated by increased expression levels of p-Akt and p-mTOR (Fig. 5b, d), reduced LC3 II-to-LC3 I ratios, increased expression of p62 (Fig. 5c, d) and reduced autophagosome amounts (Fig. 5e, f).
Fig. 5.
Thy-1 downregulation induces PI3K-Akt-mTOR activation and lung fibroblast autophagy inhibition. Western blot was performed to detect the expression levels of Thy-1 (a), phospho-AKT (p-AKT), total AKT, phospho-mTOR (p-mTOR) and total mTOR (b) in lung fibroblasts after 6 h of 1 μg/ml LPS challenge after transfection with Thy-1 shRNA or the empty vector. Representative images showing expression of LC3 I, LC3 II and P62 in lung fibroblasts after 24 h of 1 μg/ml LPS challenge after transfection with Thy shRNA or the empty vector (c). Lung fibroblasts were observed by transmission electron microscopy. White arrows indicate autophagosomes (e), the semi-quantification of autophagosomes of per cell were also shown (f). Values are mean ± SD from triplicate experiments. *p < 0.05 vs. control group; **p < 0.01 vs. control group
Thy-1 overexpression precludes LPS-induced PI3K-Akt-mTOR activation and lung fibroblast autophagy inhibition
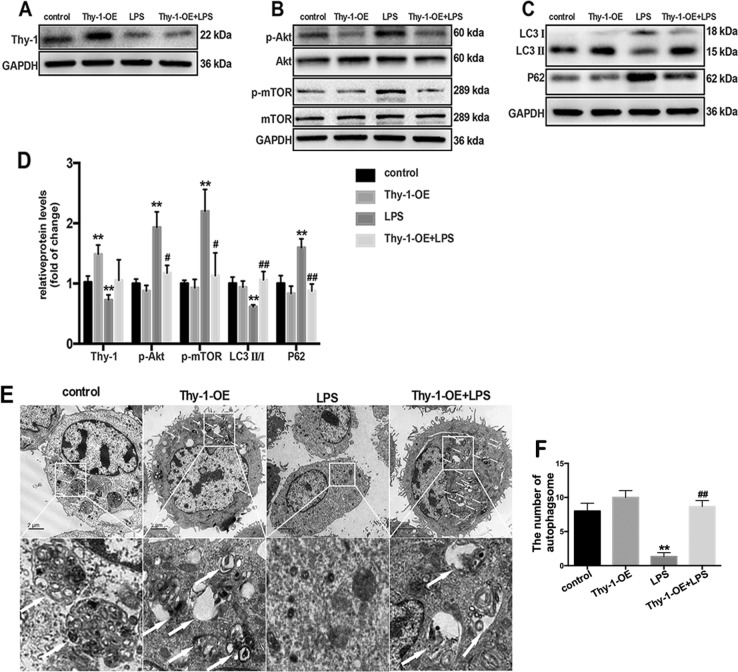
To further assess whether Thy-1 depletion alters the effect of LPS, lung fibroblasts were transfected with Thy-1-overexpression lentivirus (Thy-1-OE) to overexpress Thy-1. As shown in Fig. 6a and d, Thy-1-OE transfection prohibited LPS-induced Thy-1 depletion in lung fibroblasts, accompanied with a reversion of LPS-induced activation of the PI3K-Akt-mTOR pathway (Fig. 6b, d) and inhibition of lung fibroblast autophagy (Fig. 6c–f). These findings suggested that Thy-1 depletion mediated LPS-induced PI3K-Akt-mTOR pathway activation and lung fibroblast autophagy inhibition.
Fig. 6.
Thy-1 overexpression precludes LPS-induced PI3K-Akt-mTOR activation and lung fibroblast autophagy inhibition. Western blot was performed to detect the expression levels of Thy-1(a), phospho-AKT (p-AKT), total AKT, phospho-mTOR (p-mTOR) and total mTOR (b) in lung fibroblasts after 6 h of 1 μg/ml LPS challenge after transfection with Thy-1-OE or the empty vector. Representative images showing expression of LC3 I, LC3 II and P62 in lung fibroblasts after 24 h of 1 μg/ml LPS challenge after transfection with Thy-1-OE or empty vector (c). Lung fibroblasts were observed by transmission electron microscopy. White arrows indicate autophagosomes (e), the semi-quantification of autophagosomes of per cell were also shown (f). Values are mean ± SD (n = 3). *p < 0.05 vs. control group; **p < 0.01 vs. control group; #p < 0.05 vs. LPS group; ##p < 0.05 vs. LPS group
Thy-1 overexpression or integrin β3 inhibition prevents LPS-induced autophagy inhibition and pulmonary fibrosis
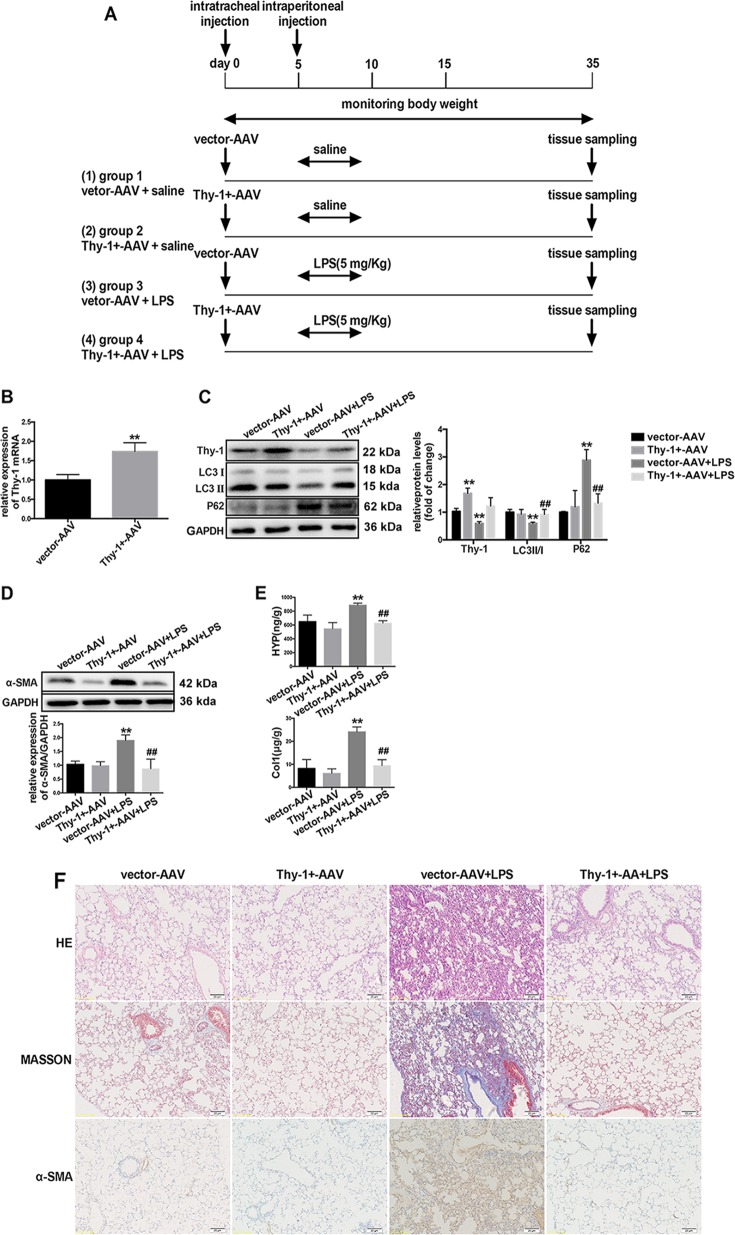
In order to investigate whether Thy-1 expression in vivo alters LPS-induced pulmonary fibrosis, Thy-1+-AAV was transfected by intratracheal injection to overexpress the Thy-1 gene in the mouse lung tissue, as shown in Fig. 7a. The mRNA expression of Thy-1 was increased significantly after Thy-1+-AAV transfection (Fig. 7b). As shown in Fig. 7c, Thy-1+-AAV transfection inhibited LPS-induced Thy-1 depletion in the lung tissue, accompanied with a reversion of LPS-induced decrease of LC3 II-to-LC3 I ratio and increased expression of P62. In addition, western blot analysis of α-SMA, hydroxyproline and collagen content measurements, pathological analysis by H&E or MASSON –staining, and α-SMA immunohistochemistry showed that Thy-1+-AAV transfection prohibited LPS-induced pulmonary fibrosis (Fig. 7d–f). These findings suggested that Thy-1 depletion mediated LPS-induced lung tissue autophagy inhibition in LPS-induced pulmonary fibrosis.
Fig. 7.
Thy-1 overexpression prevents LPS-induced autophagy inhibition and LPS-induced pulmonary fibrosis. Male C57BL/6J mice aged 6–8 weeks (n = 6/group) were treated intratracheally with AAV6-CMV-Thy-1 (5 × 1010 vg/mouse) or vector AAV, as shown in (a). Mice were treated with vector-AAV or Thy-1+-AAV, and Thy-1 mRNA levels were assessed (b). Western blot was performed to detect the expression levels of Thy-1, LC3, P62 (c) and α-SMA (d) in the lung tissue. The severity of collagen deposition was measured by assessing hydroxyproline and collagen amounts (e). The severity of pulmonary fibrosis was determined by hematoxylin-eosin (H&E) staining; collagen deposition was assessed by Masson’s trichrome staining, and α-SMA expression in the lung tissue was detected by immunohistochemistry (magnification, ×200) (f). Values are mean ± SD (n = 6). *p < 0.05 vs. control group; **p < 0.01 vs. control group; #p < 0.05 vs. LPS group; ##p < 0.05 vs. LPS group
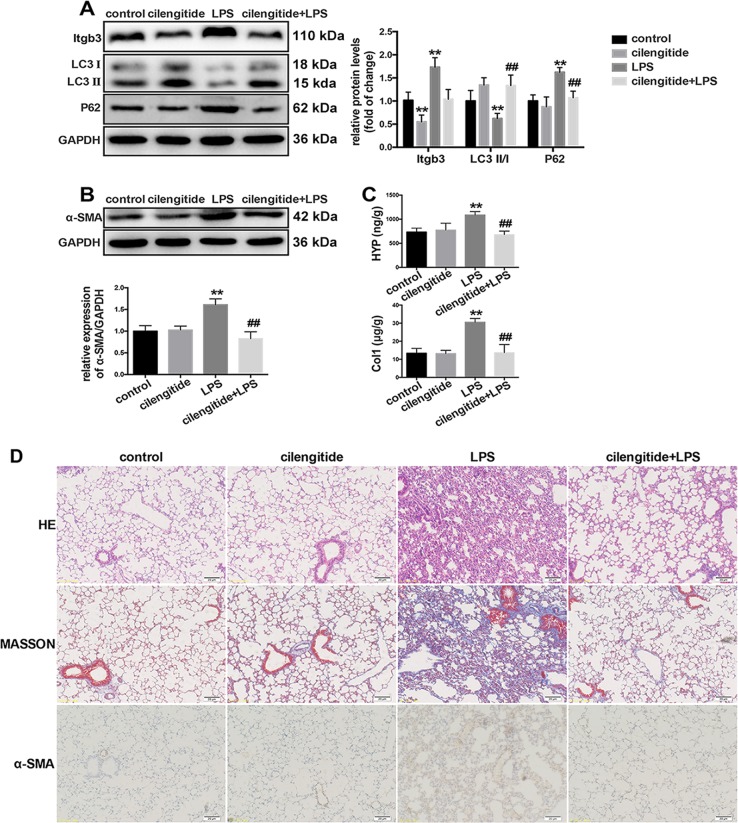
To further investigate whether pharmacologically altering integrin β3 expression and activation in vivo affects LPS-induced pulmonary fibrosis, integrin β3 was inhibited in the mouse lung tissue by intraperitoneal injection of cilengitide. As shown in Fig. 8a, cilengitide treatment prevented LPS-induced increased expression of integrin β3 and lung tissue autophagy inhibition. Cilengitide treatment also prevented LPS-induced pulmonary fibrosis (Fig. 8b–d), suggesting that integrin β3 upregulation mediated LPS-induced lung tissue autophagy inhibition in LPS-induced pulmonary fibrosis.
Fig. 8.
Integrin β3 inhibition prevents LPS-induced autophagy inhibition and LPS-induced pulmonary fibrosis. Male C57BL/6J mice aged 6–8 weeks (n = 6/group) were pretreated with cilengitide (2 mg/kg), followed by LPS (5 mg/kg) administration for consecutive 5 days. Western blot was performed to detect the expression levels of integrin β3, LC3, P62 (a) and α-SMA (b) in the lung tissue. The severity of collagen deposition was measured by assessing hydroxyproline and collagen amounts (c). The severity of pulmonary fibrosis was determined by hematoxylin-eosin (H&E) staining; collagen deposition was revealed by Masson’s trichrome staining, and α-SMA expression in the lung tissue was detected by immunohistochemistry (magnification, ×200) (d). Values are mean ± SD (n = 6). *p < 0.05 vs. control group; **p < 0.01 vs. control group; #p < 0.05 vs. LPS group; ##p < 0.05 vs. LPS group
Discussion
Although the roles of LPS-induced PI3K-Akt-mTOR pathway activation in lung fibroblast autophagy inhibition and pulmonary fibrosis have been confirmed by our previous studies, the underlying mechanisms remain unknown. This study revealed that Thy-1 depletion and integrin β3 upregulation mediated PI3K-Akt-mTOR pathway-associated inhibition of lung fibroblast autophagy in LPS-induced pulmonary fibrosis.
Autophagy is an important defense mechanism for cells, and plays a vital role in maintaining homeostasis [22, 23]. The PI3K-Akt pathway targeting mTOR mediates many physiological functions such as cell proliferation, differentiation, migration and apoptosis, and also constitutes an important signaling pathway regulating autophagy [24, 25]. Studies have shown that the PI3K-Akt pathway controlling mTOR expression negatively regulates autophagy in cells stimulated by factors such as starvation and hypoxia [26, 27]. Our previous study demonstrated that LPS activates the PI3K-Akt-mTOR pathway and inhibits autophagy in lung fibroblasts [9]. However, the mechanism by which LPS induces PI3K-Akt-mTOR pathway activation remains unclear.
Thy-1 is a glycosylphosphatidylinositol-linked cell surface glycoprotein expressed on fibroblast subpopulations and various cell types, including thymocytes, T cells, and nerve cells [28]. As a membrane-bound glycoprotein, Thy-1 is also an important regulator of cell-cell and cell-matrix interactions, activates intracellular signaling pathways, and affects a variety of cellular functions such as cell proliferation, differentiation, and survival [29, 30]. Thy-1 is inversely associated with phenotypic characteristics in fibrosis and considered a “fibrosis suppressor gene” [31]. Several studies have suggested that the absence of Thy-1 in lung fibroblasts correlates with pulmonary fibrosis [32, 33], and our previous study revealed that Thy-1-related lung fibroblast phenotype transformation is essential for LPS-induced lung fibroblast proliferation. Integrin β3 is an important adhesion molecule [34] involved in cell proliferation, survival and cancer metastasis. It has been suggested that integrin β3 may be a viable anti-fibrotic target in various fibrotic diseases. In bleomycin-induced pulmonary fibrosis, integrin β3 expression is particularly enhanced in mesenchymal stromal cells. In normal Thy-1(+) lung fibroblasts, Thy-1 interacts with integrin αvβ3 to inhibit collagen-induced cell activation [31, 32]. Meanwhile, studies have confirmed that loss of Thy-1 expression leads to the activation of integrin β3 and the downstream FAK/PI3K/ Rac1 signaling pathway [35, 36]. As shown above, LPS induced Thy-1 depletion and integrin β3 upregulation played an important role in LPS-induced PI3K-Akt-mTOR pathway activation and lung fibroblast autophagy inhibition and pulmonary fibrosis.
According to previous study, the interaction between Thy-1 and integrin β3 can affect the actvation of integrin β3 [37]. Referring to a previous research [38], we used Co-IP to analyze the interaction of Thy-1 and integrin β3. Our results revealed a strong interaction between Thy-1 and integrin β3 in lung fibroblasts, which decreased significantly after LPS challenge. Subsequently, we applied flow cytometry to detect PAC-1, a marker of integrin β3 activation, in lung fibroblasts [39, 40]. The results showed that LPS challenge led to enhanced activation of integrin β3 in lung fibroblasts. integrin β3 has been reported to be upstream of the PI3K-Akt-mTOR pathway in various cell types. Because integrin receptors do not possess a catalytic activity, the signaling pathway induced by integrin activation must be transmitted into cells through activation of integrin-associated proteins such as PI3K and FAK. This indicates PI3K-Akt-mTOR signaling or similar pathways may be activated when integrin β3 is overexpressed. Studies have shown that integrin β3 upregulation inhibits the autophagic process in cardiomyocytes by activating Akt [41], suggesting that the expression status of integrin may affect cell autophagy.
As shown above, genetic or pharmacological inhibition of integrin β3 or Thy-1 upregulation prevented LPS-induced PI3K-Akt-mTOR activation and lung fibroblast autophagy inhibition. We also found that Thy-1 overexpression or integrin β3 inhibition in vivo could prevent LPS-induced lung tissue autophagy and pulmonary fibrosis, suggesting that early intervention of Thy-1 and integrin β3 in ARDS could block the progression of pulmonary fibrosis, and is expected to improve the cure rate of patients with ARDS-associated respiratory failure, which is important for improving the prognosis of ARDS patients.
Overall, this study demonstrated that depleting Thy-1 and upregulating integrin β3 is an important mechanism and potential therapeutic target for PI3K-Akt-mTOR pathway-associated inhibition of lung fibroblast autophagy in LPS-induced pulmonary fibrosis.
Disclaimer
The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Acknowledgments
Funding:
This study was supported by grants from the National Natural Science Foundation of China (NSFC, No. 81670057 and 81870052) and Training Program Foundation for Distinguished Young Medical Professional from Shanghai Municipal Commission of Health and Family Planning (No. 2018–16).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hanxi Wan, Tingting Xie
Contributor Information
Yuan Gao, Email: rj_gaoyuan@163.com.
Zhengyu He, Phone: +86-21-58752345, Email: zhengyuheshsmu@163.com.
References
- 1.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:1904–5. doi: 10.1056/NEJMc1711824. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong L, Thickett DR, Mansell JP, Billinghurst RC, Poole AR, Millar AB. Changes in collagen turnover in early acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:1910–5. doi: 10.1164/ajrccm.160.6.9811084. [DOI] [PubMed] [Google Scholar]
- 3.Zinter MS, Delucchi KL, Kong MY, Orwoll BE, Spicer AS, Lim MJ, et al. Early plasma matrix metalloproteinase profiles: a novel pathway in pediatric acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;199:181–9. doi: 10.1164/rccm.201804-0678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196:1275–86. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei SHJ, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicko S, Kohler TC, Ayata CK, Muller T, Ehrat N, Meyer A, et al. Extracellular ATP is a danger signal activating P2X7 receptor in a LPS mediated inflammation (ARDS/ALI) Oncotarget. 2018;9:30635–48. doi: 10.18632/oncotarget.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabrera S, Maciel M, Herrera I, Nava T, Vergara F, Gaxiola M, et al. Essential role for the ATG4B protease and autophagy in bleomycin-induced pulmonary fibrosis. Autophagy. 2015;11:670–84. doi: 10.1080/15548627.2015.1034409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Fang S, Wang W, Cheng Y, Zhang Y, Liao H, et al. Macrophage-derived MCPIP1 mediates silica-induced pulmonary fibrosis via autophagy. Part Fibre Toxicol. 2016;13:55. doi: 10.1186/s12989-016-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie T, Xu Q, Wan H, Xing S, Shang C, Gao Y, et al. Lipopolysaccharide promotes lung fibroblast proliferation through autophagy inhibition via activation of the PI3K-Akt-mTOR pathway. Lab Investig J Tech Methods Pathol. 2019;99:625–33. doi: 10.1038/s41374-018-0160-2. [DOI] [PubMed] [Google Scholar]
- 10.McCullough KM, Choi D, Guo J, Zimmerman K, Walton J. Epigenetic regulation of Thy-1 gene expression by histone modification is involved in lipopolysaccharide-induced lung fibroblast proliferation. DG, et al. Molecular characterization of Thy1 expressing fear-inhibiting neurons within the basolateral amygdala. Nat Commun. 2016;7:13149. doi: 10.1038/ncomms13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Z, Wang X, Deng Y, Li W, Chen Y, Xing S, et al. Epigenetic regulation of Thy-1 gene expression by histone modification is involved in lipopolysaccharide-induced lung fibroblast proliferation. J Cell Mol Med. 2013;17:160–7. doi: 10.1111/j.1582-4934.2012.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing S, Nie F, Xu Q, Deng Y, Li W, Yang Z, et al. HDAC is essential for epigenetic regulation of Thy-1 gene expression during LPS/TLR4-mediated proliferation of lung fibroblasts. Lab Investig J Tech Methods Pathol. 2015;95:1105–16. doi: 10.1038/labinvest.2015.97. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Xu Q, Deng Y, Yang Z, Xing S, Zhao X, et al. High-mobility group box 1 accelerates lipopolysaccharide-induced lung fibroblast proliferation in vitro: involvement of the NF-kappaB signaling pathway. Lab Investig J Tech Methods Pathol. 2015;95:635–47. doi: 10.1038/labinvest.2015.44. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Gutknecht D, Anastassiadis K, Eckes B, Anderegg U, Saalbach A. Thy-1/beta3 integrin interaction-induced apoptosis of dermal fibroblasts is mediated by up-regulation of fasl expression. J Invest Dermatol. 2016;136:526–9. doi: 10.1016/j.jid.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 15.Whyte CS, Swieringa F, Mastenbroek TG, Lionikiene AS, Lance MD, van der Meijden PEJ, et al. Plasminogen associates with phosphatidylserine-exposing platelets and contributes to thrombus lysis under flow. Blood. 2015;125:2568–78. doi: 10.1182/blood-2014-09-599480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hisamoto N, Tsuge A, Pastuhov SI, Shimizu T, Hanafusa H, Matsumoto K. Phosphatidylserine exposure mediated by ABC transporter activates the integrin signaling pathway promoting axon regeneration. Nat Commun. 2018;9:3099. doi: 10.1038/s41467-018-05478-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y-Y, Zhao X-K, Yu L, Qi F, Zhai B, Gao C-Q, et al. Interaction of Src and Alpha-V integrin regulates fibroblast migration and modulates lung fibrosis in a preclinical model of lung fibrosis. Sci Rep. 2017;7:46357. doi: 10.1038/srep46357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pechkovsky DV, Scaffidi AK, Hackett TL, Ballard J, Shaheen F, Thompson PJ, et al. Transforming growth factor beta1 induces alphavbeta3 integrin expression in human lung fibroblasts via a beta3 integrin-, c-Src-, and p38 MAPK-dependent pathway. J Biol Chem. 2008;283:12898–908. doi: 10.1074/jbc.M708226200. [DOI] [PubMed] [Google Scholar]
- 19.Tian B, Lessan K, Kahm J, Kleidon J, Henke C. beta 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem. 2002;277:24667–75. doi: 10.1074/jbc.M203565200. [DOI] [PubMed] [Google Scholar]
- 20.Dong W-W, Zhang Y-Q, Zhu X-Y, Mao Y-F, Sun X-J, Liu Y-J, et al. Protective effects of hydrogen-rich saline against lipopolysaccharide-induced alveolar epithelial-to-mesenchymal transition and pulmonary fibrosis. Med Sci Monit Int Med J Exp Clin Res. 2017;23:2357–64. doi: 10.12659/MSM.900452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehghani S, Rasoulianboroujeni M, Ghasemi H, Keshel SH, Nozarian Z, Hashemian MN, et al. 3D-Printed membrane as an alternative to amniotic membrane for ocular surface/conjunctival defect reconstruction: an in vitro & in vivo study. Biomaterials. 2018;174:95–112. doi: 10.1016/j.biomaterials.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Cadwell K. Crosstalk between autophagy and inflammatory signalling pathways: balancing defence and homeostasis. Nat Rev Immunol. 2016;16:661–75. doi: 10.1038/nri.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol. 2018;19:579–93. doi: 10.1038/s41580-018-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 25.Chen N, Debnath J. IkappaB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway. Autophagy. 2013;9:1214–27. doi: 10.4161/auto.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamoureux F, Zoubeidi A. Dual inhibition of autophagy and the AKT pathway in prostate cancer. Autophagy. 2013;9:1119–20. doi: 10.4161/auto.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I, et al. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 2011;7:176–87. doi: 10.4161/auto.7.2.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neveu WA, Mills ST, Staitieh BS, Sueblinvong V. TGF-beta1 epigenetically modifies Thy-1 expression in primary lung fibroblasts. Am J Physiol Cell Physiol. 2015;309:C616–26. doi: 10.1152/ajpcell.00086.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yousefi-Rad N, Shokrgozar MA, Behdani M, Moradi-Kalbolandi S, Motamedi-Rad M, Habibi-Anbouhi M. Antigenic assessment of a recombinant human CD90 protein expressed in prokaryotic expression system. Protein Expr Purif. 2015;116:139–43. doi: 10.1016/j.pep.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Herrera-Molina R, Valdivia A, Kong M, Alvarez A, Cardenas A, Quest AFG, et al. Thy-1-interacting molecules and cellular signaling in cis and trans. Int Rev Cell Mol Biol. 2013;305:163–216. doi: 10.1016/B978-0-12-407695-2.00004-4. [DOI] [PubMed] [Google Scholar]
- 31.Cohen PY, Breuer R, Wallach-Dayan SB. A profibrotic phenotype in naive and in fibrotic lung myofibroblasts is governed by modulations in thy-1 expression and activation. Mediators Inflamm. 2018;2018:4638437. doi: 10.1155/2018/4638437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou W-Q, Wang P, Shao Q-P, Wang J. Lipopolysaccharide promotes pulmonary fibrosis in acute respiratory distress syndrome (ARDS) via lincRNA-p21 induced inhibition of Thy-1 expression. Mol Cell Biochem. 2016;419:19–28. doi: 10.1007/s11010-016-2745-7. [DOI] [PubMed] [Google Scholar]
- 33.Sanders YY, Pardo A, Selman M, Nuovo GJ, Tollefsbol TO, Siegal GP, et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:610–8. doi: 10.1165/rcmb.2007-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Hagood JS, Lu B, Merryman WD, Murphy-Ullrich JE. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem. 2010;285:22382–93. doi: 10.1074/jbc.M110.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgos-Bravo F, Figueroa NL, Casanova-Morales N, Quest AFG, Wilson CAM, Leyton L. Single-molecule measurements of the effect of force on Thy-1/alphavbeta3-integrin interaction using nonpurified proteins. Mol Biol Cell. 2018;29:326–38. doi: 10.1091/mbc.E17-03-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu W, Zhang Y, Liu X, Zhou J, Li Y, Zhou Y, et al. Sublytic C5b-9 complexes induce proliferative changes of glomerular mesangial cells in rat Thy-1 nephritis through TRAF6-mediated PI3K-dependent Akt1 activation. J Pathol. 2012;226:619–32. doi: 10.1002/path.3011. [DOI] [PubMed] [Google Scholar]
- 37.Fiore VF, Strane PW, Bryksin AV, White ES, Hagood JS, Barker TH. Conformational coupling of integrin and Thy-1 regulates Fyn priming and fibroblast mechanotransduction. J Cell Biol. 2015;211:173–90. doi: 10.1083/jcb.201505007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pankow S, Bamberger C, Calzolari D, Bamberger A, Yates JR., 3rd Deep interactome profiling of membrane proteins by co-interacting protein identification technology. Nat Protoc. 2016;11:2515–28. doi: 10.1038/nprot.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tadokoro S, Nakazawa T, Kamae T, Kiyomizu K, Kashiwagi H, Honda S, et al. A potential role for alpha-actinin in inside-out alphaIIbbeta3 signaling. Blood. 2011;117:250–8. doi: 10.1182/blood-2009-10-246751. [DOI] [PubMed] [Google Scholar]
- 40.Ghevaert C, Salsmann A, Watkins NA, Schaffner-Reckinger E, Rankin A, Garner SF, et al. A nonsynonymous SNP in the ITGB3 gene disrupts the conserved membrane-proximal cytoplasmic salt bridge in the alphaIIbbeta3 integrin and cosegregates dominantly with abnormal proplatelet formation and macrothrombocytopenia. Blood. 2008;111:3407–14. doi: 10.1182/blood-2007-09-112615. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, Li L, Gong S, Yu Y, Dai H, Cai G, et al. ss3-integrin inhibits lipopolysaccharide-induced autophagy in cardiomyocytes via the Akt signaling pathway. Cardiology. 2015;130:249–59. doi: 10.1159/000371489. [DOI] [PubMed] [Google Scholar]