Abstract
Severe acute respiratory syndrome coronavirus-2 is still active in Wuhan, China, and is spreading to the rest of the world. Recently, perioperative anesthetic management in patients with suspected or confirmed coronavirus-2 has been reported. However, little has been reported on the anesthetic management of patients undergoing aortic dissection repair in patients with suspected severe acute respiratory syndrome coronavirus-2 infection. During the outbreak in Wuhan, the authors’ team completed 4 cases of aortic dissection repair successfully in patients with suspected severe acute respiratory syndrome coronavirus-2 infection. The purpose of the present report is to summarize current knowledge and experiences on anesthetic management in this patient population and to provide clinical practice guidelines on anesthetic management and infection prevention and control in these critically ill patients.
Key Words: anesthetic management, aortic dissection, infection control, SARS-CoV-2, COVID-19
AN ONGOING epidemic by a novel coronavirus first was reported during late 2019 in Wuhan, China, and has attracted considerable attention worldwide.1, 2, 3 On January 7, 2020, the novel coronavirus was identified by the Chinese Center for Disease Control and Prevention and subsequently was named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by the World Health Organization.4 , 5 The disease caused by SARS-CoV-2 infection was named coronavirus disease 2019 (COVID-19), with clinical presentation resembling severe acute respiratory syndrome and Middle East respiratory syndrome and manifesting as cough, fever, and dyspnea.5, 6, 7, 8, 9 In particular, accumulating evidence has shown that SARS-CoV-2 exhibits effective human-to-human transmission, causing an exponential growth of new cases.6 , 10, 11, 12
The strong transmissibility of SARS-CoV-2 and a substantial number of patients who are either asymptomatic or have only mild symptoms2 , 3 , 12 present great challenges for anesthesiologists to perform anesthetic management and successful infection prevention and control. Even though studies have been published for SARS-CoV-2–related infection prevention and control during anesthesia in emergency surgery,13 unfortunately, there are no guidelines on the anesthetic management for patients with suspected SARS-CoV-2 infection undergoing long, complicated cardiac surgeries.
Herein, 4 successful cases of aortic dissection repair in patients with suspected SARS-CoV-2 infection are reported, and current knowledge on anesthetic management and infection prevention and control measures in patients undergoing aortic dissection repair with suspected SARS-CoV-2 infection is summarized. The authors aim to help perioperative healthcare providers to adopt safer anesthetic management techniques and use more effective infection prevention and control measures in these critical ill patients.
Case Characteristics
Three male patients and 1 female patient, ages 51 to 62 years, were admitted to the hospital several hours after sudden-onset chest pain. After admission, all patients were diagnosed with Standford type A aortic dissection on computed tomography scan. In addition, multiple pulmonary inflammatory changes of varying degrees were discovered on chest computed tomography in all 4 patients. None of the patients had clear complaints of respiratory symptoms. Patient 4 had moderate fever (38.6°C), patient 1 had mild fever (37.6°C), patient 2 had a borderline temperature (37.0°C), and patient 3 had no fever. Blood counts showed a reduction in lymphocyte levels and a normal or mild increase in leukocyte levels. Based on the patients’ presentation and history of contact, the possibility of SARS-CoV-2 infection were highly suspected (Table 1 ).
Table 1.
Basic Information of Patients Before Surgery
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Sex | Male | Male | Male | Female |
| Age, y | 51 | 51 | 62 | 59 |
| Patient address | Wuhan | Wuhan | Wuhan | Wuhan |
| Medical history | Hypertension; CHD |
Hypertension | None | Hypertension |
| Body temperature (°C) | 37.6 | 37 | 36.5 | 38.5 |
| BP (SBP/DBP), mmHg | 205/112 | 171/73 | 149/60 | 159/76 |
| Heart rate (beats/min) | 80 | 71 | 97 | 98 |
| Respiratory frequency (beats/min) | 18 | 19 | 17 | 18 |
| Leukocyte count (× 109/L) | 8.68 | 11.38 | 11.92 | 9.18 |
| Lymphocyte count (× 109/L) | 1.41 | 0.73 | 0.70 | 1.08 |
| Neutrophil count (× 109/L) | 6.22 | 10.36 | 10.70 | 7.26 |
| Chest x-ray and CT findings | Inflammatory changes | Inflammatory changes | Inflammatory changes | Inflammatory changes |
Abbreviations: BP, blood pressure; CT, computed tomography; CHD, coronary heart disease; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Preparation of Anesthesia and Infection Control Precautions
All medical staff adopted third-level medical protection measures, including properly wearing disposable protective clothing, medical-degree mask such as N95, disposable surgical cap, medical-degree goggles, disposable latex gloves, and disposable shoe covers. General anesthesia was induced for all patients because of the surgical procedure and the patients’ clinical conditions. To prevent cross-infection in the operating room, disposable anesthetic devices in contact with the respiratory tract, including plastic respiratory pipes, video laryngoscope lenses, filters, respiratory balloons, suction tubes, and sputum suction tubes, were discarded after single use. Rescue medications were prepared before entering the operating room and then taken to the operating room.
Anesthetic Management
All patients were monitored closely via continuous electrocardiography, noninvasive arterial blood pressure, heart rate, and peripheral oxygen saturation. Once in the operating room, 2 patients demonstrated high blood pressure, and urapidil was injected for blood pressure control before general anesthesia was administered. Invasive arterial blood pressure was monitored by radial artery puncture and catheterization with ultrasound guidance with the patient under local anesthesia.
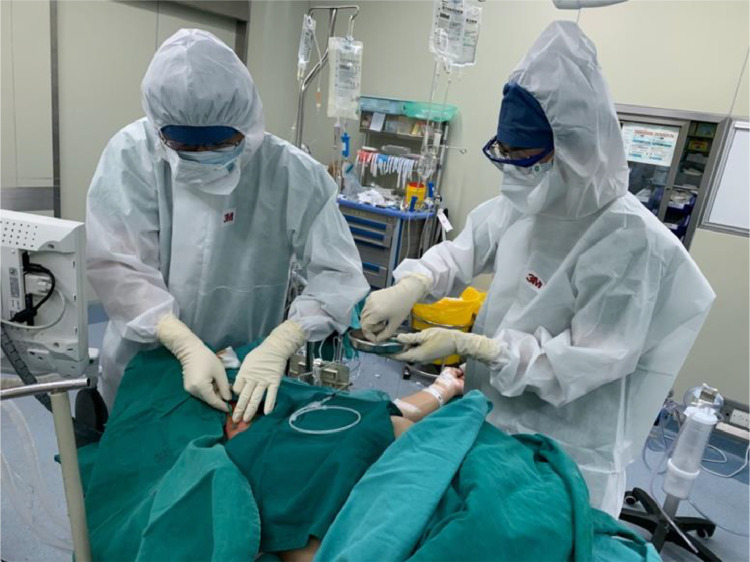
Anesthesiologists’ first layer of gloves was sterilized with a hydrogen peroxide solution, and another layer of gloves was added to prepare for anesthesia induction and intubation. High-flow, high-concentration oxygen (4-5 L min) was delivered via face mask, and anesthesia induction was performed slowly. After the patient was unconscious, high-dose opioids and adequate muscle relaxant were administrated, and the patient was started on low-tidal volume, high-frequency mechanical ventilation. None of the patients coughed during anesthesia induction. Endotracheal intubation was performed successfully with video laryngoscope. After tracheal intubation, the outer layer gloves were removed, and the inner gloves were disinfected with hydrogen peroxide solution again. Then, anesthesiologists wore a disposable surgical gown and sterile gloves to complete the internal jugular vein cannulation, and connect the cannula to the pressure measuring device (Fig 1 ). A nasal temperature probe was placed before heparinization. Finally, a clean drape was placed over the patient's head and face, and the eyes were taped shut to prevent corneal abrasions.
Fig 1.
An anesthesiologist and an assistant perform an internal jugular vein puncture and catheterization procedure.
Because of the long duration of the aortic dissection repair, medical adhesive tape was used to strengthen the connection of the different parts of the personal protective equipment, including between the goggles and the mask, between the goggles and the protective clothing cap, and between the mouth and nose of the protective clothing. During surgery, the operating room temperature and humidity level were reduced, and the protective equipment was changed every 3 to 4 hours depending on the degree of wetness in the protective clothing. Anesthesiologist relief was provided during either cardiopulmonary bypass or hemostasis (Table 2 ). Vital signs of all 4 patients were stable during the entire surgical procedure. The preoperative, intraoperative, and postoperative respiratory-related information of patients, including mode of ventilation and outcomes, is provided in Table 3 .
Table 2.
Patients’ Time in the Operating Room, Times of Replacement of Protective Equipment, and Surgical Progress During Anesthesiologist Rotation
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Time in the OR (min) | 751 | 610 | 544 | 537 |
| Anesthetic time (min) | 725 | 592 | 534 | 519 |
| Surgical time (min) | 627 | 501 | 469 | 422 |
| Times of protective equipment change | 2 | 1 | 1 | 2 |
Abbreviation: OR, operating room.
Table 3.
Preoperative, Intraoperative, and Postoperative Respiratory Parameters of Patients; Respiratory Virus, Bacteria, Mycoplasma, and Chlamydia Tests; and Clinical Diagnosis of COVID-19
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Preoperative | ||||
| SpO2 | 96% | 94% | 95% | 97% |
| Breathing pattern | Spontaneous | Spontaneous | Spontaneous | Spontaneous |
| Post-intubation SpO2 | 100% | 100% | 100% | 100% |
| Intraoperative | ||||
| Tidal volume (mL/kg) | 8-10 | 10 | 8-10 | 8-10 |
| Ventilatory frequency (beats/min) | 14 | 14 | 14 | 14 |
| Ventilation mode | PRVC | PRVC | PRVC | PRVC |
| PEEP (cm H2O) | 6 | 6-8 | 6 | 6 |
| Postoperative | ||||
| Ventilation mode/days | PCV/9 | PCV/8 | PCV/4 | PCV/3 |
| SIMV/2 | SIMV/3 | controlled | controlled | |
| spontaneous | spontaneous | |||
| SpO2 fluctuations | 80%∼96% | 84%∼95% | 87%∼96% | 85%∼91% |
| Respiratory virus, bacteria, mycoplasma, and chlamydia tests | Negative | Negative | Negative | Negative |
| Clinical diagnosis of COVID-19 | Suspected | Suspected | Confirmed | Confirmed |
Abbreviations: COVID-19, coronavirus disease 2019; PCV, pressure-controlled ventilation; PEEP, positive end-expiratory pressure; PRVC, pressure regulated volume control ventilation; SIMV, synchronized intermittent mandatory ventilation; SpO2, peripheral oxygen saturation.
Postoperatively, patients were mechanically ventilated with oxygen via a portable oxygen source and transported to an isolation intensive care unit room. After the transportation of patients, the protective equipment and other single-use equipment were discarded at a designated location in the quarantined area.
Discussion
Accumulating evidence of human-to-human transmission of SARS-CoV-22 , 6 , 11 and a substantial number of asymptomatic cases or patients with mild symptoms2 , 3 , 12 undoubtedly pose a significant challenge for anesthetic management and infection control. In particular, aortic dissection repair is a long and complicated surgery.14 Therefore, standardized anesthetic management procedures and strict infection control measures need to be developed and implemented, which is particularly important during infectious disease outbreaks. Herein, the authors report on 4 successful cases of aortic dissection repair in patients with suspected SARS-CoV-2 infection as well as anesthetic management and precaution measures in these patients with suspected COVID-19.
SARS-CoV-2 has been proven to be transmitted through aerosol or fluid from human secretions or discharges, including through coughing, sneezing, and contact with infected surfaces.6 , 11 , 15 These modalities of transmission pose a high-exposure risk for healthcare providers in the operating room, especially anesthesiologists. In addition, COVID-19 patients may present with mild symptoms or nonspecific signs in the early stage of disease but still are infectious.12 , 16 Therefore, all perioperative healthcare providers need to adopt third-level medical protection measures, including medical-degree eye-protective goggles, medical-degree mask, face shield, gloves, disposable protective clothing, and disposable outer shoe covers.13 Training personnel on the correct use of these precautions is important because incorrect use may cause potential nosocomial infections and exponential spread of SARS-CoV-2.
Considering the complexity and high risk of aortic dissection repair, direct hemodynamic monitoring with arterial and central lines is necessary. However, wearing multiple pieces of heavy protective equipment makes these procedures difficult to perform. Real-time ultrasound guidance should be used to improve the efficiency and safety. The ultrasound probe should be covered with a disposable cover to prevent probe contamination, which may lead to cross-infection.
During preoxygenation and intubation, factors such as coughing, vomiting, and desynchrony between spontaneous breathing and assisted breathing may increase the exposure risk of aerosol and oral secretions for the anesthesiologist. Therefore, adequate anesthesia and muscle relaxation must be achieved before any airway manipulation is performed. Small doses of induction drugs and high-frequency assisted ventilation with low tidal volume during anesthesia induction may reduce the incidence of these adverse reactions.
Finally, the long duration of aortic dissection repair and heavy protective equipment increase the physical burden for the anesthesiologist, and the protective efficacy of wet protective equipment will decrease significantly. Moderate hypothermia and decreased humidity in the operating room can make staff more comfortable, reduce sweating, and reduce the risk of infection. Applying anti-fog agents on protective goggles in advance can effectively reduce the fogging and water accumulation from breath and sweat evaporation. Long procedures increase the risk of perioperative aerosol infections; therefore, the authors recommend the use of medical adhesive tape to strengthen the connection between the goggles and mask, between the goggles and the protective clothing cap, and between the mouth and nose of the protective clothing to effectively prevent an insufficient seal. In addition, protective equipment should be replaced frequently to maintain the best protective function.
Conclusions
In conclusion, the authors successfully completed 4 cases of aortic dissection repair in patients with suspected SARS-CoV-2 infection and believe that the anesthetic management and infection precaution measures described herein can provide valuable information for similar conditions.
Conflict of Interest
The authors declare no conflicts of interest.
Footnotes
J. Huang and X. Chen are co-corresponding authors of this study.
This work was supported by the National Key Research and Development Project (No. 2018YFC2001802) and the National Natural Science Foundation of China (No. 81571075).
References
- 1.December I. A novel coronavirus outbreak of global health concern. Lancet. 2020;6736:1–4. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;6736:1–10. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med United States. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinese Center for Disease Control and Prevention. Available at: http://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/. Accessed March 9, 2020. [DOI] [PMC free article] [PubMed]
- 5.World Health Organization. Coronavirus disease (COVID-19) outbreak. Available at:https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed March 9, 2020.
- 6.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;6736:1–7. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Groot R.J., Baker S.C., Baric R.S. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Wit E., Van Doremalen N., Falzarano D. SARS and MERS: Recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drosten C., Gunther S., Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 10.Fuk-Woo Chan J., Yuan S., Kok K.-H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;6736:1–10. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia [E-pub ahead of print]. N Engl J Med. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed]
- 12.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [E-pub ahead of print]. JAMA. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed]
- 13.Chen X., Shang Y., Yao S. Perioperative care provider's considerations in managing patients with the COVID-19 infections. Transl Perioper Pain Med. 2020;7:216–224. [Google Scholar]
- 14.Mussa F.F., Horton J.D., Moridzadeh R. Acute aortic dissection and intramural hematoma: A systematic review. JAMA. 2016;316:754–763. doi: 10.1001/jama.2016.10026. [DOI] [PubMed] [Google Scholar]
- 15.Lu C., Liu X., Jia Z. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;6736:30313. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]