Abstract
Infections with geographically constrained dimorphic fungi cause the endemic mycoses, which include blastomycosis, coccidioidomycosis, emmonsiosis, histoplasmosis, paracoccidioidomycosis, sporotrichosis, and penicilliosis. In the last 5 years, our understanding of the epidemiology, diagnostics, and to a lesser extent management of these diseases has advanced. Specifically, the application of molecular techniques for genotyping fungal pathogens has resulted in the recognition of cryptic species within several genera, including Blastomyces, and Paracoccidioides; the reclassification of Penicillium marneffei, the agent of penicilliosis, to the genus Talaromyces; and the global emergence of dimorphic fungi of the genus Emmonsia, cause disease in immunocompromised persons. New and refined diagnostic tests are available based on the detection of circulating antigens and antibodies, mass spectrometry, and targeted gene amplification. In contrast, the development of new therapeutic options remains stalled, although isavuconazole may hold promise. Finally, advances have been made in the prospect of viable vaccines for preventing animal and human disease.
Keywords: Blastomycosis, Coccidioidomycosis, Emmonsiosis, Histoplasmosis, Paracoccidioidomycosis, Sporotrichosis, Penicilliosis, Talaromyces
Introduction
The endemic mycoses are caused by a diverse group of fungal pathogens. These agents share several characteristics including life cycles that include environmental saprophytic mold phases, and parasitic yeast-like phases; their ability, in most cases, to cause disease in immunocompetent hosts, while serving as a significant source of morbidity and mortality in immunocompromised patients; and the prerequisite of residence in or travel to a specific environmental location/niche for exposure and subsequent development of disease [1].
The last 5 years have led to refinements in our understanding of the epidemiology of these infections. Recognition of infection in regions outside the traditional regions of endemicity has recently occurred, and work is ongoing to further delineate the true geographic range of these fungal organisms. Additionally, analysis of organisms using molecular typing and whole genome sequencing has improved our understanding of genetic diversity, clarified phylogenetic relationships, and identified novel species.
Advances in diagnostic testing have also occurred with improved serologic techniques, the identification of fungal antigens useful for both diagnostic and prognostic purposes, and molecular amplification all now commercially available for a number of infections. Treatment advances have lagged behind, with few new options available. However, the development of the new broad-spectrum triazole isavuconazole provides an alternative agent to those previously available.
Blastomycosis
Epidemiology
Recent studies have advanced our understanding of the genetic diversity of the agents of blastomycosis. Meece et al. analyzed 27 polymorphic microsatellite markers of 112 clinical, veterinary, and environmental Blastomyces isolates, mostly from Wisconsin. They demonstrated that isolates could be placed in either of two distinct genetic groups [2]. Later, Brown et al. compared 7 nuclear loci among 78 clinical and environmental Blastomyces isolates representing a wider geographic range [3]. These authors confirmed distinct genetic groups among Blastomyces dermatitidis. Furthermore, they invoked molecular taxonomic criteria to suggest that one of these genetic groups represented a cryptic species, which they named Blastomyces gilchristii. Furthermore, their analysis suggested that B. gilchristii was the predominant species in northwestern Ontario and Wisconsin [3], both areas of hyperendemicity [4, 5]. The ecologic and clinical implications of this genetic distinction are currently unknown and merit further study to assess clinical, virulence, and treatment differences.
Diagnostics
An antigen enzyme immunoassay (EIA) developed for the diagnosis of blastomycosis is available, with a reported sensitivity of 89 %, but with high rates of cross-reactivity with histoplasmosis [6]. An antibody EIA to the surface protein and critical virulence factor BAD-1 was reported to be more specific (99 % vs controls without fungal infections and 94 % vs controls with histoplasmosis) and had comparative sensitivity to the antigen test (approximately 88 %) [7]. The role for these tests in clinical practice is yet to be demonstrated, but at best they may augment clinical, histopathological, and microbiological diagnostic approaches.
Therapy
The mainstay of treatment of severe blastomycosis remains lipid formulations of amphotericin B, followed by a prolonged course of triazole therapy [8, 9]. In most circumstances, itraconazole remains the preferred agent for continuation therapy, although voriconazole achieves good concentrations in the central nervous system and may have a role in the treatment of patients with central nervous system blastomycosis [10, 11]. For mild or moderate disease, itraconazole alone—given for 6 to 12 months—is usually sufficient [8].
Outcomes in acute respiratory distress syndrome (ARDS), the most feared complication of blastomycosis, remain unsatisfactory. Extracorporeal membrane oxygenation has been used successfully in extreme cases [12]. Given the overwhelming systemic inflammation associated with ARDS, optimism has been expressed for a role for corticosteroids in ARDS caused by blastomycosis, bolstered by anecdotes of success [13, 14]. More data is required before this approach can be recommended [8].
Prevention
One preventative strategy against blastomycosis that may hold promise is the development of a vaccine [15]. Th17 cells appear indispensable for vaccine-mediated immunity, and protection appears to be mediated by the adaptor molecule MyD88 [16•]. A live-attenuated vaccine lacking BAD-1 proved safe and immunogenic in dogs [17]. For now, a commercially available vaccine to prevent blastomycosis in animals or humans remains a distant, if hopeful, prospect.
Coccidioidomycosis
Epidemiology
Coccidioides immitis and Coccidioides posadasii, the etiologic agents of coccidioidomycosis, are endemic throughout the western United States, Mexico, and some areas of central and South America [18]. Recent work has focused on further elucidating the “true” range of Coccidioides, and soil samples from Washington and Utah have both been found to harbor this fungal species [19]. Genetic characterization of samples from these samples using molecular clocks suggests Coccidioides has been present in these locations for a significant amount of time rather than being newly imported [20]. The spread of the organism to these locations may be secondary to the ability of Coccidioides endospores to survive for long periods of time following aerosolization. In contrast, recent analysis of the Coccidioides genome has suggested these organisms are not soil saprophytes but have instead evolved to remain associated with their dead animal hosts in the soil—findings suggesting a possible rodent reservoir—although this hypothesis has not yet been definitely proven [21].
Phylogenetic inference and population genomic analysis of multiple Coccidioides genomes has identified distinct geographic clades in Tucson and Phoenix Arizona, with California and the Baja Peninsula representing a second clade, and Texas, Central and South America a third. These findings suggest C. posadasii is the more ancient of the two species with a southern Arizona origin and subsequent spread both north and south out of this region [22•].
Diagnostics
Despite the prevalence of coccidioidomycosis within the endemic region, the disease often remains undiagnosed or is subject to substantial delay (mean time from symptom onset to diagnosis, 5 months) [23]. Within the endemic region, close to 30 % of all community acquired pneumonia (CAP) is caused by Coccidioides [24]; however, no clear recommendations have been offered by the Infectious Diseases Society of America (IDSA) or American Thoracic Society (ATS) regarding uniform testing of all patients at risk [25]. Patients with pneumonia accompanied by a rash or a peripheral eosinophilia are more likely to have coccidioidomycosis than a bacterial etiology and these can be helpful clues [26].
Serologic methods are the mainstay of diagnosis with EIA, immunodiffusion (ID), and complement fixation (CF) frequently used. The sensitivity and specificity of each test differs and EIA testing is routinely used to “screen” those with possible coccidioidomycosis, while positive tests are confirmed with a more specific test such as ID or CF. CF testing provides a quantitative result and is useful for following patients over time. Antigen testing is now commercially available but has a limited role in the immunocompetent patient although may be useful in those with profound immunosuppression without the ability to manifest a serologic response to infection [27, 28]. Polymerase chain reaction (PCR) testing has limited usefulness in clinical diagnostics [29].
More recently, a lateral flow assay (LFA) has been developed with comparable sensitivity and specificity to other serologic methods. This test is similar in methodology to that of the cryptococcal antigen test now in wide-scale clinical use. A LFA for coccidioidomycosis allows for point-of-care diagnosis and would avoid the delay in a “send out” test in the initial evaluation of those with possible coccidioidomycosis.
Skin testing using coccidioidal antigen has returned to the market in a new formulation (Spherusol) [30]. Skin testing is useful primarily for epidemiologic surveys or to document those with existing immunity to infection although false negatives appear common (CA prison system, unpublished data). Whether a “booster effect” is present and repeat testing is necessary in those with a negative test remains unclear.
Therapy
The decision to treat primary pulmonary coccidioidomycosis remains an ongoing debate among experts in the field due to the lack of data definitively demonstrating treatment alters or shortens the course of disease [21]. When treatment is indicated, the majority of patients are treated with fluconazole for 3–6 months. Itraconazole has been proven superior for those with skeletal disease. Severe disease necessitates an amphotericin B formulation [25].
In an attempt to answer the question regarding the benefit of antifungals in those with mild-moderate pulmonary disease, the National Institutes of Health has recently sponsored a prospective double-blind study. Patients presenting with respiratory symptoms compatible with coccidioidomycosis will be randomized to CAP treatment with or without fluconazole. The primary outcome will be symptomatic improvement at day 42. Enrollment is currently underway (ClinicalTrials.gov, NCT02663674).
Emmonsiosis
Epidemiology
The global epidemiology of thermally dimorphic agents of disseminated emmonsiosis was recently reviewed [31•]. This polyphyletic group of pathogens includes at least seven separate taxa, among which only one species (Emmonsia pasteuriana) has been named [31•]. In addition, there are two other members of the genus, Emmonsia parva and Emmonsia crescens; these do not cause disseminated disease, but rather cause a distinct clinical syndrome called adiaspiromycosis. This granulomatous lung disease is caused by the host response to so-called adiaspores, enormous, sessile cells that follow transformation from inhaled conidia [32]. Infection with dimorphic Emmonsia spp. is also presumed to follow inhalation of conidia; however, instead of forming adiaspores, the conidia transform to yeast-like cells capable of replication, extra-pulmonary dissemination, and disease [31•]. The taxonomic arrangement of the group is currently in flux, complicated by the fact that the type species, E. parva, appears more related to Blastomyces spp. than to E. crescens [33].
The first thermally dimorphic Emmonsia species to be recognized was E. pasteuriana, isolated in 1994 from a human immunodeficiency virus (HIV)-infected Italian woman with a disseminated mycosis [34]. Since then, cases have been reported in immunocompromised patients from Spain [35], China [36, 37], and India [38].
On the other hand, the highest burden of emmonsiosis appears to be in South Africa, where a novel Emmonsia species was recently recognized as the cause of an acquired immunodeficiency syndrome (AIDS)-related mycosis [39]. Between 2008 (when molecular techniques were introduced to augment phenotypic identification of dimorphic fungi in South Africa) and 2015, 54 cases were diagnosed [40], although this likely represents the “tip of the iceberg.” To date, all cases have occurred in profoundly immunocompromised individuals. Earlier reports [40, 41] of disease in immunocompetent patients were, in fact, due to a second novel species of dimorphic fungus endemic to South Africa that appears to be more closely related to Blastomyces spp. [31•].
The true geographic range of these organisms is currently unknown, although cases have been reported sporadically from North America, Europe, and Asia [31•]. Our understanding of the range within sub-Saharan Africa is hampered, in part, by limited capacity for diagnostic mycology in many countries.
Clinical Features
The clinical presentation of emmonsiosis is similar to progressive disseminated histoplasmosis or penicilliosis in immunocompromised hosts [39, 40]. Cutaneous disease is most common, occurring in more than 95 % of patients at time of diagnosis [40]. Skin lesions usually include widespread papules and/or plaques, although other morphologies can also occur. The infection frequently becomes apparent as part of an unmasking immune reconstitution inflammatory syndrome (IRIS), with cutaneous lesions appearing weeks or months after initiating antiretroviral therapy (ART). In retrospect, ART was usually started—along with empiric anti-tuberculosis therapy—in response to constitution and pulmonary symptoms that likely represented protean manifestations of the disease [40]. In addition to cutaneous and lung disease, virtually any body system may become involved, including hepatic, reticuloendothelial, and gastrointestinal systems.
Diagnostics
Biopsy of affected tissue for histopathology is the most expedient way to diagnose a deep fungal infection, although features are identical to those of other dimorphic mycoses including histoplasmosis and sporotrichosis [40]. Culture of skin biopsy tissue on standard fungal media is usually diagnostic, although growth may take up to 4 weeks of incubation. The sensitivity of blood cultures is enhanced by use of mycobacterial/fungal blood culture bottles. Bone marrow cultures may also yield the diagnosis.
Chest radiographs are frequently abnormal, even when respiratory symptoms are not part of the presenting complaint. Findings are nonspecific, and pulmonary disease can easily be mistaken for bacterial or pneumocystis pneumonia, tuberculosis, or Kaposi’s sarcoma [40].
Therapy
No prospective studies have evaluated treatment strategies, and in the absence of more definitive data, the authors recommend following the IDSA guidelines for other endemic mycoses in immunocompromised hosts [8, 42]. Amphotericin B appears to be important [40], followed by suppressive itraconazole therapy until immune reconstitution. Optimal timing of ART initiation remains unknown, and clinicians should be aware of the potential for clinical worsening due to IRIS.
Histoplasmosis
Epidemiology
Histoplasma capsulatum, the dimorphic fungal pathogen that causes histoplasmosis, is widely distributed [43]. In North America, histoplasmosis is the most common endemic mycosis, with areas of known endemicity including regions along the Mississippi, Ohio, and St. Lawrence River Valleys [43]. However, autochthonous cases have been reported from outside areas of known endemicity, including Idaho, Montana, California, and Florida [44]. The geographic range of H. capsulatum extends through most of Latin America [43]. In fact, in French Guiana, histoplasmosis is the most common AIDS-related opportunistic infection [45]. Foci of endemicity also exist in sub-Saharan Africa and Asia [46].
Phylogenetic analyses of human clinical isolates have identified eight clades into which H. capsulatum strains may be grouped [47]. Interestingly, the African clade includes strains from all three varieties of H. capsulatum (var. capsulatum, the typical agent of histoplasmosis; var. duboisii—which has larger cells and causes primarily cutaneous and bone disease in limited parts of sub-Saharan Africa, sometimes called “African histoplasmosis” [46]; and var. farciminosum—the cause of epizootic lymphangitis in horses) suggesting the distinction of these varieties to be phylogenetically meaningless [47]. More recently, a ninth phylogenetic clade was identified by multilocus sequencing of veterinary isolates from cats in non-endemic areas, though no human cases with these pathogens have been reported [48]. There is data to suggest that genetic, structural, and pathogenic differences exist across clades [49].
Upon inhalation of H. capsulatum conidia into the lung, conversion to yeast forms and infection of macrophages enable immune evasion and dissemination until T cell-dependent activation of the macrophages contains the infection. In immunocompetent hosts, infection is frequently subclinical [50]. Alternatively, in patients with impaired cell-mediated immunity, infection may result in progressive disseminated histoplasmosis (PDH). Predisposing conditions most commonly include HIV (with risk a function of CD4+ lymphocyte count) [51], solid organ or bone marrow transplant recipients [52], and persons being treated with biological therapies, most notably TNF-α blocking agents [53]. Other disease forms include sub-acute histoplasmosis, chronic pulmonary or disseminated histoplasmosis, mediastinal granuloma, and fibrosing mediastinitis [50].
Diagnostics
While culture and histopathology remain mainstays in the diagnosis of histoplasmosis, antigen detection represents an important diagnostic tool. In the USA, a third generation, quantitative EIA using polyclonal antibodies against Histoplasma polysaccharide is available through MiraVista Diagnostics (MVista, Indianapolis, IN) [54]. This test has been well studied and validated in acute pulmonary histoplasmosis (with sensitivity of approximately 83 % when both urine and serum were tested, but substantially lower when testing was limited to one specimen) [55] and disseminated histoplasmosis (sensitivity >90 % for antigenuria alone) [56]. Serial measurements can also be useful for monitoring response to therapy. The MVista antigen test is limited by the lack of commercial availability of reagents, precluding testing outside of MiraVista Diagnostics’ reference laboratory. In addition to delaying turnaround times, this effectively places the test beyond reach of those outside the USA.
Recently, the Centers for Disease Control and Prevention developed an antigen capture enzyme-linked immunosorbent assay. The sensitivity and specificity of antigenuria were 81 and 95 %, respectively, among HIV-infected Guatemalan patients with PDH [57]; similar results have been found in Colombia. The availability of this test is currently limited [58].
In 2007, the Food and Drug Agency approved a polyclonal antibody-based in vitro antigen test offered commercially by ImmunoMycologics (IMMY, Norman, OK). This test was limited by poor sensitivity [59]. Subsequently, these manufacturers developed a monoclonal antibody-based test of H. capsulatum galactomannan analyte-specific reagents. This test exhibited improved performance, with a sensitivity ranging from 72–90 % (and up to 100 % in patients with PDH) and a specificity of 96–98 % [59, 60].
Molecular diagnostic techniques such as real-time PCR have shown promise for diagnosing histoplasmosis from tissue [61] and, to a lesser extent, serum [62] and urine [63]. While they have the potential to improve turnaround times compared to culture, the sensitivity from non-sterile sites remains suboptimal. Furthermore, these tests lack commercial availability that could make them widely available outside reference centers [58].
Therapy
The IDSA [42] and ATS [9] published relevant clinical management guidelines in 2007 and 2011, respectively. Both recommend liposomal amphotericin B followed by a prolonged course with a triazole (preferably itraconazole) for severe disease and immunocompromised hosts [9, 42].
The value of the continuation phase was revisited in a recent retrospective study that evaluated 97 HIV-infected persons with histoplasmosis who were on effective ART and who subsequently discontinued triazole suppressive therapy [64]. Relapse occurred in none of the 38 patients in whom discontinuation was physician-initiated, versus 21 of 59 (36 %) of persons in whom discontinuation was patient-initiated. The fact that only ¾ of persons who discontinued triazoles at advice of their physician received at least 12 months of suppressive therapy, as per IDSA guidelines, suggests that some patients may suffice with less [64].
Paracoccidioidomycosis
Epidemiology
The exact range of Paracoccidioides spp. remains unknown, although the region of endemicity extends from Mexico to Argentina and seems to spare certain countries (notably Chile, Suriname, the Guyanas, Nicaragua, Belize, and most of the Caribbean islands). Within countries where the disease is endemic, it is diagnosed only in areas with tropical and subtropical forest, abundant watercourses, mild temperatures, and high rainfall. The greatest numbers of reported cases have come from Brazil, Colombia, and Venezuela [65].
Phylogenetic analysis of Paracoccidioides brasiliensis has shown that this fungus can be divided into at least three species that appear to be confined to distinct regions [66]. These species have not been formally named, although a proposal has been made to name the highly divergent “Pb01-like” group “P. lutzii” [67, 68] and genomic comparison has indicated P. brasiliensis is related to the uncultivable pathogen Lacazia loboi [69].
Diagnostics
The most popular serologic methods for diagnosis of paracoccidioidomycosis are ID and CF. The ID test demonstrates circulating antibodies in over 90 % of cases. The CF test allows a more precise evaluation of the patient’s response to treatment but cross-reactions with H. capsulatum antigens can occur. At present, paracoccidioidomycosis antigen testing is not available as a routine diagnostic test although several reports have described the detection of P. brasiliensis antigen in urine, cerebrospinal fluid, and bronchoalveolar fluid samples [70]. Others have noted that antigen levels in serum diminish or even disappear during successful treatment [71].
Therapy
Patients with mild to moderate paracoccidioidomycosis should be treated with itraconazole rather than an alternative azole or trimethoprim-sulfamethoxazole [72]. For those with severe disease, an amphotericin B formulation should be used. Alternative triazoles have been incompletely evaluated in the treatment of paracoccidioidomycosis, although voriconazole and isavuconazole have exhibited efficacy [73, 74].
Penicilliosis
The etiologic agent of penicilliosis, previously known as Penicillium marneffii, is genetically and phenotypically remote from saprophytic Penicillium species, and was thus recently renamed Talaromyces marneffei [75]. To preserve continuity in the literature, the name of the disease remains penicilliosis.
Epidemiology
Apart from isolated cases [76, 77], penicilliosis is limited to persons living in or who have traveled to Southeast Asia or southern China [78]. Most cases occur in immunocompromised individuals. Prior to the AIDS epidemic, only a handful of cases had been described, but in the AIDS era it became the third most common opportunistic infection in northern Thailand [79]. T. marneffei has been isolated from four species of bamboo rats (Rhizomys pruinosis, Rhizomys sinensis, Rhizomys sumatrensis, and Cannomys badius) and from the earth in and around their burrows [78, 79].
Diagnostics
Diagnosis is usually made based on identification of the organism on a smear of a skin lesion, histopathology (skin, lymph node, and bone marrow biopsies giving the best yields), or culture [78, 79]. A number of serologic and molecular tests have been developed but require further validation studies before they can be recommended for routine diagnosis [80, 81]. Up to two thirds of individuals with penicilliosis test positive for galactomannan [82]. Although mass spectrometry is increasingly used in the identification of medically important fungal infections, a recent study found that none out of 28 T. marneffei isolates were identified by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) [83]. This was because the identification software did not include T. marneffei, but even once included, sensitivity was only 86 %. Nested PCR (of whole blood or tissue biopsies) has been shown to be useful in expediting the diagnosis of T. marneffei. More recently, a study found that a real-time PCR had a slightly improved sensitivity (77 %) compared to nested PCR (66 %) in the detection of T. marneffei in whole blood [80].
Advances in Therapy
Treatment should involve initial amphotericin B followed by secondary prophylaxis with itraconazole [84]. The importance of receiving both itraconazole secondary prophylaxis and ART in 103 HIV-infected individuals with culture-confirmed penicilliosis was shown in a retrospective cohort review that found that mortality was 100 % in those who did not receive ART or itraconazole secondary prophylaxis, 95 % in those receiving itraconazole but no ART, and 5 % in those receiving both [85]. In HIV-infected individuals, secondary prophylaxis should continue for a minimum of 6 months and until the CD4+ cell count is ≥100 cells/μL for at least 6 months [85].
Sporotrichosis
Typically, sporotrichosis is a subcutaneous or cutaneous infection caused by traumatic inoculation of contaminated materials. Pulmonary disease is uncommon outside of the immunosuppressed.
Epidemiology
The global epidemiology of sporotrichosis was recently reviewed [86]. In addition, a systematic review of all humans and animal cases of sporotrichosis reported in the literature from 1898 found three regions to be most endemic: Brazil (5814 cases), China (3299 cases), and South Africa (3154 cases) [87]. Sporothrix brasiliensis is confined to Brazil, Sporothrix globosa to South America and Asia, and Sporothrix schenckii sensu stricto has a worldwide distribution (Fig. 1) [86]. Of the 14,000 cases documented, most occurred within outbreaks (involving between 5 and 3069 cases). There are currently two ongoing outbreaks: (i) an outbreak of S. brasiliensis in south-east Brazil that is a zoonosis transmitted by contact with infected cats [88]. This Sporothrix is thought to multiply in the cats’ saliva and then be transferred to the claws during licking. Both scratches and bites can thus transmit S. braziliensis to humans and dogs. Phylogenetic and geographic analyses support the idea that the S. braziliensis outbreak occurred in South Eastern Brazil following a habitat shift from plants to cats [89, 90]. (ii) An outbreak of S. globosa in Jilin, north-east China, that is a sapronosis likely related to the fungus growing in fermenting offcuts from corn [87]. The increased temperature induced by fermentation is hypothesized to favor the growth of the more pathogenic yeast form. The fact that farmers typically carry large bundles of this decomposing corn in their arms bringing it into contact with their faces is thought to explain why 92 % of skin lesions in this, but not other, outbreaks have occurred on the face [87].
Fig. 1.
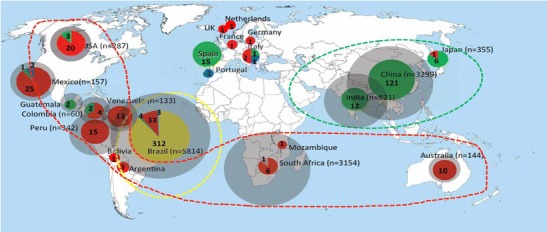
Geographic distribution of sporotrichosis caused by S. brasiliensis (yellow), S. schenckii (red), S. globosa (green), Sporothrix mexicana (blue), and Sporothrix luriei (orange). The sizes of circumference are roughly proportional to the numbers of cases/strains included in the analysis. Numbers reported within the pies denote the number of strains examined. Main endemic areas indicated by dotted lines. Reproduced with permission from [87]
S. schenckii sensu stricto which has a more global distribution and is the ancestral strain is thought to be transmitted through traumatic inoculation such as through thorns penetrating the skin. Recent outbreaks of S. schenckii sensu stricto include one from Northern Australia where wet hay was implicated as the source [91] and an outbreak in South African mineworkers where 33 % of miners examined had skin lesions compatible with sporotrichosis [92]. Untreated rotten timber supports underground were thought to be responsible.
Diagnostics
Being a predominantly cutaneous disease, the diagnosis is best made by biopsy and culture of the affected site. On occasion, biopsy and culture must be repeated. Mass spectrometry may be promising for rapid identification of Sporothrix isolates: a study established a reference database for species identification using MALDI-TOF and demonstrated excellent diagnostic accuracy for all medically important Sporothrix species [93]. Progress has also been made in the development of PCR [94] and serological tests [95] for diagnosis but clinical validation studies are required before these can be recommended for routine use.
Therapy
The last IDSA updates to the treatment guidelines for sporotrichosis were made in 2007 [96]. For severe disease, amphotericin B is indicated [96]. The therapy of choice for lymphocutaneous disease is itraconazole 200 mg/day, typically continued for 2 to 4 weeks beyond the complete resolution of all lesions; a 3- to 6-month course is usually necessary [96]. Alternatives to itraconazole include terbinafine [97] and iodide [98]. Miltefosine, a phospholipid analogue used in the treatment of leishmaniasis, has been shown to have activity against S. brazilensis strains with reduced sensitivity to itraconazole and amphotericin B [99]. There is some variation in susceptibility to antifungals between subtypes, with S. braziliensis more likely to be susceptible to itraconazole and other antifungals than S. globosa and S. schenckii sensu stricto [100].
Vaccination of cats has been proposed as a control strategy in Brazil. A cell wall-based vaccine candidate was found to confer protective immunity in a mouse model and thus provides encouragement for this strategy [101].
Conclusions
Scientific advancements regarding the endemic mycoses have occurred at both the bench-top and bedside, but still knowledge gaps abound. Research priorities should include elucidating host and pathogen factors involved in the development of disease, optimizing availability and implementation of useful diagnostic tests, particularly in developing countries, and refinement of treatment recommendations.
Compliance with Ethics Guidelines
Conflict of Interest
George Thompson reports grants and personal fees from Astellas and grants from Merck outside the submitted work.
Ilan Schwartz was supported by an R. Samuel McLaughlin—Manitoba Medical Services Foundation Research & Education Fellowship.
Chris Kenyon has received research support from Wako Diagnostics, Merck, and Astellas.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Mycology
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Kauffman CA. Endemic mycoses: blastomycosis, histoplasmosis, and sporotrichosis. Infect Dis Clin North Am. 2006;20:645–62. doi: 10.1016/j.idc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Meece JK, Anderson JL, Fisher MC, Henk DA, Sloss BL, Reed KD. Population genetic structure of clinical and environmental isolates of Blastomyces dermatitidis, based on 27 polymorphic microsatellite markers. Appl Environ Microbiol. 2011;77:5123–31. doi: 10.1128/AEM.00258-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA, Richardson SE. Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS One. 2013;8:e59237. doi: 10.1371/journal.pone.0059237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crampton TL, Light RB, Berg GM, et al. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis. 2002;34:1310–6. doi: 10.1086/340049. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease C, Prevention Blastomycosis—Wisconsin, 1986–1995. MMWR Morb Mortal Wkly Rep. 1996;45:601–3. [PubMed] [Google Scholar]
- 6.Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol. 2012;19:53–6. doi: 10.1128/CVI.05248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richer SM, Smedema ML, Durkin MM, et al. Development of a highly sensitive and specific blastomycosis antibody enzyme immunoassay using Blastomyces dermatitidis surface protein BAD-1. Clin Vaccine Immunol. 2014;21:143–6. doi: 10.1128/CVI.00597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801–12. doi: 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 9.Limper AH, Knox KS, Sarosi GA, et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 10.Ta M, Flowers SA, Rogers PD. The role of voriconazole in the treatment of central nervous system blastomycosis. Ann Pharmacother. 2009;43:1696–700. doi: 10.1345/aph.1M010. [DOI] [PubMed] [Google Scholar]
- 11.Bariola JR, Perry P, Pappas PG, et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis. 2010;50:797–804. doi: 10.1086/650579. [DOI] [PubMed] [Google Scholar]
- 12.Bednarczyk JM, Kethireddy S, White CW, et al. Extracorporeal membrane oxygenation for blastomycosis-related acute respiratory distress syndrome: a case series. Can J Anaesth. 2015;62:807–15. doi: 10.1007/s12630-015-0378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahm T, Neese S, Thornburg AT, Ober MD, Sarosi GA, Hage CA. Corticosteroids for blastomycosis-induced ARDS: a report of two patients and review of the literature. Chest. 2008;133:1478–80. doi: 10.1378/chest.07-2778. [DOI] [PubMed] [Google Scholar]
- 14.Plamondon M, Lamontagne F, Allard C, Pepin J. Corticosteroids as adjunctive therapy in severe blastomycosis-induced acute respiratory distress syndrome in an immunosuppressed patient. Clin Infect Dis. 2010;51:e1–3. doi: 10.1086/653429. [DOI] [PubMed] [Google Scholar]
- 15.Cutler JE, Deepe GS, Jr, Klein BS. Advances in combating fungal diseases: vaccines on the threshold. Nat Rev Microbiol. 2007;5:13–28. doi: 10.1038/nrmicro1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.•.Wuthrich M, Gern B, Hung CY, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest. 2011;121:554–68. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wuthrich M, Krajaejun T, Shearn-Bochsler V, et al. Safety, tolerability, and immunogenicity of a recombinant, genetically engineered, live-attenuated vaccine against canine blastomycosis. Clin Vaccine Immunol. 2011;18:783–9. doi: 10.1128/CVI.00560-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown J, Benedict K, Park BJ, Thompson GR., 3rd Coccidioidomycosis: epidemiology. Clin Epidemiol. 2013;5:185–97. doi: 10.2147/CLEP.S34434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsden-Haug N, Goldoft M, Ralston C, et al. Coccidioidomycosis acquired in Washington State. Clin Infect Dis. 2013;56:847–50. doi: 10.1093/cid/cis1028. [DOI] [PubMed] [Google Scholar]
- 20.Litvintseva AP, Marsden-Haug N, Hurst S, et al. Valley fever: finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human infection. Clin Infect Dis. 2015;60:e1–3. doi: 10.1093/cid/ciu681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson GR, 3rd, Stevens DA, Clemons KV, et al. Call for a California coccidioidomycosis consortium to face the top ten challenges posed by a recalcitrant regional disease. Mycopathologia. 2015;179:1–9. doi: 10.1007/s11046-014-9816-7. [DOI] [PubMed] [Google Scholar]
- 22.•.Engelthaler DM, Roe CC, Hepp CM, et al. Local population structure and patterns of Western Hemisphere dispersal for Coccidioides spp., the fungal cause of Valley Fever mBio 2016. This paper uses molecular epidemiology to examine the origins and diversity ofCoccidioidesspp. [DOI] [PMC free article] [PubMed]
- 23.Tsang CA, Anderson SM, Imholte SB, et al. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007–2008. Emerg Infect Dis. 2010;16:1738–44. doi: 10.3201/eid1611.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valdivia L, Nix D, Wright M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12:958–62. doi: 10.3201/eid1206.060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217–23. doi: 10.1086/496991. [DOI] [PubMed] [Google Scholar]
- 26.Stockamp NW. Thompson GR, 3rd. Infect Dis Clin North Am: Coccidioidomycosis; 2015. [DOI] [PubMed] [Google Scholar]
- 27.Thompson GR, 3rd, Bays DJ, Johnson SM, Cohen SH, Pappagianis D, Finkelman MA. Serum (1->3)-beta-D-glucan measurement in coccidioidomycosis. J Clin Microbiol. 2012;50:3060–2. doi: 10.1128/JCM.00631-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassis C, Zaidi S, Kuberski T, et al. Role of Coccidioides antigen testing in the cerebrospinal fluid for the diagnosis of coccidioidal meningitis. Clin Infect Dis. 2015;61:1521–6. doi: 10.1093/cid/civ585. [DOI] [PubMed] [Google Scholar]
- 29.Thompson GR, Sharma S, Bays DJ, et al. Coccidioidomycosis: adenosine deaminase levels, serologic parameters, culture results, and polymerase chain reaction testing in pleural fluid. Chest. 2013;143:776–81. doi: 10.1378/chest.12-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wack EE, Ampel NM, Sunenshine RH, Galgiani JN. The return of delayed-type hypersensitivity skin testing for coccidioidomycosis. Clin Infect Dis. 2015;61(5):787–91. [DOI] [PubMed]
- 31.•.Schwartz IS, Kenyon C, Feng P, et al. 50 Years of Emmonsia disease in humans: the dramatic emergence of a cluster of novel fungal pathogens. PLoS Pathog. 2015;11:e1005198. doi: 10.1371/journal.ppat.1005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anstead GM, Sutton DA, Graybill JR. Adiaspiromycosis causing respiratory failure and a review of human infections due to Emmonsia and Chrysosporium spp. J Clin Microbiol. 2012;50:1346–54. doi: 10.1128/JCM.00226-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigler L, Peterson SW. Molecular genetic analysis supports recognition of new species among Emmonsia and Blastomyces isolates. Int J Antimicrob Agents. 2009;34:s593. doi: 10.1016/S0924-8579(09)70427-3. [DOI] [Google Scholar]
- 34.Gori S, Drouhet E. Cutaneous disseminated mycosis in a patient with AIDS due to a new dimorphic. J Mycol Med. 1998;8:57–63. [Google Scholar]
- 35.Pelegrin I, Alastruey-Izquierdo A, Ayats J, Cuenca-Estrella M, Cabellos C. A second look at Emmonsia infection can make the difference. Transpl Infect Dis. 2014;16:519–20. doi: 10.1111/tid.12214. [DOI] [PubMed] [Google Scholar]
- 36.Tang XH, Zhou H, Zhang XQ, Han JD, Gao Q. Cutaneous disseminated emmonsiosis due to Emmonsia pasteuriana in a patient with cytomegalovirus enteritis. JAMA Dermatol. 2015;151:1263–4. doi: 10.1001/jamadermatol.2015.1792. [DOI] [PubMed] [Google Scholar]
- 37.Feng P, Yin S, Zhu G, et al. Disseminated infection caused by Emmonsia pasteuriana in a renal transplant recipient. J Dermatol. 2015;42:1179–82. doi: 10.1111/1346-8138.12975. [DOI] [PubMed] [Google Scholar]
- 38.Malik R, Capoor MR, Vanidassane I, et al. Disseminated Emmonsia pasteuriana infection in India: a case report and a review. Mycoses. 2016;59:127–32. doi: 10.1111/myc.12437. [DOI] [PubMed] [Google Scholar]
- 39.Kenyon C, Bonorchis K, Corcoran C, et al. A dimorphic fungus causing disseminated infection in South Africa. N Engl J Med. 2013;369:1416–24. doi: 10.1056/NEJMoa1215460. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz IS, Govender NP, Corcoran C, et al. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis. 2015;61:1004–12. doi: 10.1093/cid/civ439. [DOI] [PubMed] [Google Scholar]
- 41.Heys I, Taljaard J, Orth H. An emmonsia species causing disseminated infection in South Africa. N Engl J Med. 2014;370:283–4. doi: 10.1056/NEJMc1314277. [DOI] [PubMed] [Google Scholar]
- 42.Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–25. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 43.Bahr NC, Antinori S, Wheat LJ, Sarosi GA. Histoplasmosis infections worldwide: thinking outside of the Ohio River valley. Curr Trop Med Rep. 2015;2:70–80. doi: 10.1007/s40475-015-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedict K, Thompson GR, 3rd, Deresinski S, Chiller T. Mycotic infections acquired outside areas of known endemicity, United States. Emerg Infect Dis. 2015;21:1935–41. doi: 10.3201/eid2111.141950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nacher M, Adenis A, Adriouch L, et al. What is AIDS in the Amazon and the Guianas? Establishing the burden of disseminated histoplasmosis. AmJTrop Med Hyg. 2011;84:239–40. doi: 10.4269/ajtmh.2011.10-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antinori S. Histoplasma capsulatum: more widespread than previously thought. AmJTrop Med Hyg. 2014;90:982–3. doi: 10.4269/ajtmh.14-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasuga T, White TJ, Koenig G, et al. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol Ecol. 2003;12:3383–401. doi: 10.1046/j.1365-294X.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- 48.Arunmozhi Balajee S, Hurst SF, Chang LS, et al. Multilocus sequence typing of Histoplasma capsulatum in formalin-fixed paraffin-embedded tissues from cats living in non-endemic regions reveals a new phylogenetic clade. Med Mycol. 2013;51:345–51. doi: 10.3109/13693786.2012.733430. [DOI] [PubMed] [Google Scholar]
- 49.Edwards JA, Rappleye CA. Histoplasma mechanisms of pathogenesis—one portfolio doesn’t fit all. FEMS Microbiol Lett. 2011;324:1–9. doi: 10.1111/j.1574-6968.2011.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hage CA, Azar MM, Bahr N, Loyd J, Wheat LJ. Histoplasmosis: up-to-date evidence-based approach to diagnosis and management. Semin Respir Crit Care Med. 2015;36:729–45. doi: 10.1055/s-0035-1562899. [DOI] [PubMed] [Google Scholar]
- 51.Nacher M, Adenis A, Mc Donald S, et al. Disseminated histoplasmosis in HIV-infected patients in South America: a neglected killer continues on its rampage. PLoS Negl Trop Dis. 2013;7:e2319. doi: 10.1371/journal.pntd.0002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kauffman CA, Freifeld AG, Andes DR, et al. Endemic fungal infections in solid organ and hematopoietic cell transplant recipients enrolled in the Transplant-Associated Infection Surveillance Network (TRANSNET) Transpl Infect Dis. 2014;16:213–24. doi: 10.1111/tid.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-alpha blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis. 2015;61:409–17. doi: 10.1093/cid/civ299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Connolly PA, Durkin MM, Lemonte AM, Hackett EJ, Wheat LJ. Detection of histoplasma antigen by a quantitative enzyme immunoassay. Clin Vaccine Immunol. 2007;14:1587–91. doi: 10.1128/CVI.00071-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swartzentruber S, Rhodes L, Kurkjian K, et al. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin Infect Dis. 2009;49:1878–82. doi: 10.1086/648421. [DOI] [PubMed] [Google Scholar]
- 56.Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448–54. doi: 10.1093/cid/cir435. [DOI] [PubMed] [Google Scholar]
- 57.Scheel CM, Samayoa B, Herrera A, et al. Development and evaluation of an enzyme-linked immunosorbent assay to detect Histoplasma capsulatum antigenuria in immunocompromised patients. Clin Vaccine Immunol. 2009;16:852–8. doi: 10.1128/CVI.00066-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.neglected histoplasmosis in Latin America G. Disseminated histoplasmosis in Central and South America, the invisible elephant: the lethal blind spot of international health organizations. Aids 2016; 30: 167–70. [DOI] [PubMed]
- 59.Zhang X, Gibson B, Jr, Daly TM. Evaluation of commercially available reagents for diagnosis of histoplasmosis infection in immunocompromised patients. J Clin Microbiol. 2013;51:4095–101. doi: 10.1128/JCM.02298-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang C, Lei GS, Lee CH, Hage CA. Evaluation of two new enzyme immunoassay reagents for diagnosis of histoplasmosis in a cohort of clinically characterized patients. Med Mycol. 2015;53:868–73. doi: 10.1093/mmy/myv062. [DOI] [PubMed] [Google Scholar]
- 61.Koepsell SA, Hinrichs SH, Iwen PC. Applying a real-time PCR assay for Histoplasma capsulatum to clinically relevant formalin-fixed paraffin-embedded human tissue. J Clin Microbiol. 2012;50:3395–7. doi: 10.1128/JCM.01705-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simon S, Veron V, Boukhari R, Blanchet D, Aznar C. Detection of Histoplasma capsulatum DNA in human samples by real-time polymerase chain reaction. Diagn Microbiol Infect Dis. 2010;66:268–73. doi: 10.1016/j.diagmicrobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Scheel CM, Zhou Y, Theodoro RC, Abrams B, Balajee SA, Litvintseva AP. Development of a loop-mediated isothermal amplification method for detection of Histoplasma capsulatum DNA in clinical samples. J Clin Microbiol. 2014;52:483–8. doi: 10.1128/JCM.02739-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Myint T, Anderson AM, Sanchez A, et al. Histoplasmosis in patients with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): multicenter study of outcomes and factors associated with relapse. Medicine. 2014;93:11–8. doi: 10.1097/MD.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez R. Epidemiology of paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo. 2015;57(Suppl 19):11–20. doi: 10.1590/S0036-46652015000700004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matute DR, McEwen JG, Puccia R, et al. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol Biol Evol. 2006;23:65–73. doi: 10.1093/molbev/msj008. [DOI] [PubMed] [Google Scholar]
- 67.Teixeira MM, Theodoro RC, de Carvalho MJ, et al. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol Phylogenet Evol. 2009;52:273–83. doi: 10.1016/j.ympev.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Theodoro RC, Teixeira Mde M, Felipe MS, et al. Genus Paracoccidioides: species recognition and biogeographic aspects. PLoS One. 2012;7:e37694. doi: 10.1371/journal.pone.0037694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herr RA, Tarcha EJ, Taborda PR, Taylor JW, Ajello L, Mendoza L. Phylogenetic analysis of Lacazia loboi places this previously uncharacterized pathogen within the dimorphic Onygenales. J Clin Microbiol. 2001;39:309–14. doi: 10.1128/JCM.39.1.309-314.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marques da Silva SH, Colombo AL, Blotta MH, Lopes JD, Queiroz-Telles F, Pires de Camargo Z. Detection of circulating gp43 antigen in serum, cerebrospinal fluid, and bronchoalveolar lavage fluid of patients with paracoccidioidomycosis. J Clin Microbiol. 2003;41:3675–80. doi: 10.1128/JCM.41.8.3675-3680.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez BL, Figueroa JI, Hamilton AJ, et al. Antigenemia in patients with paracoccidioidomycosis: detection of the 87-kilodalton determinant during and after antifungal therapy. J Clin Microbiol. 1998;36:3309–16. doi: 10.1128/jcm.36.11.3309-3316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Queiroz-Telles F, Escuissato DL. Pulmonary paracoccidioidomycosis. Semin Respir Crit Care Med. 2011;32:764–74. doi: 10.1055/s-0031-1295724. [DOI] [PubMed] [Google Scholar]
- 73.Queiroz-Telles F, Goldani LZ, Schlamm HT, Goodrich JM, Espinel-Ingroff A, Shikanai-Yasuda MA. An open-label comparative pilot study of oral voriconazole and itraconazole for long-term treatment of paracoccidioidomycosis. Clin Infect Dis. 2007;45:1462–9. doi: 10.1086/522973. [DOI] [PubMed] [Google Scholar]
- 74.Thompson Iii GR, Rendon A, Santos R, et al. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. 2016 [DOI] [PMC free article] [PubMed]
- 75.Samson RA, Yilmaz N, Houbraken J, et al. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol. 2011;70:159–83. doi: 10.3114/sim.2011.70.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lo Y, Tintelnot K, Lippert U, Hoppe T. Disseminated Penicillium marneffei infection in an African AIDS patient. Trans R Soc Trop Med Hyg. 2000;94:187. doi: 10.1016/S0035-9203(00)90271-2. [DOI] [PubMed] [Google Scholar]
- 77.Patassi AA, Saka B, Landoh DE, et al. First observation in a non-endemic country (Togo) of Penicillium marneffei infection in a human immunodeficiency virus-infected patient: a case report. BMC Res Notes. 2013;6:506. doi: 10.1186/1756-0500-6-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanittanakom N, Cooper CR, Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19:95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seyedmousavi S, Guillot J, Tolooe A, Verweij PE, de Hoog GS. Neglected fungal zoonoses: hidden threats to man and animals. Clin Microbiol Infect. 2015;21:416–25. doi: 10.1016/j.cmi.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 80.Lu S, Li X, Calderone R, et al. Whole blood nested PCR and real-time PCR amplification of Talaromyces marneffei specific DNA for diagnosis. Med Mycol. 2016;54:162–8. doi: 10.1093/mmy/myv068. [DOI] [PubMed] [Google Scholar]
- 81.Chaiyaroj SC, Chawengkirttikul R, Sirisinha S, Watkins P, Srinoulprasert Y. Antigen detection assay for identification of Penicillium marneffei infection. J Clin Microbiol. 2003;41:432–4. doi: 10.1128/JCM.41.1.432-434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang YT, Hung CC, Liao CH, Sun HY, Chang SC, Chen YC. Detection of circulating galactomannan in serum samples for diagnosis of Penicillium marneffei infection and cryptococcosis among patients infected with human immunodeficiency virus. J Clin Microbiol. 2007;45:2858–62. doi: 10.1128/JCM.00050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen YS, Liu YH, Teng SH, et al. Evaluation of the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry Bruker Biotyper for identification of Penicillium marneffei, Paecilomyces species, Fusarium solani, Rhizopus species, and Pseudallescheria boydii. Front Microbiol. 2015;6:679. doi: 10.3389/fmicb.2015.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sirisanthana T, Supparatpinyo K, Perriens J, Nelson KE. Amphotericin B and itraconazole for treatment of disseminated Penicillium marneffei infection in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;26:1107–10. doi: 10.1086/520280. [DOI] [PubMed] [Google Scholar]
- 85.Son VT, Khue PM, Strobel M. Penicilliosis and AIDS in Haiphong, Vietnam: evolution and predictive factors of death. Med Mal Infect. 2014;44:495–501. doi: 10.1016/j.medmal.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 86.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53:3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y, Hagen F, Stielow B, et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia. 2015;35:1–20. doi: 10.3767/003158515X687416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanchotene KO, Madrid IM, Klafke GB, et al. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses. 2015;58:652–8. doi: 10.1111/myc.12414. [DOI] [PubMed] [Google Scholar]
- 89.Rodrigues AM, de Hoog G, Zhang Y, de Camargo ZP. Emerging sporotrichosis is driven by clonal and recombinant Sporothrix species. Emerg Microbes Infect. 2014;3:e32. doi: 10.1038/emi.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rangel-Gamboa L, Martinez-Hernandez F, Maravilla P, Arenas-Guzman R, Flisser A. Update of phylogenetic and genetic diversity of Sporothrix schenckii sensu lato. Med Mycol 2015 [DOI] [PubMed]
- 91.McGuiness S, Boyd R, McLeod C, Krause V, Ralph A. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis 2016; 16 [DOI] [PMC free article] [PubMed]
- 92.Govender NP, Maphanga TG, Zulu TG, et al. An outbreak of lymphocutaneous Sporotrichosis among mine-workers in South Africa. PLoS Negl Trop Dis. 2015;9:e0004096. doi: 10.1371/journal.pntd.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oliveira MM, Santos C, Sampaio P, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102–10. doi: 10.1016/j.resmic.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 94.Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:e0004190. doi: 10.1371/journal.pntd.0004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodrigues AM, Kubitschek-Barreira PH, Fernandes GF, de Almeida SR, Lopes-Bezerra LM, de Camargo ZP. Immunoproteomic analysis reveals a convergent humoral response signature in the Sporothrix schenckii complex. J Proteomics. 2015;115:8–22. doi: 10.1016/j.jprot.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 96.Kauffman CA, Bustamante B, Chapman SW, Pappas PG. Infectious Diseases Society of A. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255–65. doi: 10.1086/522765. [DOI] [PubMed] [Google Scholar]
- 97.Chapman SW, Pappas P, Kauffmann C, et al. Comparative evaluation of the efficacy and safety of two doses of terbinafine (500 and 1000 mg day(−1)) in the treatment of cutaneous or lymphocutaneous sporotrichosis. Mycoses. 2004;47:62–8. doi: 10.1046/j.1439-0507.2003.00953.x. [DOI] [PubMed] [Google Scholar]
- 98.Yamada K, Zaitz C, Framil VM, Muramatu LH. Cutaneous sporotrichosis treatment with potassium iodide: a 24 year experience in Sao Paulo State, Brazil. Rev Inst Med Trop Sao Paulo. 2011;53:89–93. doi: 10.1590/s0036-46652011000200006. [DOI] [PubMed] [Google Scholar]
- 99.Borba-Santos LP, Gagini T, Ishida K, de Souza W, Rozental S. Miltefosine is active against Sporothrix brasiliensis isolates with in vitro low susceptibility to amphotericin B or itraconazole. J Med Microbiol. 2015;64:415–22. doi: 10.1099/jmm.0.000041. [DOI] [PubMed] [Google Scholar]
- 100.Marimon R, Serena C, Gene J, Cano J, Guarro J. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob Agents Chemother. 2008;52:732–4. doi: 10.1128/AAC.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Portuondo DL, Batista-Duharte A, Ferreira LS, et al. A cell wall protein-based vaccine candidate induce protective immune response against Sporothrix schenckii infection. Immunobiology. 2016;221:300–9. doi: 10.1016/j.imbio.2015.10.005. [DOI] [PubMed] [Google Scholar]


