Abstract
The purpose of this study was to evaluate the significance of cerebrovascular CO2 reactivity (CO2 R) in the course and outcome of inflammatory central nervous system (CNS) diseases. Sixty-eight patients with inflammatory CNS diseases and 30 healthy volunteers were included in this prospective observational cohort study. The observational period was between January 2005 and May 2009. The CO2 R was measured by transcranial Doppler (TCD) ultrasound using the breath-holding method. We compared patients with normal CO2 R (breath-holding index [BHIm] ≥ 1.18 = BHIN group) with patients who showed impaired CO2 R (BHIm < 1.18 = BHIR group). We also analyzed the association of impaired CO2 R with the etiology, severity, and outcome of disease. When compared to the BHIN group, the patients from the BHIR group were older, had a heavier consciousness disturbance, experienced more frequent respiratory failure, and, subsequently, had worse outcomes. There were no fatalities among the 28 patients in the BHIN group. The comparison of subjects with bacterial and non-bacterial meningitis revealed no significant differences. The unfavorable outcome of disease (Glasgow Outcome Scale [GOS] score 1–3) was significantly more common in subjects with impaired CO2 R (62.5% vs. 10.7%). Logistic regression analysis was performed in order to establish the prognostic value of BHIm. The outcome variable was unfavorable outcome (GOS 1–3), while the independent variables were age, Glasgow Coma Scale (GCS) score, and BHIm. The age and BHIm showed the strongest influence on disease outcome. A decrease of BHIm for each 0.1 unit increased the risk of unfavorable outcome by 17%. Our study emphasizes the importance of CO2 R assessment in patients with inflammatory CNS diseases.
Keywords: Glasgow Coma Scale, Bacterial Meningitis, Therapeutic Hypothermia, Glasgow Outcome Scale, Central Nervous System Infection
Introduction
Despite advances in the treatment of bacterial meningitis (BM), the overall mortality as well as long-term neurological sequelae are still high. This is particularly true with pneumococcal meningitis [1–4]. The most common factors associated with poor outcome in BM are seizures, advanced age, disturbed consciousness, the presence of multiple-organ dysfunction, hypotension, APACHE II score >13, pneumococcal etiology, and delay in antimicrobial treatment [3–8].
Cerebrovascular dysregulation represents one of the most deleterious consequences of neuroinflammation [9]. The relevance of impaired cerebral blood flow (CBF) chemoregulation relies on subsequent hypoperfusion or global ‘luxury’ perfusion.
In addition, although not caused by CO2 reactivity (CO2 R) loss, severe blood–brain barrier disruption frequently occur in these patients. Therefore, the aggravation of brain edema with the use of mannitol infusion due to heavy capillary leak can be expected in such patients.
Beside osmotic diuretics and steroids, the usual treatments for increased intracranial pressure include thiopental infusion and hyperventilation [10]. The latter often has limited effectiveness if the cerebrovascular CO2 R is impaired.
The alternative and CO2 R-independent symptomatic treatments, such as therapeutic hypothermia (TH), in such patients hold promise. The effects of mild therapeutic hypothermia may target the pathophysiological mechanisms in BM because the majority of them are temperature-dependent [11, 12]. Even with this knowledge, the CO2 R has only been studied in very few patients with BM [13, 14].
Transcranial Doppler (TCD) ultrasound is widely accepted and considered to be an appropriate technique for the non-invasive assessment of cerebral arterioles’ reactivity because the changes in the mean blood flow velocities (MBFV) correlate with the changes in CBF [15, 16].
The aim of this study was to assess the cerebrovascular CO2 R measured by TCD using the breath-holding method in patients with inflammatory central nervous system (CNS) diseases.
Materials and methods
The study was approved by the hospital ethics committee and informed consent was obtained from all examinees or their next of kin.
Data collection
This prospective study was performed between January 2005 and May 2009 at the University Hospital for Infectious Diseases in Zagreb, Croatia. The following parameters were recorded in a database: age, gender, physical and neurological signs, mechanical ventilation (MV), cerebrospinal fluid (CSF) characteristics, microbiological findings in CSF and blood, mean arterial pressure (MAP), Glasgow Coma Scale (GCS) score, Glasgow Outcome Scale (GOS) score, and the mean breath-holding index (BHIm). Data were collected during the first 24 h after admission to the department and within the first 5 days of illness in all patients.
Patient selection
The patients were eligible for the study if they were 18 years of age or older and had inflammatory CNS disease. Patients were excluded if they had brain abscess, subdural empyema, nosocomial and shunt meningitis, spinal meningitis or myelitis, stroke, transitory ischemic attacks, carotid artery disease, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome (ARDS), diabetic microangiopathy, septic shock, death not related to meningitis, temporal bone ‘acoustic window’ absence, or had been treated with sodium nitroprusside.
During the same time period, 30 healthy volunteers were studied following written informed consent. All volunteers were examined and found to be healthy and without infection.
The purpose of the control group was to establish the lowest value of the BHIm in healthy volunteers.
Definitions
BM was diagnosed on the basis of clinical picture (fever, headache, neck stiffness, and consciousness disturbance with or without seizures and neurologic deficits), supportive CSF findings (pleocytosis, increased protein concentration, and decreased CSF–blood glucose ratio), and a positive CSF culture; a negative CSF culture with a positive CSF polymerase chain reaction (PCR) assay, a positive blood culture, or a positive Gram stain of a CSF sample.
Non-bacterial inflammatory CNS disease (NBM) was diagnosed on the basis of present encephalopathy with at least one of the following: fever, seizures, focal neurological findings; pleocytosis with increased protein concentration in the CSF; and a positive CSF or blood culture (fungal meningitis); virus detection by PCR assay in CSF samples; proved intrathecal antibody production and electroencephalographic or neuroimaging findings consistent with encephalitis or acute disseminated encephalomyelitis (ADEM).
The patients’ mental status was assessed using the GCS score. Recorded scores were the lowest during the first 24 h after admission to the hospital. Altered mental status was defined as GCS <15. The patients’ clinical outcome was assessed by using the GOS score at the time of discharge from the hospital.
Unfavorable clinical outcomes included death (GOS 1), vegetative state (GOS 2), and severe neurological deficit (GOS 3). GOS 5 (mild or no disability) but also GOS 4 (moderate disability) were considered to be favorable outcomes because of the extremely detrimental nature of CNS infections.
According to the disease etiology, patients were divided into bacterial (BM) and non-bacterial (NBM) groups. Thirty healthy volunteers represented the control group.
Patients were stratified according to CO2 R into ‘BHIN’ (normal CO2 R defined with BHIm ≥ 1.18) and ‘BHIR’ (impaired CO2 R defined with BHIm < 1.18 according to BHI values in healthy volunteers) groups.
Measurements
TCD measurements of CO2 R were performed during the first 24 h after admission to the hospital by using a MultiDop 4X (DWL, Sipplingen, Germany) with two 2-MHz pulsed-wave probes 1.7 cm in diameter. The software used was TCD-8 for MDX (Version 8.0, Aaslid Rune). Normal basic hemodynamic parameters (mean arterial pressure and heart rate) and PaCO2 [35–45 torr (4.67–6.0 kPa)] were confirmed in all examinees before measurements. Direct blood pressure monitoring was performed by cannilation of the radial artery. Thus, we were able to note every change in the arterial pressure which could potentially interfere with the measurements.
The left and right middle cerebral arteries (MCAs) were insonated simultaneously through the temporal bone windows at a depth of 50–55 mm. The probes were secured to the head of the patient with a specially designed spectacle frame that permitted a constant angle of insonation. The mean blood flow velocities (MBFV) were continuously recorded during normal ventilation and during breath-holding. The CO2 R was assessed using the breath-holding method.
Spontaneously breathing and compliant examinees were asked to hold their breath after normal inspiratory breath for 30 s or less if it becomes uncomfortable.
Mechanically ventilated patients were sedated and relaxed before the procedure using midazolam and vecuronium bromide, respectively. The administered drugs have no significant effect on the cerebral vasoreactivity and hemodynamics [17, 18]. In that case, the patients were disconnected from the ventilator for 30 s. Assisted controlled ventilation (ACV) mode was used in all mechanically ventilated patients.
MBFV at the start and at the end of the breath-hold period was recorded. The procedure was repeated after a 5-min rest period and the mean values from both MCAs were taken for calculation. The BHI was calculated by dividing the percentage of MBFV increase during breath-holding by the time (in seconds) of apnea. The BHIm was calculated from both MCA (left and right MCAs) breath-holding indexes.
Statistical analysis
Univariate statistics included calculation of the mean value, standard deviation, median, and interquartiles for continuous variables. Values for categorical variables were presented as frequencies.
Bivariate statistics assessed the differences between compared groups. The Mann–Whitney test was used to estimate the difference amongst continuous variables. For categorical data, the Chi-square test and Fisher’s two-tailed exact test were used when appropriate. All relevant demographic and clinical variables of the patient groups were compared.
The outcome variable was the GOS scores. The correlation between BHIm and the severity of disease was assessed using multivariate tests after bivariate analysis. Logistic regression analysis was performed to ascertain the independent predictive potential of CO2 R, after adjustment for potential confounding variables. The criteria for the inclusion of variables in the logistic regression models were based on the evidence of an association in the bivariate analysis. p-values less than 0.05 were considered to be statistically significant. The data were analyzed using the SAS 9.1 software (SAS Institute Inc., Cary, NC).
Results
Sixty-eight patients and 30 healthy volunteers were included in the study. There were no patients requiring vasopressor or inotrope support. The baseline demographics and patient characteristics are presented in Table 1.
Table 1.
Baseline demographic data and characteristics of examinees
| Control group (n = 30) | Bacterial meningitis (BM) (n = 34) | Non-bacterial meningitis (NBM) (n = 34) | p-value | |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 38 | 54 | 39 | 0.018 |
| Interquartiles | 26–55 | 44–-61 | 25–52 | |
| Gender | ||||
| Male | 15 (50%) | 26 (76.5%) | 20 (59%) | 0.081 |
| Female | 15 (50%) | 8 (23.5%) | 14 (41%) | |
| GCS | ||||
| Median | N/A | 8 | 11.5 | <0.001 |
| Interquartiles | 6–12 | 6–15 | ||
| Neurological deficit | N/A | 11 (32.3%) | 12 (35.2%) | 1.000 |
| Steroid treatment | N/A | 19 (56%) | 6 (17.6%) | 0.002 |
| Mannitol infusion | N/A | 25 (73.5%) | 20 (59%) | 0.305 |
| Respiratory failure requiring mechanical ventilation | N/A | 27 (79.4%) | 17 (50%) | 0.022 |
| Mean breath-holding index (BHIm) | ||||
| Median | 1.878 | 1.042 | 1.115 |
<0.001a 0.389b |
| Interquartiles | 1.513–2.185 | 0.675–1.377 | 0.736–1.795 | |
| Range | 1.180–2.996 | 0.113–3.02 | 0.214–2.857 | |
| Glasgow Outcome Scale (GOS) score | ||||
| GOS 1 | 5 (14.7%) | 4 (11.8%) | 0.729 | |
| GOS 2 | 2 (5.9%) | 0 | ||
| GOS 3 | N/A | 9 (26.4%) | 8 (23.5%) | |
| GOS 4 | 4 (11.9%) | 4 (11.8%) | ||
| GOS 5 | 14 (41.1%) | 18 (52.9%) | ||
aBHIm median: control group vs. all patients
bBHIm median: BM vs. NBM
Thirty-four patients with BM comprised the BM group. The etiology was confirmed in 26 (76.5%) patients. The most common pathogen was Streptococcus pneumoniae (12 patients), followed by Neisseria meningitidis (7 patients), Listeria monocytogenes (4 patients), Klebsiella pneumoniae (1 patient), Enterococcus faecalis (1 patient), and S. agalactiae (1 patient).
In the NBM group, there were 34 patients with non-BM and meningoencephalitis. The disease etiology in 12 patients with viral meningoencephalitis (35.2%) was not confirmed. The most common pathogen in the remaining patients was herpes simplex virus (6 patients), followed by tick-borne encephalitis virus (2 patients), Cryptococcus neoformans (2 patients), influenza A virus (1 patient), Mycobacterium tuberculosis (1 patient), Epstein–Barr virus (1 patient), enterovirus (1 patient), and Borrelia burgdorferi (1 patient). Five patients suffered from postinfectious meningoencephalitis and two patients had acute disseminated encephalomyelitis (ADEM).
The breath-hold period in the control group was 23.3 ± 6.5 s (median 23). We found no correlation between BHIm and the duration of breath-holding (Spearman R 0.108; p = 0.623). The possible effect of a too short breath hold to BHI values was, thus, ruled out.
Advanced age and higher incidence of respiratory failure that required mechanical ventilation were found in the BM group (54 vs. 39 years and 79.4% vs. 50%).
The most prominent difference between BM and NBM patients was the difference in the level of consciousness disturbance (GCS): BM patients had worse scores (GCS 8 vs. 11.5). Therefore, the on-admission GCS score was included in the multivariate model analyzing the predictivity of BHI for the disease outcome.
Adjuvant steroid treatment was applied in 56% patients with BM according to the current guidelines [2]. Six patients in the NBM group received short-term high-dose steroids because of severe brain edema (4 patients) and acute disseminated encephalomyelitis (2 patients).
The patients were stratified according to CO2 R and additional analysis was made. The normal CO2 R group (BHIN) was defined by BHIm ≥ 1.18. The impaired CO2 R group (BHIR) was defined by BHIm < 1.18 according to breath-holding indexes in healthy volunteers.
The BHIR group was characterized by advanced age, heavier consciousness disturbance, frequent respiratory failure, and, often, unfavorable outcome in contrast to the BHIN group (Table 2). There were no lethal outcomes amongst the 28 patients in the BHIN group.
Table 2.
Characteristics and outcome of patients according to the CO2 reactivity
| BHIN a | BHIR b | p-value | |
|---|---|---|---|
| All patients (n = 68) | |||
| n (%) | 28 (41.2) | 40 (58.8) | N/A |
| Age, years (median) | 37 | 52 | 0.002 |
| GCS (median) | 15 | 7.5 | <0.001 |
| Mechanical ventilation, n (%) | 9 (32.1) | 35 (87.5) | <0.001 |
| GOS 1–3, n (%) | 3 (10.7) | 25 (62.5) | <0.001 |
| Mortality, n (%) | 0 | 9 (22.5) | 0.008 |
| Bacterial meningitis (n = 34) | |||
| n (%) | 14 (41.2) | 20 (58.8) | N/A |
| Age, years (median) | 44.5 | 59 | <0.001 |
| GCS (median) | 13.5 | 7.5 | 0.004 |
| Mechanical ventilation, n (%) | 7 (50) | 20 (100) | <0.001 |
| GOS 1–3, n (%) | 2 (14.2) | 14 (70) | 0.004 |
| Mortality, n (%) | 0 | 5 (25) | 0.062 |
| Non-bacterial meningitis (n = 34) | |||
| n (%) | 14 (41.2) | 20 (58.8) | N/A |
| Age, years (median) | 29.5 | 43.5 | 0.306 |
| GCS (median) | 15 | 7.5 | <0.001 |
| Mechanical ventilation, n (%) | 2 (14.2) | 15 (75) | 0.001 |
| GOS 1–3, n (%) | 1 (7.1) | 11 (55) | 0.009 |
| Mortality, n (%) | 0 | 4 (20) | 0.126 |
aBHIN = BHI ≥ 1.18 (normal CO2 reactivity)
bBHIR = BHI < 1.18 (reduced CO2 reactivity)
In patients with BM and normal CO2 R, we noted a higher incidence of mechanical ventilation, advanced age, and heavier consciousness disturbance compared with patients with NBM and preserved CO2 R (Table 2). These findings indicate that, in patients with BM, neither severe consciousness disturbance nor respiratory failure necessarily imply CO2 R loss. In contrast, unconscious patients with NBM who are mechanically ventilated and have normal CO2 R are rare (14.2% vs. 50%).
There was no significant difference in the BHI between patients with BM that received dexamethasone treatment and those who did not. Normal CO2R was noted in 36.8% (7/19) of patients with steroid treatment compared with 46.6% (7/15) in the non-steroid group (p = 0.993).
The major indicator of disease severity in patients with CNS infections is GCS [3]. Because of that, the correlation of BHIm and GCS was analyzed together with other variables which may influence BHIm. We found that 80.9% (34/42) of all patients with GCS ≤10 had impaired CO2 R. In the BM and NBM groups, that was 76% (19/25) and 88.2% (15/17), respectively.
Univariate regression analysis revealed a significant correlation of BHIm with GCS (β = 0.098; p < 0.001), and BHIm with age (β = −0.012; p = 0.014).
The unfavorable outcome of disease (GOS 1–3) was noted in 28 patients (28/68; 41.1%) (Table 3). Nine patients died (five patients in the BM and four patients in the NBM groups) and the overall mortality was 13.2%. The mortality in pneumococcal meningitis was 33.3% (4/12).
Table 3.
Characteristics of the outcome groups
| GOS 1–3 (unfavorable outcome), n = 28 | GOS 4–5 (favorable outcome), n = 40 | p-value | |
|---|---|---|---|
| Age (years) | |||
| Median | 55 | 39 | <0.001 |
| Interquartiles | 44–65 | 22–52 | |
| GCS | |||
| Median | 7.5 | 13.5 | <0.001 |
| Interquartiles | 4–10 | 7.5–15 | |
| Mechanical ventilation | |||
| n (%) | 26 (92.8%) | 18 (45%) | <0.001 |
| BHIm | |||
| Median | 0.835 | 1.285 | <0.001 |
| Interquartiles | 0.551–1.044 | 0.902–1.934 | |
The BHIm was almost identical in the GOS 1–3 groups, regardless of the etiology (BHIm median 0.835 in the BM versus 0.824 in the NBM groups).
The comparison of the BM and NBM groups according to disease outcome and related BHIm revealed no significant differences (Table 4). Such findings confirmed the BHIm etiology independence. However, significant differences in BHIm were noted according to the GOS (Fig. 1).
Table 4.
Mean breath holding index (BHIm) in all patients according to disease outcome
| All patients | GOS 1 | GOS 2 | GOS 3 | GOS 4 | GOS 5 | |
|---|---|---|---|---|---|---|
| All patients | ||||||
| n (%) | 68 | 9 (13.2%) | 2 (2.9%) | 17 (25%) | 8 (11.7%) | 32 (47%) |
| BHIm: median | 1.069 | 0.667 | 0.529 | 0.983 | 1.091 | 1.313 |
| Interquartiles | 0.714–1.497 | 0.291–0.849 | 0.383–0.675 | 0.719–1.130 | 0.826–1.561 | 1.07–2.082 |
| Bacterial meningitis (BM) | ||||||
| n (%) | 34 (50%) | 5 (14.7%) | 2 (5.8%) | 9 (26.4%) | 4 (11.7%) | 14 (41.1%) |
| BHIm: median | 1.042 | 0.821 | 0.529 | 1.020 | 1.016 | 1.338 |
| Interquartiles | 0.675–1.377 | 0.341–0.849 | 0.383–0.675 | 0.719–1.075 | 0.641–1.531 | 1.111–1.673 |
| Non-bacterial meningitis (NBM) | ||||||
| n (%) | 34 (50%) | 4 (11.7%) | 0 | 8 (23.5%) | 4 (11.7%) | 18 (52.9%) |
| BHIm: median | 1.115 | 0.479 | 0.956 | 1.121 | 1.312 | |
| Interquartiles | 0.736–1.795 | 0.252–0.845 | 0.668–1.132 | 0.902–1.800 | 1.040–2.141 | |
Fig. 1.
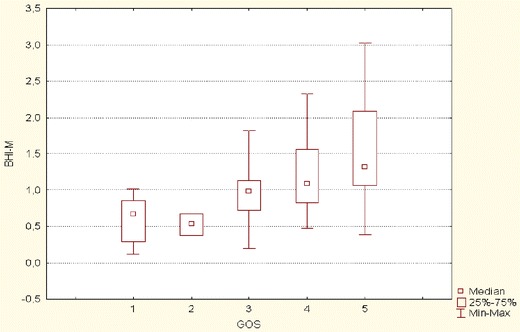
BHIm according to disease outcome (Glasgow Outcome Scale [GOS] score)
The BHIm in the favorable group (GOS 4–5) was significantly higher (1.285 vs. 0.835) than that in the unfavorable group (GOS 1–3) (Table 3). The unfavorable outcome of disease (GOS 1–3) was significantly more frequent in patients with impaired CO2 R (62.5 vs. 10.7%) (Table 2).
Logistic regression analysis was performed in order to establish the prognostic value of BHIm. The outcome variable was unfavorable outcome (GOS 1–3), while the independent variables were age, GCS, and BHIm. The appropriateness of the fitted model and its predictive utility was confirmed. Patient age and BHIm showed the strongest influence on disease outcome. A decrease of BHIm for each 0.1 unit increases the risk of unfavorable outcome by 17%. An increase of age for one year increases the risk of unfavorable outcome by 6% (Table 5). These results indicate that BHIm changes might have a better predictive value than GCS. The receiver operating characteristic (ROC) curve showed that BHIm has better explanatory value than GCS (area under the curve [AUC] 0.785 vs. 0.745).
Table 5.
Variables associated with unfavorable outcome: logistic regression analysis
| Odds ratio estimates | |||
|---|---|---|---|
| Variable | OR | 95% Wald confidence limits | |
| Age | 1.063 | 1.017 | 1.110 |
| GCS | 1.089 | 0.914 | 1.298 |
| BHIm | 4.922 | 1.161 | 20.875 |
Discussion
This prospective study assessed cerebrovascular reactivity (CO2 R) by TCD using the breath-holding method in patients with inflammatory CNS diseases. We found that impaired CO2R is independently associated with the severity and outcome of disease.
The etiology of CNS infection did not show a significant association with chemoregulation loss. However, the etiology was associated with the severity of disease and mechanical ventilation requirement. In patients with BM, we found a higher incidence of respiratory failure, advanced age, and heavier consciousness disturbance. This finding, however, does not imply obligatory chemoregulation loss in these patients. Their grave condition can be explained with reasons outside the CNS, such as multiple-organ dysfunction/failure due to severe systemic inflammatory reaction and other co-morbidities in older patients. In addition, there were no lethal outcomes in mechanically ventilated patients with normal CO2 R, regardless of the etiology. The mortality rate in mechanically ventilated patients with impaired CO2 R was similar in both groups (25% vs. 26.6%).
In contrast to previous reports, we found no significant influence of adjuvant dexamethasone treatment on CBF chemoregulation recovery [19].
The analysis of BHI according to disease outcome ascertained the association of impaired CO2 R with unfavorable outcome (GOS 1–3). According to the literature review, the impaired CBF chemoregulation is almost exclusively confined to BM [9]. That is probably because of particular interest in pneumococcal meningitis pathophysiology and the great number of experimental studies [20–24]. The lipid peroxidation and effects of matrix metalloproteinases with consequent endothelial dysfunction seems to be a plausible explanation. According to our results, the most severe CNS infections resulted in CO2 R impairment or complete CO2 R loss, which was proved to be etiology-independent. The possible mechanisms which could explain the chemoregulation loss in viral and other non-bacterial CNS infections are not known. Intracranial hypertension alone, regardless of cause, cannot explain impaired CO2 R. For example, most patients with extreme intracranial hypertension due to cerebral abscess or tumors have normal CO2 R, and the use of hyperventilation as well as mannitol infusion results in obvious short-term improvement. A similar response could be seen in patients with BM and preserved CO2 R.
We chose the breath holding method because it proved to be non-aggressive, well-tolerated, real-time, reliable, and reproducible [15, 25]. The lowest BHIm in our healthy volunteers was similar to a previously reported BHIm by Zavoreo and Demarin (1.18 vs. 1.03) [25].
Other methods such as inhalation of 5% CO2 or acetazolamide injection strongly stimulate the vasodilatation of cerebral arterioles [15, 26]. Because they also carry the risk of cerebral hyperemia and intracranial hypertension aggravation, these methods are not appropriate for the evaluation of cerebral vasoreactivity in patients with severe acute CNS infections. More advanced techniques to assess the small- and medium-sized vessel reactivity such as arterial-spin labeled magnetic resonance imaging (ASL-MRI) and blood oxygenation level-dependent (BOLD) imaging are usually unavailable.
A major disadvantage of the breath holding method is the necessity for the patients’ full cooperation if they are not mechanically ventilated. Besides disease severity, the inability of compliance in a proportion of patients is the reason for a relatively high proportion of mechanically ventilated patients in our study (64.7%; 44/68). That is the recognized limitation of our study. The second limitation of the study is a relatively small patient population. This occurred because of strict exclusion criteria and that may have resulted in selection bias.
Despite the limitations of our study, our results confirmed the importance of CO2 R assessment in patients with CNS infections. CO2 R showed good correlation with the severity and outcome of bacterial as well as non-bacterial meningitis. However, the most important value of CO2 R is the capability to define patients with chemoregulation loss immediately upon admission to the hospital. It should be stressed that it is not possible to distinguish these patients by clinical examination or with computed tomography (CT) brain scan. The effects of conventional symptomatic treatment (osmotic diuresis, thiopental, hyperventilation) are greatly dependent (or associated with) on cerebral arterioles’ vasoreactivity [27]. Patients with impaired CO2 R are candidates for therapeutic hypothermia (TH). This life-saving procedure should be started as soon as possible because conventional symptomatic treatment failure and poor outcome of disease in these patients should be anticipated.
Based on the results of this study, we made an internal guideline for TH in patients with severe CNS infections. The major criterion for TH is impaired CO2 R assessed by TCD. In patients without temporal bone ‘acoustic window’, minor criteria [optic nerve sheath diameter ≥6.0, GCS ≤8, and SjO2 (jugular bulb venous saturation) <55% or >75%] are required.
Therapeutic hypothermia during CNS infections may assist with the reduction in cerebral metabolism, cerebral blood volume (CBV), lowering of the intracranial pressure, and suppression of the inflammatory host response, allowing the maintenance of adequate cerebral perfusion pressure. According to our experience, the recovery of CO2 R cannot be expected before the fourth day of treatment. We recommend the use of TH as soon as possible and at least during the first three days after presentation. The first results of that therapeutic concept in patients with BM are very promising [28]. Therapeutic hypothermia halved the overall mortality in patients with BM and significantly decreased the mortality rates in patients with viral meningoencephalitis at our hospital during the last two years (12% vs. 24% and 9% vs. 20%, respectively; unpublished data).
Our study emphasizes the importance of CO2 R assessment in patients with CNS infections, regardless of etiology. A great predictive value as well as reliable stratification criteria in treatment strategy decisions make this method a very promising tool.
Acknowledgments
We thank Petar Mamula MD, Joel Friedlander DO, MBe, Mrs. Arijana Pavelić, and Mrs. Marija Fijucek for their help in the preparation of the manuscript.
Conflict of interest
None.
Financial support
There was no financial support for this study.
Contributor Information
D. Lepur, Phone: +385-1-2826253, Phone: +385-1-2754880, FAX: +385-1-2826255, Email: lepurix@inet.hr
M. Kutleša, Email: marko.kutlescha@gmail.com
B. Baršić, Email: bbarsic@bfm.hr
References
- 1.Lu CH, Huang CR, Chang WN, Chang CJ, Cheng BC, Lee PY, et al. Community-acquired bacterial meningitis in adults: the epidemiology, timing of appropriate antimicrobial therapy, and prognostic factors. Clin Neurol Neurosurg. 2002;104(4):352–358. doi: 10.1016/S0303-8467(02)00052-5. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J. Management of bacterial meningitis in adults: algorithm from the British Infection Society represents current standard of care. Br Med J. 2003;326(7397):996–997. doi: 10.1136/bmj.326.7397.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lepur D, Baršić B. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 2007;35(4):225–231. doi: 10.1007/s15010-007-6202-0. [DOI] [PubMed] [Google Scholar]
- 4.van de Beek D, de Gans J, Tunkel AR, Wijdicks EFM. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44–53. doi: 10.1056/NEJMra052116. [DOI] [PubMed] [Google Scholar]
- 5.Flores-Cordero JM, Amaya-Villar R, Rincón-Ferrari MD, Leal-Noval SR, Garnacho-Montero J, Llanos-Rodríguez AC, et al. Acute community-acquired bacterial meningitis in adults admitted to the intensive care unit: clinical manifestations, management and prognostic factors. Intensive Care Med. 2003;29:1967–1973. doi: 10.1007/s00134-003-1935-4. [DOI] [PubMed] [Google Scholar]
- 6.Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS, Jr, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 7.Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862–869. doi: 10.7326/0003-4819-129-11_part_1-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 8.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849–1859. doi: 10.1056/NEJMoa040845. [DOI] [PubMed] [Google Scholar]
- 9.Scheld WM, Koedel U, Nathan B, Pfister HW. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. J Infect Dis. 2002;186(Suppl 2):S225–S233. doi: 10.1086/344939. [DOI] [PubMed] [Google Scholar]
- 10.Grände PO, Asgeirsson B, Nordström CH. Volume-targeted therapy of increased intracranial pressure: the Lund concept unifies surgical and non-surgical treatments. Acta Anaesthesiol Scand. 2002;46:929–941. doi: 10.1034/j.1399-6576.2002.460802.x. [DOI] [PubMed] [Google Scholar]
- 11.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 12.Koedel U, Scheld WM, Pfister HW. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2002;2(12):721–736. doi: 10.1016/S1473-3099(02)00450-4. [DOI] [PubMed] [Google Scholar]
- 13.Møller K, Strauss GI, Thomsen G, Larsen FS, Holm S, Sperling BK, et al. Cerebral blood flow, oxidative metabolism and cerebrovascular carbon dioxide reactivity in patients with acute bacterial meningitis. Acta Anaesthesiol Scand. 2002;46:567–578. doi: 10.1034/j.1399-6576.2002.460515.x. [DOI] [PubMed] [Google Scholar]
- 14.Ashwal S, Stringer W, Tomasi L, Schneider S, Thompson J, Perkin R. Cerebral blood flow and carbon dioxide reactivity in children with bacterial meningitis. J Pediatr. 1990;117(4):523–530. doi: 10.1016/S0022-3476(05)80683-3. [DOI] [PubMed] [Google Scholar]
- 15.Markus HS, Harrison MJG. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke. 1992;23:668–673. doi: 10.1161/01.STR.23.5.668. [DOI] [PubMed] [Google Scholar]
- 16.White H, Venkatesh B. Applications of transcranial Doppler in the ICU: a review. Intensive Care Med. 2006;32(7):981–994. doi: 10.1007/s00134-006-0173-y. [DOI] [PubMed] [Google Scholar]
- 17.Strebel S, Kaufmann M, Guardiola PM, Schaefer HG. Cerebral vasomotor responsiveness to carbon dioxide is preserved during propofol and midazolam anesthesia in humans. Anesth Analg. 1994;78(5):884–888. doi: 10.1213/00000539-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Nitschmann P, Oberkogler W, Hertsig M, Schwarz S. Comparison of haemodynamic effects of rocuronium bromide with those of vecuronium in patients undergoing CABG surgery. Eur J Anaesthesiol Suppl. 1994;9:113–115. [PubMed] [Google Scholar]
- 19.Mertineit C, Samlalsingh-Parker J, Glibetic M, Ricard G, Noya FJ, Aranda JV. Nitric oxide, prostaglandins, and impaired cerebral blood flow autoregulation in group B streptococcal neonatal meningitis. Can J Physiol Pharmacol. 2000;78(3):217–227. doi: 10.1139/y99-117. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto K, Houdou S, Ando Y, Okasora T. Usefulness of ultrasonography and transcranial Doppler flowmetry in evaluation of infantile bacterial meningitis. No To Hattatsu. 1989;21(5):475–480. [PubMed] [Google Scholar]
- 21.Fassbender K, Ries S, Schminke U, Schneider S, Hennerici M. Inflammatory cytokines in CSF in bacterial meningitis: association with altered blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry. 1996;61(1):57–61. doi: 10.1136/jnnp.61.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller M, Merkelbach S, Huss GP, Schimrigk K. Clinical relevance and frequency of transient stenoses of the middle and anterior cerebral arteries in bacterial meningitis. Stroke. 1995;26(8):1399–1403. doi: 10.1161/01.STR.26.8.1399. [DOI] [PubMed] [Google Scholar]
- 23.Haring HP, Rötzer HK, Reindl H, Berek K, Kampfl A, Pfausler B, et al. Time course of cerebral blood flow velocity in central nervous system infections. A transcranial Doppler sonography study. Arch Neurol. 1993;50(1):98–101. doi: 10.1001/archneur.1993.00540010092024. [DOI] [PubMed] [Google Scholar]
- 24.Müller M, Merkelbach S, Schimrigk K. Cerebral hemodynamics in the posterior circulation of patients with bacterial meningitis. Acta Neurol Scand. 1996;93(6):443–449. doi: 10.1111/j.1600-0404.1996.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 25.Zavoreo I, Demarin V. Breath holding index in the evaluation of cerebral vasoreactivity. Acta Clin Croat. 2004;43:15–19. [Google Scholar]
- 26.Cigada M, Marzorati S, Tredici S, Iapichino G. Cerebral CO2 vasoreactivity evaluation by transcranial Doppler ultrasound technique: a standardized methodology. Intensive Care Med. 2000;26:729–732. doi: 10.1007/s001340051239. [DOI] [PubMed] [Google Scholar]
- 27.Messeter K, Nordström CH, Sundbärg G, Algotsson L, Ryding E. Cerebral hemodynamics in patients with acute severe head trauma. J Neurosurg. 1986;64:231–237. doi: 10.3171/jns.1986.64.2.0231. [DOI] [PubMed] [Google Scholar]
- 28.Lepur D, Kutleša M, Baršić B. Induced hypothermia in adult community-acquired bacterial meningitis—more than just a possibility? J Infect. 2010 doi: 10.1016/j.jinf.2010.10.001. [DOI] [PubMed] [Google Scholar]


