Abstract
Purpose of review
The expanding population of immunocompromised patients coupled with the recognition of a growing number of different species of fungi responsible for diseases in such hosts makes the diagnosis of invasive fungal infection (IFI) a challenging task. The recent advances and challenges in the diagnosis of IFI in the setting of immunocompromised hosts are reviewed. The advantages and limitations of histopathology and the role of culture-independent methods, such as those based on the use of nucleic acids applied to fresh and formalin-fixed, paraffin-embedded sections, besides culture- and non-culture-based diagnostic methods, to obtain a timely and correct diagnosis of IFI are highlighted.
Recent findings
The therapeutic implications of identifying the genus and species of the fungus present in the specimen with the molecular diagnostics applied to tissue specimens are reviewed. No method alone is efficient in correctly identifying fungi and it is essential to combine the traditional histochemical staining with molecular methods to achieve a rapid and genus-/species-specific diagnosis of IFI.
Summary
We review the recent findings and challenges in the hystopathologic diagnosis of IFI in the setting of immunocompromised hosts. Non method alone is efficient in correctly identify fungi and pathologists should combine classic staining with molecular methods to achieve a rapid and genus/species fungal diagnosis.
Keywords: Aspergillosis, Cryptococcosis, Emergomycosis, Histoplasmosis, Mucormycosis, Yeast disease
Introduction
Invasive fungal infections (IFIs) are increasingly recognized in the expanding population of immunocompromised hosts such as transplant recipients, people receiving immunosuppressive and chemotherapeutic agents, patients with human immunodeficiency virus (HIV) infection, and individuals at the extreme of life (i.e., premature infants and the elderly) [1–4]. Diagnosis of most IFIs remains a challenging task due to nonspecific clinical presentation and lack of sensitivity of traditional microbiologic methods. In recent years, the introduction of serologic tests such as the galactomannan (GMN) and 1,3-β-D-glucan (BDG) antigens as well as molecular techniques has improved the diagnosis [5]. Nevertheless, the species of fungi responsible for disease among immunocompromised patients is expanding, making the diagnosis more complicated [6]. Histopathologic examination, together with culture, is still considered the gold standard to make a definitive diagnosis of IFIs. However, due to the many pitfalls encountered in the morphological diagnosis of IFIs and the development of highly specific molecular techniques such as in situ hybridization and nucleic acid amplification, it is clear that a pathologist with a subspecialty expertise in infectious diseases is essential in hospitals dealing with large numbers of immunocompromised hosts [7–9]. The present review will focus on the morphological aspects of some of the common IFIs, with emphasis on the pitfalls in the diagnosis and the increasing role of molecular techniques applied to tissue specimens (Table 1).
Table 1.
Epidemiology, clinical aspects, and pathology of the common invasive fungal infections in immunocompromised patients
| Fungus | Geographic distribution | Hosts/risk factors | Tissue morphology | Clinical aspects | Pathology | Serum antigen | Comments |
|---|---|---|---|---|---|---|---|
| Aspergillus spp. | Ubiquitous; worldwide | Stem cell or solid organ transplantation; hematologic malignancy; HIV/AIDS infection; CGD; cancer; neutropenia; steroid therapy | Septate dichotomously branched hyaline (non-pigmented) hyphae (3–6 μm) | Invasive pulmonary disease; disseminated disease (with CNS infection) | Angioinvasion with infarction, necrosis, and hemorrhage | Galactomannan (GM) antigen (serum, BAL, CSF); (especially helpful for hematologic patients not assuming antifungal prophylaxis). Cross-reactivity with histoplasmosis | Histopathology: aspergillosis needs to be differentiated from other mycosis showing septated (Fusarium, Scedosporium) and non-septated hyphae |
| Blastomyces dermatitidis | North America (Mississippi and Ohio River Valley); Canada (the Great Lakes) | HIV/AIDS infection; hematologic malignancy; solid organ transplantation; steroid therapy | Globose yeasts (8–15 μm) with broad-based budding; occasionally smaller yeasts | Pulmonary disease (ARDS); skin, bone, and disseminated disease | Pyogranulomatous response | Blastomyces Ag* (EIA): urine (76–93% sensitivity), serum, BAL; cross-reactivity with histoplasmosis | The cell wall is best highlighted with silver stain (GMS); H&E shows a space between the capsule and the cell contents; occasionally positive with mucicarmine stain; the width of bud attachment (broad-based) useful diagnostic criteria |
| Candida spp. | Ubiquitous; worldwide | HIV/AIDS infection; hematologic malignancy; solid organ transplantation/CVC; abdominal surgery | Small yeasts (3–5 μm) with pseudohyphae | Candidemia; invasive candidiasis; esophagitis (AIDS) | Coagulative necrosis, hemorrhage, and vascular invasion without neutrophils (neutropenic patients) | 1,3-β-D-glucan (BG-panfungal); high predictive negative value in candidemia. Less useful in areas of endemic mycoses |
Candida spp. is stained with H&E, GMS, PAS; C. glabrata can be confused with Histoplasma capsulatum |
| Cryptococcus spp. | Ubiquitous; worldwide | HIV/AIDS infection; steroid therapy; solid organ transplant | Globose, encapsulated yeasts (2–15 μm) | Meningoencephalitis; disseminated disease | Massive proliferation with minimal mononuclear cell response (soap bubble lesions) | Crypto Ag (LA and LFA): high sensitivity and specificity on serum and CSF (especially in AIDS patients) | Mucicarmine-positive capsule; India ink positive (CSF); positive with melanin stain (Fontana Masson) |
| Coccidioides immitis/posadasii | Southwestern USA (Arizona, Utah, New Mexico, Texas); Mexico; Central America (Guatemala, Honduras, Guatemala); South America (Argentina, Colombia, Paraguay, Venezuela) | HIV/AIDS infection; steroids therapy; tumor necrosis factor-α blocker therapy | Globose, thick-walled spherules (20–200 μm) with multiple endospores; arthroconidia and hyphae sometimes visible | Pneumonia; disseminated disease (skin, lymph nodes, bone, joints); meningitis | Marked necrosis without granuloma and eosinophil infiltration | Coccidioides Ag*: cross-reactivity with GM and BG | The absence of spherules on histology makes it difficult to differentiate Coccidioides from other fungi (Blastomyces, Histoplasma, Candida, Pneumocystis); mature spherules stain poorly with PAS |
| Emergomyces africanus; E. pasteurianus | South Africa; India | HIV/AIDS infection; solid organ transplant | Yeast-like narrow-based budding cells | Disseminated disease (skin, blood, bone marrow) | Inflammatory infiltrate | Cross-reactivity with Histo Ag and BG | PAS-positive narrow-based budding yeasts (3–7 μm) |
| Histoplasma capsulatum var. capsulatum | Worldwide; especially frequent in the USA (Ohio, Mississippi, and St. Lawrence River valley); South America | HIV/AIDS infection; tumor necrosis factor-α blocker therapy | Small budding uninucleate yeasts (2–4 μm) | Progressive disseminated disease with pulmonary, bone marrow, liver, and splenic involvement | Scattered small granulomas (lung); macrophages filled with yeasts (lung, lymph nodes, liver, spleen, bone marrow) | Histo Ag* (serum and urine): high sensitivity in PDH; cross-reactivity with blastomycosis and talaromycosis | Cell wall highly stained with GMS and PAS; H. duboisii shows larger (8–15 μm) yeasts/need to be differentiated from B. dermatitidis, Coccidioides spp., Candida glabrata, P.jirovecii, T. marneffei, capsule-deficient cryptococci and protozoa (Leishmania spp., T. gondii, T. cruzi) |
| Mucormycosis | Ubiquitous; worldwide | Hematologic malignancy; solid organ transplantation; diabetes mellitus (ketoacidosis); deferoxamine therapy; steroid therapy | Hyaline, pauci-septate ribbon-like hyphae (right-angle branching) | Rhinocerebral disease; pulmonary and disseminated infection | Angioinvasion with hemorrhagic infarction; neutrophilic reaction with aggregates of epithelioid histiocytes | GM rarely positive | GMS and PAS stains useful to highlight fungal wall; fragmented |
| Pneumocystis jirovecii | Ubiquitous; worldwide | HIV/AIDS infection; kidney transplant; autoimmune diseases | Cysts containing intracystic bodies and trophozoite form | Interstitial pneumonia; extrapulmonary pneumocystosis (rare) | Intra-alveolar organisms with a “foamy exudate” | BG panfungal (serum): helpful in AIDS patients | Pneumocystis need to be differentiated from Histoplasma; toluidine blue stain (purple cysts). Immunofluorescence assays using monoclonal antibodies anti-Pneumocystis are specific |
| Talaromyces marneffei | Southeast Asia (Myanmar, Vietnam, Thailand; Southern China (Guangxi province)) | HIV/AIDS Infection; solid organ transplant | Elliptical yeasts (2–8 μm) with prominent transverse septum | Disseminated disease (skin, bone marrow, lung, lymph nodes) | Necrotic lesions are surrounded by histiocytes containing yeast cells | Cross-reactivity with Histo Ag | GMS stain the transverse septa thicker than the wall; organisms may appear encapsulated when stained with H&E |
HIV human immunodeficiency virus, CGD chronic granulomatous disease, CNS central nervous system, BAL bronchoalveolar lavage, CSF cerebrospinal fluid, H&E hematoxylin and eosin, GMS the Gomori methenamine silver, PAS periodic acid-Schiff, LA latex agglutination, LFA lateral flow assay, CVC central venous catheter, GM galactomannan
*All these antigen tests are available only in the USA
Aspergillosis
Aspergillus species are ubiquitous molds that traditionally cause invasive disease in severely immunocompromised hosts such as those with profound and prolonged neutropenia, those undergoing hematopoietic stem cell and solid organ transplantation, or those affected by chronic granulomatous disease (CGD) and acquired immunodeficiency syndrome (AIDS). However, new populations at risk of invasive aspergillosis (IA) have been identified in the last decade including patients with chronic obstructive lung disease, liver cirrhosis, and autoimmune disorders [10]. It has been estimated that Aspergillus spp. are responsible for more than 200,000 cases of IA annually and 0.017 to 3.7% of cases observed in intensive care units (ICU) in the USA and Europe with mortality rates in the latter ranging from 46 to 87–97% [2, 11]. On morphological grounds, the diagnosis of aspergillosis is usually obtained by the observation of thin, septate acute angle (45°) or dichotomous branching hyphae [12••]. However, it should be highlighted that other hyaline septate molds such as Fusarium spp., Scedosporium spp., and Pseudallescheria spp. may be morphologically indistinguishable. In a retrospective study conducted in the Republic of Korea on 52 cases of culture-positive aspergillosis, discordant results were observed in 17% of cases [13]. In another study conducted in Virginia, the diagnostic concordance between histopathology and culture for aspergillosis was 78% [14]. A similar prevalence (21%) of discrepant diagnoses although not only for Aspergillus spp. but also regarding other molds and yeasts were reported in a 10-year retrospective study conducted at Stanford University [15]. The authors of this study pointed out that aspergillosis should be diagnosed with caution in tissue and cytologic preparations [15]. However, their recommendation to always correlate the findings with the results of culture may not be feasible in clinical practice due to the frequent absence of a requested microbiologic culture or its unsatisfactory (less than 70%) sensitivity [16]. Sangoi and coworkers suggested to adopt a standardized report for the diagnosis of fungal infection that should include a provisional diagnosis (i.e., hyalohyphomycosis) with an appropriate accompanying comment about different possible mycoses, thus avoiding mistakes about genus identification (based on morphology) and the use of inappropriate terminology [15]. However, the increasing complexity of immunocompromised patients together with the expanding number of pathogenic filamentous fungi which show different antifungal susceptibility makes an early and accurate (genus- or species-level) identification of the causative fungal pathogen challenging to optimize drug treatment. Molecular diagnostic methods applied either to fresh or formalin-fixed paraffin-embedded (FFPE) tissue specimens have been increasingly used to help in the identification of different fungi responsible for invasive diseases [9, 17–19].
The majority of fungal polymerase chain reaction (PCR)-based assays target multicopy genes of ribosomal DNA (18S, 28S, and 5.8S) and the intervening internal transcribed spacer (ITS) regions, allowing the design of universal primers in order to amplify DNA from a large number of fungi [20]. In 2007, Lau et al. developed a panfungal PCR targeting the ITS1 region of ribosomal DNA (rDNA) gene cluster with subsequent sequencing of fungal DNA [17]. They showed a sensitivity of 93.6% in identifying fungal pathogens among culture-proven cases and of 64.3% among only histology-proven IFI. Interestingly, among cases of aspergillosis, the sequence allowed the identification of Neosartorya pseudofischeri instead of the phylogenetically closely related Aspergillus fumigatus as determined by culture. However, Lau et al. indicated the limitations regarding the use of panfungal PCR and observed the turnaround time of 4–5 days when using FFPE specimens and estimated 10–20% of misidentified organisms according to the fungal sequences deposited in GenBank [17].
More recently, Salehi and coworkers, using a multiple real-time quantitative PCR (qPCR) targeting the ITS2 region of rDNA, found an overall sensitivity of 64% for the identification of fungi at the species or genus level [21•]. However, 16% of the histopathologically diagnosed cases of aspergillosis according to real-time qPCR were fusariosis (5) and mucormycosis (1). The latter diagnosis had important therapeutic implications since treatment of mucormycosis differs in a substantial way from that of aspergillosis. In this regard, it should be highlighted that in the study by Dekio et al. conducted among immunocompromised pediatric patients with histology-proven IFIs, the communication of histologic diagnosis resulted in changes in antifungal therapy in 64% of patients [16]. Another important issue with relevant therapeutic implications about aspergillosis is related to the diffusion of azole-resistant Aspergillus spp. [22]. Voriconazole has been established as the first-choice treatment for IA. However, since the late 2000s, several reports from Europe documented the emergence of clinical isolates of A. fumigatus with azole resistance that was associated with treatment failure [23, 24]. Alterations in the cyp51A gene (the azole target in the fungal ergosterol synthesis) tandem repeats and mutations in the gene itself are more frequently associated with azole resistance. Detection of azole-resistant aspergillosis is complicated by the fact that cultures are negative in up to 50% of patients with pulmonary lesions and in vitro susceptibility testing are not routinely available. In this context, van den Linden and coworkers demonstrated the feasibility of rapid detection of the more frequent mutation associated with azole resistance (TR34/L98H) directly on FFPE tissue specimens by a specific real-time PCR [25].
Blastomycosis
Blastomyces dermatitidis is the dimorphic fungus (the perfect stage named Ajellomyces dermatitidis) responsible of blastomycosis (once known as Chicago disease) endemic in North America (Mississippi, Ohio, and St. Lawrence River valley) and the Canadian provinces that border the Great Lakes [26]. Although generally observed among immunocompetent patients, blastomycosis causes more severe disease (with multi-organ involvement and adult respiratory distress syndrome (ARDS) or central nervous system (CNS) involvement) and more frequently associated with fatal outcome among immunocompromised patients [27]. Pulmonary involvement with alveolar or mass-like infiltrate mimicking malignancy is the most common presentation of blastomycosis but essentially, any organ may be affected (skin and subcutaneous tissue, skeletal system, genitourinary tract, CNS, myocardium) with unusual manifestations. Morphological diagnosis of blastomycosis by examination of cytologic or tissue samples is generally entertained when single, broad-budding yeasts (that measure 8 to 15 μm in diameter) are visible by hematoxylin and eosin (H&E) or Papanicolaou stain [12, 28]. The thick refractile cell wall of the fungus stains is positive on silver stains (the Gomori methenamine silver (GMS) or periodic acid-Schiff (PAS) stain) but negative (or weakly positive) for mucicarmine [28]. Although a definitive diagnosis of blastomycosis requires a positive culture, it must be highlighted that fungal cultures are sometimes missed in view of the clinical suspicion of malignancy and the long turnaround time for culture (2–4 weeks). In a recent retrospective study conducted in Chicago, 24% of patients who underwent concomitant histopathology and culture received a diagnosis only by histology [29]. The major advantage of histopathology is the timely diagnosis of blastomycosis (1–2 days from the date of biopsy) facilitating an early antifungal treatment; however, it should be noted that 11% of culture-proven cases are missed by tissue examination and about 9% are misdiagnosed on histopathology because other fungi like Coccidioides immitis, Candida albicans, and Aspergillus grew on culture [29]. Therefore, it has been suggested that the pathology report should contain the description of the budding pattern with a list of yeasts with similar morphologic appearance [12••].
Candidiasis
Candidiasis among immunocompromised patients can present as either superficial mucocutaneous (non-invasive) candidiasis (i.e., oral thrush and esophageal candidiasis) especially in HIV-infected patients or an invasive disease (i.e., fungemia, intra-abdominal candidiasis, hepatosplenic candidiasis) in neutropenic hemato-oncologic patients and non-neutropenic critically ill patients [2, 30]. According to a recent case-control study, esophageal candidiasis (EC) is the most common cause of infectious esophagitis (88% of all cases with a known etiology) with an overall incidence of 5.2% [31]. A 16-fold underestimate of EC may be observed in endoscopic diagnosis alone versus histology with mistaken diagnosis on endoscopy as eosinophilic esophagitis or Barrett’s esophagus [31]. In the study by Alsomali et al., pseudohyphae infiltrating detached or intact squamous epithelium (considered the minimum criterion for EC diagnosis) was observed on H&E in 92.5% of patients and therefore these authors suggest the use of PAS/diastase to confirm the diagnosis only in negative cases presenting with ulcer and hyper-pink parakeratosis or when EC is suspected on clinical grounds [31]. The application of this strategy could allow 81% reduction in costs. In the diagnosis of invasive candidiasis (IC), the diagnostic sensitivity of blood cultures (that are considered the gold standard) is estimated to be less than 50% [32, 33]. In view of the overall crude mortality of 40–60% of IC, especially in the presence of sepsis or septic shock, alternative rapid and easily performed methods such as serum BDG or the application of technologies such as MALDI-TOF or T2 magnetic resonance have been proposed in order to expedite both diagnosis and treatment [34]. The role of histopathology in the diagnosis of acute IC is limited except in chronic disseminated disease with unusual localizations [17]. The authors illustrated a case report where a panfungal PCR with subsequent sequencing of the PCR products identified the fungus as Candida glabrata in an immunocompromised patient with a biopsy diagnosis of histoplasmosis versus candidiasis (Fig. 1) [17].
Fig. 1.
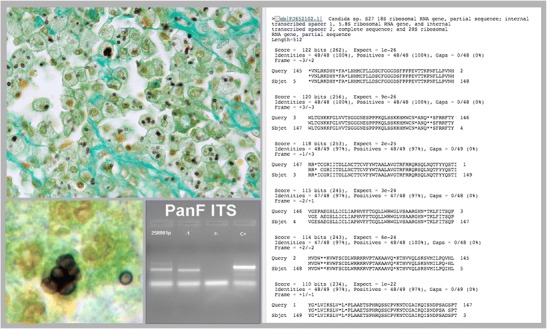
Biopsy showing a subcutaneous granuloma. The Grocott stain shows small yeast-like cells compatible with Histoplasma capsulatum and Candida glabrata (on the left) (original magnification × 40, inset × 100). Panfungal PCR that amplifies the internal transcriber spacer (ITS-1) region of the rDNA gene (panel to the left) that after the sequence matched with Candida spp. (panel on the right). The final diagnosis was Candida glabrata
Coccidioidomycosis
Coccidioidomycosis is caused by two species of dimorphic fungi (Coccidioides immitis and C. posadasii) and is endemic in several states of North America (California, Arizona, Texas, New Mexico, Utah, Nevada) as well as in South American nations (Mexico, Guatemala, Honduras, Argentina, Brazil, Paraguay, Bolivia, and Venezuela) [35]. More than 60% of healthy subjects exposed to the fungus do not develop symptoms whereas the remainder develops a community-acquired pneumonia. Immunocompromised patients (i.e., HIV-seropositive subjects, those receiving anti-tumor necrosis factor (TNF)-α therapy, or allogenic transplant recipients) living in endemic areas are at risk of either acute or reactivated infections with pulmonary and/or disseminated disease [35, 36]. The classic pathologic hallmark of coccidioidomycosis is the presence of spherules of various size (depending of their maturation stage) containing multiple endospores and are easily observed with H&E [12••]. The mature spherulae are pathognomonic and easily diagnosed by expert pathologists but young spherules may be mistakenly confused with several fungi like Histoplasma, Blastomyces, Candida, Emmonsia, or Pneumocystis [37]. A recent series from Arizona examining the cytological diagnosis of coccidioidomycosis over a 10-year period showed that majority of the specimens were from lung, either fine-needle aspirates (FNA) or bronchoalveolar lavage (BAL) [38]. Conventional Papanicolaou smears gave more pink staining of spherules in comparison with rapid Papanicolaou. In addition, budding and hyphal forms were more frequently seen in BAL specimens whereas immature spherules predominated in pulmonary FNA specimens. Mesothelial and bronchial cells may at times show mild cytologic atypia and GMS stain was particularly helpful for the identification of very small spherules in BAL specimens [38]. Recently, two recent series of coccidioidomycosis with unusual presentation in pleural and skeletal presence of spherules was lower in pleural coccidioidomycosis (mean density < 1/10 high-power field (HPF)) than in skeletal coccidioidomycosis (mean density 4.8/HPF) and in the latter, it was higher in immunocompromised patients (median 4.3/HPF versus 1.6/HPF) [39, 40]. Granulomatous inflammation was present in both locations of the disease but was universal in the pleural form while they were observed in only one third of patients with the skeletal localization. A real-time PCR designed to target the ITS2 region of Coccidiodes spp. showed a 100% sensitivity and 98.4% specificity for the diagnosis of coccidioidomycosis on respiratory specimens, when compared with culture [41]. The sensitivity of the assay dropped significantly (73.4%) when used on paraffin-embedded tissue samples. By contrast, Montone et al. showed on a small number of FFPE pulmonary tissue specimens that in situ hybridization (ISH) using locked nucleic acid probes targeting ribosomal RNA sequences of Coccidioides spp. can rapidly confirm the diagnosis in spite of negative concurrent culture [42].
Cryptococcosis
Cryptococcosis represents one of the most common opportunistic invasive fungal disease worldwide with the highest burden among HIV/AIDS patients especially in sub-Saharan Africa [43]. However, an updated estimate in 2017 indicated a 70% reduction of the global cases among HIV-infected patients with still a high number of cases (278,000). In fact, cryptococcal meningitis accounts for 15% of AIDS-related mortality [44]. Interestingly, the recent whole-genome sequencing studies suggest to split Cryptococcus neoformans into two species (C. neoformans and C. deneoformans) and C. gattii into five species (C. gattii, C. bacillisporus, C. deuterogattii, C. tetragatti, and C. decagattii) although consensus between researchers in the field has not yet been achieved [45, 46•, 47, 48]. The morphology of Cryptococcus spp. is that of a spherical to oval encapsulated yeast with a narrow-based budding. The polysaccharide capsule is stained by mucicarmine, PAS, and alcian blue whereas GMS stains the fungal wall [12••]. In general, diagnosis of cryptococcal meningitis or disseminated disease in immunocompromised patients is easily achieved by using India ink stain (on cerebrospinal fluid), culture and latex agglutination, enzyme immunoassay, or lateral flow assays (as point-of care) targeting the cryptococcal antigen [49, 50]. The latter shows high sensitivity and specificity but may be negative in localized infections and in the presence of acapsular cryptococci [51]. Moreover, when the mucin capsule is absent, it can be difficult to distinguish Cryptococcus from Candida glabrata and Histoplasma capsulatum, especially in necrotic tissues [12••]. Recently, Indian researchers proposed to use the acid-fast property associated with the mycolic acid in the cell wall of Histoplasma spp. to differentiate it from Cryptococcus [52, 53]. Ranjan et al. examining FNA cytology showed that Histoplasma stained as blue-colored yeasts whereas Cryptococcus displayed only clear haloes with the Ziehl-Neelsen (ZN) stain [52]. In the study by Rajeshwari et al., 46.5% of tissue specimens with a diagnosis of histoplasmosis stained positive with ZN stain [53].These authors found that cryptococci stained with ZN showed magenta color that can be differentiated from the bright pink cytoplasmic staining of Histoplasma [53].
Several studies have evaluated post-mortem findings of cryptococcosis in AIDS patients showing the frequent dissemination of the disease besides the CNS with an average of 3.8 organs per patient involved [54, 55]. Excluding CNS, the organs more frequently involved are lungs, lymph nodes, kidney, liver, spleen, and adrenal glands. Interestingly, the agreement rates of pre-mortem and post-mortem diagnosis was more than 95% in an Italian study but only 64.4% in a Brazilian one [54, 56].
Emergomycosis (Emmonsiosis)
Emergomycosis (formerly emmonsiosis) refers to a disseminated infection caused by a newly recognized dimorphic fungus originally named Emmonsia pasteuriana when first isolated in an Italian woman with AIDS [57]. Skin biopsies initially misinterpreted as histoplasmosis showed yeast-like cells with pleomorphic features resembling either H. capsulatum or Blastomyces dermatitidis but without the broad bud base of the latter fungus. Subsequently, other cases of Emmonsia pasteuriana and a novel Emmonsia-like fungus were reported from Spain, India, China, and South Africa [58–60]. Actually, both fungi were classified by means of morphological and phylogenetic analyses as members of the Ajellomycetaceae in the new genus Emergomyces and named Emergomyces pasteurianus and E. africanus sp. nov (formerly Emmonsia sp. 5) [60]. New Emergomyces species have been isolated in Germany and Canada and this genus should be considered an emerging opportunistic pathogen [61, 62]. Skin and lung are the more frequently affected sites in disseminated emmonsiosis among HIV-infected patients but any organ may be involved. It is clinically misdiagnosed as tuberculosis, histoplasmosis, or other diseases [62]. Histopathology showing yeast-like cells can mimic histoplasmosis, blastomycosis, and sporotrichosis, with in vitro culture providing the correct diagnosis.
Histoplasmosis
Histoplasmosis caused by Histoplasma capsulatum var. capsulatum has a worldwide distribution although it is erroneously considered endemic almost exclusively in the Mississippi and Ohio River Valleys in the USA [63, 64]. Impaired cell-mediated immunity as observed among HIV/AIDS patients, solid organ, or bone marrow transplant recipients and those treated with TNF-α blocking agents is the known predisposing condition associated with progressive disseminated histoplasmosis (PDH) [4, 65–67]. Characteristic histopathologic features are clusters of yeasts phagocytized by macrophages or the presence of small (2–4 μm) narrow-based budding yeasts in the extracellular space. The cell wall is highlighted by GMS and PSA staining [12••, 68]. Morphologically, the differential diagnosis of histoplasmosis includes several fungi (B. dermatitidis, Coccidioides spp., Cryptococcus spp., Pneumocystis jirovecii, Talaromyces marneffei, and Candida glabrata) as well as protozoa (Leishmania spp., Trypanosoma cruzi, and Toxoplasma gondii) [12••, 69, 70, 71•]. Fine-needle aspiration biopsy (FNAB) is especially used to diagnose and differentiate granulomatous infections such as histoplasmosis from sarcoidosis and neoplasia when lung mass lesions or mediastinal adenopathy are observed on computed tomography imaging [72–74]. A retrospective study examining 500 lung biopsies and resections containing granulomas from seven countries showed that sarcoidosis (27%) and mycobacteria and fungal infections (25%) were the most commonly identified causes [75]. Moreover, fungi were mostly detected by histology, with histoplasmosis as the most frequent one and fungal infections being more common in the USA (19 versus 4%) than in other countries [75].
Gailey et al. in their study of histoplasmosis [58] and sarcoidosis (44) frequent bland necrosis, no or rare granulomas (< 2 per slide), foreign body-type giant cells, and yeasts (62% of cases) using GMS-stained material indicated the diagnosis of histoplasmosis. [72]. In PDH, the sensitivity of culture to identify the fungus is about 74%. Given the fact that cultures are not always performed and the difficulty to rule out other microorganisms, molecular methods can aid the diagnosis. A real-time PCR targeted to a 99-bp portion of the 100-kDa-specific protein gene showed a sensitivity of 88.9% and a specificity of 100% when tested on nine FFPE culture-positive specimens [76]. However, the handling and the long storage of the paraffin blocks with possible contamination with spores of environmental fungi limit the use of FFPE for the correct identification at the species level [77].
Mucormycosis
Mucormycosis (formerly zygomycosis) is an emerging mold infection especially in developing countries affecting immunocompromised patients including those with uncontrolled diabetes [78–80]. Rhizopus, Mucor, and Lichtheimia are the species responsible for around 80% of cases but other species (Rhizomucor, Cunninghamella, Actinomucor, etc.) may be implicated in causing disease [80]. Ribbon-like hyphae, with no or few septations, and right angle branching are considered the hallmark histopathologic characteristics of mucormycosis [12••]. Angioinvasion frequently associated with intravascular thrombosis and coagulative necrosis is observed in patients with malignancy and mucormycosis [81]. Although culture is considered the gold standard for correct species identification of Mucorales, the yield is low.
Differentiation of mucormycosis from aspergillosis and other hyaline septate molds is crucial due to the difference in response to antifungal drugs. The tissue morphology alone can be misleading [82]. Immunohistochemistry (IHC) on FFPE tissue showed 100 and 87% sensitivity in cases of proven mucormycosis and aspergillosis, respectively [82]. However, 25% of patients with a probable diagnosis of invasive pulmonary aspergillosis showed positive IHC for mucormycosis. A real-time qPCR targeting the ITS region of rDNA showed a 62% sensitivity in cases with histopathologic diagnosis of mucormycosis but in 11% of the specimens, the species identified were Aspergillus flavus [21•]. Another study using a semi-nested PCR for the diagnosis of mucormycosis and aspergillosis exhibited 79.3% sensitivity [83].
Pneumocystosis
Pneumocystis jirovecii is the human species of an atypical fungus that causes pneumonia and only rarely extrapulmonary disseminated disease among immunocompromised hosts [84]. Recognized since the beginning of the AIDS epidemic as one of the more frequent AIDS-defining diseases, pneumocystosis is increasingly diagnosed among kidney transplant patients, individuals with autoimmune diseases, and those receiving TNF-α [85]. Bronchoalveolar lavage fluid is the usual diagnostic specimen employed to make the diagnosis of pulmonary pneumocystosis by cytopathology because a culture method for this fungus is lacking. The organism can be overlooked with H&E and the presence of eosinophilic alveolar casts is considered very important to perform a GMS staining to highlight the characteristic thin-walled cysts (2–5 μm). Fluorescent staining with monoclonal antibodies increases the sensitivity in diagnosis but it is not always available [86]. In a recent study, the use of toluidine blue staining showed a sensitivity ranging from 71.4 to 85.7%, depending on the subset analyzed, but results were similar to histopathology [87] Pneumocystis PCR has been shown to be the most sensitive method with a fourfold increase of annual detections of pneumocystosis cases when compared with toluidine blue staining [87]. To differentiate colonization from true infection, Damiani et al. proposed the combination of a qPCR cut-off (1.6 × 103 and 2 × 104 copies/μL) on BAL and a BG serum threshold (100 pg/mL). [88]. In the morphologic differentiation of P. jirovecii and H. capsulatum with granulomatous reaction, in the absence of budding (H. capsulatum), the shape (spherical versus oval) and size of the intracystic bodies (capsular dot) are the best clue to avoid misdiagnosis [89].
Talaromycosis (Formerly Penicilliosis)
Talaromyces (formerly Penicillium) marneffei is a dimorphic fungus, endemic in Southeast Asia, Southern China, and Northeastern India. It is responsible for a disseminated disease among immunocompromised hosts (especially HIV-positive patients) including travelers [90–92]. Biopsies are usually obtained from skin, bone marrow, and lymph nodes and are helpful in making a rapid histopathologic diagnosis based on the characteristic yeast-like cells that divide by binary fission and show a typical transverse septum on GMS [93–95].
Autopsy Studies
Although the number of autopsies is declining worldwide, there is agreement that autopsy studies are of pivotal importance in the knowledge of the evolving epidemiology of IFIs as well as in identifying major discrepancies between intravitam clinical and post-mortem diagnoses [56, 96, 97, 98•]. Autopsy studies regarding IFIs performed in different categories of immunocompromised hosts have been recently reviewed by Dignani showing that during life, half of IFIs are missed and the median prevalence of IFIs at autopsy is 9% [98•]. However, several issues need to be addressed, namely the concomitant role of conventional or molecular microbiology in correctly identifying fungi at autopsy [98•, 99]. A study conducted in Thailand found an 89.5% concordance between histopathology and panfungal PCR with fusariosis misinterpreted as aspergillosis [100].
Conclusions
The identification of fungal pathogens by histopathology remains an important means to rapidly make a diagnosis of IFIs among immunocompromised patients. However, the pitfalls encountered in the correct identification of fungi at the species level are an important factor that can influence adequate treatment in the era of expanding antifungal armamentarium. The use of molecular methods (i.e., panfungal PCR, species-specific PCR, quantitative real-time PCR) is emerging as a useful approach to improve the diagnosis.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Clinical Pathology
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Antinori S, Ridolfo AL, Fasan M, Magni C, Galimberti L, Milazzo L, Sollima S, Adorni F, Giuliani G, Galli M, Corbellino M, Parravicini C. AIDS-associated cryptococcosis: a comparison of epidemiology, clinical features and outcome in the pre- and post-HAART eras. Experience of a single centre in Italy. HIV Med. 2009;10(1):6–11. doi: 10.1111/j.1468-1293.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- 2.Colombo AL, de Almeida Junior JN, Slavin MA, Chen SC, Sorrell TC. Candida and invasive mould diseases in non-neutropenic critically ill patients and patients with haematological cancer. Lancet Infect Dis. 2017;17(11):e344–e356. doi: 10.1016/S1473-3099(17)30304-3. [DOI] [PubMed] [Google Scholar]
- 3.Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signalling pathways. Clin Infect Dis. 2017;66(1):140–148. doi: 10.1093/cid/cix687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajurel K, Dhakal R, Deresinski S. Histoplasmosis in transplant recipients. Clin Transpl. 2017;31:10. doi: 10.1111/ctr.13087. [DOI] [PubMed] [Google Scholar]
- 5.Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev. 2014;27(3):490–526. doi: 10.1128/CMR.00091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidel D, Duran Graeff LA, Vehreschild MJ, Wisplinghoff H, Ziegler M, Vehreschild JJ, et al. FungiScope™: global emerging fungal infection registry. Mycoses. 2017;60(8):508–516. doi: 10.1111/myc.12631. [DOI] [PubMed] [Google Scholar]
- 7.Guarner J. Incorporating pathology in the practice of infectious disease: myths and reality. Clin Infect Dis. 2014;59(8):1133–1141. doi: 10.1093/cid/ciu469. [DOI] [PubMed] [Google Scholar]
- 8.Procop GW, Wilson M. Infectious disease pathology. Clin Infect Dis. 2001;32(11):1589–1601. doi: 10.1086/320537. [DOI] [PubMed] [Google Scholar]
- 9.Martinez RM. Genes in your tissue: probe identification and sequencing microbial targets from formalin-fixed, paraffin-embedded tissue. Clin Microbiol Newslett. 2014;36(18):139–147. doi: 10.1016/j.clinmicnews.2014.08.003. [DOI] [Google Scholar]
- 10.Maertens JA, Blennow O, Duarte RF, Munoz P. The current management landscape: aspergillosis. J Antimicrob Chemother. 2016;71(Suppl 2):ii23–ii29. doi: 10.1093/jac/dkw393. [DOI] [PubMed] [Google Scholar]
- 11.Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45–68. doi: 10.1183/13993003.00583-2015. [DOI] [PubMed] [Google Scholar]
- 12.Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Yun NR, Kim K-H, Jeon JH, Kim EC, Chung DH, Park WB, Oh MD. Discrepancy between histology and culture in filamentous fungal infections. Med Mycol. 2010;48(6):886–888. doi: 10.3109/13693780903512835. [DOI] [PubMed] [Google Scholar]
- 14.Shah AA, Hazen KC. Diagnostic accuracy of histopathologic and cytopathologic examination of Aspergillus species. Am J Clin Pathol. 2013;139(1):55–61. doi: 10.1309/AJCPO8VTSK3HRNUT. [DOI] [PubMed] [Google Scholar]
- 15.Sangoi AR, Rogers WM, Longacre TA, Montoya JG, Baron EJ, Banaei N. Challenges and pitfalls of morphologic identification of fungal infections in histologic and cytologic specimens. A ten-year retrospective review at a single institution. Am J Clin Pathol. 2009;131(3):364–375. doi: 10.1309/AJCP99OOOZSNISCZ. [DOI] [PubMed] [Google Scholar]
- 16.Dekio F, Bhatti TR, Zhang SX, Sullivan KV. Positive impact of fungal histopathology on immunocompromised pediatric patients with histology-proven invasive fungal infection. Am J Clin Pathol. 2015;144(1):61–67. doi: 10.1309/AJCPEMVYT88AVFKG. [DOI] [PubMed] [Google Scholar]
- 17.Lau A, Chen S, Sorrell T, Carter D, Malik R, Martin P, Halliday C. Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens. J Clin Microbiol. 2007;45(2):380–385. doi: 10.1128/JCM.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moncada PA, Budvytiene I, Ho DH, Deresinski SC, Montoya JG, Banaei N. Utility of DNA sequencing for direct identification of invasive fungi from fresh and formalin-fixed specimens. Am J Clin Pathol. 2013;140(2):203–208. doi: 10.1309/AJCPNSU2SDZD9WPW. [DOI] [PubMed] [Google Scholar]
- 19.Munoz-Cadavid C, Rudd S, Zaki SR, Patel M, Moser SA, Brandt ME, Gomez BL. Improving molecular detection of fungal DNA in formalin-fixed paraffin-embedded tissues: comparison of five tissue DNA extraction methods using panfungal PCR. J Clin Microbiol. 2010;48(6):2147–2153. doi: 10.1128/JCM.00459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliday CL, Kidd SE, Sorrell TC, Chen SC. Molecular diagnostic methods for invasive fungal disease: the horizon draws nearer? Pathology. 2015;47(3):257–269. doi: 10.1097/PAT.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 21.Salehi E, Hedayati MT, Zoll J, Rafati H, Ghasemi M, Doroudinia A, et al. Discrimination of aspergillosis, mucormycosis, fusariosis, and scedosporiosis in formalin-fixed paraffin-embedded tissue specimens by use of multiple real-time quantitative PCR assays. J Clin Microbiol. 2016;14:2798–2803. doi: 10.1128/JCM.01185-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhary A, Sharma C, Meis JF. Azole-resistant aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216(S3):S436–S444. doi: 10.1093/infdis/jix210. [DOI] [PubMed] [Google Scholar]
- 23.van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattson E, Debets-Ossenkopp YJ, et al. Clinical implications of azole resistance to Aspergillus fumigatus, the Netherlands, 2007–2009. Emerg Infect Dis. 2011;17(10):1846–1854. doi: 10.3201/eid1710.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard SJ, Cerar D, Anderson MJ, Albarraq A, Fischer MC, Pasqualotto AC, et al. Frequency and evolution of azole resistance in Aspergillus fumigatus associated treatment failure. Emerg Infect Dis. 2009;15(7):1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Linden JW, Snelders E, Arends JP, Daenen SM, Melchers WJ, Verweij PE. Rapid diagnosis of azole-resistant aspergillosis by direct PCR using tissue specimens. J Clin Microbiol. 2010;48(4):1478–1480. doi: 10.1128/JCM.02221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKinnell JA, Pappas GA. Blastomycosis: new insights into diagnosis, prevention and treatment. Clin Chest Med. 2009;30(2):227–239. doi: 10.1016/j.ccm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Pappas PG, Threlkeld MG, Bedsole GD, Cleveland KO, Gelfand MS, Dismukes WE. Blastomycosis in immunocompromised patients. Medicine. 1993;72(5):311–325. doi: 10.1097/00005792-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Taxy J. Blastomycosis: contributions of morphology to diagnosis. A surgical pathology, cytopathology and autopsy pathology study. Am J Surg Pathol. 2007;31(4):615–623. doi: 10.1097/01.pas.0000213389.47913.b8. [DOI] [PubMed] [Google Scholar]
- 29.Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34(2):256–261. doi: 10.1097/PAS.0b013e3181ca48a5. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y, Nagata N, Shimbo T, Nishijima T, Watanabe K, Aoki T, Sekine K, Okubo H, Watanabe K, Sakurai T, Yokoi C, Mimori A, Oka S, Uemura N, Akiyama J. Upper gastrointestinal symptoms predictive of Candida esophagitis and erosive esophagitis in HIV and non-HIV patients: an endoscopy based cross-sectional study of 6011 patients. Medicine. 2015;94(47):e2138. doi: 10.1097/MD.0000000000002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsomali MI, Arnold MA, Frankel WL, Graham RP, Hart PA, Lam-Himlin DM, Naini BV, Voltaggio L, Arnold CA. Challenges to “classic” esophageal candidiasis. Looks are usually deceiving. Am J Clin Pathol. 2017;147(1):33–42. doi: 10.1093/ajcp/aqw210. [DOI] [PubMed] [Google Scholar]
- 32.Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284–1292. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 33.Antinori S, Milazzo L, Sollima S, Galli M, Corbellino M. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Intern Med. 2016;34:21–28. doi: 10.1016/j.ejim.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Bassetti M, Garnacho-Montero J, Calandra T, Kullberg B, Dimopoulos G, Azoulay E, Chakrabarti A, Kett D, Leon C, Ostrosky-Zeichner L, Sanguinetti M, Timsit JF, Richardson MD, Shorr A, Cornely OA. Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intensive Care Med. 2017;43(9):1225–1238. doi: 10.1007/s00134-017-4731-2. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, Orbach MJ, Galgiani JN. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev. 2013;26(3):505–525. doi: 10.1128/CMR.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, Williams PL, Infectious Diseases Society of America Coccidioidomycosis. Clin Infect Dis. 2005;41(9):1217–1223. doi: 10.1086/496991. [DOI] [PubMed] [Google Scholar]
- 37.Saubolle MA. Laboratory aspects in the diagnosis of coccidioidomycosis. Ann N Y Acad Sci. 2007;1111:310–314. doi: 10.1196/annals.1406.049. [DOI] [PubMed] [Google Scholar]
- 38.Aly FZ, Millius R, Sobonya R, Aboul-Nasr K, Klein R. Cytologic diagnosis of coccidioidomycosis. Spectrum of findings in Southern Arizona patients over a 10 year period. Diagn Cytopathol. 2016;44(3):195–200. doi: 10.1002/dc.23419. [DOI] [PubMed] [Google Scholar]
- 39.Shekhel TA, Ricciotti RW, Blair JE, Colby TV, Sobonya RE, Larsen BT. Surgical pathology of pleural coccidioidomycosis: a clinicopathological study of 38 cases. Hum Pathol. 2014;45(5):961–969. doi: 10.1016/j.humpath.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Ricciotti RW, Shekhel TA, Blair JE, Colby TV, Sobonya RE, Larsen BT. Surgical pathology of skeletal coccidioidomycosis. A clinical and histopathologic analysis of 25 cases. Am J Surg Pathol. 2014;38(12):1672–1680. doi: 10.1097/PAS.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 41.Binnicker MJ, Buckwalter SP, Eisberner JJ, Stewart RA, McCullogh AE, Wohlfiel SL, et al. Detection of Coccidioides species in clinical specimens by real-time PCR. J Clin Microbiol. 2007;45(1):173–178. doi: 10.1128/JCM.01776-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montone KT, Litzky LA, Feldman MD, Peterman H, Mathis B, Baliff J, Kaiser LR, Kucharczuk J, Nachamkin I. In situ hybridization for Coccidioides immitis 5.8S ribosomal RNA sequences in formalin-fixed, paraffin-embedded pulmonary specimens using a locked nucleic acid probe. A rapid means for identification in tissue section. Diagn Mol Pathol. 2010;19(2):99–104. doi: 10.1097/PDM.0b013e3181b3aa55. [DOI] [PubMed] [Google Scholar]
- 43.Park BJ, Wannemuehler KA, Maraton BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 44.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17(8):873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol. 2016;14(2):106–117. doi: 10.1038/nrmicro.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol. 2015;78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, et al. The case for adopting the “species complex” nomenclature fort the etiologic agents of cryptococcosis. mSphere. 2017;2:e00357–e00316. doi: 10.1128/mSphere.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagen F, Lumbsch HT, Arsic Arsenijevic V, Badali H, Bertout S, Billmyre RB, et al. Importance of resolving fungal nomenclature: the case of multiple pathogenic species in the Cryptococcus genus. mSphere. 2017;2:e00238–e00217. doi: 10.1128/mSphere.00238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antinori S, Radice A, Galimberti L, Magni C, Fasan M, Parravicini C. The role of cryptococcal antigen assay in diagnosis and monitoring of cryptococcal meningitis. J Clin Microbiol. 2005;43(11):5828–5829. doi: 10.1128/JCM.43.11.5828-5829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang H, Fan L, Rajbanshi B, Xu JF. Evaluation of a new cryptococcal antigen lateral flow immunoassay in serum, cerebrospinal fluid and urine for the diagnosis of cryptococcosis: a meta-analysis and systematic review. PLoS One. 2015;10(5):e0127117. doi: 10.1371/journal.pone.0127117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chastain DB, Guarner J, Franco-Paredes C. Cryptococcal antigen negative meningoencephalitis in HIV/AIDS. Diagn Microbiol Infect Dis. 2017;89(2):143–145. doi: 10.1016/j.diagmicrobio.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Ranjan R, Jain D, Singh L, Iyer VK, Sharma MC, Mathur SR. Differentiation of histoplasma and cryptococcus in cytology smears: a diagnostic dilemma in severely necrotic cases. Cytopathology. 2015;26(4):244–249. doi: 10.1111/cyt.12180. [DOI] [PubMed] [Google Scholar]
- 53.Rajeshwari M, Xess I, Sharma MC, Jain D. Acid-fastness of Histoplasma in surgical pathology practice. J Pathol Transl Med. 2017;51(5):482–487. doi: 10.4132/jptm.2017.07.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antinori S, Galimberti L, Magni C, Casella A, Vago L, Mainini F, Piazza M, Nebuloni M, Fasan M, Bonaccorso C, Vigevani G, Cargnel A, Moroni M, Ridolfo A. Cryptococcus neoformans infection in a cohort of Italian AIDS patients: natural history, early prognostic parameters, and autopsy findings. Eur J Clin Microbiol Infect Dis. 2001;20(10):711–717. doi: 10.1007/s100960100616. [DOI] [PubMed] [Google Scholar]
- 55.Torres RG, Etchebehere RM, Adad SJ, Micheletti AR, Ribeiro BM, Silva LE, et al. Cryptococcosis in acquired immunodeficiency syndrome patients clinically confirmed and/or diagnosed at necropsy in a teaching hospital in Brazil. Am J Trop Med Hyg. 2016;95(4):781–785. doi: 10.4269/ajtmh.16-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antinori S, Nebuloni M, Magni C, Fasan M, Adorni F, Viola A, Corbellino M, Galli M, Vago G, Parravicini C, Ridolfo a. Trends in the postmortem diagnosis of opportunistic invasive fungal infections in patients with AIDS: a retrospective study of 1,630 autopsies performed between 1984 and 2002. Am J Clin Pathol. 2009;132(2):221–227. doi: 10.1309/AJCPRAAE8LZ7DTNE. [DOI] [PubMed] [Google Scholar]
- 57.Gori S, Drouhet E, Gueho E, Huerre M, Lofaro A, et al. Cutaneous disseminated mycosis in a patient with AIDS due to a new dimorphic fungus. J Mycol Med. 1998;8:57–63. [Google Scholar]
- 58.Schwartz IS, Kenyon C, Feng P, Govender NP, Dukik K, Sigler L, Jiang Y, Stielow JB, Muñoz JF, Cuomo CA, Botha A, Stchigel AM, de Hoog GS. 50 years of Emmonsia disease in humans: the dramatic emergence of a cluster of novel fungal pathogens. PLoS Pathog. 2015;11(11):e1005198. doi: 10.1371/journal.ppat.1005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kenyon C, Bonorchis K, Corcoran C, Meintjes G, Locketz M, Lehloenya R, Vismer HF, Naicker P, Prozesky H, van Wyk M, Bamford C, du Plooy M, Imrie G, Dlamini S, Borman AM, Colebunders R, Yansouni CP, Mendelson M, Govender NP. A dimorphic fungus causing disseminated infection in South Africa. N Engl J Med. 2013;369(15):1416–1424. doi: 10.1056/NEJMoa1215460. [DOI] [PubMed] [Google Scholar]
- 60.Dukik K, Munoz JF, Jiang Y, Feng P, Sigler L, Stielow JB, et al. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales) Mycoses. 2017;60(5):296–309. doi: 10.1111/myc.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang O, Kenyon C, De HG, Guo L, Fan H, Liu H, et al. A novel dimorphic pathogen, Emergomyces orientalis (Onygenales) , agent of disseminated infection. Mycoses. 2017;60(5):310–319. doi: 10.1111/myc.12583. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz IS, Govender NP, Corcoran C, Dlamini S, Prozesky H, Burton R, Mendelson M, Taljaard J, Lehloenya R, Calligaro G, Colebunders R, Kenyon C. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis. 2015;61(6):1004–1012. doi: 10.1093/cid/civ439. [DOI] [PubMed] [Google Scholar]
- 63.Bahr N, Antinori S, Wheat L, Sarosi G. Histoplasmosis infections worldwide: thinking outside of the Ohio River valley. Curr Trop Med Rep. 2015;2(2):70–80. doi: 10.1007/s40475-015-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antinori S. Histoplasma capsulatum: more widespread than previously thought. Am J Trop Med Hyg. 2014;90(6):982–983. doi: 10.4269/ajtmh.14-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wheat LJ, Azar MM, Bahr N, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin N Am. 2016;30(1):207–227. doi: 10.1016/j.idc.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Hage CA, Azar MM, Bahr N, Loyd J, Wheat LJ. Histoplasmosis: up-to-date evidence-based approach to diagnosis and management. Semin Respir Crit Care Med. 2015;36:738–754. doi: 10.1055/s-0035-1562899. [DOI] [PubMed] [Google Scholar]
- 67.Vergidis P, Avery RK, Wheat LJ, Dotson JL, Assi MA, Antoun SA, Hamoud KA, Burdette SD, Freifeld AG, McKinsey DS, Money ME, Myint T, Andes DR, Hoey CA, Kaul DA, Dickter JK, Liebers DE, Miller RA, Muth WE, Prakash V, Steiner FT, Walker RC, Hage CA. Histoplasmosis complicating tumor necrosis factor-alpha blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis. 2015;61(3):409–417. doi: 10.1093/cid/civ299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guarner J. Human immunodeficiency virus and fungal infections. Sem Diagn Pathol. 2017;34:325–331. doi: 10.1053/j.semdp.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Gupta N, Arora S, Rajwanshi A, Nijhawan R, Srinivasan R. Histoplasmosis: cytodiagnosis and review of literature with special emphasis on differential diagnosis on cytomorphology. Cytopathology. 2010;21(4):240–244. doi: 10.1111/j.1365-2303.2009.00693.x. [DOI] [PubMed] [Google Scholar]
- 70.Sharma S, Gupta P, Gupta N, Lai A, Behera D, Rajwanshi A. Pulmonary infections in immunocompromised patients: the role of image-guided fine needle aspiration cytology. Cytopathology. 2017;28(1):46–54. doi: 10.1111/cyt.12359. [DOI] [PubMed] [Google Scholar]
- 71.Azar MM, Hage CH. Laboratory diagnostics for histoplasmosis. J Clin Microbiol. 2017;55:1612–1620. doi: 10.1128/JCM.02430-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gailey MP, Keeney ME, Jensen CS. A cytomorphometric analysis of pulmonary and mediastinal granulomas: differentiating histoplasmosis from sarcoidosis by fine-needle aspiration. Cancer Cytopathol. 2015;123(1):51–58. doi: 10.1002/cncy.21491. [DOI] [PubMed] [Google Scholar]
- 73.Field AS, Geddie WR. Role of fine needle aspiration biopsy cytology in the diagnosis of infection. Diagn Cytopathol. 2016;44(12):1024–1038. doi: 10.1002/dc.23568. [DOI] [PubMed] [Google Scholar]
- 74.Mukhopadhyay S, Gal AA. Granulomatous lung disease. An approach to the differential diagnosis. Arch Pathol Lab Med. 2010;134(5):667–690. doi: 10.5858/134.5.667. [DOI] [PubMed] [Google Scholar]
- 75.Mukhopadhyay S, Farver CF, Vaszar LT, Dempsey OJ, Popper HH, Mani H, Capelozzi VL, Fukuoka J, Kerr KM, Zeren EH, Iyer VK, Tanaka T, Narde I, Nomikos A, Gumurdulu D, Arava S, Zander DS, Tazelaar HD. Causes of pulmonary granulomas: a retrospective study of 500 cases from seven countries. J Clin Pathol. 2012;65(1):51–57. doi: 10.1136/jclinpath-2011-200336. [DOI] [PubMed] [Google Scholar]
- 76.Koepsell SA, Hinrichs SH, Iwen PC. Applying a real-time PCR assay for Histoplasma capsulatum to clinically relevant formalin-fixed paraffin-embedded human tissue. J Clin Microbiol. 2012;50(10):3395–3397. doi: 10.1128/JCM.01705-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frickmann H, Loderstaedt U, Racz P, Tenner-Racz K, Eggert P, Haeupler A, Bialek R, Hagen RM. Detection of tropical fungi in formalin-fixed, paraffin-embedded tissue: still an indication for microscopy in times of sequence-based diagnosis? BioMed Res Intern. 2015;2015:938721. doi: 10.1155/2015/938721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwon-Chung KJ. Taxonomy of fungi causing Mucormycosis and Entomophthoramycosis (Zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis. 2012;54(S1):S8–15. doi: 10.1093/cid/cir864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meis JF, Chakrabarti A. Changing epidemiology of an emerging infection: zygomycosis. Clin Microbiol Infect. 2009;15(Suppl.5):10–14. doi: 10.1111/j.1469-0691.2009.02973.x. [DOI] [PubMed] [Google Scholar]
- 80.Gomez MZ, Lewis RE, Kontoyannis DP. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtemia species. Clin Microbiol Rev. 2011;24(2):411–445. doi: 10.1128/CMR.00056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ben-Ami R, Luna M, Lewis RE, Walsh TJ, Kontoyannis DP. A clinicopathological study of pulmonary mucormycosis in cancer patients: extensive angioinvasion but limited inflammatory response. J Infect. 2009;59:134–138. doi: 10.1016/j.jinf.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jung J, Park YS, Sung H, Song JS, Lee SO, Choi SH, Kim YS, Woo JH, Kim SH. Using immunohistochemistry to assess the accuracy of histomorphologic diagnosis of aspergillosis and mucormycosis. Clin Infect Dis. 2015;61(11):1664–1670. doi: 10.1093/cid/civ660. [DOI] [PubMed] [Google Scholar]
- 83.Drogari-Apiranthitou M, Panayiotides I, Galani I, Konstantoudakis S, Arvanitidis G, Spathis A, Gouloumi AR, Tsakiraki Z, Tsiodras S, Petrikkos G. Diagnostic value of a semi-nested PCR for the diagnosis of mucormycosis and aspergillosis from paraffin-embedded tissue: a single-center experience. Pathol Res Prac. 2016;212(5):393–397. doi: 10.1016/j.prp.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 84.Ng V, Yajko D, Hadley W. Extrapulmonary pneumocystosis. Clin Microbiol Rev. 1997;10(3):401–418. doi: 10.1128/cmr.10.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fillatre P, Decaux O, Jouneau S, Revest M, Gacouin A, Robert-Gangneux F, et al. Incidence of Pneumocystis jiroveci pneumonia among groups at risk in HIV-negative patients. Am J Med. 2014;127:1241.e11–7. 10.1016/j.amjmed.2014.07.010. [DOI] [PubMed]
- 86.Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 87.Doyle L, Vogel S, Procop GW. Pneumocystis PCR: it is time to make PCR the test of choice. Open Forum Infect Dis. 2017;4(4):ofx193. doi: 10.1093/ofid/ofx193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Damiani C, Le Gal S, Da Costa C, Virmaux M, Nevez G, Totet A. Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1→3)-β-D-glucan for differential diagnosis of Pneumocystis pneumonia and Pneumocystis colonization. J Clin Microbiol. 2013;51(10):3380–3388. doi: 10.1128/JCM.01554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hartel PH, Shilo K, Klassen-Fischer M, Neafie RC, Ozbudak IH, Galvin JR, Franks TJ. Granulomatous reaction to Pneumocystis jirovecii. Clinicopathologic review of 20 cases. Am J Surg Pathol. 2010;34(5):730–734. doi: 10.1097/PAS.0b013e3181d9f16a. [DOI] [PubMed] [Google Scholar]
- 90.Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017;17(11):e334–e343. doi: 10.1016/S1473-3099(17)30303-1. [DOI] [PubMed] [Google Scholar]
- 91.Chan JF, Chan TS, Gill H, Lam FY, Trendell-Smith NJ, Sridhar S, et al. Disseminated infections with Talaromyces marneffei in non-AIDS patients given monoclonal antibodies against CD20 and kinase inhibitors. Emerg Infect Dis. 2015;21(7):1101–1106. doi: 10.3201/eid2107.150138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Antinori S, Gianelli E, Bonaccorso C, Ridolfo AL, Croce F, Sollima S, Parravicini C. Disseminated Penicillium marneffei infection in an HIV-positive Italian patient and a review of cases reported outside endemic regions. J Travel Med. 2006;13(3):181–188. doi: 10.1111/j.1708-8305.2006.00039.x. [DOI] [PubMed] [Google Scholar]
- 93.Wong KF. Marrow penicilliosis: a readily missed diagnosis. Am J Clin Pathol. 2010;134(2):214–218. doi: 10.1309/AJCPWVBQCW13DJLO. [DOI] [PubMed] [Google Scholar]
- 94.Qiu Y, Liao H, Zhang J, Zhong X, Tan C, Lu D. Differences in clinical characteristics and prognosis of Penicilliosis among HIV-negative patients with or without underlying disease in Southern China: a retrospective study. BMC Infect Dis. 2015;15(1):525. doi: 10.1186/s12879-015-1243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lim D, Lee YS, Chang AR. Rapid diagnosis of Penicillium marneffei infection by fine needle aspiration cytology. J Clin Pathol. 2006;59(4):443–444. doi: 10.1136/jcp.2004.024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alsharif M, Cameron SE, Young JA, Savik K, Henriksen JC, Gulbahce HE, et al. Time trends in fungal infections as a cause of death in hematopoietic stem cell transplant recipients. Am J Clin Pathol. 2009;132(5):746–755. doi: 10.1309/AJCPV9DC4HGPANKR. [DOI] [PubMed] [Google Scholar]
- 97.Colombo TE, Nunes Soares MM, D’Avilla SC, Nogueira MC, de Almeida MT. Identification of fungal diseases at necropsy. Pathol Res Pract. 2012;208(9):549–552. doi: 10.1016/j.prp.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 98.Dignani MC. Epidemiology of invasive fungal diseases on the basis of autopsy reports. F1000Prime Rep. 2014;6:81. doi: 10.12703/P6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rickerts V. Identification of fungal pathogens in formalin-fixed, paraffin-embedded tissue samples by molecular methods. Fungal Biol. 2016;120(2):279–287. doi: 10.1016/j.funbio.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 100.Ruangritchankul K, Chindamporn A, Worasilchai N, Poumsuk U, Keelawat S, Bychlov A. Invasive fungal disease in university hospital: a PCR-based study of autopsy cases. Int J Exp Pathol. 2015;8:14840–14852. [PMC free article] [PubMed] [Google Scholar]


