Abstract
Dobrava-Belgrade virus (DOBV) is a hantavirus that causes a disease in humans known as hemorrhagic fever with renal syndrome. Hallmarks of hantaviral infections are increased vascular permeability due to dysregulation of the endothelial cell barrier and acute thrombocytopenia. In order to gain insight into the immune response in DOBV infections, the serum levels of 27 cytokines in 24 hospitalized Greek HFRS patients were evaluated. Compared to the control group, significantly higher IL-1ra, IL-6, IL-8, IL-9, IL-10, GM-CSF, IP-10, MIP-1b, TNF-α and VEGF levels were found in severe cases, while in non-severe cases, IL-13 and TNF-α levels were significantly higher (p < 0.05). In all groups, IP-10 was increased and RANTES was decreased. Significant and time- (after onset of illness) dependent differences among fatal, severe and non-severe cases were seen. VEGF was positively associated with disease severity. A strong immune response was seen during the first week of illness, especially in severe cases, while the response in non-severe cases was weaker and delayed. The Th1 response was strong in non-severe cases and weak in the fatal case, while a mixed Th1/Th2 immune response was seen in the survivors of severe disease.
Keywords: Fatal Case, Hemorrhagic Fever With Renal Syndrome, VEGF Level, Hantavirus Pulmonary Syndrome, Puumala Virus
Introduction
Hantaviruses (genus Hantavirus, family Bunyaviridae) cause two clinical syndromes in humans: hemorrhagic fever with renal syndrome (HFRS) in Asia and Europe and hantavirus pulmonary syndrome (HPS) in the Americas [6]. Hallmarks of both syndromes are increased vascular permeability due to dysregulation of the endothelial cell barrier and acute thrombocytopenia. Humans become infected after inhalation of aerosolized excreta of persistently infected (but asymptomatic) rodents. The most severe form of HFRS in Europe is caused by Dobrava-Belgrade virus (DOBV), and especially by the genotypes associated with Apodemus flavicollis and Apodemus ponticus rodents, which serve as natural reservoir hosts of the virus [8, 11, 19]. After an incubation period of 2-42 days (usually 2 weeks), HFRS starts with a febrile, flu-like illness lasting 3–7 days, followed by hypotension and oliguria (or even anuria) that may result in fatal shock. Glomerular and tubular disorders lead to kidney dysfunction and acute renal failure [10]; hemodialysis is often required. Recovery occurs after a polyuric phase, which usually starts in the second week of illness. Hemorrhagic manifestations may appear towards the end of the febrile phase, while renal failure occurs during the hypotensive phase; pulmonary involvement is present in several cases, and sometimes acute respiratory distress syndrome (ARDS) develops [1, 19].
Hantaviruses infect and replicate predominantly within vascular endothelial cells [27, 28]. Since no cytopathogenic effect is seen in endothelial cells, it has been suggested that the underlying hyperpermeability mechanisms are more complex, that humoral and cellular components of the adaptive and innate immunity contribute to hantavirus-induced disruption of the endothelial barrier, and that the extent of vascular and renal dysfunction determines the severity of the disease [24]. The observation of overproduction of inflammatory cytokines, known as a “cytokine storm”, gave rise to the hypothesis that it may play a major role in the pathogenesis of HFRS [7]. There have been several studies indicating the potential key role of some cytokines and chemokines in hantavirus infections and their correlation to disease severity [12–14, 26, 28]. However, studies focused specifically on DOBV pathogenesis are lacking, and the currently available studies on the immune response in DOBV infections are based on a limited number of cytokines [9, 12, 22, 25]. In order to investigate the immune response in patients with acute DOBV infection, we analyzed the levels of 27 cytokines, chemokines, and growth factors during the acute phase of the disease.
Materials and methods
Patients and healthy subjects
Thirty serum samples collected during 2003-2015 from 24 hospitalized Greek HFRS patients (21 males) were included in the study. The patients’ ages ranged from 21 to 70 years (median age, 34.5 years). The samples were collected 5-13 days (mean 8.9 days) after onset of the symptoms.
Fever, myalgia, and malaise were present in all patients, while the majority of them had acute renal failure (elevated creatinine and urea levels) and thrombocytopenia (platelets <100,000/mm3). One case was fatal. Four serial samples were available from this case (taken on the 5th, 7th, 18th and 22nd day of illness); the first two were included in the general analysis, since an inclusion criterion was that the samples be taken up to 14 days after the onset of the illness. Among the 23 survivors, 13 had severe disease: three were admitted to the Internal Care Unit (ICU), three had symptoms of the respiratory system (two had acute respiratory distress syndrome, ARDS), six had hemorrhagic manifestations, one had rhabdomyolysis, and 10 underwent hemodialysis.
Based on the week of illness, the samples from the survivors were divided into two groups: group A included 11 samples taken from nine HFRS patients during the first week of illness, and group B included 17 samples from 15 patients during the second week of illness (Table 1). Serum samples from 16 apparently healthy individuals (nine males), aged 18-65 years (median age, 45 years) were included in the study as a control group. All samples were kept at -70 °C until analysis.
Table 1.
Serum samples included in the study grouped according to the course of the disease and the week of illness
| Course | 1st week | 2nd week | Total |
|---|---|---|---|
| Non-severe | 7 | 6 | 13 |
| Severe | 4 | 11 | 15 |
| Total | 11 | 17 | 28 |
Laboratory diagnosis
Laboratory diagnosis was performed using serological and molecular methods. For the detection of IgM and IgG antibodies, commercial ELISA kits (Anti-Hanta Virus Pool 1 “Eurasia”, Euroimmun Medizinische Labordiagnostika AG, Luebeck, Germany) and in-house indirect immunofluorescence assays were used. Serum samples were additionally analyzed applying a bunyavirus immunoblot IgM and IgG test (recomLine Bunyavirus IgG/IgM, Mikrogen GmbH, Neuried, Germany) with recombinant antigens of four serotypes of hantaviruses (DOBV, Hantaan, Puumala and Seoul viruses). RT-nested PCR was applied on RNA extracted from patients’ serum or blood samples using a QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany) [18]. All PCR products were sequenced in a 3130 ABI Genetic Analyser, and the obtained nucleotide sequences were compared to the corresponding sequences available in GenBank database using the Basic Local Alignment Search Tool (http://www.ncbi.nlm.nih.gov/BLAST/).
Quantification of serum cytokines
The serum levels of 27 cytokines were measured using a Bio-Plex™ Suspension Array System (Bio-Rad Laboratories, CA), following the manufacturer’s instructions. The cytokines tested were IL-1b, IL-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin, basic fibroblast growth factor (FGF-basic), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), IFN-γ, IFN-inducible protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), MIP-1b, platelet-derived growth factor-ββ (PDGF-ββ), regulated-on-activation normal T-cell expressed and secreted (RANTES), TNF-α and VEGF. The ratio IL10/TNF-α was used as a measure of the balance between the T helper type 1 (Th1) and Th2 immune responses.
Statistical analysis
Statistical analysis was performed using SPSS 22 (IBM Inc.). The evaluation of differences in cytokine levels between the patient and control groups was done using the nonparametric Mann-Whitney U test. The Mann-Whitney U test or Kruskal-Wallis test was conducted in order to evaluate the significance of independent continuous variables between HFRS groups. Binary logistic regression analysis was applied to define associations between the course of the disease and cytokine levels. The significance level was set at a p-value of <0.05.
Results
Using serological and molecular methods, it was shown that all HFRS cases were caused by DOBV. The RT-nested PCR was positive in 19 out of 24 (79.2 %) cases, and all of the sequences that were obtained were similar to that of DOBV associated with A. flavicollis [18].
The mean values of the 27 cytokines in non-severe, severe and fatal HFRS cases and in the control group are shown in Table 2. Compared to the control group, severe cases had significantly higher IL-1ra, IL-6, IL-8, IL-9, IL-10, GM-CSF, IP-10, MIP-1b, TNF-α and VEGF levels, while non-severe cases had significantly higher IL-13 and TNF-α levels (p < 0.05). In both groups, RANTES was significantly lower than in the control group (p < 0.05). The two samples from the fatal case had decreased levels of several cytokines, including IL-2, INF-γ, PDGF, and VEGF, while they had the highest levels of IP-10. Significant differences between fatal and severe cases were seen in the levels of IL-2, IL-13, IL-15, GM-CSF, IFN-γ, VEGF (lower levels in the fatal case), and IP-10 (higher levels in the fatal case). IL-1ra, IL-6, IL-8, IL-9, IL-10, IL-13, IL-15, GM-CSF, IFN-γ, IP-10, MIP1a, MIP-1b, and VEGF were significantly higher in severe HFRS cases than in non-severe cases (p < 0.05). The mean IL-10/TNF-α ratio was higher in the fatal case (4.74), moderate (2.25) in the severe cases, and low (0.81) in the non-severe cases. Th1 predominated in the non-severe cases (IL-10/TNF-α ratio <1, p < 0.05) (Table 2).
Table 2.
Mean values (pg/ml) of the 27 cytokines in non-severe (NS), severe (S) and fatal (F) HFRS patients and in the control (C) group. Significant p-values between groups are shown (Mann-Whitney test). N, number of samples
| Cytokine | NS N = 13 |
S N = 15 |
F N = 2 |
C N = 16 |
p
NS-C |
p
S-C |
p
F-C |
p
NS-S |
p
NS-F |
p
S-F |
|---|---|---|---|---|---|---|---|---|---|---|
| L-1b | 3.12 | 7.36 | 1.69 | 3.18 | .046 | |||||
| IL-1ra | 244.89 | 4292.12 | 498.97 | 127.66 | .035 | .038 | ||||
| IL-2 | 21.70 | 73.02 | 0 | 3.47 | .027 | .036 | ||||
| IL-4 | 3.55 | 13.58 | 3.14 | 5.33 | .044 | |||||
| IL-5 | 7.66 | 15.65 | 20.89 | 12.42 | ||||||
| IL-6 | 25.95 | 161.96 | 145.26 | 7.69 | .013 | .046 | .021 | |||
| IL-7 | 9.00 | 27.86 | 2.74 | 11.68 | .046 | |||||
| IL-8 | 18.28 | 183.65 | 87.21 | 12.44 | .004 | .046 | .001 | .027 | NS | |
| IL-9 | 112.16 | 151.66 | 25.02 | 29.12 | .020 | .003 | ||||
| IL-10 | 19.39 | 113.56 | 91.65 | 8.91 | .002 | .046 | .001 | |||
| IL-12 | 72.55 | 42.26 | 18.98 | 23.52 | ||||||
| IL-13 | 9.09 | 36.20 | 0.52 | 8.25 | .040 | .033 | .007 | .044 | ||
| IL-15 | 12.03 | 45.83 | 0 | 10.91 | .040 | .036 | ||||
| IL-17 | 103.17 | 61.60 | 14.92 | 77.01 | .046 | |||||
| Eotaxin | 79.81 | 248.40 | 56.67 | 46.03 | ||||||
| FGF | 50.35 | 73.81 | 15.69 | 35.39 | ||||||
| G-CSF | 27.91 | 73.49 | 28.48 | 17.63 | ||||||
| GM-CSF | 88.32 | 344.75 | 0 | 9.05 | .006 | .050 | .027 | .025 | ||
| IFN-γ | 122.31 | 926.21 | 29.54 | 98.91 | .009 | .037 | ||||
| IP-10 | 4536.56 | 10558.36 | 40226.34 | 691.52 | .029 | .033 | .036 | .027 | .025 | |
| MCP-1 | 71.65 | 238.45 | 121.73 | 29.53 | ||||||
| MIP-1a | 15.63 | 32.27 | 3.17 | 6.29 | .033 | .005 | ||||
| MIP-1b | 152.04 | 264.53 | 133.05 | 36.21 | .002 | .046 | .036 | |||
| PDGF | 4259.85 | 8185.56 | 1089.80 | 1738.61 | .015 | .033 | ||||
| RANTES | 30629.31 | 6523.32 | 4888.93 | 40006.12 | .004 | .000 | .033 | |||
| TNF-α | 47.59 | 110.03 | 19.35 | 14.26 | .035 | .016 | ||||
| VEGF | 86.85 | 424.30 | 3.87 | 21.68 | .001 | .000 | .025 | |||
| IL10/TNFα | 0.81 | 2.25 | 4.74 | .023 | .041 |
To find out the most significant factors associated with severe disease, logistic regression analysis was performed. Univariate logistic regression analysis showed that high IL-8, IL-10 and VEGF levels were significantly associated with severe disease (Table 3). In multivariate logistic regression analysis, VEGF was positively associated with disease severity (OR, 1.011; 95 % CI, 1.002-1.020; p, 0.018).
Table 3.
Univariate logistic regression analysis in HFRS patients: significant cytokines related to severity of the disease
| Cytokine | Severe N = 15 |
Non-severe N = 13 |
OR (95 % CI) | p-value |
|---|---|---|---|---|
| IL-8 | 183.65 | 18.28 | 1.055 (1.001-1.110) | .044 |
| IL-10 | 113.56 | 19.39 | 1.048 (1.011-1.086) | .010 |
| VEGF | 424.30 | 86.85 | 1.010 (1.003-1.017) | .004 |
The mean values for cytokines are shown. N, number of samples
Regarding the time after onset of the disease, significant differences in cytokine levels were seen between group A (first week of illness) and group B (second week of illness) (Fig. 1). Specifically in the first week of illness, the mean levels of IL-1b, IL-1ra, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-17, eotaxin, FGF, TNF-α and VEGF were significantly higher in severe cases than in non-severe cases, while IL-8, IL-10 and VEGF were also higher during the second week of illness (p < 0.05).
Fig. 1.
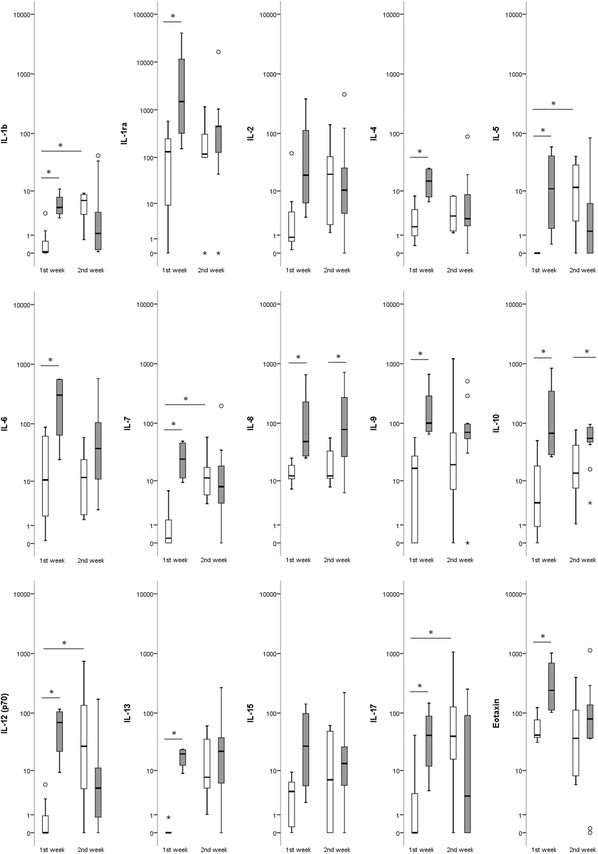
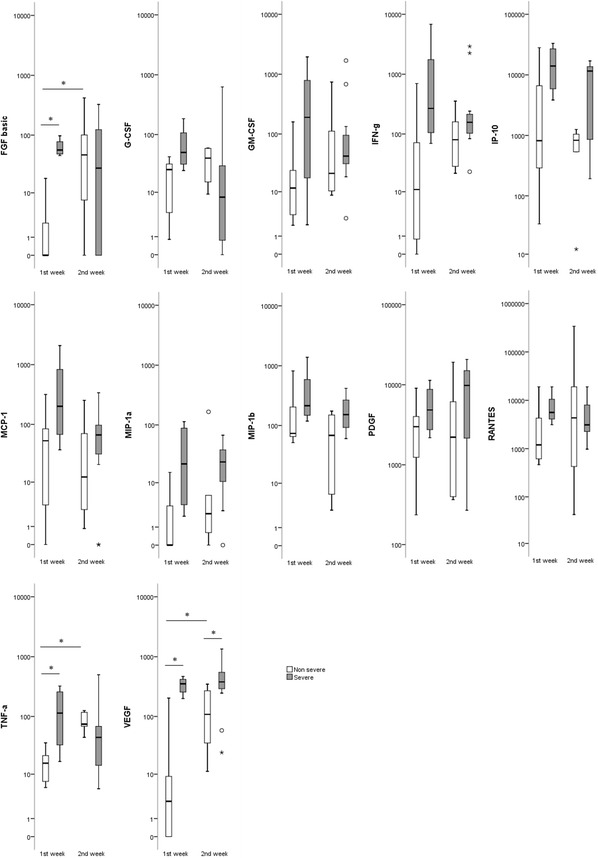
Box plots showing the serum cytokine levels (pg/ml) in patients with DOBV infection in the first (A) and second (B) week of illness. The vertical line with whiskers shows the range of values. Spots show individual outlier samples. Asterisks indicate statistical significance (p < 0.05; Mann-Whitney U test)
It is of interest that the significantly affected cytokines in the first and second week of illness in severe cases were different from those in the non-severe cases. Specifically, IL-1ra, IL-4, IL-13, G-CSF, eotaxin, IP-10, MCP-1, and PDGF were increased significantly compared to the control group in the first week in severe cases, while IL-6, IL-8, IL-9, IL-10, GM-CSF and MIP-1b were increased in the first week in severe cases and remained significantly increased in the second week; TNF-α and VEGF were increased in the first week in severe cases and remained significantly increased in the second week, while in the non-severe cases, they were increased only in the second week; RANTES was significantly lower in both weeks in the severe cases, while in the non-severe cases, it was significantly lower in the first week and increased significantly in the second week (p < 0.05).
Discussion
It is generally accepted that most features of viral hemorrhagic fevers are caused by innate immune responses, since the systemic spread of virus to macrophages and dendritic cells leads to the release of mediators that modify vascular function and have procoagulant activity [4]. A cytokine storm has been suggested to play a major role in the pathogenesis of HFRS [7]. The present study was focused on patients with DOBV infection. Significant differences in the cytokine levels were seen between non-severe, severe, and fatal cases, which were time-dependent (time after onset of the symptoms). A strong immune response was seen during the first week of illness, especially in the severe cases, while the response in the non-severe cases was weaker and delayed.
TNF-α is an early-phase pro-inflammatory cytokine. It was elevated in both severe and non-severe survivors, while it was not in the fatal case. Elevated IL-6, IL-8, IL-10, IP-10, and MIP-1b levels were seen in the fatal and severe cases, differing significantly from the non-severe cases. In previous studies, IL-6, IL-10, TNF-α have been related to severe and/or fatal cases [2, 12, 13, 17, 22]. Increased VEGF levels were seen in the survivors (with severe or non-severe disease), being higher in those with severe disease, while extremely low VEGF levels (below the control group) were seen in both samples from the fatal case (Table 2). Two additional serial samples (third and fourth) were available from the fatal case: VEGF remained low in the third serial sample and became undetectable in the fourth sample which was taken one day before death. Logistic regression analysis showed that VEGF was the cytokine significantly associated with disease severity. The higher VEGF level in severe cases can be explained by the increased need for endothelium repair in these cases. The elevation was seen even from the first week of illness and increased further in the second week, while in non-severe cases, the elevation was seen only in the second week of illness. The association of VEGF with repair of vascular damage in HFRS has been reported previously [25].
RANTES was decreased in all HFRS cases, including the fatal one. Given that platelets are the major reservoir of RANTES in the peripheral circulation, the noted low levels may be related to decreased production due to the thrombocytopenia seen during the disease. Downregulation of cytokines associated with platelet numbers and function consistent with thrombocytopenia has been reported also for HPS patients [16]. Furthermore, RANTES has been also negatively correlated with the outcome of the disease in patients with Crimean-Congo hemorrhagic fever (CCHF) [20].
The Th1 immune response plays a key role in antiviral immunity, favoring viral clearance. This response was decreased in the fatal case of the present study, since IL-2, IL-12, TNF-α, and IFN-γ levels were extremely low, and the Th2/Th1 ratio was high (4.74), suggestive of predominance of the Th2 response. In contrast, these cytokines were increased in the group of the survivors (with severe or non-severe disease), suggesting an early and effective Th1 activation, which resulted in a favorable outcome. However, the Th2/Th1 ratio differed significantly between severe and non-severe cases (2.25 and 0.81, respectively, p < 0.05). Based on these results, we concluded that the Th1 response was high in the non-severe cases and low in the fatal case, while a mixed Th1/Th2 immune response was seen in the survivors of severe disease. A mixed Th1/Th2 immune response has also been reported for HPS patients, and it has been shown that the magnitude of Th1 response effector cytokines is correlated to HPS severity [3]. An association between a mild form of HFRS caused by Puumala virus and elevated levels of IFN-γ and IL-12 has also been observed, which led the authors to suggest that the administration of exogenous IFN-γ and IL-12 may provide antiviral benefits for the treatment of HFRS and thus warrants further investigation [7]. The level of IL-13, which is a cytokine produced by T lymphocytes, was also extremely low in the fatal case, significantly lower than in survivors with severe disease. It is of interest that the MIP-1a level was also decreased (below that of the control group) in the fatal case, while MIP-1b was on average fourfold higher than that of the control group (but lower than in the severe cases) (Table 1). In fact, MIP-1a and MIP-1b chemokines attract distinct populations of lymphocytes with MIP-1a having greater effects than MIP-1b, particularly on B cells [23].
The proinflammatory chemokine IP-10 was increased in all HFRS cases, with the highest levels being observed in the fatal case. Expression of IP-10 is seen in many Th1-type inflammatory diseases, and it is thought to play an important role in recruiting activated T cells to sites of tissue inflammation [5]. A correlation of IP-10 with the viral load in CCHF patients has been reported recently; given that the viral load is critical for the outcome of the disease, it was suggested that IP-10 plays an important role in the pathogenesis of the disease [21]. Furthermore, the most significant change in cytokine expression in HFRS cases caused by another hantavirus, Puumala virus, was observed for IP-10, which increased tenfold over the control value at the onset and remained elevated through the oliguric phase, progressively decreasing to a value approximately fivefold over controls by the convalescent phase [2].
In conclusion, the present study showed that the cytokine patterns differ between severe and non-severe DOBV HFRS cases and that an early and efficient Th1 response is critical for the patient’s survival. The immune response is the result of numerous interactions and networks of several factors, including cytokines. Systems biology approaches are currently being used to curate the innate immunity interactome and to provide insight into the cross-talk between innate immunity pathways and other biological processes [15]. Similar approaches could enable a better understanding of the immune response in HFRS that may help to guide future studies for drug design.
Acknowledgments
We thank Elpida Gavana for the excellent technical assistance.
Compliance with ethical standards
Conflict of interest
K. Tsergouli declares that she has no conflict of interest. A. Papa declares that she has no conflict of interest.
Ethical approval
The study was retrospective, all samples were de-identified, and informed consent from the patients was not obtained. The study was approved by the Ethics Committee of the Medical School of Aristotle University of Thessaloniki, Greece.
References
- 1.Avsic-Zupanc T, Petrovec M, Furlan P, Kaps R, Elgh F, Lundkvist A. Hemorrhagic fever with renal syndrome in the Dolenjska region of Slovenia—a 10-year survey. Clin Infect Dis. 1999;28:860–865. doi: 10.1086/515185. [DOI] [PubMed] [Google Scholar]
- 2.Baigildina AA, Khaiboullina SF, Martynova EV, Anokhin VA, Lombardi VC, Rizvanov AA. Inflammatory cytokines kinetics define the severity and phase of nephropathia epidemica. Biomarkers Med. 2015;9:99–107. doi: 10.2217/bmm.14.88. [DOI] [PubMed] [Google Scholar]
- 3.Borges AA, Campos GM, Moreli ML, Moro Souza RL, Saggioro FP, Figueiredo GG, Livonesi MC, Moraes Figueiredo LT. Role of mixed Th1 and Th2 serum cytokines on pathogenesis and prognosis of hantavirus pulmonary syndrome. Microbes Infect. 2008;10:1150–1157. doi: 10.1016/j.micinf.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Bray M. Pathogenesis of viral hemorrhagic fever. Curr Opin Immunol. 2005;17:399–403. doi: 10.1016/j.coi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 6.Jonsson CB, Figueiredo LT, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev. 2010;23:412–441. doi: 10.1128/CMR.00062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khaiboullina SF, Martynova EV, Khamidullina ZL, Lapteva EV, Nikolaeva IV, Anokhin VV, Lombardi VC, Rizvanov AA. Upregulation of IFN-gamma and IL-12 is associated with a milder form of hantavirus hemorrhagic fever with renal syndrome. Eur J Clin Microbiol Infect Dis. 2014;33:2149–2156. doi: 10.1007/s10096-014-2176-x. [DOI] [PubMed] [Google Scholar]
- 8.Klempa B, Avsic-Zupanc T, Clement J, Dzagurova TK, Henttonen H, Heyman P, Jakab F, Kruger DH, Maes P, Papa A, Tkachenko EA, Ulrich RG, Vapalahti O, Vaheri A. Complex evolution and epidemiology of Dobrava-Belgrade hantavirus: definition of genotypes and their characteristics. Arch Virol. 2012;158:521–529. doi: 10.1007/s00705-012-1514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korva M, Saksida A, Kejzar N, Schmaljohn C, Avsic-Zupanc T. Viral load and immune response dynamics in patients with haemorrhagic fever with renal syndrome. Clin Microbiol Infect. 2013;19:E358–E366. doi: 10.1111/1469-0691.12218. [DOI] [PubMed] [Google Scholar]
- 10.Krautkramer E, Grouls S, Stein N, Reiser J, Zeier M. Pathogenic old world hantaviruses infect renal glomerular and tubular cells and induce disassembling of cell-to-cell contacts. J Virol. 2011;85:9811–9823. doi: 10.1128/JVI.00568-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruger DH, Tkachenko EA, Morozov VG, Yunicheva YV, Pilikova OM, Malkin G, Ishmukhametov AA, Heinemann P, Witkowski PT, Klempa B, Dzagurova TK. Life-threatening Sochi virus infections, Russia. Emerg Infect Dis. 2015;21:2204–2208. doi: 10.3201/eid2112.150891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyriakidis I, Papa A. Serum TNF-alpha, sTNFR1, IL-6, IL-8 and IL-10 levels in hemorrhagic fever with renal syndrome. Virus Res. 2013;175:91–94. doi: 10.1016/j.virusres.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Libraty DH, Makela S, Vlk J, Hurme M, Vaheri A, Ennis FA, Mustonen J. The degree of leukocytosis and urine GATA-3 mRNA levels are risk factors for severe acute kidney injury in Puumala virus nephropathia epidemica. PLoS One. 2012;7:e35402. doi: 10.1371/journal.pone.0035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linderholm M, Ahlm C, Settergren B, Waage A, Tarnvik A. Elevated plasma levels of tumor necrosis factor (TNF)-alpha, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J Infect Dis. 1996;173:38–43. doi: 10.1093/infdis/173.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Lynn DJ, Chan C, Naseer M, Yau M, Lo R, Sribnaia A, Ring G, Que J, Wee K, Winsor GL, Laird MR, Breuer K, Foroushani AK, Brinkman FS, Hancock RE. Curating the innate immunity interactome. BMC Syst Biol. 2010;4:117. doi: 10.1186/1752-0509-4-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morzunov SP, Khaiboullina SF, St Jeor S, Rizvanov AA, Lombardi VC. Multiplex analysis of serum cytokines in humans with hantavirus pulmonary syndrome. Front Immunol. 2015;6:432. doi: 10.3389/fimmu.2015.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Outinen TK, Makela SM, Ala-Houhala IO, Huhtala HS, Hurme M, Paakkala AS, Porsti IH, Syrjanen JT, Mustonen JT. The severity of Puumala hantavirus induced nephropathia epidemica can be better evaluated using plasma interleukin-6 than C-reactive protein determinations. BMC Infect Dis. 2010;10:132. doi: 10.1186/1471-2334-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papa A, Johnson AM, Stockton PC, Bowen MD, Spiropoulou CF, Alexiou-Daniel S, Ksiazek TG, Nichol ST, Antoniadis A. Retrospective serological and genetic study of the distribution of hantaviruses in Greece. J Med Virol. 1998;55:321–327. doi: 10.1002/(SICI)1096-9071(199808)55:4<321::AID-JMV11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Papa A. Dobrava-Belgrade virus: phylogeny, epidemiology, disease. Antiviral Res. 2012;95:104–117. doi: 10.1016/j.antiviral.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Papa A, Tsergouli K, Yagci Caglayik D, Bino S, Como N, Uyar Y, Korukluoglu G. Cytokines as biomarkers of Crimean-Congo hemorrhagic fever. J Med Virol. 2015;88:21–27. doi: 10.1002/jmv.24312. [DOI] [PubMed] [Google Scholar]
- 21.Papa A, Yagci Caglayik D, Christova I, Tsergouli K, Korukluoglu G, Uyar Y. Crimean-Congo hemorrhagic fever: CXCL10 correlates with the viral load. J Med Virol. 2015;87:899–903. doi: 10.1002/jmv.24141. [DOI] [PubMed] [Google Scholar]
- 22.Saksida A, Wraber B, Avsic-Zupanc T. Serum levels of inflammatory and regulatory cytokines in patients with hemorrhagic fever with renal syndrome. BMC Infect Dis. 2011;11:142. doi: 10.1186/1471-2334-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schonrich G, Kruger DH, Raftery MJ. Hantavirus-induced disruption of the endothelial barrier: neutrophils are on the payroll. Front Microbiol. 2015;6:222. doi: 10.3389/fmicb.2015.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsergouli K, Papa A. Vascular endothelial growth factor levels in Dobrava/Belgrade virus infections. Viruses. 2013;5:3109–3118. doi: 10.3390/v5123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Li XL, Dai Y, Qiu ZF, Li TS. The dynamics of T lymphocyte subsets in hemorrhagic fever with renal syndrome. Zhonghua Nei Ke Za Zhi. 2008;47:654–657. [PubMed] [Google Scholar]
- 27.Yanagihara R, Silverman DJ. Experimental infection of human vascular endothelial cells by pathogenic and nonpathogenic hantaviruses. Arch Virol. 1990;111:281–286. doi: 10.1007/BF01311063. [DOI] [PubMed] [Google Scholar]
- 28.Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, Feddersen RM, Zumwalt RE, Miller GL, Khan AS, et al. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]


