Abstract
Purpose
To describe the clinical features, risk factors for severe disease and effectiveness of oseltamivir in patients with 2009 pandemic influenza A (H1N1) virus infection.
Methods
In a prospective, cross-sectional, multicentre study, data on 540 patients with confirmed 2009 H1N1 infection from seven Austrian hospitals were collected using a standardised online case-history form.
Results
The median age of the patients was 19.3 years (range 26 days–90.8 years); point-of-care testing yielded false-negative results in 60.2% of the 176 cases tested. The most common symptoms were fever, cough, fatigue and headache. Overall, 343 patients (63.5%) were hospitalised, 49 (9.1%) were admitted to an intensive care unit (ICU) and 14 (4.1%) died. Case fatality rates were highest (9.1%) in those aged 65 years or older. Factors significantly associated with a higher risk for ICU admission included age, neurological disease, adipositas, and both interstitial pathology and lobular pathology on chest X-ray. No association with pregnancy, malignancy or immunosuppressive therapy was detected. Antiviral treatment significantly reduced the duration of fever by 0.66 days and lowered the risk of ICU admission, but had no significant benefit on survival.
Conclusions
During the 2009 H1N1 influenza pandemic, elderly or obese patients and those with neurological disease had an increased risk for severe H1N1 infection in Austria. Pregnancy was not associated with a higher risk for severe disease in the later phase of the 2009 H1N1 pandemic. Antiviral treatment provided a minimal effect on the symptoms of influenza but reduced the risk of admission to an ICU.
Keywords: Influenza, Pandemic, H1N1, Risk factor
Introduction
In early April 2009, a new influenza virus emerged in Mexico and California and quickly spread worldwide by human-to-human transmission. Early reports of disproportionately high mortality in young adults in Mexico [1] followed after various reports of pandemic type A (H1N1) influenza infections with variable disease severity but lower overall mortality than that of the initial Mexican epidemic [2–7]. On 11 June 2009, the World Health Organization (WHO) raised the influenza pandemic alert to level 6, the highest level, on the basis of the widespread incidence of H1N1 cases observed globally, while noting that the current pandemic could be characterised as moderate in severity [8]. The virus of this influenza pandemic, the first in the 21st century, appeared to primarily affect children, young adults and pregnant woman, as well as those with underlying lung or cardiac disease conditions [9].
In Austria, the first case of infection with pandemic H1N1 virus was notified on 29 April 2009. Containment measures were implemented in accordance with the Austrian national plan for pandemic influenza and included active case finding and tracing of contacts, airport entry controls, isolation of patients and contacts, and antiviral treatment and prophylaxis [10]. These measures were pertinent in reaction to the first appearance of the new virus in Europe. In the initial phase of the pandemic in Austria, all cases of H1N1 infection were associated with travel and autochthonous transmission was widely impeded [11], although this might have been due to factors in addition to the control measures [12].
Due to the long-term nature of the pandemic and because of preliminary reports of the moderate severity of the pandemic as observed in European countries, in August 2009, Austria moved from containment to a mitigation strategy 4 months after the first Austrian case was reported. In this phase of the pandemic, hospitalisation was no longer compulsory for healthcare institutions [10].
After an initial vaccination campaign among healthcare workers, a nationwide swine flu vaccination campaign with the bivalent vaccine Celvapan was launched on 9 November 2009. At the same time, scepticism over the overall safety and importance of the vaccination was increasing among the Austrian population. Despite multiple campaigns by public health authorities, only about 300,000 persons (approximately 3.6% of Austrian residents) were vaccinated against pandemic influenza during the 2009–2010 influenza season [13].
In early November 2009, the number of H1N1 infections skyrocketed in Austria, with more than 30,000 new cases weekly, tapering off up to spring 2010 [10]. The estimated total number of patients infected with H1N1 in the 2009–2010 season was 350,000–400,000, which was similar to the numbers observed in seasonal influenza. A total of 1,569 patients were hospitalised, among whom 40 persons died [10]. Apart from two patients with confirmed type B influenza infection, type A H1N1 was the exclusive virus prevalent in Austria, having displaced other circulating influenza viruses [11].
There have been a few published analyses of clinical presentation and outcome of pandemic H1N1 influenza, mainly from the Americas, Australia and New Zealand [14–17]. In central Europe, apart from preliminary descriptions of the initial phase of the pandemic, data on characteristics and risk factors for severe disease in the first influenza pandemic of the 21st century are scarce [18–20]. We have, therefore, analysed the clinical characteristics and outcomes of in- and outpatients with pandemic H1N1 influenza in Austria.
Methods
We conducted a prospective multicentre cross-sectional study to obtain data on the impact of the H1N1 pandemic in Austria. The participating institutions included three out of four university hospitals (Medical University of Vienna, Salzburg Medical University, Innsbruck Medical University), the Military Hospital of Vienna and three regional hospitals (Hospital of the Barmherzigen Schwestern Ried im Innkreis, Public Hospital Kirchdorf an der Krems, Hospital Elisabethinen Linz). Only patients with H1N1 infection confirmed by real time reverse transcriptase polymerase chain reaction (RT-PCR) assay or by Quick test were included in the analysis. The study protocol was approved by the Ethics Committee of the Medical University of Vienna.
Demographic characteristics, clinical presentation, coexisting medical conditions and vaccination status (seasonal influenza, pandemic H1N1 virus, pneumococcus) were obtained in a total of 540 cases of confirmed H1N1 infection. Obesity was defined as body mass index (BMI) ≥ 35 in adults 18 years of age or older or a BMI percentile of 95–100 in children between the ages of 2 and 17 years (BMI calculated as the weight in kilograms divided by the square of the height in metres). Obesity was not defined for pregnant woman or children under the age of 2 years. Presenting laboratory findings and chest X-ray findings on the admission radiograph were also collected. Outcome measures included duration of fever, need for intensive care unit (ICU) admission and death during the hospital stay. All data were reported on a standardised online case-history form (k-ontext®, Key Solutions, Vienna, Austria).
Continuous data are presented as medians with range or as mean ± standard deviation (SD), as appropriate. For categorical variables, the percentage of patients in each category was calculated. For descriptive analysis, we made no assumptions about missing data and adjusted proportions to the number of patients with available data. Univariate analyses were used to compare in- and outpatients with ICU patients or fatal cases. Fisher’s exact test was used to compare proportions of categorical data and the Wilcoxon rank-sum test was used for continuous variables.
We used multivariable logistic regression for the identification of independent predictors of ICU admission. The dependent variable was admission to ICU (yes vs. no). For potential independent predictors, we chose variables based on earlier reports and on differences in the univariate analysis, where we set the P-value at 0.25. Likelihood ratio tests were used to test for linear effects and model improvement. We aimed at the most parsimonious model. The Hosmer–Lemeshow test was used to assess goodness of model fit for the final model. We used web-based data collection and found considerable item non-response, rendering reasonable multivariate modelling impossible. However, it is reasonable to assume that pathological conditions were reported and that item non-response could be mainly explained by the absence of the specific condition. This is obvious for pathologies observed on chest X-rays and evident for pregnancy, where no input option was provided for male cases. Thus, missing values were not random and it appeared reasonable to assume that they equated to absence of the specific condition and, therefore, were set at zero for the multivariable analysis.
SPSS (release 18.0) and Stata 11 for Mac (Stata Corp, College Station, TX, USA) were used for all statistical analyses. Reported P-values are two-sided and, unless otherwise stated, a P-value < 0.05 was considered to be statistically significant.
Results
Clinical characteristics
This report describes data on 540 human cases of infection with 2009 H1N1 virus collected from seven hospitals in Austria. Among these patients, 343 were admitted to the hospitals representing 22% of the total numbers hospitalised with 2009 H1N1 influenza and whose cases were reported to the Austrian epidemiological registry [10].
Infection was confirmed in 516 patients (95.6%) using a real time RT-PCR assay specific for 2009 H1N1 influenza; a positive influenza rapid test was confirmed in the remaining 24 patients. Among 176 PCR-positive cases also evaluated by point-of-care testing for influenza A, 70 (39.8%) were positive, the sensitivity of the test being found higher in children (17/22; 77.3%) than in adults (53/154; 34.4%).
Dates of onset of illness in the patient group overall ranged from 20 September 2009 to 2 February 2010 (Fig. 1). The median age of the patients was 19.3 years (range 26 days–90.8 years) (Table 1); 225 patients (41.7%) were children under the age of 18 years and 313 (58.3%) were adults.
Fig. 1.
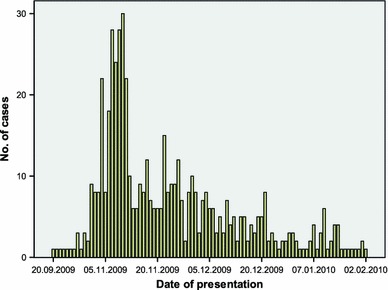
Epidemiological curve of the dates of presentation
Table 1.
Demographic characteristics of 540 patients infected with 2009 pandemic H1N1 influenza virus in Austria (September 2009–February 2010)
| Characteristic | All cases | Hospitalised patients | ICU patients | Fatal casesa |
|---|---|---|---|---|
| All patients | 540 (100) | 343/540 (63.5) | 49/540 (9.1) | 14/341 (4.1) |
| Female | 203 (37.6) | 102/203 (50.2) | 21/203 (10.3) | 4/100 (4.0) |
| Male | 337 (62.4) | 241/337 (71.5) | 28/337 (8.3) | 10/241 (4.1) |
| Age groupb | ||||
| 0–23 months | 33 (6.1) | 24/33 (72.7) | 3/33 (9.1) | 0/24 (0.0) |
| 2–4 years | 27 (5.0) | 14/27 (51.9) | 1/27 (3.7) | 0/14 (0.0) |
| 5–9 years | 64 (11.9) | 14/64 (21.5) | 1/64 (1.6) | 0/14 (0.0) |
| 10–17 years | 101 (18.7) | 29/101 (28.7) | 3/101 (3.0) | 0/29 (0.0) |
| 18–29 years | 166 (30.7) | 140/166 (84.3) | 3/166 (1.8) | 1/140 (0.7) |
| 30–64 years | 119 (22.0) | 92/119 (77.3) | 26/119 (21.8%) | 8/90 (8.9) |
| ≥65 years | 30 (5.6) | 30/30 (100.0) | 12/30 (40.0) | 5/30 (16.7) |
Data are presented as no. (%). Because of rounding, the percentages may not total 100
aDeath was recorded for inpatients only and was not available for two patients
bThe median age of the patients was 19.3 years (range 26 days–90.8 years). Percentages of inpatients, ICU patients and fatal cases refer to all cases within the age groups
Of the 540 patients evaluated, 133 (24.6%) had an underlying medical condition (Table 2). Information on pregnancy status was available for 147 patients, among whom 15 (15.2%) were pregnant (pregnancy data input option was not provided for male cases). One of the pregnant patients was in the first trimester, four were in the second and six were in the third; the gestational duration of four patients was not known. Two of the 15 pregnant women had an underlying condition. Obesity was reported on admission in 16 of 299 adults (5.1%) and 2 of 223 children (0.9%).
Table 2.
Underlying medical conditions among patients with pandemic H1N1 infection according to age group
| Underlying condition | All patients | Patients <18 years old | Patients ≥18 years old |
|---|---|---|---|
| Any | 133/540 (24.6) | 29/225 (12.9) | 104/315 (32.9) |
| Pregnancya | 15/147 (15.2) | 0/77 (0.0) | 15/70 (27.3) |
| Asthma | 13/540 (2.4) | 1/225 (0.4) | 12/315 (3.8) |
| Chronic obstructive pulmonary disease | 25/540 (4.6) | 1/225 (0.4) | 24/315 (7.6) |
| Neuromuscular disorder | 3/540 (0.6) | 1/225 (0.4) | 2/315 (0.6) |
| Guillain–Barré syndrome | 0/540 (0) | 0/225 (0.0) | 0/315 (0.0) |
| Other neurological disease | 16/540 (3) | 7/225 (3.1) | 9/315 (2.9) |
| Obesity (BMI >35) | 18/540 (3.3) | 2/225 (0.9) | 16/315 (5.1) |
| Cardiovascular disease | 32/540 (5.9) | 2/225 (0.9) | 30/315 (9.5) |
| Diabetes mellitus | 23/540 (4.3) | 0/225 (0.0) | 23/315 (7.3) |
| Chronic liver disease | 10/540 (1.9) | 1/225 (0.4) | 9/315 (2.9) |
| Malignancy | 12/540 (2.2) | 1/225 (0.4) | 11/315 (3.5) |
| Haematological malignancy | 11/540 (2.0) | 5/225 (2.2) | 6/315 (1.9) |
| Immunosuppressive therapy | 23/540 (4.3) | 3/225 (1.3) | 20/315 (6.3) |
| Other | 39/540 (7.2) | 11/225 (4.9) | 28/315 (8.9) |
Data are presented as no./total no. (%)
aData input option on pregnancy was provided for female patients only
None of the patients evaluated had received prophylactic antiviral treatment. Among 122 patients for whom information on influenza vaccination was available, 14 had been vaccinated with a single dose of Celvapan. None of the patients had received the recommended booster vaccination. Two of 71 patients had been vaccinated against seasonal influenza.
Symptoms at presentation are outlined in Table 3. Information on raised body temperature was available for 340 patients: 17.6% presented with body temperature between 37.3 and 38°C, 55.6% presented with 38.1 to 39°C, and 26.8% presented with >39°C. Noteworthy, fever over 38°C was recorded in more than 80% of the patients and, thus, resulted to be the most sensitive parameter. The mean duration of fever was 3 days (range 1–16); the mean duration in patients who received oseltamivir was significantly shorter than in patients who did not receive antiviral treatment (2.59 vs. 3.25 days, P = 0.01). Other common symptoms at presentation included cough, recorded in 268 of 334 patients (80.2%), and fatigue, reported in 70.4% of patients, including 91 of 95 children (95.8%) and 147 of 243 adults (60.5%). Myalgia and/or arthralgia were reported in 50.0% of the children and 47.5% of the adults. Nausea and vomiting were recorded in 25 of 49 children (51%) and 24 of 189 adults (12.7%); diarrhoea was present in 25% of children and 8.6% of the adults.
Table 3.
Symptoms at presentation in patients with 2009 pandemic H1N1 infection according to age group
| Symptom | All patients | Patients <18 years old | Patients ≥18 years old |
|---|---|---|---|
| Elevated temperature (°C) | |||
| 37.3–38 | 60/340 (17.6) | 10/94 (10.6) | 50/246 (20.3) |
| 38.1–39 | 189/340 (55.6) | 58/94 (61.7) | 131/246 (53.3) |
| >39 | 91/340 (26.8) | 26/94 (27.7) | 65/246 (26.4) |
| Cough | 268/334 (80.2) | 74/85 (87.1) | 194/249 (77.9) |
| Rhinorrhoea | 112/283 (39.6) | 49/71 (69.0) | 63/212 (29.7) |
| Headache | 182/273 (66.7) | 41/51 (80.4) | 141/222 (63.5) |
| Fatigue | 238/338 (70.4) | 91/95 (95.8) | 147/243 (60.5) |
| Myalgia, arthralgia | 109/228 (47.8) | 15/30 (50.0) | 94/198 (47.5) |
| Chills | 61/211 (28.9) | 17/34 (50.0) | 44/177 (24.9) |
| Diarrhoea | 29/244 (11.9) | 12/47 (25.5) | 17/197 (8.6) |
| Nausea, vomiting | 49/238 (20.6) | 25/49 (51.0) | 24/189 (12.7) |
| Chest pain | 31/211 (14.7) | 3/25 (12.0) | 28/186 (15.1) |
Data are presented as no./total no. (%)
Diagnostic findings at presentation
The results of laboratory examination and chest X-rays at presentation are outlined in Table 4. Of 391 patients for whom these data were available, 86 presented with anaemia (22.0%), 33 with leukopaenia (8.4%) and 78 with leukocytosis (22.3%). Thrombocytopaenia was present in 57 of 390 patients (15.5%); lymphopaenia was reported in 42 out of 77 children (54.5%) and 29 out of 96 adults (30.2%). A raised C-reactive protein (CRP) value was reported in 275 of 384 patients (71.6%).
Table 4.
Diagnostic findings at presentation in patients with 2009 pandemic H1N1 infection
| All patients | Patients <18 years old | Patients ≥18 years old | |
|---|---|---|---|
| Selected laboratory abnormalities | |||
| Laboratory results available | 395/540 (73.1) | 100/225 (44.4) | 295/315 (93.7) |
| Leukocyte count | |||
| <4,000/μl | 33/391 (8.4) | 11/99 (11.1) | 22/292 (7.5) |
| 4,000–10,000/μl | 271/391 (69.3) | 62/99 (62.6) | 209/292 (77.1) |
| >10,000/μl | 78/391 (22.3) | 26/99 (25.3) | 81/292 (20.9) |
| Platelet count | |||
| <150,000/mm3 | 57/390 (14.6) | 15/98 (15.3) | 42/292 (14.4) |
| Haemoglobin <12 g/dl | 86/391 (22.0) | 26/97 (26.8) | 60/294 (20.4) |
| Lymphocyte count | |||
|
<3,000/mm3 in children <1,500/mm3 in adults |
71/173 (41.0) | 42/77 (54.5) | 29/96 (30.2) |
| GOT >40 U/l | 84/287 (29.3) | 14/30 (46.7) | 70/257 (27.2) |
| GPT >45 U/l | 63/285 (22.1) | 4/29 (10.2) | 59/256 (23.0) |
| Creatinine >1.2 mg/dl | 0/234 (0.0) | 0/33 (0.0) | 0/201 (0.0) |
| Creatine kinase >200 U/l | 54/196 (27.6) | 3/9 (33.3) | 51/187 (27.3) |
| CRP >1 mg/dl | 275/384 (71.6) | 57/94 (60.6) | 218/290 (75.2) |
| CRP mg/dl (mean ± SD) | 7.92 ± 10.2, n = 275 | 8.14 ± 15.1, n = 57 | 7.86 ± 8.5, n = 218 |
| Radiographic findings | |||
| Chest X-ray available | 285/540 (52.8) | 43/225 (19.1) | 242/315 (76.8) |
| Without pathological findings | 84/285 (29.5) | 8/43 (18.6) | 76/242 (31.4) |
| Interstitial pathology | 31/285 (10.9) | 9/43 (20.9) | 22/242 (9.1) |
| Lobular pathology | 63/285 (22.1) | 11/43 (25.6) | 52/242 (21.5) |
Data are presented as no./total no. (%)
GOT glutamate oxaloacetate transaminase, GPT glutamic pyruvic transaminase, CRP C-reactive protein
A total of 285 patients had a chest X-ray on presentation: among these, 84 (29.5%) showed no pathological findings, 94 (33.0%) had findings suggestive for pneumonia or acute respiratory distress syndrome (ARDS), 63 (22.1%) demonstrated lobular findings and 31 (10.9%) had alveolar pathology. The median age of these patients was 42.1 years (range 6 months–90.8 years) and 64.4% had an underlying medical condition.
Hospital admission
Of the 540 patients reviewed in this study, 343 (63.5%) were admitted to hospital, the admission rates being highest in adults aged 65 years or older (Table 1).
Among the 343 inpatients, underlying medical conditions were present in 127 (37%), of whom 27 of 81 were children (33.3%) and 100 of 262 were adults (38.2%). Overall, 18.9% of hospitalised patients had at least two such conditions and, among the 30 patients aged 65 years or older, 90% had an underlying medical condition.
The reasons for admission are shown in Table 5: these included containment strategy for 114 patients (33.2%), respiratory failure in 129 patients (37.6%), haemodynamic failure in 36 (10.5%), cerebral failure in 8 (2.3%), pregnancy in 8 (2.3%) and relevant comorbidity (patient at risk) in 39 (11.4%). In 14 cases (4.1%), acquisition of the 2009 H1N1 virus infection was nosocomial. The median duration of hospitalisation was 7 days (range 1–78, excluding patients admitted to ICUs).
Table 5.
Reasons for hospital admission according to age group in patients with 2009 pandemic H1N1 infection
| All patients (n = 343) | Patients <18 years old (n = 81) | Patients ≥18 years old (n = 262) | |
|---|---|---|---|
| Containment strategy | 114 (33.2) | 6 (7.5) | 108 (41.2) |
| Nosocomial acquisition | 14 (4.1) | 2 (2.5) | 12 (4.6) |
| Respiratory impairment | 129 (37.6) | 36 (44.4) | 93 (35.5) |
| Haemodynamic impairment | 36 (10.5) | 8 (9.9) | 28 (10.7) |
| Cerebral impairment | 8 (2.3) | 5 (6.2) | 3 (1.1) |
| Pregnancy | 8 (2.3) | 0 (0) | 8 (3.1) |
| Risk patient | 39 (11.4) | 8 (9.9) | 31 (11.8) |
Data are presented as no. (%)
Smoking status was available for 293 patients: 73 out of 75 smokers were hospitalised.
Complications in hospitalised patients
Complications were reported for 83 (37.1%) of the 343 patients who were admitted to hospital and included bacterial pneumonia in 50 (14.6%) patients, sepsis in 10 (2.9%), enteritis in 9 (0.2%) and myositis in 9 (0.2%).
Eight patients with septicaemia had positive blood cultures: a 56-year-old man with concomitant cholangitis caused by Enterococcus faecium and extended-spectrum beta-lactamases (ESBL)-positive Escherichia coli infection; a 41-year-old man with Staphylococcus aureus infection; a 54-year-old man with group A streptococcus infection; a 73-year-old woman with perforation of the colon and Enterococcus faecalis infection; a 85-year-old woman with peritonitis after perforated sigmoid diverticulitis and Enterococcus and Candida tropicana infection; a 43-year-old man with Acinetobacter and group A streptococcus infection; a 66-year-old man with Streptococcus pneumoniae infection, pneumonia and pleura-empyema; an 8-year-old boy with monosomy 1p and E. coli infection.
Infections that were identified from sources other than blood cultures included S. pneumoniae in four patients (ages 3, 40, 43 and 49 years) with pneumonia; Staphylococcus spp. in three (ages 48, 57 and 58 years) with pneumonia; Pneumocystis pneumonia in a 53-year-old man with ARDS and bronchiolitis obliterans; Candida spp. and Klebsiella in a 77-year-old man with pneumonia; group A streptococcus in a patient with putrid rhinitis.
ICU admissions
In total, 49 (14.3%) of the 343 inpatients were admitted to an ICU. Omitting the 114 who were admitted because of containment strategies, intensive care was necessary in 21.4% of the inpatients. The median age of those admitted to an ICU was 51.6 years (range 4 months–85 years). Rates of ICU admission were highest in patients aged 65 years or older (40%) (Table 1). Among the 49 ICU admissions, 39 (79.6 %) had an underlying medical condition. The median age of the ten previous healthy patients requiring intensive care was 51 years (range 38–63). All patients aged 65 years or older (n = 12) had at least one underlying medical condition. The most common comorbidities were cardiovascular disease (32.7%), obesity (18.4%), neurological diseases (18.4%) and diabetes mellitus (16.3%). One patient with diabetes was pregnant.
Reasons for ICU admission were provided for 42 patients and included respiratory impairment in 37 (62.7%), haemodynamic impairment in 18 (30.5%) and cerebral impairment in 4 (6.8%).
Of the 49 patients admitted to an ICU, 36 (73.5%) required mechanical ventilation for a median duration of 15 days (range 1–76), 6 (12.2%) required extracorporeal membrane oxygenation (ECMO) for a median duration of 12 days (range 10–12), 8 (18.3%) required dialysis for a median duration of 9 days (range 4–72). Of the eight patients requiring haemodialysis, two patients had myositis and raised creatine kinase levels and one patient had raised creatine kinase levels. Twenty-one patients (42.9%) had a clinical diagnosis of pneumonia and 8 (16.3%) had a clinical diagnosis of sepsis.
Treatment
Overall, 177 of the 197 outpatients (89.8%) and 243 of the 343 inpatients (70.8%) received treatment with oseltamivir. Data on the time elapsed between the onset of symptoms and the initiation of therapy were not available.
Information on antibiotic treatment was provided for 151 of the 343 inpatients (44.3%), among whom 130 (86.1%) received parenteral antibiotics with a median of one antibiotic (range 1–7). More than one intravenous antibiotic was used in 39.2% of the patients. Parenteral antibiotics included macrolides in 31 patients, cephalosporins in 29, piperacillin/tazobactam in 29, aminopenicillin/clavulanic acid in 26, linezolid in 15 and ciprofloxacin/levofloxacin in 14. Antibiotics with activity against Pseudomonas were used mainly in patients requiring intensive care. For oral treatment, the most common antibiotics used were macrolides and aminopenicillin/clavulanic acid. Twenty-one patients were treated with both oral and intravenous antibiotics.
Of the patients who had radiographic findings of interstitial or alveolar changes, 85.9% received intravenous antibiotics, 5.9% received oral antibiotics and 16.6% received both; 67.1% of these patients were treated with oseltamivir.
Systemic corticosteroids were administered in 37 (15.2%) of 243 inpatients for whom this information was available: 29 (10.2%) received hydrocortisone, 8 (2.8%) received other corticosteroids.
Among the patients treated with systemic corticosteroids, 83.8% had an underlying medical condition, the most common being asthma or chronic obstructive pulmonary disease (COPD) (11.6%), immunosuppression (10.5%), cardiovascular disease (9.3%) and diabetes mellitus (9.3%).
Outcomes
Of the 343 patients who were hospitalised, 329 (95.9%) were discharged and 14 (4.1%) died. Among those who died, 10 were female (71.4%), 13 had been admitted to an ICU and 11 required mechanical ventilation. The median age of patients who died was 55.4 years (range 26–85). Case fatality rates were highest (9.1%) in those aged 65 years or older (Table 1). Twelve of the patients who died (85.7%) had an underlying medical condition: cardiovascular diseases in six, asthma or COPD in three, diabetes in three, obesity (BMI >35) in two, neurological disorders in two and other chronic diseases in six. Eleven of these patients (78.6%) had two or more underlying medical conditions. Nine of the 14 patients had received antiviral drugs and all had antibiotic treatment.
Autopsy results were available from 7 out of 14 patients who died. In two patients, diffuse alveolar damage (DAD) with fibrinous exudates, hyaline membranes and interstitial neutrophilic infiltrates was reported. Another patient with clinically diagnosed with S. aureus pneumonia, who developed sepsis with multiorgan failure and finally died because of cerebral mass bleeding, autopsy showed DAD with additional abscess formation.
In addition, for one patient with underlying chronic lung disease who died because of respiratory insufficiency, pathology findings revealed organised pneumonia in all lobes of both lungs. Two patients died because of myocardial infarction. One of these patients demonstrated pathologic-anatomical findings of DAD with interstitial inflammation, alveolar oedema and pulmonary congestion, the other suffered from sepsis with multiorgan failure showing interstitial and alveolar oedema and bilateral pleural effusions.
In one patient dying because of sepsis with multiorgan failure, no signs of lung inflammation were observed in the autopsy.
Risk factors for ICU admission
Risk factors for ICU admission are shown in Table 6. Patients who were admitted to an ICU were significantly more likely to present with thoracic pain, together with fatigue and chills.
Table 6.
Comparison of in- and outpatients not admitted to an intensive care unit (ICU) and patients who were admitted to an ICU
| ICU patients (n = 49) | Non-ICU patients (n = 491) | P-value | |
|---|---|---|---|
| Age, mean (SD) | 46.9 (23.6) | 22.6 (18.2) | <0.0001 |
| Female | 21/49 (42.9) | 182/491 (37.1) | 0.44 |
| Smoker | 11/34 (32.4) | 64/257 (24.9) | 0.40 |
| Pregnancy | 1/20 (5) | 14/127 (13.2) | 0.69 |
| Duration of fever | 5.4 (3.6) | 3 (1.9) | <0.0001 |
| Previous seasonal influenza virus vaccination | 0/8 (0) | 2/63 (3.2) | 0.99 |
| Previous H1N1 virus vaccination | 0/14 (0) | 14/108 (13.0) | 0.37 |
| Previous pneumococcus vaccination | 5/40 (12.5) | 1/6 (16.7) | 0.99 |
| Positive H1N1 PCR | 47/48 (97.9) | 469/474 (99.0) | 0.44 |
| Positive H1N1 rapid test | 4/10 (40.0) | 66/166 (39.8) | 0.99 |
| Oseltamivir treatment | 28/49 (57.1) | 392/491 (79.8) | 0.001 |
| Fatal cases | 13/47 (27.7) | 1/294 (0.3) | <0.001 |
| Comorbidities | |||
| Asthma | 2/49 (4.1) | 11/491 (2.2) | 0.33 |
| COPD | 3/49 (6.1) | 22/491 (4.5) | 0.49 |
| Neuromuscular disease | 0/49 (0.0) | 3/491 (1.0) | 0.99 |
| Neurological disease | 9/49 (18.4) | 7/491 (1.4) | <0.001 |
| Obesity | 9/49 (18.4) | 9/491 (1.8) | <0.001 |
| Cardiovascular disease | 16/49 (32.7) | 16/491 (3.3) | <0.001 |
| Diabetes mellitus | 8/49 (16.3) | 15/491 (3.1) | <0.001 |
| Chronic liver disease | 3/49 (6.1) | 7/491 (1.4) | 0.05 |
| Malignancy | 2/49 (4.1) | 10/491 (2.0) | 0.30 |
| Haematological malignancy | 1/49 (2.0) | 10/491 (2.0) | 0.99 |
| Immunosuppression | 3/49 (6.1) | 20/491 (4.1) | 0.46 |
| Symptoms at presentation | |||
| Body temperature (°C) | |||
| 37.3–38 | 10/37 (27.0) | 50/303 (16.5) | 0.12 |
| 38.1–39 | 19/37 (51.4) | 170/303 (56.1) | 0.60 |
| >39.0 | 8/37 (21.6) | 83/303 (27.4) | 0.56 |
| Fatigue | 26/27 (96.3) | 212/311 (68.2) | 0.001 |
| Myalgia | 6/10 (60.0) | 103/218 (43.6) | 0.53 |
| Chills | 7/13 (53.9) | 54/198 (27.3) | 0.06 |
| Thoracic pain | 10/19 (52.6) | 21/192 (10.9) | <0.001 |
| Diagnostic findings at presentation | |||
| Chest X-ray | |||
| Chest X-ray available | 47/49 (95.9) | 238/491 (48.5) | <0.001 |
| Without pathological findings | 5/47 (10.6) | 79/238 (33.2) | 0.001 |
| Interstitial pathology | 11/47 (23.4) | 20/238 (8.4) | 0.01 |
| Lobulary pathology | 30/47 (63.8) | 33/238 (13.9) | <0.001 |
| Laboratory findings | |||
| Leukocyte count <4,000/μl | 5/49 (10.2) | 28/342 (8.2) | 0.59 |
| Leukocyte count 4,000–10,000/μl | 27/49 (55.1) | 244/342 (71.4) | 0.03 |
| Leukocyte count >10,000/μl | 17/49 (34.7) | 70/342 (20.5) | 0.04 |
| Thrombocytopaenia | 10/49 (20.4) | 47/341 (13.8) | 0.28 |
| Anaemia | 22/49 (44.9) | 64/342 (18.7) | <0.001 |
| Lymphopaenia children | 1/2 (50.0) | 41/75 (54.7) | 0.90 |
| Lymphopaenia adults | 9/13 (69.2) | 20/83 (24.1) | 0.001 |
| GOT | 30/44 (68.2) | 54/243 (22.2) | <0.001 |
| GPT | 21/44 (47.7) | 42/241 (17.4) | <0.001 |
| Creatine kinase >200 U/l | 18/31 (58.1) | 36/165 (21.8) | <0.001 |
| CRP mg/dl, mean (SD) | 15.5 (11.5) | 6.7 (9.5) | <0.001 |
| Reason for admission | |||
| Containment strategy | 0/49 (0) | 114/491 (23.2) | <0.001 |
| Nosocomial acquisition | 2/49 (4.1) | 12/491 (2.4) | 0.37 |
| Respiratory impairment | 37/49 (75.5) | 92/491 (18.7) | <0.001 |
| Haemodynamic impairment | 18/49 (36.7) | 18/491 (3.7) | <0.001 |
| Cerebral impairment | 4/49 (8.2) | 4/491 (0.8) | 0.003 |
| Pregnancy | 1/49 (2.0) | 7/491 (1.4) | 0.54 |
| Risk patient | 6/49 (12.2) | 33/491 (6.7) | 0.15 |
| Complications during hospital stay | |||
| Neuropathia | 1/49 (2.0) | 0/295 (0.0) | 0.14 |
| Myocarditis | 1/49 (2.0) | 1/295 (0.3) | 0.27 |
| Myositis | 9/49 (18.4) | 0/295 (0.0) | <0.001 |
| Enteritis | 3/49 (6.1) | 6/295 (2.03) | 0.12 |
| Bacterial pneumonia | 21/49 (42.9) | 29/295 (9.83) | <0.001 |
| Sepsis | 9/49 (16.3) | 2/295 (0.7) | <0.001 |
| Pulmonary embolism | 2/49 (4.08) | 0/295 (0.0) | 0.02 |
Data are presented as no./total no. (%) for categorical data or as mean ± SD for continuous data (no. of patients in parentheses)
GOT glutamate oxaloacetate transaminase, GPT glutamic pyruvic transaminase, CRP C-reactive protein
Comorbidities significantly associated with ICU admission included obesity, neurological disease, cardiovascular disease and diabetes mellitus. No association with pregnancy, malignancy or immunosuppressive therapy was detected.
Diagnostic findings on admission that were found to be significantly associated with ICU admission included anaemia, high CRP levels, elevated liver enzymes and radiographically confirmed pneumonia.
Antiviral treatment significantly reduced the duration of fever (by 0.66 days, P = 0.01) and lowered the risk of ICU admission (P = 0.001), but had no significant benefit on survival (P = 0.56) (Table 7).
Table 7.
Influence of antiviral treatment with oseltamivir on the duration of fever, risk of severe disease and survival in patients hospitalised with 2009 pandemic H1N1 infection
| Oseltamivir | No antiviral treatment | P-value | |
|---|---|---|---|
| Duration of fever (days) | 2.59 (159 patients) | 3.25 (57 patients) | 0.01 |
| ICU admission | 28/420 (6.7%) | 21/120 (17.5%) | 0.001 |
| Death | 9/241 (3.7%) | 5/100 (5.0%) | 0.56 |
A multivariable model for critical care requirement included the variables age (decades), antiviral treatment with oseltamivir, neurological comorbidity, interstitial or lobular pathology on chest X-ray, and obesity. All factors demonstrated a significant association (Table 8). Although the overall population was young, the increase in risk for critical care requirement for each additional decade of age was 4%.
Table 8.
Results of logistic regression analyses of demography, comorbidities, clinical presentation and diagnostic findings at admission for critical care requirement in patients hospitalised with 2009 pandemic H1N1 infection
| Odds ratio | 95% confidence interval | P-value | |
|---|---|---|---|
| Age (increasing by each decade) | 1.04a | 1.02–1.06 | <0.001 |
| Oseltamivir treatment (yes vs. no) | 0.325 | 0.14–0.74 | 0.008 |
| Neurologic comorbidity (yes vs. no) | 19.11 | 3.92–93.22 | <0.001 |
| Obesity (yes vs. no) | 3.97 | 1.11–14.21 | 0.03 |
| Chest X-ray: interstitial pathology (yes vs. no) | 4.25 | 1.40–12.87 | 0.01 |
| Chest X-ray: lobular pathology (yes vs. no) | 13.24 | 5.92–29.63 | <0.001 |
Multivariate analysis based on the observation of 539 cases. Hosmer–Lemeshow χ2(6) = 5.66, P > χ2 = 0.4625
aReflects a 4% increase in risk for critical care requirement for each additional decade of age
Discussion
We report on a large case series of in- and outpatients with 2009 pandemic H1N1 influenza virus infection that included more than 20% of all patients hospitalised with this infection in Austria during the period 2009–2010. In our study population, half of the patients were <19 years of age and half of the hospitalisations affected persons under the age of 21 years; only 5.6% were 65 years of age or older. Our findings are in line with the growing volume of literature showing that the age distribution of 2009 pandemic H1N1 influenza disease was markedly different from that observed in seasonal influenza, where hospitalisation is more common among persons 65 years of age or older and in children under the age of 5 years [21].
A potential explanation for this observation is that patients in the older age group have an antibody that is cross-reactive with 2009 H1N1 virus at much higher rates than which is found in younger patients [22–24]. It has been further presumed that children are more likely to be exposed to the infection in schools and that young febrile persons are more likely to be tested than older adults with influenza, who often do not have fever [25].
Despite persons aged 65 years or older having lower infection rates, in our study, they were the group most likely to be admitted to an ICU or to die. These findings concur with early reports from Mexico and later reports worldwide [26]. Comorbidity may have contributed to 2009 pandemic H1N1 mortality in elderly persons: 60% of cases over the age of 60 years had heart or respiratory disease [26]. Similarly, we found a clear linear trend between the number of comorbidities and ICU admission; in the present case series, more than 90% of the hospitalised patients and all patients admitted to the ICU aged 65 years or older had an underlying medical condition. In contrast, all previous healthy patients requiring intensive care were under the age of 65 years.
The relative absence of comorbidities in our case series suggests that the population primarily affected by severe H1N1 infection during the 2009 pandemic were young and relatively healthy adults.
Nevertheless, the fact that severe disease has arisen in such a population, with a high probability of survival given the availability of appropriate resources, has important societal implications [27]. Although the increased demand for ICU care and ECMO was foreseeable [15, 16, 27–30], the capacity to care for critically ill patients was seriously challenged and ECMO services in Austria were at the limit [31]. Despite international warnings and recommendations regarding the management of intensive care services, neither specific procedures for the management of increased numbers of ICU cases nor improved regional coordination for ICU care was established in Austria.
According to Austrian health officials, approximately 300,000 persons received a vaccination against the 2009 pandemic H1N1 virus, which corresponds to only about 3% of the population. Concerning the vaccination rate among healthcare workers in Austria, no official data are available. At the Medical University of Salzburg, 62.7% of the physicians and 26.6% of the nursing staff received at least a single dose of the bivalent vaccine Celvapan. At the Medical University of Vienna, comprising 8,955 employees, 3,670 doses of Celvapan were used, which would correspond to 1,835 employees (20.5%) receiving the recommended two doses of the vaccine. This strikingly low vaccination rates resulted from a lack of enthusiasm in the general public and in some healthcare professionals, and highlights the need for effective strategies to counter anti-vaccination messages.
In contrast to seasonal influenza, obesity has been identified as a risk factor for severe 2009 pandemic H1N1 infection in several reports [16, 19, 27, 32]. In the present study population, the prevalence of obesity was low, being 5.1% in adults and 0.9% in children. Nonetheless, obesity was significantly related to severe disease requiring ICU admission, although not with fatality amongst our H1N1 patients.
Pregnancy is a well-established risk factor for severe influenza disease. During the 2009 influenza pandemic, pregnant women accounted for up to 7–10% of hospitalised patients [16, 17, 33], 6–9% of ICU patients [15, 27] and 6–10% of patients who died [16, 34], compared to 1–2% of pregnancies among a healthy population. This is also consistent with influenza-associated excess deaths among pregnant women during the pandemics of 1917–1918 and 1957–1958 [35].
However, in the later phases of the 2009 H1N1 pandemic, its effect on pregnant women was less than initially anticipated, which may be attributable to worldwide recommendations for pregnant women to be vaccinated against the 2009 H1N1 strain and advice to facilitate early access to antiviral treatment for pregnant women with symptoms of flu [36]. Presentation with mild or moderate symptoms was frequent among the 15 pregnant women in our series, although rapid clinical progression and deterioration did occur: one woman in the third trimester with diabetes mellitus and respiratory failure required a Caesarean section, ICU admission and mechanical ventilation for 21 days. In our analysis, pregnancy was not associated with a higher hospitalisation rate or the need for ICU admission. No data are available on the rate of H1N1 vaccination among pregnant women in Austria.
In contrast to seasonal influenza, early reports of severe cases of 2009 pandemic H1N1 infection showed no evidence of concomitant bacterial pneumonia [37], although in one smaller study, early-onset secondary bacterial pneumonia due to S. aureus was observed at a high frequency of 22% [38]. In the present analysis, concomitant laboratory-confirmed bacterial pneumonia was reported in only nine patients, the most common pathogen isolated being S. pneumoniae. These findings might lead to the perception that bacterial coinfections played a limited role during the 2009 H1N1 pandemic. On the other hand, a recent study on bacterial pneumonia in fatal cases of the pandemic indicates that, as observed during previous influenza pandemics, bacterial pneumonia considerably contributed to death [39]. Thus, a low number of clinically documented bacterial lung infections might reflect the difficulty of establishing specific bacterial diagnoses in persons with suspected bacterial coinfection rather than reflecting a truly low incidence [40]. Accordingly, in the present analysis, 14.6% of the hospitalised patients, 42.9% of the patients requiring intensive care and 38.5% of fatal cases had a clinical diagnosis of bacterial pneumonia. Our data suggest that bacterial lung infections occurred among patients with 2009 pandemic H1N1 infection and may underline the importance of pneumococcal vaccination for persons at increased risk of pneumococcal pneumonia.
In this context, higher CRP values at presentation were significantly associated with a higher risk for critical care requirement. Generally, CRP values are higher in patients with bacterial infections than in those with non-bacterial infection, and high levels are associated with mortality [19, 41]. These findings are in accordance with previous reports of raised CRP levels as a risk factor for severe H1N1 influenza disease and highlight the potential seriousness of this parameter.
Of interest, fever ≥38°C was recorded in more than 80% of the patients and was, thus, the most sensitive parameter. However, this result should be considered with caution, because information on raised body temperature was provided for only 340 (63%) out of 540 patients. Under the assumption that this item non-response can be mainly explained by the absence of the specific condition, our data accords to previous reports of low prevalence of fever among patients with 2009 pandemic H1N1 infection [9, 19].
Our data on the 2009 H1N1 pandemic further underline important issues in diagnostic testing and treatment: rapid influenza tests yielded false-negative results in 60.2% of cases tested. This is in line with other reports of low sensitivity of point-of-care tests for 2009 pandemic H1N1 infection [42, 43]. The sensitivity was higher in children, who shed higher viral loads for longer durations compared with adults [44]. Thus, reliance on the results of rapid tests may be misleading and could result in the delayed diagnosis of H1N1 infection.
In our study population, antiviral treatment was administered to 70% of the inpatients and 89.8% of the outpatients. Although no information was obtained on the time between the onset of symptoms and the initiation of therapy, antiviral therapy reduced the symptoms of influenza. The benefit was, however, modest, with a reduction in the duration of fever of 0.6 days. No benefit in terms of survival could be identified. Nonetheless, antiviral treatment significantly reduced the risk of admission to an ICU. Thus, our data indicate that antiviral treatment may be beneficial for patients with 2009 pandemic H1N1, regardless of the time of symptom onset.
The limitations of our study should be noted. Participation in the study was voluntary and was, therefore, subject to reporting bias. In addition, as data were collected only on patients seeking emergency medical care, the results may not truly represent cases of milder disease or patients who were not tested for H1N1 infection. Further, data were collected from pre-existing medical records. Some variables, such as presenting symptoms, may have been subject to bias in terms of questioning or interpretation by healthcare practitioners.
In summary, although the majority of the described cases were mild, severe illness, including pneumonia and ARDS, was observed. Underlying medical conditions were common in the 540 cases we evaluated, but severe illness was also identified among previously healthy persons. Elderly, obese and patients with neurologic disease had an increased risk for severe H1N1 infection during the 2009 pandemic in Austria. Antiviral treatment provided a minimal effect on the symptoms of influenza, but reduced the risk of admission to an ICU.
Acknowledgments
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
On behalf of the Austrian Clinical Influenza Study Group. Collaborators: Milo Halabi, Walter Hasibeder, Holger Flick, Lukas Gansinger, Martina Voith, Gregor Chmelizek, Viola Maass, Wolfgang Sperl, Langenhorst Udo, Ingrid Pretsch, Richard Greil, Michael Grundbichler, Thomas Staudinger, Peter Schellongowski.
W. Poeppl and M. Hell contributed equally.
References
- 1.Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–679. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 2.Cao B, Li XW, Mao Y, Wang J, Lu HZ, Chen YS, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361:2507–2517. doi: 10.1056/NEJMoa0906612. [DOI] [PubMed] [Google Scholar]
- 3.Gilsdorf A, Poggensee G. Influenza A(H1N1)v in Germany: the first 10,000 cases. Euro Surveill. 2009;14:pii=19318. doi: 10.2807/ese.14.34.19318-en. [DOI] [PubMed] [Google Scholar]
- 4.Kelly H, Grant K. Interim analysis of pandemic influenza (H1N1) 2009 in Australia: surveillance trends, age of infection and effectiveness of seasonal vaccination. Euro Surveill. 2009;14. pii=19288. [DOI] [PubMed]
- 5.Michaelis M, Doerr HW, Cinatl J., Jr An influenza A H1N1 virus revival—pandemic H1N1/09 virus. Infection. 2009;37:381–389. doi: 10.1007/s15010-009-9181-5. [DOI] [PubMed] [Google Scholar]
- 6.Nishiura H, Castillo-Chavez C, Safan M, Chowell G. Transmission potential of the new influenza A(H1N1) virus and its age-specificity in Japan. Euro Surveill. 2009;14(22):pii=19227. doi: 10.2807/ese.14.22.19227-en. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Surveillance for pediatric deaths associated with 2009 pandemic influenza A (H1N1) virus infection—United States, April–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:941–947. [PubMed] [Google Scholar]
- 8.Chen M. World now at the start of 2009 influenza pandemic. World Health Organization (WHO). 2009. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
- 9.Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 10.Austrian Federal Ministry of Health. Ein Jahr Neue Grippe—Die Maßnahmen des Gesundheitsministeriums. 2010. http://www.bmg.gv.at/home/Presse/Pressemeldungen/Ein_Jahr_Neue_Grippe_Die_Massnahmen_des_Gesundheitsministeriums.
- 11.Redlberger-Fritz M, Popow-Kraupp T. Rückblick auf die erste Welle der Influenza A/H1N1v-Pandemie 2009/2010. Virusepidemiologische Information 05/10. 2010. http://www.virologie.meduniwien.ac.at/home/upload/vei/2010/0510s.pdf.
- 12.Depoortere E, Mantero J, Lenglet A, Kreidl P, Coulombier D. Influenza A(H1N1)v in the southern hemisphere—lessons to learn for Europe? Euro Surveill. 2009;14. pii=19246. [DOI] [PubMed]
- 13.Austrian Agency for Health and Food Safety (AGES). Zur Situation in Österreich. 2010. http://www.ages.at/ages/gesundheit/mensch/neue-grippe-ah1n1.
- 14.Zarychanski R, Stuart TL, Kumar A, Doucette S, Elliott L, Kettner J, et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182:257–264. doi: 10.1503/cmaj.091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb SA, Pettilä V, Seppelt I, Bellomo R, Bailey M, Cooper DJ, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 16.Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 17.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 18.Santa-Olalla Peralta P, Cortes-García M, Vicente-Herrero M, Castrillo-Villamandos C, Arias-Bohigas P, Pachon-del Amo I, et al. Risk factors for disease severity among hospitalised patients with 2009 pandemic influenza A (H1N1) in Spain, April–December 2009. Euro Surveill. 2010;15. pii=19667. [DOI] [PubMed]
- 19.Nguyen-Van-Tam JS, Openshaw PJ, Hashim A, Gadd EM, Lim WS, Semple MG, et al. Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May–September 2009) Thorax. 2010;65:645–651. doi: 10.1136/thx.2010.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassetti A, Parisini A, Calzi A, Pallavicini FM, Cassola G, Artioli S, et al. Risk factors for severe complications of the novel influenza A (H1N1): analysis of patients hospitalized in Italy. Clin Microbiol Infect. 2011;17:247–250. doi: 10.1111/j.1469-0691.2010.03275.x. [DOI] [PubMed] [Google Scholar]
- 21.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 22.Fisman DN, Savage R, Gubbay J, Achonu C, Akwar H, Farrell DJ, et al. Older age and a reduced likelihood of 2009 H1N1 virus infection. N Engl J Med. 2009;361:2000–2001. doi: 10.1056/NEJMc0907256. [DOI] [PubMed] [Google Scholar]
- 23.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 24.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–1108. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson KG. Clinical features of influenza. Semin Respir Infect. 1992;7:26–37. [PubMed] [Google Scholar]
- 26.Vaillant L, La Ruche G, Tarantola A, Barboza P. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009;14. pii=19309. [DOI] [PubMed]
- 27.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 28.Domínguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 29.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 30.White DB, Angus DC. Preparing for the sickest patients with 2009 influenza A(H1N1) JAMA. 2009;302:1905–1906. doi: 10.1001/jama.2009.1539. [DOI] [PubMed] [Google Scholar]
- 31.Staudinger T. New flu (H1n1): phantom or intensive medicine super-GAU—a view from the Austrian reality. Wien Klin Wochenschr. 2010;122:3–5. doi: 10.1007/s00508-010-1304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donaldson LJ, Rutter PD, Ellis BM, Greaves FE, Mytton OT, Pebody RG, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ. 2009;339:5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.[No authors listed] Transmission dynamics and impact of pandemic influenza A (H1N1) 2009 virus. Wkly Epidemiol Rec. 2009;84:481–484. [PubMed] [Google Scholar]
- 34.Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 35.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 36.Lapinsky SE. Critical illness as a result of influenza A/H1N1 infection in pregnancy. BMJ. 2010;340:c1235. doi: 10.1136/bmj.c1235. [DOI] [PubMed] [Google Scholar]
- 37.Cui W, Zhao H, Lu X, Wen Y, Zhou Y, Deng B, et al. Factors associated with death in hospitalized pneumonia patients with 2009 H1N1 influenza in Shenyang, China. BMC Infect Dis. 2010;10:145. doi: 10.1186/1471-2334-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsigrelis C, Mohammad M, Fraimow HS, Dellinger RP, Marchesani D, Reboli AC. Secondary bacterial pneumonia due to Staphylococcus aureus complicating 2009 influenza A (H1N1) viral infection. Infection. 2010;38:237–239. doi: 10.1007/s15010-010-0009-0. [DOI] [PubMed] [Google Scholar]
- 39.Gill JR, Sheng ZM, Ely SF, Guinee DG, Beasley MB, Suh J, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134:235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–1074. [PubMed] [Google Scholar]
- 41.Keshet R, Boursi B, Maoz R, Shnell M, Guzner-Gur H. Diagnostic and prognostic significance of serum C-reactive protein levels in patients admitted to the department of medicine. Am J Med Sci. 2009;337:248–255. doi: 10.1097/MAJ.0b013e31818af6de. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention (CDC) Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) Virus—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:826–829. [PubMed] [Google Scholar]
- 43.Vasoo S, Stevens J, Singh K. Rapid antigen tests for diagnosis of pandemic (Swine) influenza A/H1N1. Clin Infect Dis. 2009;49:1090–1093. doi: 10.1086/644743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden FG, et al. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–1032. doi: 10.1086/598513. [DOI] [PMC free article] [PubMed] [Google Scholar]


