Abstract
Objective
To investigate the potential therapy targets and pharmacological mechanism of traditional Chinese medicine (TCM) Xihuang pill in liver cancer based on network pharmacology.
Methods
Drug ingredients-target network was constructed based on the target sets of Xihuang pill and liver cancer. The overlapping genes between Xihuang pill targets and liver cancer-related molecular targets were investigated using comparative analysis. Moreover, the PPI network and module was constructed based on overlapping genes and hub nodes, respectively, followed by the pathway enrichment analysis.
Results
A drug ingredients-target network was established with 1184 nodes and 11035 interactions. Moreover, a total of 106 overlapping genes were revealed between drug targets and liver cancer molecular targets. Furthermore, a PPI network and 4 modules were further investigated based on overlapping genes, respectively. These hub nodes such as VEGFA and EGFR were mainly enriched in GO functions including positive regulation of MAP kinase activity, activation of protein kinase activity, regulation of MAP kinase activity, and pathways like proteoglycans in cancer, bladder cancer, and estrogen signaling.
Conclusion
VEGFA and EGFR might be potential therapy targets of Xihuang pill in liver cancer. Furthermore, the effect of Xihuang pill on liver cancer might be realized by targeting VEGFA and EGFR in pathways like proteoglycans in cancer and estrogen signaling.
1. Introduction
As the second leading cause of death, liver cancer has caused a wide social burden for a long period of time [1]. Although partial surgical resection is the optimal therapy strategy for patients with liver cancer, the recurrence rates after surgery are still very high [2, 3]. Thus, exploring the effective clinical treatment for liver cancer is necessary.
Traditional Chinese medicine (TCM) has been widely used for clinical treatment of various tumors such as liver cancer [4, 5]. Xihuang pill is a complementary and alternative medicine that has been used in TCM due to the inhibition for tumor cell proliferation [6]. Xihuang pill is composed of Ru Xiang (olibanum), Mo Yao (Commiphora myrrha), She Xiang (Moschus), and Niu Huang (calculus bovis), which exert multiple antitumor effects [7]. A previous study shows that Xihuang pill has an anticancer effect on breast tumor [8], gastric cancer, and primary liver cancer [7]. Actually, Xihuang pill could improve quality of life and clinical manifestations of advanced primary liver cancer patients [9]. A previous study indicates that there is a reversal effect of serum containing Xihuang pill on the multidrug resistance of liver cancer cells via P-glytoprotein pathway [10]. Although Xihuang pill is effective in treatment of liver cancer, the drug targets and pharmacological mechanisms of Xihuang pill on liver cancer are still unclear.
The network pharmacology analysis is a useful tool for further understanding of the drug action [11], which may provide insights into how we can improve drug discovery for complex diseases [12]. In the current study, the potential mechanism of Xihuang pill on the treatment of liver cancer was analyzed by a network-based systematic study (as shown in Supplementary ). Briefly, the drug ingredients-target network was constricted based on the target sets of Xihuang pill and liver cancer. Then, the overlapping genes were investigated using comparative analysis. Moreover, the networks of protein-protein interaction (PPI) and modules were constructed based on overlapping genes and hub nodes, followed by the GO and KEGG pathway enrichment analysis. We hoped to explore the potential therapy targets of Xihuang pill in the treatment of liver cancer, which can provide the basis for the pharmacological mechanism study of Xihuang pill.
2. Materials and Methods
2.1. Identification of Ingredients of Drug
The chemical ingredients of Xihuang pill were collected from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) Database (http://lsp.nwsuaf.edu.cn/tcmsp.php) [13] and the Traditional Chinese Medicine Integrated Database (TCMID, http://www.megabionet.org/tcmid/) [14]. According to the relevant parameters of the pharmacokinetic properties, the ingredients were screened according to oral bioavailability (OB) and drug-likeness (DL) values and those ingredients with DL ≥ 0.18 and OB ≥ 30% were selected as the active ingredients [15, 16].
2.2. Drug Target
Usually, the ingredients of drugs play a role in related biological functions via targets. To predict the targets of ingredients of Xihuang pill, the small molecular structure information of active ingredients in Xihuang pill was retrieved on the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) [17]. Subsequently, the targets were screened with a screening online tool called the Swiss Target Prediction (http://www.swisstargetprediction.ch/) [18]. Finally, all the target information was standardized using UniProt (http://www.UniProt.org/).
2.3. Drug Ingredients-Target Network Construction
The drug ingredients-target network was constructed by Cytoscape software (version: 3.0.0, http://chianti.ucsd.edu/cytoscape-3.0.0/) [11]. The topological parameters of the network, including the degree were analyzed using Degree Centrality (DC) based on CytoNCA software [19].
2.4. Liver Cancer Targets
The main source of disease targets for liver cancer was obtained from IPA (http://www.ingenuity.com). The targets of liver cancer were retrieved after deleting duplicate data. Then, the target datasets were compared by Geneweaver (http://www.geneweaver.org) [20], and the overlapping targets of both liver cancer and the drug ingredients were considered potential targets of Xihuang pill for the treatment of liver cancer.
2.5. PPI Network Construction and Module Analysis
According to STING database (http://www.string-db.org/) [21] with the species limited to “Homo sapiens” and the confidence score >0.9, the protein interaction pairs associated with overlapping target genes between Xihuang pill and liver cancer were screened. And the PPI network was constructed via utilizing the network visualization software Cytoscape (version: 3.0.0, http://chianti.ucsd.edu/cytoscape-3.0.0/) [11], and the topological properties of the PPI network were analyzed through NetworkAnalyzer (default settings). Furthermore, MCODE (version 1.5.1) [22] was used to screen the significant enriched modules from the PPI network.
2.6. Hub Gene Investigation in PPI Network
The hub genes in PPI network were further investigated by a Cytoscape plugin cytoHubba [23], and 11 network topology parameters, including Degree, Edge Percolated Ingredients, Maximum Neighborhood Ingredients, Density of Maximum Neighborhood Ingredients, Maximal Clique Centrality Bottleneck, EcCentricity, Closeness, Radiality, Betweenness, and Stress were selected for importance calculation. After screening the core nodes in PPI network, the rank sum ratio (RSR) [24] was used to rank the nodes synthetically to get the core nodes of the network. The more important the output of a node, the higher the value of this node in the network.
2.7. Enrichment Analysis for the Hub Genes
In order to reveal the potential biological function of Xihuang pill in the treatment of liver cancer, GO (Gene Ontology) functions [25] and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway [26] enrichment analysis were performed. Specially, GO terms were grouped into the following three categories: biological process (BP), molecular function (MF), and cellular component (CC) [25]. And the KEGG analysis was conducted according to the enrichment analysis tool of the Database for Annotation, Visualization and Intergrated Discovery (DAVID, version: 6.8) software [27]. A p value <0.05 was considered as the cutoff criterion.
3. Results
3.1. Drug Ingredients-Target Network Analysis
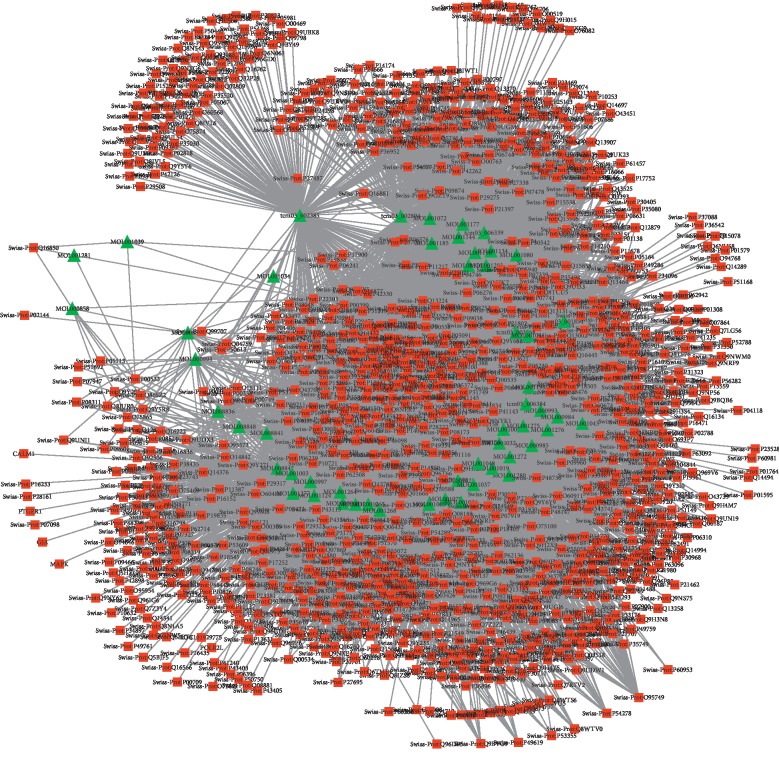
A total of 53 ingredients and 1131 targets of Xihuang pill, as well as 566 molecular targets of liver cancer, were obtained in the current study. Based on these data, the drug ingredients-target network was constructed. As shown in Figure 1, the network consists of 1184 nodes and 11035 interactions (see details in Supplementary ). Moreover, the top 50 nodes including 3 targets were further selected to construct the module of the drug ingredients-target network (Figure 2; Supplementary ).
Figure 1.
The drug ingredients-target network in the current study. The red square represented the target; the green triangle represented the drug ingredient; the line between two nodes represented the interaction.
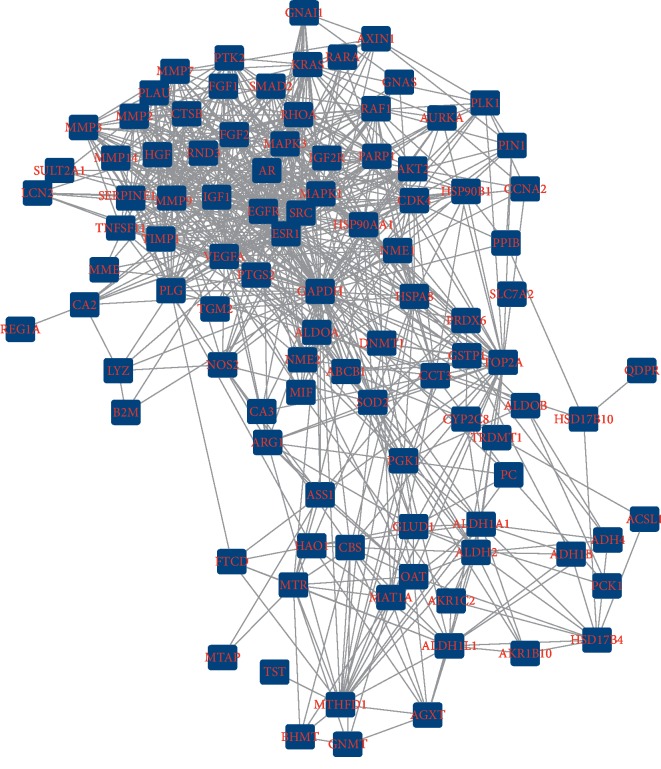
Figure 2.
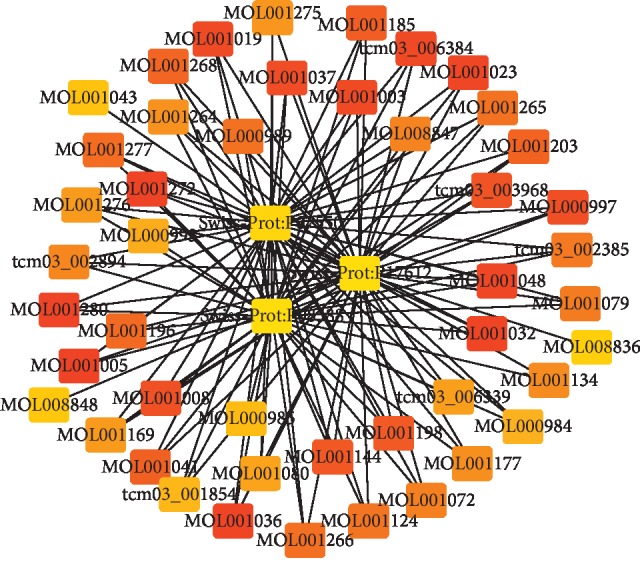
The module constructed by the TOP 50 nodes from the drug ingredients-target network. The triangle represented the ingredients of drug, and the square represented the targets of disease. The darker the color, the more significant it is.
3.2. Overlapping Genes between Drug Targets and Liver Cancer Molecular Targets
After the comparative analysis, the overlapping genes, which might be potential drug therapy target for Xihuang pill in liver cancer, between drug targets and liver cancer therapy targets were obtained. The result showed that a total of 106 overlapping genes (Attachment 1), including VEGFA, EGFR, ESR1, PLG, and MAPK3, were revealed in the current study.
Additionally, the KEGG pathway enrichment analysis showed that these overlapping genes were mainly enriched in pathways, such as metabolic pathways, pathways in cancer, proteoglycans in cancer, estrogen-signaling pathway, and HIF-1-signaling pathway. The top 20 pathways enriched by overlapping genes were listed in Table 1.
Table 1.
The top 20 pathways enriched by the overlapping genes between Xihuang pill targets and liver cancer molecular targets.
| ID | Pathway description | Count | FDR |
|---|---|---|---|
| 1100 | Metabolic pathways | 35 | 2.81E − 16 |
| 5200 | Pathways in cancer | 24 | 4.18E − 19 |
| 5205 | Proteoglycans in cancer | 18 | 4.38E − 15 |
| 4151 | PI3K-Akt-signaling pathway | 15 | 4.16E − 09 |
| 4915 | Estrogen-signaling pathway | 13 | 9.30E − 14 |
| 4015 | Rap1-signaling pathway | 13 | 1.04E − 09 |
| 4066 | HIF-1-signaling pathway | 12 | 9.29E − 12 |
| 4510 | Focal adhesion | 11 | 1.12E − 07 |
| 4014 | Ras-signaling pathway | 11 | 2.20E − 07 |
| 1230 | Biosynthesis of amino acids | 10 | 9.36E − 11 |
| 5218 | Melanoma | 10 | 9.36E − 11 |
| 5215 | Prostate cancer | 10 | 5.39E − 10 |
| 4068 | FoxO-signaling pathway | 10 | 1.29E − 08 |
| 5206 | MicroRNAs in cancer | 10 | 5.79E − 08 |
| 5219 | Bladder cancer | 8 | 4.16E − 10 |
| 4370 | VEGF-signaling pathway | 8 | 1.34E − 08 |
| 5212 | Pancreatic cancer | 8 | 1.64E − 08 |
| 4914 | Progesterone-mediated oocyte maturation | 8 | 1.12E − 07 |
| 4912 | GnRH-signaling pathway | 8 | 2.20E − 07 |
| 5210 | Colorectal cancer | 7 | 2.20E − 07 |
Count, the number of genes enriched in certain pathways; FDR, false discovery rate.
3.3. PPI Network Analysis
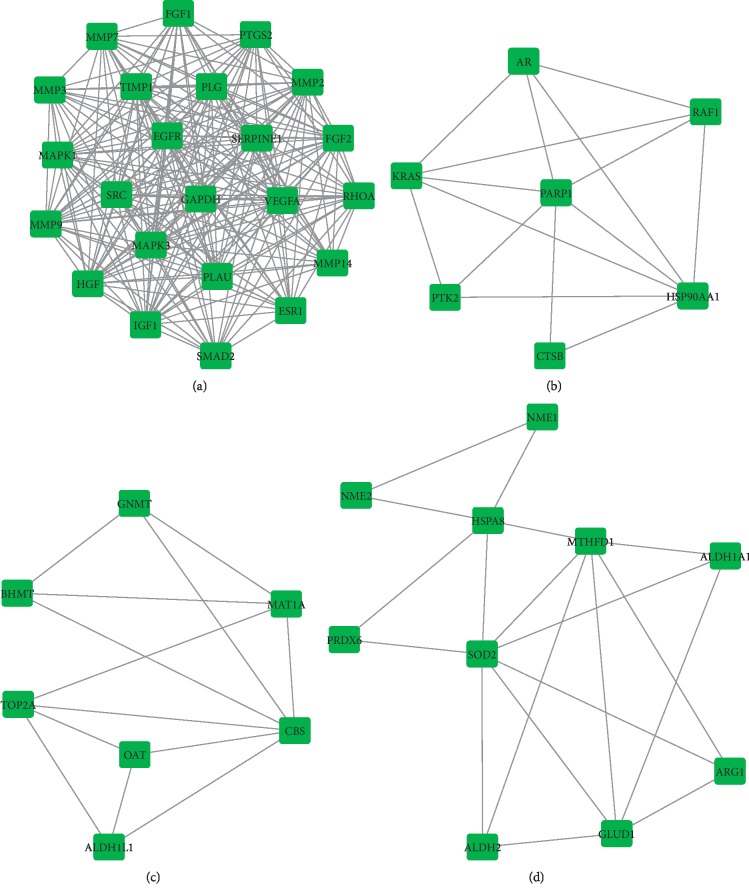
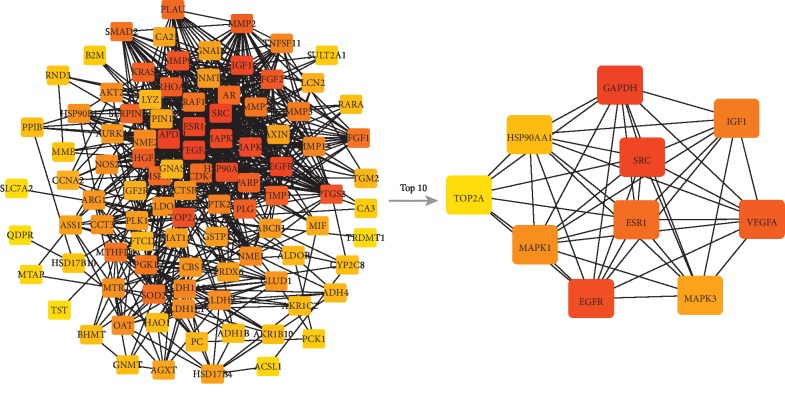
Based on the potential pharmacodynamic target of Xihuang pill for liver cancer, a PPI network was constructed by using the STRING. The result showed that there were 102 nodes and 766 interactions in the network (Figure 3). Moreover, a total of 4 modules including module 1 (score = 9.913), module 2 (score = 2.143), module 3 (score = 1.5), and module 4 (score = 1.333) were further investigated from PPI network using MOCDE (Figures 4(a)–4(d)).
Figure 3.
The protein-protein interaction network. The PPI network constructed by the potential therapy targets of Xihuang pill on liver cancer; the square represented the targets; the line between two nodes represented the interaction.
Figure 4.
The modules networks of PPI. The square represented the targets; the line between two nodes represented the interaction. (a) Module 1. (b) Module 2. (c) Module 3. (d) Module 4.
3.4. Hub Nodes Investigation
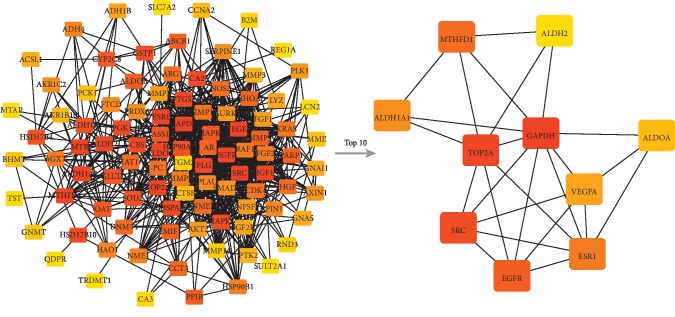
According to the PPI network, the core nodes of the network were screened based on the characteristics of the network's topological structure (Figures 5 and 6). The top 20 important nodes including VEGFA (RSR = 0.1218), EGFR (RSR = 0.1382), ESR1 (RSR = 0.1427), PLG (RSR = 0.1436), and MAPK3 (RSR = 0.1464) were summarized in Table 2. Moreover, the association among top 5 hub nodes, drug ingredients, and CTM was listed in Table 3.
Figure 5.
The results of hub nodes in current PPI network. The ranking of hub nodes based on median. From red to yellow represented a decline in importance; the line between two nodes represented the interaction.
Figure 6.
The results of hub nodes in current PPI network. The ranking of hub nodes based on degree. From red to yellow represented a decline in importance; the line between two nodes represented the interaction.
Table 2.
The top 20 core targets genes in the drug-target gene network.
| Name | RSR |
|---|---|
| VEGFA | 1.22E − 01 |
| EGFR | 1.38E − 01 |
| ESR1 | 1.43E − 01 |
| PLG | 1.44E − 01 |
| MAPK3 | 1.46E − 01 |
| IGF1 | 1.49E − 01 |
| GAPDH | 1.54E − 01 |
| SRC | 1.55E − 01 |
| MAPK1 | 1.84E − 01 |
| HGF | 1.88E − 01 |
| PTGS2 | 1.90E − 01 |
| MMP9 | 2.08E − 01 |
| FGF2 | 2.17E − 01 |
| TIMP1 | 2.25E − 01 |
| MMP2 | 2.25E − 01 |
| SERPINE1 | 2.33E − 01 |
| HSP90AA1 | 2.35E − 01 |
| TOP2A | 2.44E − 01 |
| RHOA | 2.47E − 01 |
RSR, the rank sum ratio.
Table 3.
The drug ingredients corresponding to hub nodes.
| Hub nodes | Code | CTM | Ingredients |
|---|---|---|---|
| VEGFA | Swiss-Prot:P15692 | Niuhuang | MOL008847 |
| VEGFA | Swiss-Prot:P15692 | Ruxiang | MOL001280 |
| VEGFA | Swiss-Prot:P15692 | Ruxiang | MOL001264 |
| VEGFA | Swiss-Prot:P15692 | Ruxiang | MOL001266 |
| VEGFA | Swiss-Prot:P15692 | Ruxiang | MOL001265 |
| VEGFA | Swiss-Prot:P15692 | Ruxiang | MOL001268 |
| VEGFA | Swiss-Prot:P15692 | Ruxiang | MOL001277 |
| VEGFA | Swiss-Prot:P15692 | Moyao | MOL001003 |
| VEGFA | Swiss-Prot:P15692 | Moyao | MOL000997 |
| VEGFA | Swiss-Prot:P15692 | Moyao | MOL001023 |
| VEGFA | Swiss-Prot:P15692 | Moyao | MOL001005 |
| VEGFA | Swiss-Prot:P15692 | Moyao | MOL000989 |
| VEGFA | Swiss-Prot:P15692 | Moyao | MOL001124 |
| EGFR | Swiss-Prot:P00533 | Niuhuang | MOL008847 |
| EGFR | Swiss-Prot:P00533 | Ruxiang | MOL000858 |
| EGFR | Swiss-Prot:P00533 | Ruxiang | MOL001281 |
| EGFR | Swiss-Prot:P00533 | Moyao | MOL001003 |
| EGFR | Swiss-Prot:P00533 | Moyao | MOL000989 |
| EGFR | Swiss-Prot:P00533 | Moyao | MOL001039 |
| ESR1 | Swiss-Prot:P03372 | Shexiang | tcm03_006384 |
| ESR1 | Swiss-Prot:P03372 | Shexiang | tcm03_006339 |
| ESR1 | Swiss-Prot:P03372 | Shexiang | tcm03_001854 |
| ESR1 | Swiss-Prot:P03372 | Shexiang | tcm03_003968 |
| ESR1 | Swiss-Prot:P03372 | Niuhuang | MOL008836 |
| ESR1 | Swiss-Prot:P03372 | Ruxiang | MOL001276 |
| ESR1 | Swiss-Prot:P03372 | Ruxiang | MOL001272 |
| ESR1 | Swiss-Prot:P03372 | Ruxiang | MOL001275 |
| ESR1 | Swiss-Prot:P03372 | Ruxiang | MOL001264 |
| ESR1 | Swiss-Prot:P03372 | Ruxiang | MOL001266 |
| ESR1 | Swiss-Prot:P03372 | Ruxiang | MOL001265 |
| ESR1 | Swiss-Prot:P03372 | Ruxiang | MOL001268 |
| ESR1 | Swiss-Prot:P03372 | Ruxiang | MOL001277 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001203 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001198 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001003 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL000997 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001032 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001036 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001048 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001023 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001037 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001019 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001005 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001008 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001041 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001196 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL000989 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001079 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001124 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001134 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001072 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL001080 |
| ESR1 | Swiss-Prot:P03372 | Moyao | MOL000993 |
| PLG | Swiss-Prot:P00747 | Shexiang | tcm03_002894 |
| PLG | Swiss-Prot:P00747 | Shexiang | tcm03_002385 |
| MAPK3 | Swiss-Prot:P27361 | Ruxiang | MOL001266 |
| MAPK3 | Swiss-Prot:P27361 | Ruxiang | MOL001265 |
| MAPK3 | Swiss-Prot:P27361 | Ruxiang | MOL001268 |
| MAPK3 | Swiss-Prot:P27361 | Moyao | MOL000997 |
| MAPK3 | Swiss-Prot:P27361 | Moyao | MOL001036 |
| MAPK3 | Swiss-Prot:P27361 | Moyao | MOL001023 |
| MAPK3 | Swiss-Prot:P27361 | Moyao | MOL001037 |
| MAPK3 | Swiss-Prot:P27361 | Moyao | MOL001019 |
| MAPK3 | Swiss-Prot:P27361 | Moyao | MOL001124 |
CTM, Chinese traditional medicine; VEGFA, vascular endothelial growth factor A; EGFR, epidermal growth factor receptor; ESR1, estrogen receptor 1; PLG, plasminogen; MAPK3, mitogen-activated protein kinase 3.
3.5. Investigation of Pathways Enriched by Hub Nodes
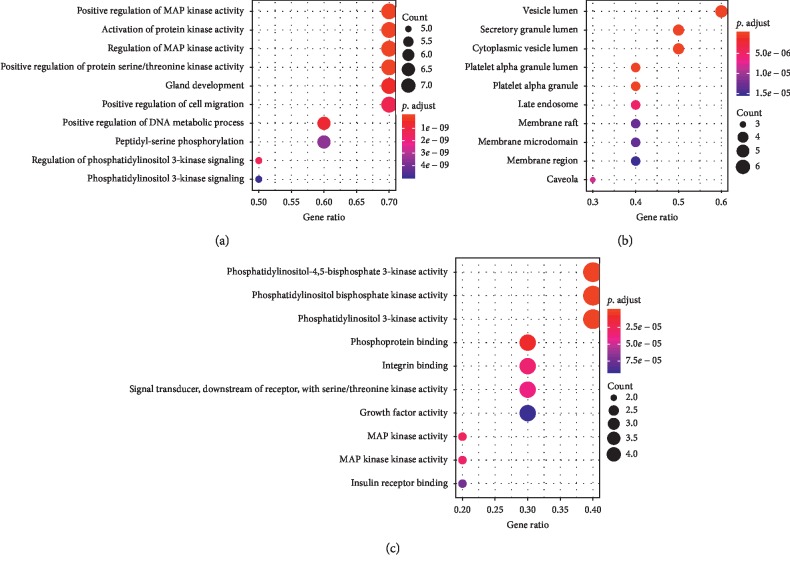
The top 5 hub nodes including VEGFA, EGFR, ESR1, PLG, and MAKP3 were used for further GO function and pathway enrichment analyses. The result showed that the hub genes could be assigned to different GO terms for BP, CC, and MF categories. The prominent functions enriched by hub genes were positive regulation of MAP kinase activity, activation of protein kinase activity, regulation of MAP kinase activity and phosphatidylinositol bisphosphate kinase activity, of which the top 10 functions for GO terms are presented in Figure 7. Moreover, these hub nodes were mainly enriched in pathways like proteoglycans in cancer (ID: 05205; count = 4; FDR = 8.93E-06), bladder cancer (ID: 05219; count = 3; FDR = 8.93E-06), and estrogen-signaling pathway (ID: 04915; count = 3; FDR = 6.48E-05). The top 10 pathways are listed in Table 4.
Figure 7.
Gene ontology (GO) term enrichment for hub genes. GO terms including biological process (BP), cellular component (CC), and molecular function (MF) enriched by hub genes, respectively.
Table 4.
The top 10 pathways enriched by the top 5 hub nodes in PPI network.
| ID | Description | Count | FDR |
|---|---|---|---|
| 05205 | Proteoglycans in cancer | 4 | 8.93E − 06 |
| 05219 | Bladder cancer | 3 | 8.93E − 06 |
| 05212 | Pancreatic cancer | 3 | 2.40E − 05 |
| 04915 | Estrogen-signaling pathway | 3 | 6.48E − 05 |
| 04066 | HIF-1-signaling pathway | 3 | 7.06E − 05 |
| 04014 | Ras-signaling pathway | 3 | 3.80E − 04 |
| 04015 | Rap 1-signaling pathway | 3 | 3.80E − 04 |
| 04320 | Dorso-ventral axis formation | 2 | 3.80E − 04 |
| 04510 | Focal adhesion | 3 | 3.80E − 04 |
FDR, false discovery rate; Count, the number of nodes enriched in certain pathway.
4. Discussion
Liver cancer is one of the most lethal cancers having worldwide prevalence, and the effect of clinical treatment for this disease is not satisfactory [28]. The current study, for the first time, explored the potential therapy targets of Xihuang pill on liver cancer based on network pharmacology analysis. The results showed that a drug ingredients-target network was established with 1184 nodes and 11035 interactions. Moreover, a total of 106 overlapping genes were revealed between drug targets and liver cancer molecular targets. Furthermore, a PPI network and 4 modules networks were further investigated based on overlapping genes and hub nodes, respectively. These hub nodes such as VEGFA, EGFR, ESR1, PLG, and MAPK3 were mainly enriched in pathways like proteoglycans in cancer, bladder cancer, and estrogen-signaling pathway.
Vascular endothelial growth factor (VEGF) family plays a major role in angiogenesis, which are essential for both healing of injured tissue and proliferation of carcinoma cells [29]. The previous meta-analysis indicates that there is a prognostic role for VEFGA in various cancers including cervical cancer, gastric cancer, epithelial ovarian cancer, and liver cancer [30–33]. Taniguchi et al. indicates that VEGF promotes proliferation of hepatocytes through reconstruction of liver sinusoids by proliferation of sinusoidal endothelial cells [33]. Importantly, VEGF is an important drug target of various TCM [34, 35]. A previous study shows that the TCM Fuzhenghuayu has a decoction effect on VEGF secretion in hepatic stellate cells [36]. However, the association between VEGF and Xihuang pill in liver cancer is unknown. Moreover, epidermal growth factor receptor (EGFR) is a transmembrane protein associated with the epidermal growth factor family [37]. The compartmentalization and biological function of EGFR in liver plasma membrane have been focused [38]. A previous study indicates that there is an increment of serum EGFR level in liver cancer patients [39]. Actually, there is a dual role of EGFR in liver injury and regeneration after acetaminophen overdose in mice [40]. Zhu et al. indicate that the EGFR status is independently correlated with TCM treatment in non-small-cell lung cancer patients [41]. Although previous study has confirmed the relation between EGFR expression and TCM active compounds using cell membrane chromatography [42], the effect of Xihuang pill on EGFR expression in liver cancer is still unclear. In the current study, VEGFA and EGFR were not only overlapping genes revealed between Xihuang pill target sets and liver cancer target sets but also hub nodes in PPI modules. Thus, we speculated that genes such as VEGFA and EGFR might be potential therapy targets of Xihuang pill in liver cancer.
Proteoglycans perform multiple functions in cancer and angiogenesis due to their polyhedric nature [43]. The deterioration of liver function is accompanied by an increase in the amount of chondroitin sulfate proteoglycans [44]. The alteration of proteoglycan composition interferes with the physiologic function of the liver on several levels [45]. Actually, the biological function of proteoglycans has been widely investigated in VEGF-induced diseases [46, 47]. A previous study shows that the activation of EGFR contributes to the inhibition of axon regeneration [48]. Furthermore, estrogen plays a vital role in the progression of liver cancer [49]. Kim et al. indicate that estrogen-related receptor γ is upregulated in liver cancer [50]. Previous studies show that VEGF and EGFR expression are all associated with estrogen receptor status in patients with cancer [51, 52]. Meanwhile, cytoplasmic expression of estrogen receptor can predict poor outcome of EGFR-TKI therapy in metastatic lung adenocarcinoma [53]. In this study, the pathway analysis showed that the potential targets such as VEGFA and EGFR were mainly enriched in pathways including proteoglycans in cancer and estrogen-signaling pathway. Thus, we speculated that the effect of Xihuang pill on liver cancer might be realized by targeting VEGFA and EGFR in pathways like proteoglycans in cancer and estrogen signaling. However, there were some limitations in this study such as small sample size and lack of verification analysis. Thus, a further verification study based on a large sample size is needed.
In conclusion, VEGFA and EGFR might be potential therapy targets of Xihuang pill in liver cancer. Furthermore, the effect of Xihuang pill on liver cancer might be realized by targeting VEGFA and EGFR in pathways like proteoglycans in cancer and estrogen signaling.
Acknowledgments
This work was supported by Longgang District Special Funds for Economic and Technological Development of Shenzhen City (LGKCYLWS2019000876).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Additional Points
Highlights. (1) VEGFA and EGFR might be potential therapy targets of Xihuang pill in liver cancer. (2) Proteoglycans in cancer and estrogen signaling might be associated with liver cancer. (3) Totally, 106 genes were explored between drug targets and liver cancer targets
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Supplementary Figure 1: workflow of network pharmacology analysis. Supplementary File 1: all nodes and interactions in drug ingredients-target network. Supplementary File 2: the top 50 nodes in drug ingredients-target network.
References
- 1.Iburg K. M. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;385(9963):117–171. doi: 10.1016/s0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omata M., Cheng A. L., Kokudo N., et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatology International. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tejeda-Maldonado J., García-Juárez I., Aguirre-Valadez J., et al. Diagnosis and treatment of hepatocellular carcinoma: an update. World Journal of Hepatology. 2015;7(3):362–376. doi: 10.4254/wjh.v7.i3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin W. F., Lu J. Y., Cheng B. B., Ling C. Q. Progress in research on the effects of traditional Chinese medicine on the tumor microenvironment. Journal of Integrative Medicine. 2017;15(4):282–287. doi: 10.1016/s2095-4964(17)60345-5. [DOI] [PubMed] [Google Scholar]
- 5.Liao Y. H., Lin C. C., Li T. C., Lin J. G. Utilization pattern of traditional Chinese medicine for liver cancer patients in Taiwan. BMC Complementary & Alternative Medicine. 2012;12(1):p. 146. doi: 10.1186/1472-6882-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng X., Zhang M., Wang J., Lv S. Study of qualitative and quantitative methods for Xihuang pills. Chinese Journal of Pharmaceutical Analysis. 2011;31(7):1410–1413. [Google Scholar]
- 7.Guo Q., Lin J., Liu R., et al. Review on the applications and molecular mechanisms of Xihuang pill in tumor treatment. Evidence-Based Complementary and Alternative Medicine. 2015;2015:10. doi: 10.1155/2015/854307.854307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan G., Wang W., Wang L., et al. Anti-breast cancer effects and mechanisms of Xihuang pill on human breast cancer cell lines. Journal of Traditional Chinese Medicine. 2013;33(6):770–778. doi: 10.1016/s0254-6272(14)60011-x. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Z. Q. Clinical observation on Xihuang pill in treating 23 cases of advanced primary hepatic cancer. China Journal of Traditional Chinese Medicine & Pharmacy. 2010;1 in Chinese. [Google Scholar]
- 10.Gui-zhi L., Yi D., Ren-bao H., Zhu-ting T., Meng-qun Y., Fei Z. In vitro study on the reversal effect of serum containing Xihuang pill on the multidrug-resistance of hepatic cancer cells via P-glytoprotein pathway. Chinese Journal of Integrated Traditional and Western Medicine on Digestion. 2016;2 in Chinese. [Google Scholar]
- 11.Zhang R. Z., Yu S. J., Bai H., Ning K. TCM-Mesh: the database and analytical system for network pharmacology analysis for TCM preparations. Scientific Reports. 2017;7(1):p. 2821. doi: 10.1038/s41598-017-03039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Cao Y., Zhang H., et al. Network pharmacology-based strategy for predicting active ingredients and potential targets of Yangxinshi tablet for treating heart failure. Journal of Ethnopharmacology. 2018;219:359–368. doi: 10.1016/j.jep.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Ru J., Li P., Wang J., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6(1):p. 13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue R., Fang Z., Zhang M., Yi Z., Wen C., Shi T. TCMID: traditional Chinese medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Research. 2012;41(D1):D1089–D1095. doi: 10.1093/nar/gks1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee A. Y., Park W., Kang T.-W., Cha M. H., Chun J. M. Network pharmacology-based prediction of active compounds and molecular targets in Yijin-Tang acting on hyperlipidaemia and atherosclerosis. Journal of Ethnopharmacology. 2018;221:151–159. doi: 10.1016/j.jep.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Xu X., Zhang W., Huang C., et al. A novel chemometric method for the prediction of human oral bioavailability. International Journal of Molecular Sciences. 2012;13(6):6964–6982. doi: 10.3390/ijms13066964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S., Thiessen P. A., Bolton E. E., et al. PubChem substance and compound databases. Nucleic Acids Research. 2016;44(D1):D1202–D1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gfeller D., Michielin O., Zoete V. Shaping the interaction landscape of bioactive molecules. Bioinformatics. 2013;29(23):3073–3079. doi: 10.1093/bioinformatics/btt540. [DOI] [PubMed] [Google Scholar]
- 19.Yu Tang M. L., Wang J., Pan Yi, Wu F.-X. CytoNCA: a cytoscape plugin for centrality analysis and evaluation of biological networks. BioSystems. 2014;11:p. 5. doi: 10.1016/j.biosystems.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Baker E. J., Jay J. J., Bubier J. A., Langston M. A., Chesler E. J. GeneWeaver: a web-based system for integrative functional genomics. Nucleic Acids Research. 2012;40(D1):D1067–D1076. doi: 10.1093/nar/gkr968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szklarczyk D., Morris J. H., Cook H., et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Research. 2017;45(D1):D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bader G. D., Hogue C. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. 2003;4(1):p. 2. doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin C. H., Chen S. H., Wu H. H., Ho C. W., Ko M. T., Lin C. Y. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Systems Biology. 2014;8(S4):p. S11. doi: 10.1186/1752-0509-8-s4-s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H., Jiang W. Q. Research on two-stage approach based on rank-sum ratio in multi-attribute decision condition. Mathematics in Practice & Theory. 2015;5(10):199–203. [Google Scholar]
- 25.Ashburner M., Ball C. A., Blake J. A., et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature Genetics. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanehisa M., Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Research. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang D. W., Sherman B. T., Lempicki R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 28.Boix L., Mariño Z., Torres F., Forns X., Bruix J., Reig M. Liver cancer emergence associated with antiviral treatment: an immune surveillance failure? Seminars in Liver Disease. 2017;37(2):109–118. doi: 10.1055/s-0037-1601349. [DOI] [PubMed] [Google Scholar]
- 29.Roskoski R. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacological Research. 2017;120:116–132. doi: 10.1016/j.phrs.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J., Liu J., Zhu C., et al. Prognostic role of vascular endothelial growth factor in cervical cancer: a meta-analysis. Oncotarget. 2017;8(15):24797–24803. doi: 10.18632/oncotarget.15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhuang M., Peng Z., Wang J., Su X. Vascular endothelial growth factor gene polymorphisms and gastric cancer risk: a meta-analysis. Journal of BUON: Official Journal of the Balkan Union of Oncology. 2017;22(3):p. 714. [PubMed] [Google Scholar]
- 32.Komatsu H., Oishi T., Itamochi H., et al. Serum vascular endothelial growth factor-A as a prognostic biomarker for epithelial ovarian cancer. International Journal of Gynecological Cancer. 2017;27(7):1325–1332. doi: 10.1097/IGC.0000000000001027. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi E., Sakisaka S., Matsuo K., Tanikawa K., Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. Journal of Histochemistry & Cytochemistry. 2001;49(1):121–129. doi: 10.1177/002215540104900112. [DOI] [PubMed] [Google Scholar]
- 34.Guo J. W., Chen C., Huang Y., Li B. Combinatorial effects of Naomai Yihao capsules and vascular endothelial growth factor gene-transfected bone marrow mesenchymal stem cells on angiogenesis in cerebral ischemic tissues in rats. Journal of Traditional Chinese Medicine. 2012;32(1):87–92. doi: 10.1016/s0254-6272(12)60038-7. [DOI] [PubMed] [Google Scholar]
- 35.Qi Z.-X., Chen L. Effect of Chinese drugs for promoting blood circulation and eliminating blood stasis on vascular endothelial growth factor expression in rabbits with glucocorticoid-induced ischemic necrosis of femoral head. Journal of Traditional Chinese Medicine. 2009;29(2):137–140. doi: 10.1016/s0254-6272(09)60050-9. [DOI] [PubMed] [Google Scholar]
- 36.Liu C., Jiang C. M., Liu C. H., Liu P., Hu Y. Y. Effect of Fuzhenghuayu decoction on vascular endothelial growth factor secretion in hepatic stellate cells. Hepatobiliary & Pancreatic Diseases International. 2002;1(2):207–210. [PubMed] [Google Scholar]
- 37.Wee P., Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers. 2017;9(12):p. 52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y., Posner B. I., Balbis A. Compartmentalization of epidermal growth factor receptor in liver plasma membrane. Journal of Cellular Biochemistry. 2009;107(1):96–103. doi: 10.1002/jcb.22105. [DOI] [PubMed] [Google Scholar]
- 39.Sung T. I., Wang Y. J., Chen C. Y., Hung T. L., Guo H. R. Increased serum level of epidermal growth factor receptor in liver cancer patients and its association with exposure to arsenic. Science of the Total Environment. 2012;424:74–78. doi: 10.1016/j.scitotenv.2012.02.079. [DOI] [PubMed] [Google Scholar]
- 40.Bhushan B., Chavan H., Borude P., et al. Dual role of epidermal growth factor receptor in liver injury and regeneration after acetaminophen overdose in mice. Toxicological Sciences. 2017;155(2):363–378. doi: 10.1093/toxsci/kfw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y. J., Zhang H. B., Liu L. R., et al. Yin-cold or yang-heat syndrome type of traditional Chinese medicine was associated with the epidermal growth factor receptor gene status in non-small cell lung cancer patients: confirmation of a TCM concept. Evidence-Based Complementary and Alternative Medicine. 2017;2017:7. doi: 10.1155/2017/7063859.7063859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He H., Han S., Zhang T., Zhang J., Wang S., Hou J. Screening active compounds acting on the epidermal growth factor receptor from Radix scutellariae via cell membrane chromatography online coupled with HPLC/MS. Journal of Pharmaceutical and Biomedical Analysis. 2012;62(6):196–202. doi: 10.1016/j.jpba.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 43.Iozzo R. V., Sanderson R. D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. Journal of Cellular & Molecular Medicine. 2011;15(5):1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner O. H., Zoremba M., Gressner A. M. Gene expression of syndecans and betaglycan in isolated rat liver cells. Cell & Tissue Research. 1996;285(1):11–16. doi: 10.1007/s004410050615. [DOI] [PubMed] [Google Scholar]
- 45.Baghy K., Tátrai P., Regős E., Kovalszky I. Proteoglycans in liver cancer. World Journal of Gastroenterology. 2016;22(1):379–393. doi: 10.3748/wjg.v22.i1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jan S. L., Hayashi M., Kasza Z., et al. Functional overlap between chondroitin and heparan sulfate proteoglycans during VEGF-induced sprouting angiogenesis. Arteriosclerosis, Thrombosis & Vascular Biology. 2012;32(5):1255–1263. doi: 10.1161/atvbaha.111.240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beckouche N., Bignon M., Lelarge V., et al. The interaction of heparan sulfate proteoglycans with endothelial transglutaminase-2 limits VEGF165-induced angiogenesis. Science Signaling. 2015;8(385):p. ra70. doi: 10.1126/scisignal.aaa0963. [DOI] [PubMed] [Google Scholar]
- 48.Koprivica V., Cho K. S., Park J. B., et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310(5745):106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- 49.Villa E. Role of estrogen in liver cancer. Women’s Health. 2008;4(1):41–50. doi: 10.2217/17455057.4.1.41. [DOI] [PubMed] [Google Scholar]
- 50.Kim J. H., Choi Y. K., Byun J. K., et al. Estrogen-related receptor γ is upregulated in liver cancer and its inhibition suppresses liver cancer cell proliferation via induction of p21 and p27. Experimental & Molecular Medicine. 2016;48(3):p. e213. doi: 10.1038/emm.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuckar D., Dekanic A., Stifter S., et al. VEGF expression is associated with negative estrogen receptor status in patients with breast cancer. International Journal of Surgical Pathology. 2006;14(1):49–55. doi: 10.1177/106689690601400109. [DOI] [PubMed] [Google Scholar]
- 52.Tanjak P., Thiantanawat A., Watcharasit P., Satayavivad J. Genistein reduces the activation of AKT and EGFR, and the production of IL6 in cholangiocarcinoma cells involving estrogen and estrogen receptors. International Journal of Oncology. 2018;53(1):177–188. doi: 10.3892/ijo.2018.4375. [DOI] [PubMed] [Google Scholar]
- 53.Ding X., Li L., Tang C., et al. Cytoplasmic expression of estrogen receptor β may predict poor outcome of EGFR-TKI therapy in metastatic lung adenocarcinoma. Oncology Letters. 2018;16(2):2382–2390. doi: 10.3892/ol.2018.8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: workflow of network pharmacology analysis. Supplementary File 1: all nodes and interactions in drug ingredients-target network. Supplementary File 2: the top 50 nodes in drug ingredients-target network.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.