Abstract
Background
The aim of this study was to estimate the utility of a preoperative model of end-stage liver disease (MELD) score and Child-Turcotte-Pugh (CTP) score in predicting the prognosis after othotopic liver transplantation (OLT) for chronic severe hepatitis B (CSHB) and explore the prognostic factors.
Methods
The outcome of 137 patients who underwent OLT using donors after cardiac death (DCDs) for CSHB in our center was reviewed retrospectively. Survival analysis was performed using the Kaplan-Meier method; the log-rank test was used for univariate analysis; and the Cox proportional hazards regression model was used for prognostic factors screening.
Results
The overall mortality rate was 33.6% (46/137); and 1-month, 6-month, 1-year, and 5-year patient survival rates were 75.8, 72.0, 71.0, and 60.1%, respectively. Most patients (33/46) died during the first month after OLT. The area under the curve values generated by the receiver operating characteristics curves were 0.82 [95% confidence interval (CI) 0.72–0.92] and 0.68 (95% CI 0.58–0.79), respectively (P < 0.01), for the MELD and CTP models in predicting 1-month mortality after OLT. Patients with a preoperative MELD score <33.8 or a CTP score <12.5 had significantly better prognosis than those with higher scores (P < 0.05). Other mortality predictors include hepatic encephalopathy, preoperative infection, serum creatinine ≥1.5 mg/dl.
Conclusions
The MELD score was more efficient than the CTP score for evaluating the short-term prognosis in patients with CSHB undergoing OLT using DCDs, which should be taken into consideration during graft allocation.
Keywords: Hepatic Encephalopathy, Hepatorenal Syndrome, Cold Ischemia Time, Hepatic Artery Thrombosis, Poor Graft Function
Introduction
Chronic severe hepatitis (CSH) can cause irreversible hepatic failure resulting from severe impairment of liver function. It is associated with a mortality rate of >70% if liver transplantation (LT) is not available [1]. The main causes of CSH are alcoholic cirrhosis and viral hepatitis C in Western countries [2], whereas it is viral hepatitis B in China. The current policy for determining priority for organ allocation is based on the model for end-stage liver disease (MELD). Patients with the highest MELD score, which predicts the greatest risk for dying without LT, would be given the highest priority [3]. However, the correlation between either the preoperative MELD or the Child-Turcotte-Pugh (CTP) score and outcome after LT using donors after cardiac death (DCDs) for chronic severe hepatitis B (CSHB) in mainland China has not yet been evaluated adequately [4]. The primary objective of this study was to test the hypothesis that the severity of liver disease based on the MELD or CTP score is important for predicting outcome after LT for CSHB. The secondary objective was to reexamine other potential factors that may be associated with poor patient survival.
Patients and methods
Between August 2002 and November 2007, a series of 137 patients underwent 143 orthotopic liver transplantation (OLT) for CSHB in our center. The reasons for re-OLT included primary nonfunction (PNF) or initial poor graft function in four cases and hepatic artery thrombosis (HAT) in two cases. The diagnoses conformed to the Prevention and Treatment Program for Viral Hepatitis [5] formulated at the 10th National Workshop on Viral Hepatitis held in 2000 in Xi’an, China. The diagnostic standard for CSHB includes (1) basic conditions: the period of hepatitis B surface antigen (HBsAg) positivity exceeds 6 months, and the level of serum bilirubin on the liver failure index exceeds 10 times the normal level (17.1 μmol/l) (i.e., the level is >171 μmol/l; (2) additional conditions: the presence of at least one of the following liver failure indexes: (a) prothrombin activity <40%; (b) hepatic encephalopathy; (c) ascites; (d) progressive reduction in liver size; and (e) hepatorenal syndrome.
None of the patients required mechanical ventilation within 1 week before OLT. Clinic and laboratory data within 48 hours preceding OLT were collected retrospectively from a computerized database and by reviewing the patients medical charts. We calculated for each patient the MELD score based on serum bilirubin and creatinine levels and the INR (prothrombin) [6]. From the outset, the INR was included in the reporting of the prothrombin time for all patients. We also retrospectively determined the CTP score, which is based on five variables: hepatic encephalopathy (HE), ascites, bilirubin, albumin levels, and the INR [7]. HE was present if the patient received medical treatment including l-ornithine, l-aspartate, or lactulose or had clinical manifestations of at least stage 2 HE based on the criteria by Gitlin [8]. The presence and degree of ascites were assessed by diuretic requirements as well as by abdominal imaging studies including ultrasonography (US) or computed tomography (CT) scans.
The donors in our study were all DCDs. None of the donors included in this study were prisoners who died as a result of execution. The hospital committee of ethical issues reviewed their written applications and supporting documents to make sure that they were well informed and made the decision by their own or their near relatives before voting for permission. According to our regulations, the clinical team began the procedure after receiving the written permissions.
Briefly, patients were withdrawn from organ-perfusion support inside the operating room, and systemic heparin (12,500 units) was administered prior to extubation, with another 25,000 units mixed into the first bag of preservation solution. After cardiac arrest, rapid cannulation of the abdominal aorta and portal vein were performed, cold perfusion of organs using modified HC-A solution (3000 ml) was administrated through each catheter; then, another 1000–2000 ml of University of Wisconsin (UW) solution was used when necessary for portal perfusion at the back table. The common bile duct was also infused directly with several injections of cold normal saline. Donor warm ischemia time (DWIT) was defined as the time interval between cardiac arrest of the donor to the perfusion of the abdominal aorta, and cold ischemia time (CIT) was defined as the time interval from perfusion of organs during the recovery to reperfusion of the liver in the recipient. The mean donor age, DWIT, and CIT were 45 ± 16 years, 3.3 ± 2.0 min, and 7.4 ± 3.3 h, respectively. The proportion of sex/serotype mismatch were 21.9 and 13.1%, respectively.
After OLT, the tacrolimus-based triple immunosuppressive protocols were applied. Lamivudine and hepatitis B immunoglobulin (HBIG) were used for anti-virus treatment, in which HBIG was first given during the anhepatic phase in a dose of 4000 IU, and anti-HBs antibody levels were maintained at a level higher than 100 IU by periodic HBIG bolus infusions. Patients were then followed every month for half a year and every 2 months thereafter. From the second year after OLT, the follow-up interval was prolonged to 3–6 months. All results were recorded by the same staff.
SPSS13.0 software was adopted for statistical analysis. Survival analysis was performed using the Kaplan–Meier method; and the log-rank test was used to compare survival probabilities. The Cox proportional hazards regression model was used for prognostic factors screening. Receiver operating characteristics (ROC) curves [9] were generated for the CTP and MELD scores using short-term mortality after OLT as the endpoint. The area under the curve (AUC) generated by connected ROC curves with the 95% confidence interval (CI) was used as a measure of the ability of each model to predict mortality after OLT and was compared by nonparametric methods [10]. AUC between 0.8 and 0.9 indicates excellent diagnostic accuracy, and a model with an AUC >0.7 should be considered clinically useful. For all analysis, a value of P < 0.05 was considered statistically significant.
Results
The baseline clinical and laboratory characteristics of the 137 patients are summarized in Table 1. A total of 23 patients experienced 25 episodes of serious infections within 1 month before OLT (22 pneumonia, 3 spontaneous bacterial peritonitis). The median MELD and CTP scores were 27.0 (range 13.4–54.8) and 13 (range 10–14), respectively. All the patients had a CTP score of at least 10, corresponding to Child’s class C cirrhosis.
Table 1.
Baseline clinic and laboratory data for 137 patients undergoing OLT
| Clinical data before OLT | Number (%) |
|---|---|
| Ascites | 100 (73) |
| Hepatic encephalopathya | 56 (41) |
| Renal insufficiency (creatinine > 1.5 mg/dl) | 22 (16) |
| Variceal bleeding | 20 (15) |
| TIPS | 0 |
| Infection within 1 month before OLT | 23 (16.8) |
| Laboratory data before OLT | Median (range) |
|---|---|
| Total bilirubin (mg/dl) | 30 (2.3–63.7) |
| Albumin level (g/dl) | 3.0 (2.1–4.0) |
| Alanine aminotransferase (U/l) | 244 (34–1234) |
| γ-Glutamyltransferase (U/l) | 345 (85–1088) |
| AFP (μg/l) | 10 (1–3044) |
| International normalized ratio | 2.8 (0.7–32.8) |
| Serum sodium (mmol/l) | 133 (124–147) |
| MELD score | 27.0 (13.4–54.8) |
| CTP score | 13 (10–14) |
The median age of the patients was 46 years (range 23–67 years). There were 122 men and 15 women
OLT orthotopic liver transplantation, TIPS transjugular intrahepatic portosystemic shunt, AFP α-fetoprotein, MELD model for end-stage disease, CTP Child-Turcotte-Pugh
aBased on the criteria of Gitlin [8]. None of our patients was in stage 3 or 4 coma before OLT. Subtle signs of encephalopathy (stage 1) were not included in our definition
At the endpoint of follow-up (range 1–66 months), no patient was lost. The patients 1-month, 6-month, 1-year, and 5-year survival rates were 75.8, 72.0, 71.0, and 60.1%, respectively, for the entire cohort (Fig. 1). The graft 1-month, 6-month, 1-year, and 5-year survival rates were 74.3, 70.6, 65.2 and 56.6%, respectively. There were 46 deaths within the first 5 years after OLT, with the main cause of death being multiorgan failure (MOF) followed by liver failure, acute respiratory distress syndrome (ARDS), and disseminated intravascular coagulation (DIC) (Table 2). In a subgroup study, 72.0% (33/46) of the patients died within the first month and 89.1% (41/46) patients within the first year after OLT. Among these deaths, 63.6% (21/33) in the first month were due to MOF associated with sepsis or poor graft function; and two deaths beyond the 6-month interval but within 1 year after OLT were due to hepatitis C virus (HCV) infection and cryptogenic liver failure.
Fig. 1.
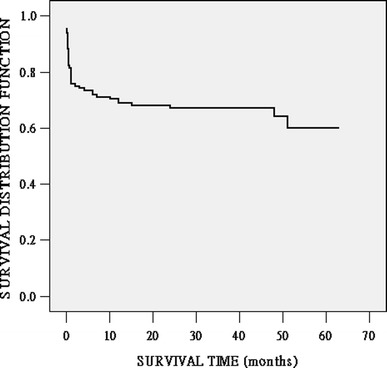
Kaplan–Meier survival for up to 5 years after orthotopic liver transplantation (OLT) for the entire cohort of 137 patients. The curve declined sharply during the first month after OLT and became consistently stable thereafter. The 1-month, 6-month, 1-year, and 5-year patient survival rates after OLT were 75.8, 72.0, 71.0, and 60.1%, respectively
Table 2.
Causes of 46 deaths during the first 5 years after OLT
| Cause of death | No. (%) |
|---|---|
| MOF-PNF | 2 (4.3) |
| MOF-sepsis, IPF | 4 (8.7) |
| MOF-sepsis, pneumonia | 20 (43.5) |
| MOF-sepsis, PSP | 3 (6.5) |
| Liver failure-rejectiona | 2 (4.3) |
| Liver failure, unknown type | 1 (2.2) |
| Liver failure-HAT/biliary complications | 6 (13.0) |
| Hemorrhage-DIC | 3 (6.5) |
| ARDS | 5 (10.9) |
OLT othotopic liver transplantation, MOF multiorgan failure, PNF primary nunfunction, IPF initial poor graft function, PSP preoperative spontaneous peritonitis, HAT hepatic artery thrombosis, DIC diffuse intravascular coagulation, ARDS acute respiratory distress syndrome
aSevere rejection in these two patients occurred after hepatitis C virus (HCV) infection treated with interferon
Biliary complications (BC) occurred in 20 (14.6%) patients; 6 (30.0%) developed graft failure due to HAT, and 2 of them underwent retransplantation within 1 month after OLT. Bile duct complications consisted of intrahepatic strictures along with anastomotic structure (1 patient), isolated anastomotic strictures (9 patients), and bile leaks (4 patients). All of these 14 patients, whose grafts did not fail, were managed with endoscopic retrograde cholangiopancreatography (ERCP) plus stenting or drainage. Univariate and multivariate analysis showed that the BC were probably associated with the use of donors >60 years of age (P = 0.042 and 0.050).
Figure 2 showed the ROC curves generated for the MELD and CTP models. The AUC values were 0.82 (95% CI 0.72–0.92) and 0.68 (95% CI 0.58–0.79) (P < 0.01) for the MELD and CTP models in predicting 1-month mortality after OLT. The larger AUC value for the MELD model suggested that it was a stronger predictor of mortality than the CTP model. Similar results were observed for the MELD and CTP models in predicting 6-month and 1-year mortality after OLT, but the AUC values were relatively smaller. According to the Youden index [11], patients with a preoperative MELD score <33.8 or a CTP score <12.5 had a significantly better prognosis than those with higher scores. The mean survival time was 48.71 ± 2.54 months (95% CI 43.74–53.69) for patients with a MELD score <33.8 versus 23.55 ± 5.02 months (95% CI 13.72–33.38) for other patients with a higher MELD score. In the former group, the actuarial survival rates were 87.1, 84.1, 80.0, and 67.8% at 1 month, 6 months, 1 year, and 5 years, respectively. The corresponding survival figures were 43.0, 37.3, 37.3, and 37.3%, respectively, in the latter group (P < 0.01 by the log-rank test) (Fig. 3). A CTP score of ≥12.5 was also a significant predictor of mortality: The patients 1-month, 6-month, 1-year, and 5-year survival rates were 70.0, 64.9, 61.9, and 54.5%, respectively, whereas the corresponding survival figures were 91.7%, 91.7%, 88.6%, and 71.1%, respectively, for patients with a lower CTP score (P = 0.01 by the log-rank test) (Fig. 4).
Fig. 2.
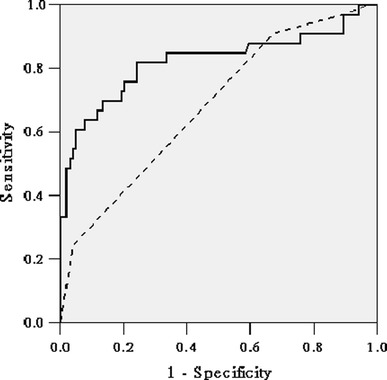
Receiver operating characteristic (ROC) curves for the model for end-stage disease (MELD) and Child-Turcotte-Pugh (CTP) prognostic models in predicting 1-month mortality rates after OLT. Solid line: MELD score; dotted line: CTP score. For the entire cohort of 137 patients, the area under the curve (AUC) values were 0.82 [95% confidence interval (95% CI) 0.72–0.92] for MELD and 0.68 (95% CI 0.58–0.79) for CTP. P < 0.01 for the difference between the AUC values for MELD and CTP
Fig. 3.
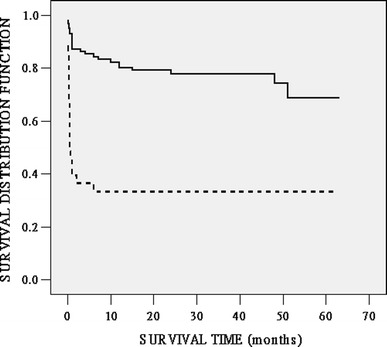
Kaplan–Meier survival function for up to 5 years after OLT according to the preoperative MELD score. Solid line: 101 patients with MELD scores < 33.8; dotted line: 36 patients with MELD scores of ≥ 33.8. The difference in survival rates between these two subgroups was statistically significant (P < 0.01 by the log-rank test)
Fig. 4.
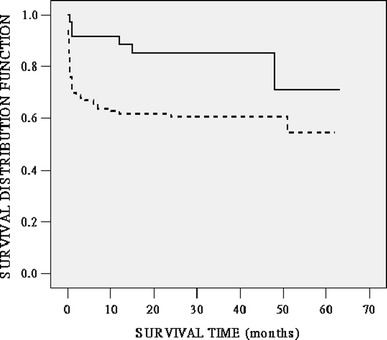
Kaplan–Meier survival function for up to 5 years after OLT according to the preoperative CTP score. Solid line: 40 patients with CTP scores < 12.5; dotted line: 97 patients with CTP scores ≥ 12.5. The difference in survival rates between these two subgroups was statistically significant (P = 0.01 by the log-rank test)
In addition to the MELD and CTP scores, the univariate analysis by log-rank test identified hepatic encephalopathy, preoperative infection, serum creatinine (Scr), total bilirubin, and INR as prognostic predictors (data not shown), whereas sex, total bilirubin, albumin, serum Na+, serum α-fetoprotein (AFP), alanine aminotransferase, γ-glutamyltransferase, INR, proportion of sex/serotype mismatch, and cold/warm ischemia time were not statistically significant. Cox proportional hazards regression analysis showed that independent predictors of 5-year mortality after OLT included the presence of hepatic encephalopathy (P = 0.045), preoperative infection (P = 0.018), Scr ≥ 1.5 mg/dl (P = 0.022), MELD ≥ 33.8 (P = 0.001), and CTP ≥ 12.5 (P = 0.037) (Table 3). Statistical significance was marginal with the recipient and donor age (P = 0.056, 0.052, respectively) and ascites (P = 0.069). Because most patients died during the early stage after OLT, Cox proportional hazards regression analysis was conducted again, and the same five independent predictors for 1-month and 1-year mortality were confirmed.
Table 3.
Cox multivariate analysis of predictors of patients mortality after OLT
| Predictor variables | Hazard ratio (95% CI) | P |
|---|---|---|
| Recipient variablesa | ||
| Age > 50 years | 2.031 (0.875–4.174) | 0.056 |
| Ascites | 1.377 (0.250–2.487) | 0.069 |
| Hepatic encephalopathy | 2.817 (0.180–3.556) | 0.045 |
| Preoperative infection | 4.799 (2.341–13.552) | 0.018 |
| Creatinine ≥ 1.5 mg/dl | 3.980 (1.025–9.294) | 0.022 |
| MELD ≥ 33.8 | 5.600 (3.130–6.017) | 0.001 |
| CTP ≥ 12.5 | 3.110 (1.500–7.023) | 0.037 |
| Donor variables | ||
| Age > 60 years | 2.714 (0.470–7.259) | 0.052 |
aNone of the recipients required mechanical ventilation within 1 week before OLT
OLT orthotopic liver transplantation, MELD model for end-stage disease, CTP Child-Turcotte-Pugh
Discussion
The incidence of viral hepatitis B infection in the Chinese population is about 10% [12]. Every year, 1–3% of patients deteriorate into CSH, where liver functions—particularly detoxification, synthetic functions, and metabolic regulation—are impaired to different degrees and may result in life-threatening complications such as hepatic encephalopathy, bleeding, and hepatorenal syndrome [13]. So far, treatment is mainly combined medications [14, 15], artificial liver support [16], and OLT, which has obviously improved the prognosis [1].
In an era of a critical shortage of graft material, one of the most important issues facing the liver transplant community is how to allocate the grafts appropriately. A logical approach to patients with CSHB is stratification according to the risk of dying after OLT. Srikureja et al. [17] found that the MELD score was a better prognostic model than the CTP score in patients with alcoholic hepatitis. Different from the West, the main etiology for chronic liver failure in China is hepatitis B, but the impact of the preoperative MELD or CTP score on survival after OLT for CSHB has not yet been evaluated adequately.
According to our results, the 5-year patient mortality rate was 33.6%, which was significantly lower than that for conventional medical treatment for CSH, for which the reported 3-month mortality rate was 47.4% [18]. Interestingly, the survival curve declined sharply during the first month after OLT and became consistently stable thereafter (Fig. 1), indicating that most patients died during the peroperative period. As expected, those patients who died had significantly higher MELD (35.17 ± 11.00 vs. 26.76 ± 6.76, p < 0.001) and CTP (13.12 ± 0.65 vs. 12.59 ± 0.78, p = 0.001) scores than those of survivors. This was supported by Yao et al. [19], who found patients with a MELD score ≥ 25 or a CTP score ≥ 10 had poorer prognosis after re-OLT. Recently, Wang et al. [4] found the MELD and CTP scores for the patients who died were significantly higher than those for the survivors. In their study, all patients demonstrated clinical features of chronic type B hepatitis; patients with fulminant hepatic failure or subacute fulminant hepatitis as a result of acute hepatitis B infection were not included. The ROC analysis identified the best cutoff point for them to be 25.67 (AUC 0.841; sensitivity 85.7%; specificity 60.0%) and 11.5 (AUC 0.747; sensitivity 85.7%; specificity 54.3%), respectively, to predict postoperative short-term survival and 3-month morbidity among patients with acute-on-chronic hepatitis B liver failure (which is defined as a syndrome with severe liver dysfunction and at least grade II encephalopathy for patients with chronic liver disease) undergoing OLT.
These results revealed that patients with more advanced liver disease, based on either a high preoperative MELD score or CTP score, had a worse postoperative outcome than patients with less advanced disease. Certainly, there are complex technical and medical factors, in addition to disease severity alone, that may be associated with early mortality after OLT. For example, the potential bias and subjectivity in retrospectively determining individual CTP scores, primarily with respect to the degree of ascites and hepatic encephalopathy [20]. However, the MELD score seemed to be more preferable in our current study. First, MELD gives a continuous liver disease severity score that can be easily applied to rank patients on the waiting list according to their risk of dying over a defined period of time. It also ranks patients more specifically according to the severity of their disease [3]. In our study, the patients with a MELD score ≥ 33.8 had significantly lower short-term (1-month and 6-month) survival rates than did patients with a CTP score ≥ 12.5, which indicated that a high MELD score had a more compacted correlation with early death after OLT [6, 21].
Many studies have verified hepatic encephalopathy, serum creatinine, and preoperative infection as independent factors that influence the mortality of patients with severe hepatitis [22–26]. Considering that most patients died of MOF in our study due to sepsis caused by a preoperative pulmonary infection or spontaneous peritonitis or respiratory failure caused by ARDS, the peroperative management against infection should be enhanced. Our results also differed from several other studies that found advanced recipient age [26–28], serum sodium level [18], and bilirubin level [27–29] to be significant predictors of mortality after OLT. The limited sample of patients and possible selection bias are the most likely explanations for these differences.
More recently, de Vera et al. [30] concluded that compared to brain death donors similar patient survival can be achieved with DCDs under favorable conditions, including donor age ≤ 60 years, DWIT ≤ 15 minutes, and CIT ≤ 8 hours. In their study, primary nonfunction/delayed graft function (PNF/DGF) and BC cumulatively accounted for 67% of early graft failures, and recurrent disease (30%) and BC (20%) mainly caused late (> 1 year after OLT) graft failure. However, the incidence of BC in our study was significantly lower than that of theirs (14.6% vs. 25%), and it caused only 13% (6/46) graft loss during the early stage after OLT. This was probably due to the fact that most DCDs in our study enjoyed shorter DWIT and CIT, which were considered to be the risk factors for BC and graft failure [30–32]. However, the lack of a standard definition of the DWIT has been problematic [33], making studies that utilize UNOS data difficult to interpret, confounding comparisons of individual DCD studies, and preventing valid recommendations of a DWIT ceiling above which would predict poor outcomes. Our definition of DWIT was unambiguous—from the time of cardiac arrest of the donor to perfusion of the abdominal aorta. We did not use a blood pressure threshold below which would define the beginning of the DWIT as others have [34, 35], as tissues are still hypoxic in DCDs who maintain a blood pressure but cease to ventilate. However, further reducing both DWIT and CITas well as possibly reducing the donor age might improve results [28].
In addition, Tojimbara et al. [36] demonstrated in a rat model of non-heart-beating donors (NHBDs) LT that preflush with low-viscosity solution decreases the vascular resistance, avoids ischemia-reperfusion injury, and improves survival. A clinical study [37] indicated that high-viscosity preservation solutions cause an increased incidence of biliary strictures compared with low-viscosity solutions. This is probably caused by inadequate clearance of the small peribiliary capillaries, a phenomenon to which liver grafts from NHBDs may be particularly vulnerable because of the stagnation of blood during warm ischemia [38]. Therefore, the main usage of low-viscosity modified HC-A solution and the common bile duct infusion with cold normal saline for DCDs in our study probably contributed to the low incidence of BC.
Conclusions
Nearly three-fourths of the patients with CSHB died during the first month after OLT, which was mainly caused by MOF. The MELD score was superior to the CTP score in predicting 1-month survival after OLT. In an era when grafts are a scarce resource, utilizing the MELD scoring system for pretransplant assessment is recommended for patients with CSHB, and the DCD has become an important source of grafts. Peroperative management against hepatic encephalopathy, infection, and hepatorenal syndrome should be enhanced to improve prognosis. However, to gain more precise results requires long-term follow-up in a larger sample size or even multicenter research.
Acknowledgment
We thank Gui-Hua Wang for providing technical support in preparing this paper.
References
- 1.Marrero J, Martinez FJ, Hyzy R. Advances in critical care hepatology. Am J Respir Crit Care Med. 2003;168:1421–1426. doi: 10.1164/rccm.200303-361UP. [DOI] [PubMed] [Google Scholar]
- 2.Ostapowicz G, Lee WM. Acute hepatic failure: a Western perspective. J Gastroenterol Hepatol. 2000;15:480–488. doi: 10.1046/j.1440-1746.2000.02074.x. [DOI] [PubMed] [Google Scholar]
- 3.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 4.Wang ZX, Yan LN, Wang WT, et al. Impact of pretransplant MELD score on posttransplant outcome in orthotopic liver transplantation for patients with acute-on-chronic hepatitis B liver failure. Transplant Proc. 2007;39:1501–1504. doi: 10.1016/j.transproceed.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 5.Chinese Society of Infectious Diseases and Chinese Society of Hepatology, CMA Prevention and treatment program for viral hepatitis. Chin J Infect Dis. 2001;1:56–62. [Google Scholar]
- 6.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 7.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 8.Gitlin N. Hepatic encephalopathy. In: Zakim D, Boyer TD, editors. Hepatology: a textbook of liver disease. 3. Philadelphia: Saunders; 1996. pp. 605–617. [Google Scholar]
- 9.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 10.De Long ER, De Long DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristics curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 11.Schisterman EF, Faraggi D, Reiser B, et al. Youden Index and the optimal threshold for markers with mass at zero. Stat Med. 2008;27:297–315. doi: 10.1002/sim.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Department of Communicable Diseases Surveillance and Response: Hepatitis B. WHO/CDS/CSR/LYO/2002.2: Hepatitis B. WHO, Geneva
- 13.Polson J, Lee WM, American Association for the Study of Liver Disease AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 14.Heidelbaugh JJ, Sherbondy M. Cirrhosis and chronic liver failure. Part II. Complications and treatment. Am Fam Physician. 2006;74:767–776. [PubMed] [Google Scholar]
- 15.Xu JH, Yu YY, Si CW, et al. Promoting hepatic growth factor in the treatment of heavy type hepatitis and severe chronic hepatitis: a multicenter clinical study. Hepatobiliary Pancreat Dis Int. 2004;3:381–385. [PubMed] [Google Scholar]
- 16.Patzer JF, II, Lopez RC, Zhu Y, et al. Bioartificial liver assist devices in support of patients with liver failure. Hepatobiliary Pancreat Dis Int. 2002;1:18–25. [PubMed] [Google Scholar]
- 17.Srikureja W, Kyulo NL, Runyon BA, et al. MELD score is a better prognostic model than Child-Turcotte-Pugh score or discriminant function score in patients with alcoholic hepatitis. J Hepatol. 2005;42:700–706. doi: 10.1016/j.jhep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Yuan GY, Tang KC, et al. Prognostic factors for chronic severe hepatitis and construction of a prognostic model. Hepatobiliary Pancreat Dis Int. 2008;7:40–44. [PubMed] [Google Scholar]
- 19.Yao FY, Saab S, Bass NM, et al. Prediction of survival after liver retransplantation for late graft failure based on preoperative prognostic scores. Hepatology. 2004;39:230–238. doi: 10.1002/hep.20005. [DOI] [PubMed] [Google Scholar]
- 20.Forman LM, Lucey MR. Predicting the prognosis of chronic liver disease: an evolution from Child to MELD. Hepatology. 2001;33:473–475. doi: 10.1053/jhep.2001.22481. [DOI] [PubMed] [Google Scholar]
- 21.Sumskiene J, Kupcinskas L, Pundzius J, et al. Prognostic factors for short and long-term survival in patients selected for liver transplantation. Medicina (Kaunas) 2005;41:39–46. [PubMed] [Google Scholar]
- 22.Lai N, Guo SH, Zhang DZ, et al. A single factor analysis of the prognosis of 301 hepatitis failure cases and a study of a scoring system on their prognostic assessment. Zhonghua Gan Zang Bing Za Zhi. 2005;13:586–589. [PubMed] [Google Scholar]
- 23.Ding HG, Xiang HP, Shan J, et al. A scoring model for predicting the prognosis of severe viral hepatitis. Chin Med J (Engl) 2005;118:249–251. [PubMed] [Google Scholar]
- 24.Farmer DG, Anselmo DM, Ghobrial RM, et al. Liver transplantation for fulminant hepatic failure: experience with more than 200 patients over a 17-year period. Ann Surg. 2003;237:666–675. doi: 10.1097/00000658-200305000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duran FG, Piqueras B, Romero M, et al. Pulmonary complications following orthotopic liver transplant. Transpl Int. 1998;11(Suppl 1):S255–S259. doi: 10.1111/j.1432-2277.1998.tb01127.x. [DOI] [PubMed] [Google Scholar]
- 26.Li XM, Ma L, Yang YB, et al. Analyses of prognostic indices of chronic liver failure caused by hepatitis virus. World J Gastroenterol. 2005;11:2841–2843. doi: 10.3748/wjg.v11.i18.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong T, Devlin J, Rolando N, et al. Clinical characteristics affecting the outcome of liver retransplantation of the liver. Transplantation. 1997;64:878–882. doi: 10.1097/00007890-199709270-00015. [DOI] [PubMed] [Google Scholar]
- 28.Rosen HR, Madden JP, Martin P. A model to predict survival following liver retransplantation. Hepatology. 1999;29:365–370. doi: 10.1002/hep.510290221. [DOI] [PubMed] [Google Scholar]
- 29.Doyle HR, Morelli F, McMichael J, et al. Hepatic retransplantation: an analysis of risk factors associated with outcome. Transplantation. 1996;61:1499–1505. doi: 10.1097/00007890-199605270-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Vera ME, Lopez-Solis R, Dvorchik I, et al. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant. 2009;9:773–781. doi: 10.1111/j.1600-6143.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee KW, Simpkins CE, Montgomery RA, et al. Factors affecting graft survival after liver transplantation from donation after cardiac death donors. Transplantation. 2006;82:1683–1688. doi: 10.1097/01.tp.0000250936.73034.98. [DOI] [PubMed] [Google Scholar]
- 32.Zheng S, Feng X, Qing D, et al. The tolerance time limits of biliary tracts of liver grafts subjected to warm ischemia and cold preservation: an experimental study in swine. Transplant Proc. 2008;40:1629–1634. doi: 10.1016/j.transproceed.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 33.Bernat JL, D D’Alessandro AM, Port FK, et al. Report of a national conference on donation after cardiac death. Am J Transplant. 2006;6:281–291. doi: 10.1111/j.1600-6143.2005.01194.x. [DOI] [PubMed] [Google Scholar]
- 34.Chan EY, Olson LC, Kisthard JA, et al. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604–610. doi: 10.1002/lt.21361. [DOI] [PubMed] [Google Scholar]
- 35.Muiesan P, Girlanda R, Jassem W, et al. Single-center experience with liver transplantation from controlled non-heartbeating donors. Ann Surg. 2005;242:732–738. doi: 10.1097/01.sla.0000186177.26112.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tojimbara T, Wicomb WN, Garcia-Kennedy R, et al. Liver transplantation form non-heart beating donors in rats: influence of viscosity and temperature of initial flushing solutions on graft function. Liver Transpl Surg. 1997;3:39–45. [PubMed] [Google Scholar]
- 37.Pirenne J, Van Gelder F, Coosemans W, et al. Type of donor aortic preservation solution and not cold ischemia time is a major determinant of biliary strictures after liver transplantation. Liver Transpl. 2001;7:540–545. doi: 10.1053/jlts.2001.24641. [DOI] [PubMed] [Google Scholar]
- 38.Monbaliu D, Crabbé T, Roskams T, et al. Livers from non-heart-beating donors tolerate short periods of warm ischemia. Transplantation. 2005;79:1226–1230. doi: 10.1097/01.TP.0000153508.71684.99. [DOI] [PubMed] [Google Scholar]


