Abstract
Introduction
Rapid worldwide spread of Coronavirus Disease 2019 (COVID-19) has resulted in a global pandemic.
Objective
This review article provides emergency physicians with an overview of the most current understanding of COVID-19 and recommendations on the evaluation and management of patients with suspected COVID-19.
Discussion
Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for causing COVID-19, is primarily transmitted from person-to-person through close contact (approximately 6 ft) by respiratory droplets. Symptoms of COVID-19 are similar to other viral upper respiratory illnesses. Three major trajectories include mild disease with upper respiratory symptoms, non-severe pneumonia, and severe pneumonia complicated by acute respiratory distress syndrome (ARDS). Emergency physicians should focus on identifying patients at risk, isolating suspected patients, and informing hospital infection prevention and public health authorities. Patients with suspected COVID-19 should be asked to wear a facemask. Respiratory etiquette, hand washing, and personal protective equipment are recommended for all healthcare personnel caring for suspected cases. Disposition depends on patient symptoms, hemodynamic status, and patient ability to self-quarantine.
Conclusion
This narrative review provides clinicians with an updated approach to the evaluation and management of patients presenting to the emergency department with suspected COVID-19.
Keywords: Coronavirus Disease, COVID-19, Infectious disease, Pulmonary
1. Introduction
On January 30, 2020, the World Health Organization (WHO) designated an outbreak of a novel coronavirus not seen before in humans to be a “public health emergency of international concern” (PHEIC); this was followed by the declaration of a pandemic on March 11, 2020 [1,2]. Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), previously referred to as 2019-nCoV, is the virus responsible for causing Coronavirus Disease 2019 (COVID-19) [[3], [4], [5], [6], [7]]. The pandemic traces its early beginnings to the report of a cluster of 27 unexplained pneumonia cases in late December 2019 originating from a seafood and live animal market in Wuhan, Hubei Province, China [[8], [9], [10], [11]]. From the outset, the causative agent was thought to be viral, with most patients reporting fever or dyspnea [9,11]. With unprecedented numbers of individuals under travel restrictions or quarantine, worldwide spread, and no known cure or vaccine yet available, COVID-19 has proven a formidable adversary [12,[13], [14], [15]].
The Ebola Virus Disease (EVD) outbreak of 2014 in West Africa provided valuable lessons with regards to emergency preparedness, personal protective equipment use, and triage processes and underscored the important role that emergency physicians play on the frontlines of emerging infectious diseases [[16], [17], [18]]. We describe the virology, epidemiology, clinical presentation, radiographic and laboratory findings, current testing protocols, and management of patients presenting with COVID-19 to the emergency department (ED). In this review article, we provide emergency physicians with best practices based on the rapidly evolving body of literature surrounding COVID-19.
2. Discussion
2.1. Virology
SARS-CoV-2 is a member of the coronavirus family, named for the crown-like appearance of spikes on the virus surface [5,19]. Other members of the coronavirus family include Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and SARS-CoV-1, as well as coronaviruses responsible for the common cold (Fig. 1, Fig. 2 ) [5,6,8,19]. Like MERS-CoV and SARS-CoV-1, SARS-CoV-2 is a betacoronavirus and is likely associated with an animal reservoir (e.g., bats) [6,8,14]. While an exact animal source has not been confirmed for COVID-19, many of the early cases in China were linked to a live animal and seafood market [6,14,20,21].
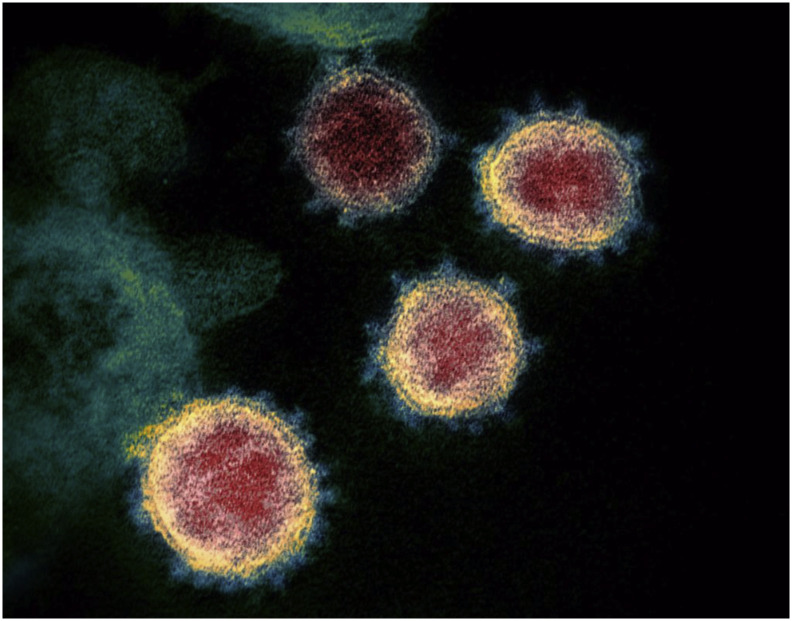
Fig. 1.
Transmission electronic microscope image of COVID-19 illustrating the characteristic crown-like spikes on the outer rim.
Source: National Institute of Allergy and Infectious Diseases-Rocky Mountain Laboratories (NIAID-RML). Novel Coronavirus SARS-CoV-2.; 2020. https://www.flickr.com/photos/niaid/49534865371/. Accessed February 18, 2020.
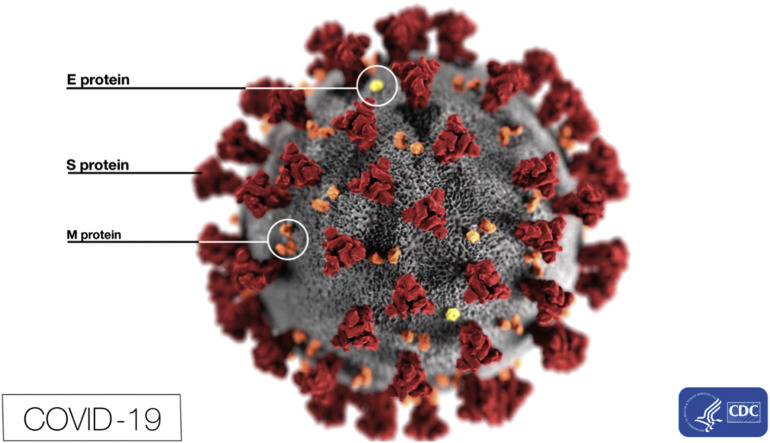
Fig. 2.
This electron microscope image depicts the spikes on the outer surface of the COVID-19 in addition to several protein particles.
Eckert A, Higgins D, Centers for Disease Control and Prevention. ID# 23313; 2020. https://phil.cdc.gov/Details.aspx?pid=23313. Accessed February 18, 2020.
2.2. Epidemiology
The majority of initial COVID-19 cases were associated with travel to Hubei Province, China; however, a growing number of cases due to person-to-person transmission have been reported both in and outside of China (Fig. 3 ) [8,14,22,23]. Up to 94% of COVID-19 cases were reported to originate from Hubei Province in December 2019; as of March 2020, the greatest number of new cases are now being reported in Italy, Spain, Germany, and the United States (U.S.) (Fig. 3, Fig. 4 ) [[24], [25], [26]].
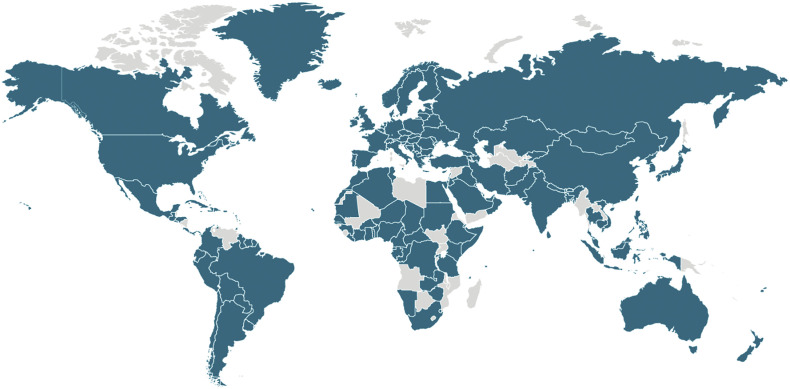
Fig. 3.
Countries with at least 1 confirmed case of COVID-19 as of March 21, 2020.
Available from https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/world-map.html.
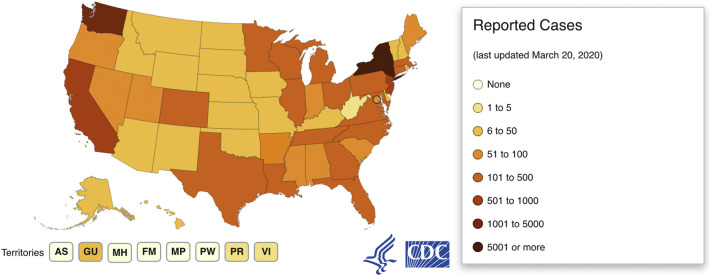
Fig. 4.
United States cases as of March 21, 2020.
Available from https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
Based on what is known about other coronaviruses, experts believe COVID-19 primarily spreads from person-to-person through close contact (approximately 6 ft) by respiratory droplets [4,8,14,23,27]. Transmission of the virus through contaminated surfaces or fomites with subsequent contact with the eyes, nose, or mouth may also occur [14,23,27]. Patients are felt to be at highest risk of spreading the illness when they are most symptomatic [23,27]. Limited data support viral shedding in asymptomatic patients while increased levels of viral shedding may be more pronounced in those critically ill [[28], [29], [30]]. Current epidemiologic patterns of COVID-19 in China indicate that it is highly contagious with sustained spread; the extent of person-to-person transmission within the U.S. was initially limited but has progressed now to community transmission in many parts of the country [23]. The current R0 or basic reproduction number is estimated to be >2.2; for every case of COVID-19 identified in the population, >2 additional cases are possible in the absence of adequate isolation. [20,[31], [32], [33]]
In an early epidemiologic analysis of 425 COVID-19 cases in Wuhan, China, the median patient age was 59 years, and 56% were male [20]. In the largest study to date of COVID-19, comprising over 72,000 patient records (up to February 11, 2020) from China, 86.6% of patients were 30–79 years of age [34]. While 80.9% of these cases were reported to be mild, the overall case fatality rate was 2.3% [34]. Few pediatric cases of COVID-19 have been reported, with patients aged 0–19 years representing just 2.1% of all cases [34,35]. Approximately 3.8% of laboratory confirmed cases of COVID-19 occurred in healthcare personnel, and 14.6% of these cases were either severe or critical [34]. To be classified as severe, the following characteristics were required: PaO2/FiO2 < 300, oxygen saturation ≤ 93%, presence of >50% lung infiltrates within 24–48 h, respirations ≥30 breaths/min, or dyspnea [34]. Critical patients, defined as those with septic shock, multiple organ dysfunction/failure, and/or respiratory failure, accounted for approximately 5% of the study population with a case fatality rate of 49.0% [34,24]. The highest case fatality rate was observed in those older than 80 years (14.8%) [34]. Patients without comorbidities had a case fatality rate of just 0.9%, in contrast to those with comorbid conditions such as cardiovascular disease (10.5%) [34].
Caution should be exercised in interpreting early findings, as underreporting and variable testing practices have been a concern with COVID-19 [36,37]. Case fatality rates in Hubei Province have been reported to be 18% (95% confidence interval [CI] 11–18), while those outside mainland China range from 1.2–5.1% [38]. Mortality rates have been calculated to be as high as 11–15% [39,40]. When compared to other recent epidemics such as SARS (9.56) or EVD (39.53), the average case fatality rate (4.2%) for COVID-19 is much lower (Table 1 ) [41,42]. In comparison, the 2009 H1N1 influenza pandemic and 2017 influenza season were responsible for approximately 220 times more cases [41,42].
Table 1.
Comparison of epidemiological characteristics of recent global epidemics as of March 21, 2020 [41,4].
| Disease | Case fatality rate | Deaths | Cases |
|---|---|---|---|
| COVID-19 (2019) | 4.2 | 11,299 | 272,166 |
| Influenza (2017)a | N/A | 145,000 | 54,481,000 |
| Ebola (2014) | 39.53 | 11,323 | 28,646 |
| H1N1 (2009) | 0.1 | 18,449 | 60,800,000 |
| SARS (2003) | 9.56 | 774 | 8096 |
Estimated global burden of disease from influenza-related lower respiratory tract infections; excludes cases with alternative causes (i.e. cardiac).
Based on what is known about similar coronaviruses, the longest potential incubation period for COVID-19 is thought to be 14 days from initial exposure [4,6]. The mean incubation period is 5.2 days (95% CI 4.1–7.0) but can range from 2 to 14 days [4,20,43]. Co-infections occur in 22–33% of patients and may be higher in critical patients [44,45].
2.3. Special populations
Risk factors for severe COVID-19 disease include advanced age, chronic medical conditions, immunocompromise, and cancer [46].
Data regarding pregnancy and COVID-19 are limited [47]. Pregnant women and fetuses may be more vulnerable to COVID-19 infection compared to the general population [48]. There are case reports of pregnant women diagnosed with COVID-19 complicated by adverse outcomes including preterm birth [47]. Historically, infants born to mothers with other coronaviruses such as MERS-CoV or SARS-CoV-1 have been small for gestational age or preterm [47]. Newborn infants are also an at-risk population [48].
Occupational exposure to pathogens is an inherent risk of working in healthcare settings [49]. During the 2003 SARS outbreak, 9% of healthcare professionals (HCPs) in Toronto, Canada participating in endotracheal intubation of infected patients became infected themselves [50]. In another study, 77% of SARS patients in Toronto had ties to the hospital setting, and 51% of cases were HCPs [51]. During the H1N1 influenza pandemic, HCPs were significantly more likely to develop infection (odds ratio [OR] 2.08, 95% CI 1.73–2.51) with a pooled prevalence of 6.3% [52]. As COVID-19 has disproportionately affected HCPs, emergency physicians must be vigilant about potential exposure risks and adhere to appropriate infection prevention precautions [53,54]. In Italy, anywhere from 8 to 30% of HCPs have been infected with SARS-CoV-2 [55,56].
2.4. Clinical presentation
The symptoms of COVID-19 are similar to other viral upper respiratory illnesses and include fever, cough, fatigue, and dyspnea [6,8,43]. The differential diagnosis for COVID-19 should be tailored to the patient and their presenting symptoms and comorbidities. Influenza, respiratory syncytial virus (RSV), other viral illnesses, and bacterial pneumonia should be considered, as well as other pulmonary diseases (ie, pulmonary embolism). Completing a thorough yet focused history and physical examination and obtaining collateral history from family members are vital. Aside from pulmonary symptoms, patients with COVID-19 may initially present with more vague complaints including diarrhea, lethargy, myalgias, and nausea [40,57]. Patients may also experience headache, confusion, vomiting, pleurisy, sore throat, sneezing, rhinorrhea, and nasal congestion [40,58]. A case series of 41 patients (median age 49.0 years) with COVID-19 from Wuhan, China found the most commonly reported symptoms were cough (76%), fever (98%), or dyspnea (55%) [39]. In the same case series, patients also reported myalgias/fatigue (44%), productive cough (28%), headache (8%), hemoptysis (5%), and diarrhea (3%) [39]. In a nationwide study of COVID-19 cases from across China, the most common presenting symptoms included cough (68%), fever (44%), fatigue (38%), sputum production (34%), and shortness of breath (19%) [59]. Fever was not a predominant symptom at the time of initial presentation. In patients with more severe disease, dyspnea may be present in 37% of patients and progress to acute lung injury in 15% of patients [60]. One study of 204 patients with confirmed COVID-19 suggests 48.5% of patients have gastrointestinal (GI) symptoms [61]. These symptoms may include anorexia (83.8%), diarrhea (29.3%), vomiting (0.8%), and abdominal pain (0.4%). Seven of the 204 patients had only GI symptoms with no respiratory symptoms [61].
Atypical presentations of infection in general may be more likely in the elderly and immunocompromised, who may not mount a febrile response [62,63]. To increase sensitivity and identify potential COVID-19 patients sooner, the U.S. Centers for Disease Control and Prevention (CDC) recommends using a temperature cutoff of 100.0 Fo [64]. Patients older than 60 years of age and those with comorbidities may also present with more severe disease compared to other populations [58].
Three major trajectories for COVID-19 have been described: mild disease with upper respiratory symptoms, non-severe pneumonia, and severe pneumonia complicated by acute respiratory distress syndrome (ARDS) necessitating aggressive resuscitative measures [65]. Anywhere from 17 to 29% of patients may develop ARDS [39,40]. Other complications of COVID-19 include secondary bacterial infection, acute kidney injury, septic shock, ventilator-associated pneumonia, and cardiac injury [39,40].
2.5. Initial approach to COVID-19 in the emergency department
An emergency medicine approach to COVID-19 should focus on identifying and isolating patients at risk for infection, informing hospital infection prevention and local public health authorities, and engaging infectious disease and other specialists early in care. The World Health Organization (WHO) has established case and contact definitions for COVID-19 to standardize global surveillance (Table 2 ). Most patients with confirmed COVID-19 have had a subjective or confirmed fever and/or symptoms of acute respiratory illness (e.g., cough, difficulty breathing) [66].
Table 2.
World Health Organization (WHO) case and contact definitions for global surveillance of COVID-19.
| Suspected case A. A patient with acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g., cough, shortness of breath), AND a history of travel to or residence in a location reporting community transmission of COVID-19 disease during the 14 days prior to symptom onset; OR B. A patient with any acute respiratory illness AND having been in contact with a confirmed or probable COVID-19 case (see definition of contact) in the last 14 days prior to symptom onset; OR C. A patient with severe acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g., cough, shortness of breath; AND requiring hospitalization) AND in the absence of an alternative diagnosis that fully explains the clinical presentation. |
| Probable case A. A suspect case for whom testing for the COVID-19 virus is inconclusive. OR B. A suspect case for whom testing could not be performed for any reason. |
| Confirmed case A person with laboratory confirmation of COVID-19 infection, irrespective of clinical signs and symptoms. See laboratory guidance for details: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technicalguidance/laboratory-guidance |
| Contact A contact is a person who experienced any one of the following exposures during the 2 days before and the 14 days after the onset of symptoms of a probable or confirmed case:
|
Note: For confirmed asymptomatic cases, the period of contact is measured as the 2 days before through the 14 days after the date on which the sample was taken which led to confirmation.
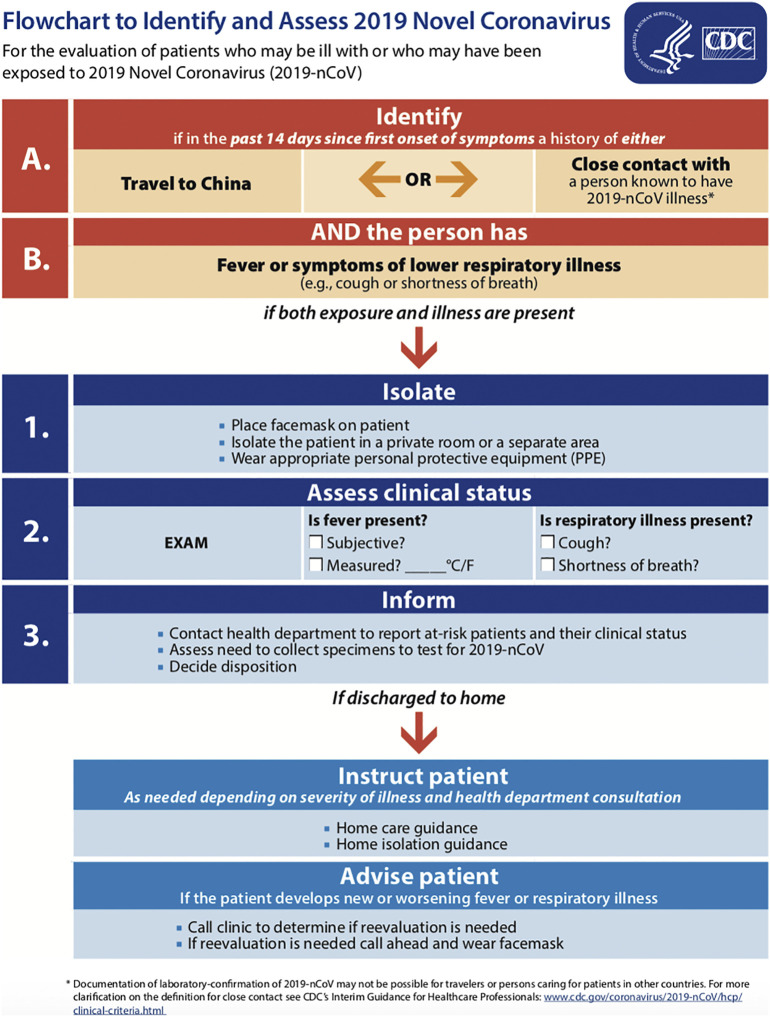
In concert with clinician judgment regarding patient presentations compatible with COVID-19, CDC guidelines prioritize patients from defined populations for further evaluation and testing as persons under investigation (PUI) (Table 3 ). These criteria are not exhaustive, and patients with an unestablished etiology or equivocal history of exposure may be considered for further testing on an individual basis [67]. Confirmed local COVID-19 cases in the setting of known community transmission should reduce the threshold for further COVID-19 evaluation in the ED. Collaboration with local and state public health departments is strongly recommended [62,67]. A PUI should be asked to wear a facemask to reduce risk of transmission to others in the immediate vicinity. Fig. 5 details CDC recommendations for identifying and assessing suspected COVID-19.
Table 3.
Patient populations that should be prioritized for evaluation of COVID-19 in the setting of compatible signs and symptoms. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html
| 1. Hospitalized patients who have signs and symptoms compatible with COVID-19 in order to inform decisions related to infection control. 2. Other symptomatic individuals such as, older adults and individuals with chronic medical conditions and/or an immunocompromised state that may put them at higher risk for poor outcomes (e.g., diabetes, heart disease, receiving immunosuppressive medications, chronic lung disease, chronic kidney disease). 3. Any persons including healthcare personnela, who within 14 days of symptom onset had close contactb with a suspect or laboratory-confirmedc COVID-19 patient, or who have a history of travel from affected geographic areasd within 14 days of their symptom onset. |
|
Notes: aFor healthcare personnel, testing may be considered if there has been exposure to a person with suspected COVID-19 without laboratory confirmation. Because of their often extensive and close contact with vulnerable patients in healthcare settings, even mild signs and symptoms (e.g., sore throat) of COVID-19 should be evaluated among potentially exposed healthcare personnel. Additional information is available in CDC’s Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19). bClose contact is defined as— a) being within approximately 6 feet (2 meters) of a COVID-19 case for a prolonged period of time; close contact can occur while caring for, living with, visiting, or sharing a healthcare waiting area or room with a COVID-19 case – or – b) having direct contact with infectious secretions of a COVID-19 case (e.g., being coughed on) If such contact occurs while not wearing recommended personal protective equipment or PPE (e.g., gowns, gloves, NIOSH-certified disposable N95 respirator, eye protection), criteria for PUI consideration are met. cDocumentation of laboratory-confirmation of COVID-19 may not be possible for travelers or persons caring for COVID-19 patients in other countries. dAffected areas are defined as geographic regions where sustained community transmission has been identified. For a list of relevant affected areas, see CDC’s Coronavirus Disease 2019 Information for Travel. |
Fig. 5.
Flowchart to Identify and Assess 2019 Novel Coronavirus from the CDC.
Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/2019-nCoV-identify-assess-flowchart-508.pdf. Accessed February 26, 2020.
2.5.1. Pre-hospital setting
Emergency medical services (EMS) directors and public health authorities working in conjunction with the CDC will need to modify emergency preparedness strategies to address COVID-19 [68]. Emergency medical dispatchers should consider whether callers describing risk factors and symptoms concerning for COVID-19 should be identified as a potential PUI [30,68]. If so, EMS personnel arriving on-scene as well as HCPs at the receiving hospital should be notified immediately to ensure proper personal protective equipment (PPE) use and confirm that appropriate isolation facilities are available [30,68]. Once contact is made with the patient, initial triage and assessment should be done at least 6 ft or 2 meters away and minimized until the PUI dons a facemask [68]. In addition to limiting the number of EMS personnel in the patient compartment, those providing any direct patient care should follow standard, droplet (surgical mask), and contact precautions (gown and gloves) while wearing eye protection (face shield or goggles) [68]. Airborne precautions (N95 respirator) should be employed if the patient is critically ill and/or if an aerosol-generating procedure is anticipated during transport. Ideally, transport vehicles with isolated compartments or high efficiency particulate air (HEPA) filtration should be used, and the patient should be transferred directly to a treatment room on arrival at the receiving healthcare facility [68]. After the patient has been transported and EMS documentation is being completed, patient compartment doors should be left open to allow proper ventilation [68]. When cleaning the vehicle, disposable gown, gloves, surgical mask, and face shield should be worn [68]. Routine cleaning should be followed by application of a hospital-grade disinfectant, preferably one approved by the U.S. Environmental Protection Agency (EPA) for use against emerging viral pathogens including SARS-CoV-2 [68].
2.5.2. Emergency department setting
Patients presenting with symptoms concerning for COVID-19 to the ED should be separated from other patients by at least 6 ft or 2 m and asked to wear a facemask [62,69]. Ideally, stable COVID-19 PUIs would be identified at time of check-in or triage and then placed in a private room with the door closed [30,64]. Critically ill patients and those requiring aerosol-generating procedures should be placed in an airborne infection isolation room (AIIR), also known as a negative pressure isolation room, with HEPA filtration of the recirculated air [30,69]. Once a PUI is identified, the appropriate health department or agency and institutional personnel should be notified in an expeditious manner [62,67]. Movement into and out of the patient's treatment room should be limited to only essential HCPs involved in patient care [69]. While in the room, the PUI may remove their facemask [69]. However, it is reasonable to ask the patient to wear a facemask during interactions with HCPs (e.g., performing a physical examination) in the room as tolerated to contain respiratory droplets generated from coughing.
HCPs should either use alcohol-based hand sanitizer or wash their hands with soap and water before and after contact with a COVID-19 PUI [62,69]. They should be trained in the appropriate use of PPE per hospital guidelines, including techniques to safely doff equipment protecting mucous membranes [69]. When caring for a stable PUI, HCPs should adhere to droplet (surgical mask), contact (gown and gloves), and standard precautions with the addition of eye protection (face shield or goggles) [8,57,62,64,69]. If a PUI is critically ill or an aerosol-generating procedure (e.g., endotracheal intubation, suctioning of the airway, sputum induction) is necessary, HCPs should escalate to airborne precautions with the use of a fitted N95 respirator in place of a surgical mask [62,69,70]. Reusable respirators such as powered air purifying respirators (PAPRs) may also be used, but should be disinfected and maintained appropriately [69]. Patients with a history of COVID-19 exposure presenting with non-infectious symptoms may be evaluated and treated in adherence to standard precautions alone [64].
If portable studies (e.g., plain radiography) cannot be completed within the patient's room or the patient requires transport elsewhere within the ED or hospital by wheelchair or stretcher, HCPs should don appropriate PPE [64,70]. Healthcare professionals at the destination or receiving location should be made aware of the patient's arrival and likewise don appropriate PPE [64,71]. Patients leaving their treatment room should wear a facemask, be dressed in a clean hospital gown (when possible), perform hand hygiene, and be educated in proper respiratory hygiene [71].
Personnel cleaning empty PUI rooms should follow droplet, contact, and standard precautions with eye protection as infectious particles may be present [63]. It is unclear how long SARS-CoV-2 remains in the air, but drawing parallels from other airborne disease such as tuberculosis can be helpful, particularly if an aerosol-generating procedure has been performed [63]. Frequently used surfaces should be cleaned at least twice daily with implementation of standard institutional cleaning procedures [71].
2.5.3. Considerations for airway management
Intubation is a high-risk procedure due to the aerosolization of respiratory droplets [30,72]. Rescue intubations should be avoided whenever possible as complete adherence to PPE may be inadequate in a time-sensitive critical scenario [72]. Society of Critical Care Medicine (SCCM) Surviving Sepsis COVID-19 guidelines recommend performing endotracheal intubation under airborne precautions, including use of a fitted N95 respirator and placement of the patient in an AIIR [70]. Based on prior cases of HCPs infected with SARS-CoV-1 while using N95 respirators, some experts recommend using a PAPR [72]. The most experienced provider should intubate [70,72]. To reduce inadvertent contamination by touching one's face or hair, a full face shield and head cover is recommended if a PAPR is not used [30,72]. Wrist exposure can be minimized by using longer-sleeved gloves or vertically taping gloves to the gown [30]. Applying tape circumferentially makes removing PPE more difficult and does not have added benefit [30]. Shoe covers should be avoided, as they can lead to accidental self-contamination. Instead, impermeable shoes that can be appropriately decontaminated should be worn [30]. If available, coveralls with or without a hood may be used, but processes and training in safe doffing should be established beforehand as HCPs may be less experienced in using these PPE ensembles [30].
HCPs may consider double gloving and positioning waste and other transport receptacles close by to limit droplet and/or contact transmission when securing contaminated equipment for disposal or reprocessing [72]. Preoxygenation should be optimized with non-aerosol generating strategies including head of bed elevation, jaw thrust, and use of positive end expiratory pressure valves. Fiberoptic laryngoscopy should be avoided unless absolutely necessary as atomization of anesthetic will cause the virus to become aerosolized [72]. Preoxygenation for at least 5 min with 100% oxygen before performing rapid sequence intubation (RSI) may be used with nasal cannula, though this may increase the risk of contamination [30,72]. To reduce this risk, a surgical mask can be placed on the patient over the device. Non-invasive positive pressure ventilation (NIPPV) may increase risk of aerosolization and is not recommended for preoxygenation [30,72]. A high efficiency hydrophobic filter should be used between the facemask and the rest of the respiratory circuit [72]. Video laryngoscopy is preferred to direct laryngoscopy to increase distance between the intubator and the patient [30,70]. A closed system should be utilized for suctioning. Once the intubation is complete, the emergency physician should immediately place the laryngoscope in their outer glove along with all other equipment used for intubation in a double zip-locked plastic bag [72]. The presence of a high-efficiency particulate air (HEPA) filter should be verified in the expiratory limb of the mechanical ventilator prior to patient use.
2.6. Laboratory and radiographic findings
The CDC has developed a real time reverse transcription polymerase chain reaction (RT-PCR) assay for detecting SARS-CoV-2 in upper and lower respiratory specimens obtained from COVID-19 PUIs [73]. [74] A nasopharyngeal swab specimen should be collected for testing [75]. For lower respiratory tract specimens, sputum can be obtained from patients with productive cough, otherwise bronchoalveolar lavage or tracheal aspirate can be substituted [75]. Serum samples are not necessary [75,76].
There are few data available regarding sensitivity and specificity for the test, but false negatives may be seen in asymptomatic individuals or those early in the course of their disease who may not have high viral burden [27]. Patients who test negative for COVID-19 using a sample taken while they were symptomatic likely do not have the disease [6]. However, the sensitivity of RT-PCR has been reported to range from 66% to 80% [77]. A single negative RT-PCR should not be used to exclude the diagnosis, especially if the patient is in the early stages of the disease with no severe symptoms. A patient with negative RT-PCR with continued suspicion of COVID-19 should be isolated and rechecked several days later. Molecular testing (e.g., respiratory virus panel) for alternative diagnoses such as influenza should be considered for all PUIs [67]. However, co-infection with other viruses may occur.
Anemia, lymphopenia, hypoxemia, abnormal kidney and liver function, elevated creatine kinase and D-dimer, thrombocytopenia, and increased lactate dehydrogenase can be present [39,40,58]. Lymphocytopenia can occur in up to 80% of patients [60]. Interestingly, one study found procalcitonin was elevated in just 6% of patients, while other inflammatory markers like serum ferritin and C-reactive protein were elevated [40]. Troponin and brain natriuretic peptide may be elevated in those with cardiac involvement and should be obtained in patients with suspected myocardial infarction or heart failure [78].
Advanced imaging such as computed tomography (CT) is not required for diagnosis and may create additional infection prevention challenges in the ED. However, if obtained, CT may demonstrate several findings. Lung findings may be present on imaging before patients develop clinical manifestations. In a case series of patients from Wuhan, China admitted with COVID-19, 100% had chest CT findings consistent with pneumonia [39]. Patients may also have radiographic ground-glass opacities [58]. Another study of 99 COVID-19 patients found 75% of patients had bilateral pneumonia, 25% had unilateral pneumonia, and 14% had mottling and ground-glass opacities on chest X-ray and CT imaging [40].
Ultrasound can be utilized as well, as it is repeatable and reliable, has no radiation, and is inexpensive. Ultrasound findings depend on the stage and severity of the disease, and it cannot detect lesions deeper in the lung. Patients with COVID-19 typically demonstrate an irregular/thickened pleural line, scattered/confluent B lines, consolidations of various sizes, and both non-translobar and translobar consolidations on lung ultrasound [79]. Pleural effusions are typically small and localized if they are present, and abnormalities are typically found in multiple lung zones.
2.7. Treatment
Currently, no specific treatments exist nor are recommended for patients with COVID-19 [4,15,62]. Several vaccines are under study, including DNA-based, vector-based, and protein based vaccines [80]. Supportive care is the mainstay of treatment, preferably with acetaminophen [4,15]. If pneumonia is present on imaging or the patient is critically ill, antibiotics are recommended. Patients presenting with respiratory insufficiency in the setting of potential COVID-19 infection should be given supplementary oxygen to maintain an oxygenation saturation ≥90% but no higher than 96% [62,70]. Up to 76% of patients require oxygen therapy [40]. For those with acute hypoxemic respiratory failure who require intubation, endotracheal intubation should be performed. For those with hypoxemic respiratory failure who do not require intubation but who do not improve with conventional oxygen therapies, high flow nasal cannula (HFNC) is recommended over noninvasive positive pressure ventilation (NIPPV) [70]. If HFNC is not available and there is no urgent need for intubation, a trial of NIPPV ventilation is recommended with frequent reassessment, though NIPPV increases the risk of aerosolization [70]. NIPPV may result in patient improvement. The SCCM does not make a clear recommendation for helmet NIPPV compared to mask NIPPV [70]. While most recommend avoiding NIPPV due to the risk of aerosolization, it can be utilized safely if high risk patients are cohorted and clinicians use appropriate PPE [81]. Patients who decline despite use of HFNC or NIPPV should be intubated [70]. If intubation is indicated, airborne precautions should be used with the patient ventilated using tidal volumes of 4–8 mL/kg of predicted body weight and plateau pressures <30 cm H2O [62]. If available, patients with severe ARDS may benefit from prone ventilation >12 h per day [62,70].
Over-resuscitation with intravenous fluids should be avoided, which can potentially worsen oxygenation [62]. Even when COVID-19 is suspected as the cause of the patient's symptoms, the WHO recommends administering empiric antibiotics and a neuraminidase inhibitor within 1 h of identifying sepsis [62]. Early recognition of septic shock is critical, with management of sepsis focusing on intravenous fluid resuscitation and antibiotics [62]. A conservative resuscitation strategy with buffered/balanced crystalloids is recommended for those in shock, and hypotonic crystalloids should be avoided [62,70]. Vasopressors, preferentially norepinephrine, are indicated for persistent shock with a goal MAP of 60–65 mmHg [62,70]. For those with continued shock despite norepinephrine, vasopressin should be added, rather than increasing norepinephrine dose [70]. If cardiac dysfunction is present and there is persistent hypoperfusion, dobutamine is recommended [70]. Systemic steroids (hydrocortisone 200 mg per day) should be considered in those with vasopressor-refractory shock or for those with another indication for steroids such as chronic obstructive pulmonary disease exacerbation [22,62,70]. Without delaying antibiotic administration, bacterial blood cultures should be obtained [62].
Clinical trials of investigational drugs and antivirals are underway, although none are currently approved by the U.S. Food and Drug Administration (FDA) (Table 4 ) [4,46]. Remdesivir has demonstrated activity against MERS-CoV and SARS-CoV in vitro and animal models [82,83]. An in vitro study found that remdesivir and chloroquine inhibit viral infection, but further study is required [84,85]. Results from a single study of over 100 COVID-19 patients found chloroquine was superior to control in reducing pneumonia exacerbation, improving imaging findings and virus-negative conversion, and shortening the course of the disease [86]. A study evaluating lopinavir-ritonavir found no improvement in patient survival or differences in detectable viral RNA [87]. Hydroxychloroquine and azithromycin are also under study [88]. In a single prospective, observational study of 36 patients with COVID-19, those receiving hydroxychloroquine demonstrated higher rates of viral load reduction/disappearance, though no patient centered outcomes were assessed [88]. Other medications under study include tocilizumab and favipiravir [89,90]. There are no clear data supporting harm or benefit with angiotensin converting enzyme inhibitors or ACE receptor blockers (ARBs) [91,92]. Unless authorized through a clinically approved trial or Monitored Emergency Use of Unregistered Interventions Framework (MEURI), unlicensed treatments should not be administered [62]. Continuous renal replacement therapy (CRRT), extracorporeal membrane oxygenation (ECMO), and immunoglobulin have been utilized for management, but have not been definitively shown to be beneficial [40].
Table 4.
COVID-19 therapies under study.
| Medication | Dosing |
|---|---|
| Remdesivir | 200 mg for 1 day, then 100 mg IV every day for 9 days |
| Lopinavir/Ritonavir | 400–100 mg PO BID for 14 days |
| Chloroquine | 500 mg PO BID for 10 days |
| Hydroxychloroquine | 400 mg PO BID for 1 day, then 200 mg PO BID for 4 days |
| Tocilizumab | 8 mg/kg in 100 mL of 0.9% NS IV over 60 min |
| Favipiravir | 1600 mg PO BID for one day, then 600 mg PO BID for 6 days |
Abbreviations: mg – milligrams, IV -intravenous, BID – twice per day, PO – per os, NS – normal saline, mL – milliliters, kg – kilogram.
2.8. Disposition
Patients with severe symptoms, hypoxemia requiring oxygen supplementation, or high risk for clinical deterioration (i.e. pneumonia on radiograph, severe comorbidities) may require admission for further management and monitoring. Patients with mild symptoms and no significant comorbidities without concern for deterioration of clinical condition may be candidates for discharge, self-quarantine for two weeks, and home monitoring [93]. These patients must have the ability to be safely isolated at home to prevent transmission to others and be carefully monitored [22]. Social distancing is a vital component of reducing the spread of the virus, comprised of limiting events, mass gatherings, and even small group meeting [94]. Individuals should remain 6 ft or 2 meters apart from other individuals. Health departments should be involved early in the care of these patients and can assist with decisions regarding disposition, further surveillance, and testing, especially until confirmatory test results are available [57,62]. Emergency physicians should counsel these patients to return for worrisome symptoms including new or worsening pulmonary complaints and fever [57,62]. Development of a clinical pathway among emergency physicians, infectious disease specialists, and health departments is critical to safely evaluate COVID-19 PUIs in the community.
3. Conclusion
COVID-19 is a novel coronavirus that has affected an unprecedented number of people to date. Patients typically present with a combination of fever or cough and have a history of exposure to either a close contact with COVID-19 or travel to an affected geographic area. While most patients will have mild disease, some may develop severe complications including ARDS and multi-organ failure, with some succumbing to the disease. Special consideration should be given to those at the extremes of age, the immunocompromised, or pregnant women. No curative treatment is currently approved. Emergency physicians should obtain a detailed travel history from all patients and suspect COVID-19 in patients presenting with symptoms of an acute upper respiratory illness and fever. Early recognition and isolation of a patient with COVID-19 in the ED may help decrease exposure to other patients and healthcare personnel. Future research is necessary to expand our collective knowledge of COVID-19 and optimize patient outcomes.
Meetings
None.
Grants/financial support
None.
Author contributions
SC, SL, AK, and BL conceived the idea for this manuscript and contributed substantially to the writing and editing of the review.
Declaration of competing interest
None.
Acknowledgements
This manuscript did not utilize any grants, and it has not been presented in abstract form. This clinical review has not been published, it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. This review does not reflect the views or opinions of the U.S. government, Department of Defense, U.S. Army, U.S. Air Force, Brooke Army Medical Center, or SAUSHEC EM Residency Program.
References
- 1.World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov). Published January 30, 2020. Accessed February 18, 2020.
- 2.World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Published March 11, 2020. Accessed March 20, 2020.
- 3.World Health Organization. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Published February 11, 2020. Accessed March 08, 2020.
- 4.World Health Organization. Q&A on coronaviruses. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Published February 11, 2020. Accessed March 08, 2020.
- 5.New images of Novel Coronavirus SARS-CoV-2 now available|NIH: National Institute of Allergy and Infectious Diseases. https://www.niaid.nih.gov/news-events/novel-coronavirus-sarscov2-images. Accessed March 18, 2020.
- 6.CDC. 2019 ovel Coronavirus (2019-nCoV) frequently asked questions and answers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/faq.html. Published February 11, 2020. Accessed March 18, 2020.
- 7.Naming the coronavirus disease (COVID-2019) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. Accessed March 12, 2020.
- 8.CDC. HAN Archive - 00426|Health Alert Network (HAN). https://emergency.cdc.gov/han/han00426.asp. Published February 11, 2020. Accessed March 19, 2020.
- 9.News Scan for Dec 31 CIDRAP. 2019. http://www.cidrap.umn.edu/news-perspective/2019/12/news-scan-dec-31-2019
- 10.Taylor DB. A timeline of the coronavirus. The New York Times. https://www.nytimes.com/2020/02/13/world/coronavirus-timeline.html. Published February 13, 2020. Accessed March 18, 2020.
- 11.CHP closely monitors cluster of pneumonia cases on Mainland. https://www.info.gov.hk/gia/general/201912/31/P2019123100667.htm
- 12.Salcedo A, Cherelus G. Coronavirus travel restrictions, across the globe. The New York Times. https://www.nytimes.com/article/coronavirus-travel-restrictions.html. Published March 20, 2020. Accessed March 20, 2020.
- 13.CNN JG, AW 780 million people in China face travel restrictions over coronavirus outbreak. CNN. https://www.cnn.com/2020/02/16/asia/coronavirus-covid-19-death-toll-update-intl-hnk/index.html
- 14.CDC. 2019 Novel Coronavirus (2019-nCoV) situation summary. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/summary.html. Published February 11, 2020. Accessed March 18, 2020.
- 15.CDC. 2019 Novel Coronavirus (2019-nCoV) prevention & treatment. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/prevention-treatment.html. Published February 11, 2020. Accessed March 19, 2020.
- 16.Molinari N.-A.M., LeBlanc T.T., Stephens W. The impact of a case of Ebola virus disease on Emergency Department visits in Metropolitan Dallas-Fort Worth, TX, July, 2013–July, 2015: An interrupted time series analysis. PLoS Curr. 2018:10. doi: 10.1371/currents.outbreaks.e62bdea371ef5454d56f71fe217aead0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewlett A.L., Varkey J.B., Smith P.W., Ribner B.S. Ebola virus disease: preparedness and infection control lessons learned from two biocontainment units. Curr Opin Infect Dis. 2015;28(4):343–348. doi: 10.1097/QCO.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Identify, Isolate, Inform: Emergency Department Evaluation and Management for Patients Under Investigation (PUIs) for Ebola Virus Disease (EVD)|Emergency Services|Clinicians|Ebola (Ebola Virus Disease)|CDC. https://www.cdc.gov/vhf/ebola/clinicians/emergency-services/emergency-departments.html. Published August 30, 2019. Accessed February 18, 2020.
- 19.Coronavirus|Human Coronavirus Types|CDC. https://www.cdc.gov/coronavirus/types.html. Published February 16, 2020. Accessed March 12, 2020.
- 20.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of Novel Coronavirus–infected pneumonia. N Engl J Med. 2020;0(0) doi: 10.1056/NEJMoa2001316. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC. Coronavirus Disease 2019 (COVID-19): animals and Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/prepare/animals.html. Published March 16, 2020. Accessed March 20, 2020.
- 22.McIntosh K. In: Coronavirus disease 2019 (COVID-19) Hirsch M., Bloom A., editors. Date; February 2020. https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19/print [Google Scholar]
- 23.CDC. 2019 Novel Coronavirus (2019-nCoV) transmission. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html. Published February 11, 2020. Accessed March 12, 2020.
- 24.WHO Director-General's remarks at the media briefing on COVID-2019 outbreak on 17 February. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-covid-2019-outbreak-on-17-february-2020
- 25.World Health Organization. Coronavirus disease 2019 (COVID-19) situation report – 60. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200320-sitrep-60-covid-19.pdf?sfvrsn=8894045a_2. Published March 20, 2020. Accessed March 20, 2020.
- 26.CDC. 2019 Novel Coronavirus (2019-nCoV) cases in the U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-in-us.html. Published March 20, 2020. Accessed March 20, 2020.
- 27.Coronavirus Disease 2019 Transcript for CDC Media Telebriefing. Centers for Disease Control and Prevention. https://wwwdev.cdc.gov/media/releases/2020/s0215-Diamond-Princess-Repatriation.html. Published February 18, 2020. Accessed March 19, 2020.
- 28.Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper eespiratory specimens of infected patients. N Engl J Med. 2020;0(0) doi: 10.1056/NEJMc2001737. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. February 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth Can Anesth. February 2020 doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.del Rio C., Malani P.N. JAMA; 2020. COVID-19—new insights on a rapidly changing epidemic. February. [DOI] [PubMed] [Google Scholar]
- 32.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance. 2020;25(4):2000058. doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rettner R. How does the new coronavirus compare with the flu? livescience.com. https://www.livescience.com/new-coronavirus-compare-with-flu.html. Published March 19, 2020. Accessed March 11, 2020.
- 34.Zhang Y. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Wkly. 2020;2:1–10. [PMC free article] [PubMed] [Google Scholar]
- 35.Feb 17 LS|NE|CN|, 2020. More outbreak details emerge as COVID-19 cases top 70,000. CIDRAP. http://www.cidrap.umn.edu/news-perspective/2020/02/more-outbreak-details-emerge-covid-19-cases-top-70000 Accessed March 19, 2020.
- 36.Szabo L. Facts vs. fears: five things to help weigh your Coronavirus risk. Kais Health News. February 2020. https://khn.org/news/facts-vs-fears-five-things-to-help-weigh-your-coronavirus-risk/
- 37.Imai N., Dorigatti I., Cori A., Donnelly C., Riley S., Ferguson N.M. Report 2: estimating the potential total number of novel Coronavirus cases in Wuhan City. China. 2020;6 [Google Scholar]
- 38.Dorigatti I., Okell L., Cori A., et al. Report 4: severity of 2019-novel coronavirus (nCoV) https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200219-sitrep-30-covid-19.pdf?sfvrsn=3346b04f_2 Available at.
- 39.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker E, The Vaccine Centre, London School of Hygiene & Tropical Medicine. Covid 2019 tracker. https://vac-lshtm.shinyapps.io/ncov_tracker/. Published 2020. Accessed March 21, 2020.
- 42.Troeger C.E., Blacker B.F., Khalil I.A., et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;7(1):69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CDC. 2019 Novel Coronavirus (2019-nCoV) symptoms. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html. Published February 11, 2020. Accessed March 19, 2020.
- 44.Wang M., Wu Q., Xu W., et al. Clinical diagnosis of 8274 samples with 2019-novel coronavirus in Wuhan. medRxiv. February 2020;2020 doi: 10.1101/2020.02.12.20022327. (02.12.20022327) [DOI] [Google Scholar]
- 45.Shah N. Higher co-infection rates in COVID19. Medium. https://medium.com/@nigam/higher-co-infection-rates-in-covid19-b24965088333. Published March 19, 2020. Accessed March 20, 2020.
- 46.CDC. 2019 Novel Coronavirus (2019-nCoV) clinical care. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Published February 11, 2020. Accessed March 19, 2020.
- 47.CDC. 2019 Novel Coronavirus (2019-nCoV) pregnant women. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/pregnancy-faq.html. Published February 11, 2020. Accessed March 19, 2020.
- 48.Qiao J. What are the risks of COVID-19 infection in pregnant women? The Lancet. 2020;0(0) doi: 10.1016/S0140-6736(20)30365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.CDC - Health Care Workers, Infectious Agents - NIOSH workplace safety and health topic. https://www.cdc.gov/niosh/topics/healthcare/infectious.html. Published November 6, 2018. Accessed March 12, 2020.
- 50.C Km, B R., C Mg, O Bj, O Ba. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth J Can Anesth. 2006;53(2):122–129. doi: 10.1007/bf03021815. [DOI] [PubMed] [Google Scholar]
- 51.Booth C.M., Matukas L.M., Tomlinson G.A., et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 52.Lietz J., Westermann C., Nienhaus A., Schablon A. The occupational risk of influenza A (H1N1) infection among healthcare personnel during the 2009 pandemic: a systematic review and meta-analysis of observational studies. PLoS ONE. 2016;11(8) doi: 10.1371/journal.pone.0162061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American College of Emergency Physicians. Two emergency physicians in critical condition. ACEP. https://www.acep.org/corona/covid-19-articles/a-statement-from-acep-president-william-jaquis-md-facep/. Published March 14, 2020. Accessed March 21, 2020.
- 54.2 ER doctors at rush Oak Park Hospital test positive for Coronavirus. NBC Chic. March 2020. https://www.nbcchicago.com/news/local/2-doctors-at-rush-oak-park-hospital-test-positive-for-coronavirus/2240158/. Accessed March 21, 2020.
- 55.Italy has a world-class health system. The coronavirus has pushed it to the breaking point. NBC News. https://www.nbcnews.com/health/health-news/italy-has-world-class-health-system-coronavirus-has-pushed-it-n1162786. Accessed March 20, 2020.
- 56.Mar 16 MVB|NW|CN|, 2020. Doctors: COVID-19 pushing Italian ICUs toward collapse. CIDRAP. http://www.cidrap.umn.edu/news-perspective/2020/03/doctors-covid-19-pushing-italian-icus-toward-collapse Accessed March 20, 2020.
- 57.CDC. 2019 Novel Coronavirus (2019-nCoV) flowchart for healthcare professionals. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/identify-assess-flowchart.html. Published February 11, 2020. Accessed March 19, 2020.
- 58.Chan J.F.-W., Yuan S., Kok K.-H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;0(0) doi: 10.1056/NEJMoa2002032. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan L., Mu M., Ren H.G., et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. 2020. https://journals.lww.com/ajg/Documents/COVID_Digestive_Symptoms_AJG_Preproof.pdf Published March 5. Available at. [DOI] [PMC free article] [PubMed]
- 62.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
- 63.CDC. Coronavirus Disease 2019 (COVID-19) Interim Infection Prevention and Control Recommendations. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html. Published February 11, 2020. Accessed March 20, 2020.
- 64.CDC. 2019 Novel Coronavirus (2019-nCoV) healthcare infection prevention and control FAQs for COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-prevention-control-faq.html. Published February 11, 2020. Accessed March 19, 2020.
- 65.Heymann D.L., Shindo N. COVID-19: what is next for public health? The Lancet. 2020;395(10224):542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.CDC. Coronavirus Disease 2019 (COVID-19): evaluating and testing persons for Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published February 11, 2020. Accessed March 21, 2020.
- 67.CDC. 2019 Novel Coronavirus (2019-nCoV) evaluating and reporting PUI. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published February 11, 2020. Accessed March 19, 2020.
- 68.CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
- 69.CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
- 70.Alhazzani W., Møller M.H., Arabi Y.M., et al. Vol. 101. 2020. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Public Health Agency of Canada. Infection prevention and control for coronavirus disease (COVID-19): interim guidance for acute healthcare settings. aem. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/interim-guidance-acute-healthcare-settings.html#a4.18. Published February 24, 2020. Accessed February 27, 2020.
- 72.Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
- 73.CDC. 2019 Novel Coronavirus (2019-nCoV) Testing. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html. Published February 11, 2020. Accessed March 19, 2020.
- 74.CDC. 2019 Novel Coronavirus (2019-nCoV) information for laboratories COVID-19 requests for diagnostic panels and virus. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/lab/tool-virus-requests.html. Published February 11, 2020. Accessed March 19, 2020.
- 75.CDC. Coronavirus Disease 2019 (COVID-19) interim guidelines for collecting, handling, and testing clinical specimens from persons under investigation (PUIs) for Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Published February 11, 2020. Accessed March 20, 2020.
- 76.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. March 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., et al. Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 Feb;26:200642. doi: 10.1148/radiol.2020200642. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Januzzi J. Troponin and BNP use in COVID-19. Latest in cardiology. http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2020%2f03%2f18%2f15%2f25%2ftroponin-and-bnp-use-in-covid19. Published March 18, 2020. Accessed March 20, 2020.
- 79.Peng Q., Wang X., Zhang L. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. March 2020 doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.World Health Organization. DRAFT landscape of COVID-19 candidate vaccines. World Health Organization. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1. Published March 20, 2020. Accessed March 21, 2020.
- 81.Episode 38 - COVID-19 Update An interview with Andrea Duca, MD. EBMedicine. 2020. https://www.ebmedicine.net/topics/infectious-disease/COVID-19/podcast Published March 19. Available at.
- 82.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. February 2020 doi: 10.1074/jbc.AC120.013056. jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. March 2020 doi: 10.1128/AAC.00399-20. AAC.00399-20, aac;AAC.00399-20v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 86.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020 Mar 16;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 87.Cao B., Wang Y., Wen D., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. March 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gautret P., Lagier J.-C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. March 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Tocilizumab vs CRRT in management of cytokine release syndrome (CRS) in COVID-19 (TACOS) https://clinicaltrials.gov/ct2/show/NCT04306705?term=Tocilizumab&cond=COVID19&draw=2
- 90.Favipiravir combined with tocilizumab in the treatment of Corona Virus Disease. 2019. https://clinicaltrials.gov/ct2/show/NCT04310228?term=favipiravir&cond=covid19&draw=2&rank=2
- 91.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. March 2020:1–5. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;0(0) doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.World Health Organization. Home care for patients with suspected novel coronavirus (nCoV) infection presenting with mild symptoms and management of contacts. https://www.who.int/publications-detail/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts. Published February 4, 2020. Accessed February 24, 2020.
- 94.CDC. Coronavirus Disease 2019 (COVID-19): interim US guidance for risk assessment and public health management of persons with potential Coronavirus Disease 2019 (COVID-19) exposures: geographic risk and contacts of laboratory-confirmed cases. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html. Published March 7, 2020. Accessed March 20, 2020.