Summary
Purpose
To investigate the clinical and imaging characteristics of computed tomography (CT) in novel coronavirus pneumonia (NCP) caused by SARS-CoV-2.
Materials and methods
A retrospective analysis was performed on the imaging findings of patients confirmed with COVID-19 pneumonia who had chest CT scanning and treatment after disease onset. The clinical and imaging data were analyzed.
Results
Fifty patients were enrolled, including mild type in nine, common in 28, severe in 10 and critically severe in the rest three. Mild patients (29 years) were significantly (P<0.03) younger than either common (44.5 years) or severe (54.7) and critically severe (65.7 years) patients, and common patients were also significantly (P<0.03) younger than severe and critically severe patients. Mild patients had low to moderate fever (<39.1 °C), 49 (98%) patients had normal or slightly reduced leukocyte count, 14 (28%) had decreased counts of lymphocytes, and 26 (52%) patients had increased C-reactive protein. Nine mild patients were negative in CT imaging. For all the other types of NCP, the lesion was in the right upper lobe in 30 cases, right middle lobe in 22, right lower lobe in 39, left upper lobe in 33 and left lower lobe in 36. The lesion was primarily located in the peripheral area under the pleura with possible extension towards the pulmonary hilum. Symmetrical lesions were seen in 26 cases and asymmetrical in 15. The density of lesion was mostly uneven with ground glass opacity as the primary presentation accompanied by partial consolidation and fibrosis.
Conclusion
CT imaging presentations of NCP are mostly patchy ground glass opacities in the peripheral areas under the pleura with partial consolidation which will be absorbed with formation of fibrotic stripes if improved. CT scanning provides important bases for early diagnosis and treatment of NCP.
Keywords: Novel coronavirus pneumonia, Covid-19, SARS-CoV-2, Computed tomography, Imaging finding
Introduction
The outbreak of an epidemical pneumonia with initially unknown reasons in December 2019 in Wuhan, China was ultimately found to be caused by a new virus, the “2019 novel Coronavirus” (2019-nCoV) or SARS-CoV-2 formally named by the World Committee on Virus Classification,1, 2, 3 and the disease caused by this virus was named as COVID-19 by the World Health Organization.4 , 5 This virus has a characteristic crown representing the spike peplomers on the viral envelope, with strong infectivity and general susceptibility to people of all ages and across the globe. On the 8th February 2020, the disease was named as “Novel Coronavirus Pneumonia (NCP)” in China.6 By February 16, 2020, China had reported a cumulative total of 70,548 patients being infected with SARS-CoV-2, 57,934 cases currently treated (10,644 severe cases), 1770 deaths, 10,844 cases being discharged from hospitals, 7264 suspected cases and 546,016 cases in close contact including 150,539 people being isolated and closely watched. The SARS-CoV-2 is primarily propagated through respiratory droplets and close contact, with the incubation period usually between 1 and 14 days and the typical symptoms after onset of fever, dry cough, fatigue and gradual appearance of dyspnea. People carrying this virus are the source of infection even in the incubation period, and early diagnosis of this disease or carrier of the virus is crucial to preventing it from further spread. However, confirmation of infection of this virus requires presence of the virus nucleic acid detected in swabs, secretions and sputum from the respiratory tract, blood or stools.7 Nonetheless, detection of the virus nucleic acid may not be convenient, and early diagnosis of the NCP is essential for timely isolation and treatment of the patient. Computed tomography (CT) is easily available and can be used to screen patients for rapid confirmation of SARS-CoV-2 infected NCP because CT, especially high resolution CT, has the advantages of high spatial resolution, freedom of disturbance from other structures outside the scanning plane and ability to display every details of the lesion in multiple planes and directions. This study was performed to analyze the clinical and CT imaging features in patients with SARS-CoV-2 infected NCP or COVID-19 and to familiarize radiologists and doctors with the common clinical and CT imaging findings of this disease for quick recognition, rapid isolation and treatment of the patient.
Materials and methods
This retrospective study was conducted in January and February 2020, and the ethics committee of our hospital waived written informed consent because of its retrospective and emergent nature and evaluation of only the imaging and clinical data of the patients, involving no potential risk. Inclusion criteria were patients infected with 2019-nCoV, positive nucleic acid of the virus and CT scanning of the lung.
When patients were admitted, CT pulmonary scanning was performed for all patients with the high-resolution LightSpeed VCT CT64 scanner (GE MEDICAL SYSTEMS, China Branch, Beijing, China). The patient was in the supine position and scanned with breath holding at the end of inhaling. The scanning range was from the thoracic entrance to the level of posterior costophrenic anglem with the scanning parameters of tube voltage 120 kV, with the automatic milliampere technology (40–250 mA), noise index (NI) 25, pitch 0.984:1, matrix 512 × 512, slice thickness 5 mm, window width/level 2000/- 600 HU for the lung window, 350/40 HU for mediastinal window, and slice thickness 0.625 mm for reconstruction of the lung window in the axial position.
Imaging analysis was performed by three experienced radiologists with over 10-year experience, and in case of disagreement, they would consult to reach an agreement. The CT imaging was analyzed according to the following parameters: lesion distribution: left lung (upper or lower lobe), right lung (upp, middle or lower lob) and single or multiple lesions within each lobe; lesion location: peripheral, central or involving both peripheral and central locations; lesion density: ground glass opacity, consolidation and mixed type of ground glass opacity and consolidation; thickness of interlobular and intralobular septa, enlarged lymph nodes within the mediastinum and pleural effusion.
Based on the fifth edition of the China Guidelines for the Diagnosis and Treatment Plan of Novel Coronavirus (2019-nCoV) Infection by the National Health Commission (Trial Version 5),6 the NCP was classified into four types: mild with slight clinical symptoms but no imaging presentations of pneumonia; common with fever, respiratory symptoms and imaging presentations of pneumonia; severe type with any of the following: respiratory distress with RR> 30 times/minutes, oxygen saturation at rest <93%, or PaO2/FiO2<300 mmHg (1 mmHg=0.133 kPa); critically severe type with any of the following: respiratory failure needing mechanical ventilation, shock, or combination with other organ failure needing ICU intensive care.
Statistical analysis
The statistical analysis was performed with the SPSS software (Version 19.0, IBM, Chicago, IL, USA). Continuous data were presented as mean± SD (standard deviation) and tested with paired t-test. Categorical data were presented as number (%) and tested with Chi Square test or Fisher's exact test. The significant P value was set at < 0.05.
Results
Basic features of NCP
Fifty patients with NCP caused by infection of the SARS-CoV-2 virus were enrolled and had high-resolution pulmonary CT scanning, including mild type in nine, common in 28, severe in 10 and critically severe in the rest three (Table 1 ). There were 29 (58%) male patients and 21 (42%) female patients, with an age range of 3–85 years (mean 43.9 ± 16.8). Five (10%) patients were below the age of 18 years, 30 (60%) between 18 and 50 years and 15 (30%) over 50 years. The mean age and age range were 29 and 3–47 years for mild patients, 44.5 and 17–62 years for common, 54.7 and 34–85 years for severe, and 65.7 and 50–79 years for critically severe, respectively. Mild patients were significantly (P<0.03) younger than either common or severe and critically severe patients, and common patients were also significantly (P<0.03) younger than severe and critically severe patients.
Table 1.
Demography and clinical classification of patients.
| Variables | Total (n or%) | Mild | Moderate | Severe | Critically severe |
|---|---|---|---|---|---|
| Case no. | 50 | 9(18%) | 28(56%) | 10(20%) | 3(6%) |
| Sex | M(29 or 58%) | 7(14%) | 15(30%) | 7(14%) | 0 |
| F(21 or 42%) | 2(4%) | 13(26%) | 3(6%) | 3(6%) | |
| Age (y) | <18 (5 or 10%) | 4(8%) | 1(2%) | 0 | 0 |
| 18–50 (30 or 60%) | 5(10%) | 17(34%) | 7(14%) | 1(2%) | |
| >50 (15 or 30%) | 0 | 10(20%) | 3(6%) | 2(4%) | |
| Epidemical history | Wuhan or nearby (30 or 60%) | 8(16%) | 16(32%) | 6(12%) | 0 |
| Close contact (18 or 36%) | 1(2%) | 11(22%) | 4(8%) | 2(4%) | |
| No definite causes | 0 | 1(2%) | 0 | 1(2%) | |
| Fever | 37.3–38 °C (22 or 44%) | 3(6%) | 16(32%) | 3(6%) | 0 |
| 38.1–39 °C (16 or 32%) | 3(6%) | 8(16%) | 3(6%) | 2(4%) | |
| >39 °C (5 or 10%) | 0 | 3(6%) | 2(4%) | 0 | |
| Cough | 20 (40%) | 3(6%) | 11(22%) | 4(8%) | 2(4%) |
| Expectoration | 7 (14%) | 1(2%) | 4(8%) | 1(2%) | 1(2%) |
| Sore throat | 4 (8%) | 1(2%) | 2(4%) | 1(2%) | 0 |
| Headache | 5 (10%) | 0 | 3(6%) | 1(2%) | 1(2%) |
| Fatigue | 8 (16%) | 0 | 2(4%) | 5(10%) | 1(2%) |
| Muscle ache | 8 (16%) | 0 | 4(8%) | 3(6%) | 1(2%) |
| Chest tightness and dyspnea | 4 (8%) | 0 | 0 | 3(6%) | 1(2%) |
| Gastrointestinal reaction | 1 (2%) | 0 | 1(2%) | 0 | 0 |
| Normal or decreased leukocyte count | 49 (98%) | 8(16%) | 28(56%) | 10(20%) | 3(6%) |
| Decreased lymphocyte count | 14 (28%) | 2(4%) | 6(12%) | 4(8%) | 2(4%) |
| Increased C-reactive protein | 26 (52%) | 2(4%) | 16(32%) | 5(10%) | 3(6%) |
In disease exposure history, thirty (60%) patients had been to Wuhan or the nearby regions, 18 (36%) in close contact with patients of NCP and 2 (4%) with no definite exposure history.
Clinical presentations of NCP
Clinical presentations of NCP were fever, cough, expectoration, fatigue, headache, gastrointestinal discomfort, dyspnea and muscle ache. The body temperature was below 37.3 °C in seven patients (14%), between 37.3 °C and 38 °C in 22 (44%), between 38 °C and 39 °C in 16 (32%), and over 39 °C in 5 (10%). Cough was in 20 (40%) patients, expectoration in 7 (14%), fatigue in 8 (16%), headache in five (10%), sore throat in four (8%), gastrointestinal discomfort in one (2%), dyspnea in four (8%) and muscle ache in 8 (16%). Mild patients had low to moderate fever (<39.1 °C), 49 (98%) patients had normal or slightly reduced leukocyte count, 14 (28%) had decreased counts of lymphocytes, and 26 (52%) patients had increased C-reactive protein (Table 1).
Lesion distribution in imaging
Among 50 patients infected with SARS-CoV-2, nine mild patients were negative in CT pulmonary imaging (Fig. 1 ), including five cases below the age of 18 years, suggesting that children, teenagers and younger patients were mostly mild. Among 41 common, severe and critically severe patients who had positive pulmonary imaging (Table 2 , Figs. 2 –6), the lesion was in the right upper lobe in 30 cases, right middle lobe in 22, right lower lobe in 39, left upper lobe in 33 and left lower lobe in 36, suggesting bilateral lower lobes as the mostly infected while the right middle lobe the least infected. Most of the patients had involvement of over two lobes, and single lobe involvement was only in two cases in the right lower lobe, with the lesion within lobes being mostly multiple (Table 3 ). Severe and critically severe NCP involved most commonly 4–5 lobes and most significantly (P<0.05) bilateral lower and upper lobes compared with common NCP (Table 3, Table 4 ). The lesion was primarily located in the peripheral area under the pleura with possible extension towards the pulmonary hilum in big lesions (Table 5 ). Symmetrical lesions were seen in 26 cases and asymmetrical in 15.
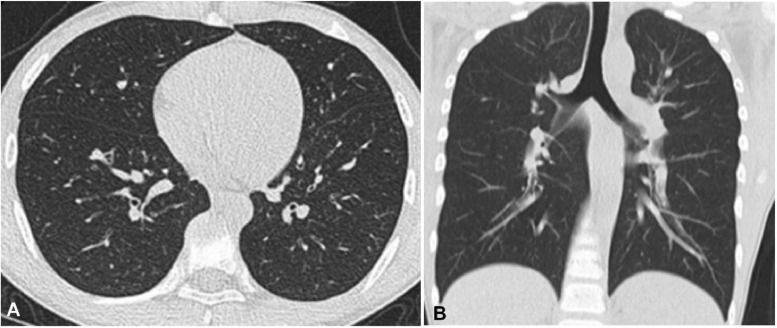
Fig. 1.
Mild novel coronavirus pneumonia in a 13-year-old man who had intermittent fever for three days before admission. Plain computed tomographic scan of the lung (A, axial plane, and B, coronal plane) in the lung window showed no obvious abnormality in the lungs.
Table 2.
Location of lesions in 41 common and severe/critically severe NCP [n(%)].
| Lobe | Case no.(%) | Moderate (n = 28) |
Severe and critically severe (n = 13) |
||
|---|---|---|---|---|---|
| Single lesion (%) | Multiple(%) | Single (%) | Multiple (%) | ||
| Right upper lobe | 30(73.2) | 6(33.3) | 12(66.7) | 2(16.7) | 10(83.3) |
| Right middle lobe | 22(53.7) | 4(30.8) | 9(69.2) | 2(22.2) | 7(77.8) |
| Right lower lobe | 39(95.1) | 6(23.1) | 20(76.9) | 0(0.0) | 13(100.0) |
| Left upper lobe | 33(80.5) | 6(28.6) | 15(71.4) | 2(16.7) | 10(83.3) |
| Left lower lobe | 36(87.8) | 3(13.0) | 20(87.0) | 2(16.7) | 11(84.6) |
Note: NCP, novel coronavirus pneumonia.
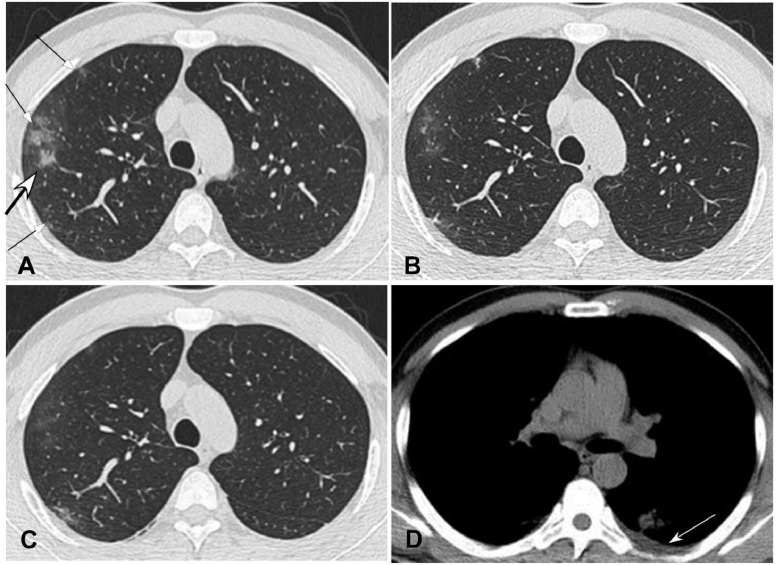
Fig. 2.
Common novel coronavirus pneumonia in a 37-year-old man with fever for six days and cough for two days before admission. A. Axial plane of computed tomography scan in the lung window showed multiple irregular pieces (arrows) of ground glass opacity under the pleura with consolidation (bigger arrow) and thickened interlobular sept in the right upper lobe. B. Seven days after treatment, the extent of the lesions decreased with fibrosis formation. C. Ten days after treatment, the extent of disease further shrank with decreased density. D. Axial plane in the mediastinal window revealed a small amount of pleural effusion (arrow).
Fig. 6.
Critically severe novel coronavirus pneumonia (NCP) in a 50-year-old woman with fever, cough, dizziness and fatigue for five days before admission. A&B. Computed tomography axial (A) and coronal (B) plane revealed multiple lesions of ground glass opacity accompanied with consolidation. The lesions extended towards the pulmonary hilum and had air bronchogram and thickened interlobular septa. C&D. Five days after treatment, the extent of disease shrank with decreased density but stripes of fibrosis.
Table 3.
Lung lobes involved in 41 common and severe/critically severe NCP [n(%)].
| Lobes involved | Moderate (n = 28) | Severe and critically severe (n = 13) |
|---|---|---|
| Single lobe | 2(7.1%) | 0(0%) |
| Two lobes | 4(14.3%) | 1(7.7%) |
| Three lobes | 5(17.9%) | 0(0%) |
| Four lobes | 9(32.1%) | 3(23.1%) |
| Five lobes | 8(28.6%) | 9(69.2%) |
Note: NCP, novel coronavirus pneumonia.
Table 4.
Lung lobes involved in 41 common and severe/critically severe NCP [n(%)].
| Lobes involved | Common (n = 28) | Severe/critically severe (n = 13) | χ2 | P |
|---|---|---|---|---|
| Bilateral upper lobes | 15 (53.6) | 12 (92.3) | 4.327 | 0.038 |
| Bilateral lower lobes | 12 (42.9) | 13 (100.0) | 12.183 | <0.001 |
Note: NCP, novel coronavirus pneumonia.
Table 5.
CT imaging of 41 common and severe/critically severe NCP [n(%)].
| CT imaging | Moderate (n = 28) | Severe/critically severe (n = 13) | χ2 | P |
|---|---|---|---|---|
| Within lobes | ||||
| Peripheral | 27(96.4) | 12(92.3) | -* | 0.539 |
| Central | 14(50.0) | 5(38.5) | 0.475 | 0.491 |
| Peripheral involving central | 12(42.9) | 11(78.6) | 4.805 | 0.028 |
| Symmetrical | 15(53.6) | 11(84.6) | 2.471 | 0.116 |
| Density and inner features | ||||
| Ground glass opacity | 21(75.0) | 9(69.2) | 0.001 | 0.993 |
| consolidation | 6(21.4) | 9(69.2) | 6.805 | 0.009 |
| Mixed ground glass opacity and consolidation | 15(53.6) | 10(76.9) | 2.035 | 0.154 |
| Thickened intralobular septa | 21(75.0) | 9(69.2) | 0.001 | 0.993 |
| Thickened interlobular septa | 20(71.4) | 13(100.0) | -* | 0.040 |
| Air bronchogram | 15(53.6) | 7(53.8) | 0.000 | 1.000 |
| Other features | ||||
| Pleural effusion | 2(7.1) | 2(15.4) | 0.069 | 0.793 |
| Enlarged mediastinal nodes | 1(3.6) | 0(0.0) | 0.000 | 1.000 |
Fisher exact probability method; NCP, novel coronavirus pneumonia; CT, computed tomography.
CT imaging presentations
On CT imaging, common NCP was mostly at the early stage with bilaterally scattered irregular patches of ground glass opacity. Some lesions might have a mixed pattern of consolidation in the center and ground glass opacity in the peripheral like a “halo sign”. The lesion density was mostly non-uniform with air bronchogram and thickened interlobular or intralobular septa. In severe or critically severe patients, multiple patches or an integrated larger patch of ground glass opacity, consolidation or mixed consolidation and ground glass opacity might present in bilateral lungs, with these three imaging manifestations being possibly presented in one patient at the same time. Consolidation and thickened interlobular septa were presented in severe or critically severe patients more than common ones, suggesting progression of the disease. The lesion was mostly located in the peripheral area under the pleura but might extend towards the center in bigger lesions, and peripheral lesions with involvement of the center were mostly seen in severe and critically severe patients. Four to ten days after treatment in this series, repeated CT scanning revealed that most lesions were absorbed and improved with reduced extent, decreased density and formation of fibrotic stripes (Fig. 2 –6 ). In one common patient, the lesion in the right lower lobe was improved four days after treatment, however, a new lesion appeared in the left lower lobe which was improved after treatment for three additional days (Fig. 3). This might indicate that the lesion might change rapidly in a short period of time and repeated CT scanning could provide basis for clinical diagnosis and treatment.
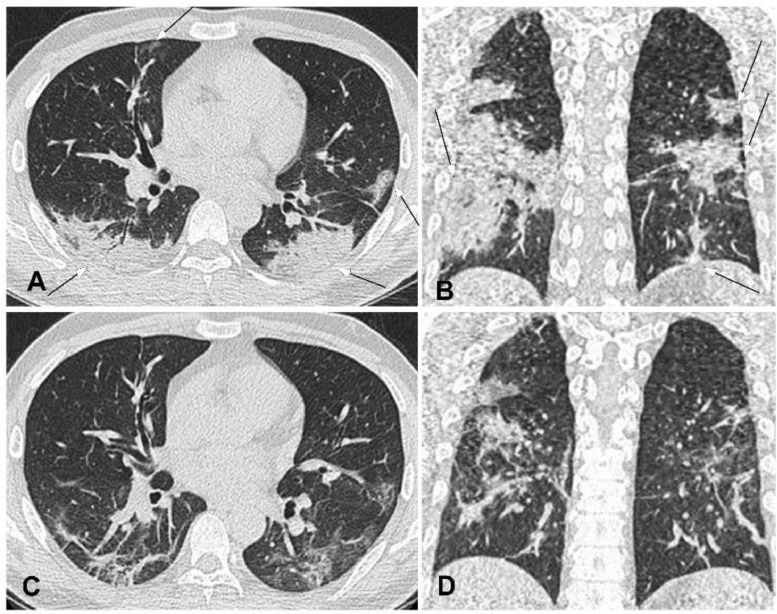
Fig. 4.
Severe novel coronavirus pneumonia in a 34-year-old man with fever and cough for ten days before admission. A&B. Computed tomography axial (A) and coronal (B) plane revealed multiple lesions of ground glass opacity, consolidation and fibrosis with symmetrical distribution in bilateral lungs, with the lesion extending towards the pulmonary hilum. Air bronchogram was observed within the lesion. C&D. Four days after treatment, the extent of lesion shrank with decreased density and formation of fibrosis.
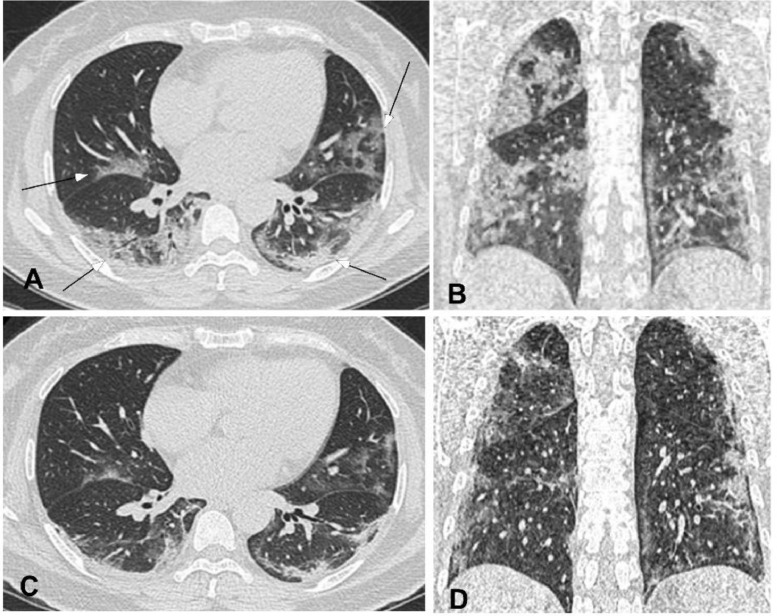
Fig. 5.
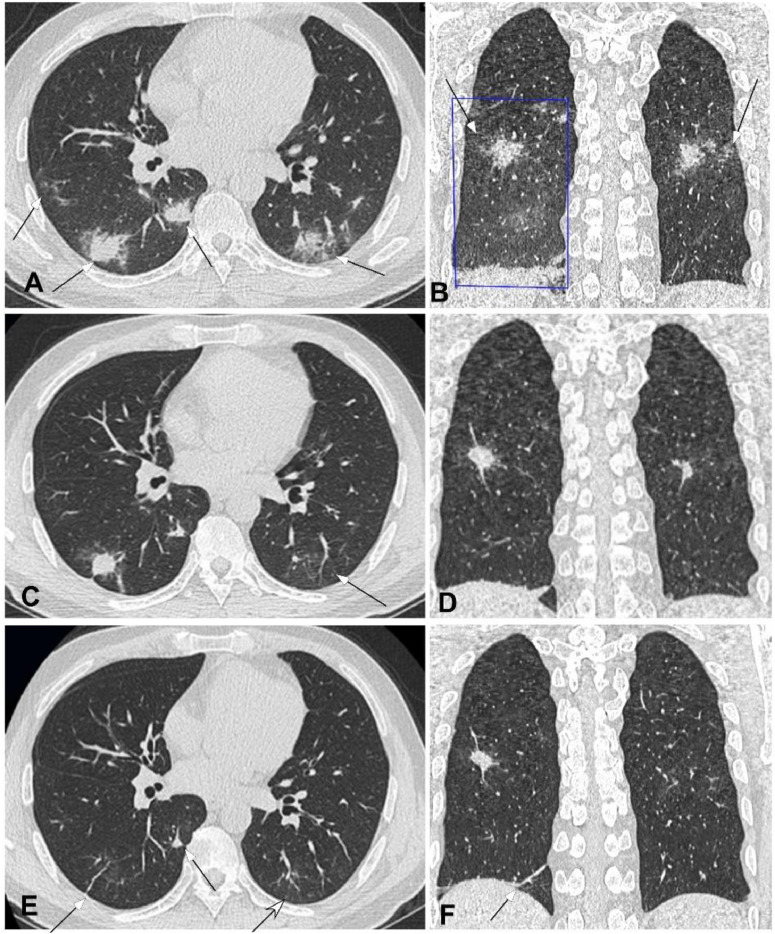
Severe novel coronavirus pneumonia in a 48-year-old man with fever for seven days before admission. A&B. Computed tomography axial (A) and coronal (B) plane revealed multiple lesions (arrows) of ground glass opacity accompanied with consolidation under or near the pleura in bilateral lower lobes, with air bronchogram and thickened interlobular septa. A large piece of ground glass opacity (square box) could also be seen in the right lower lobe (B). C&D. Seven days after treatment, the right lesion was significantly reduced with formation of fibrotic stripes, and the left lesion was also absorbed with decreased density like ground glass opacity (arrow in C). E&F. Ten days after treatment, bilateral lesions were mostly absorbed with only some nodules and stripes of fibrosis left (arrows).
Fig. 3.
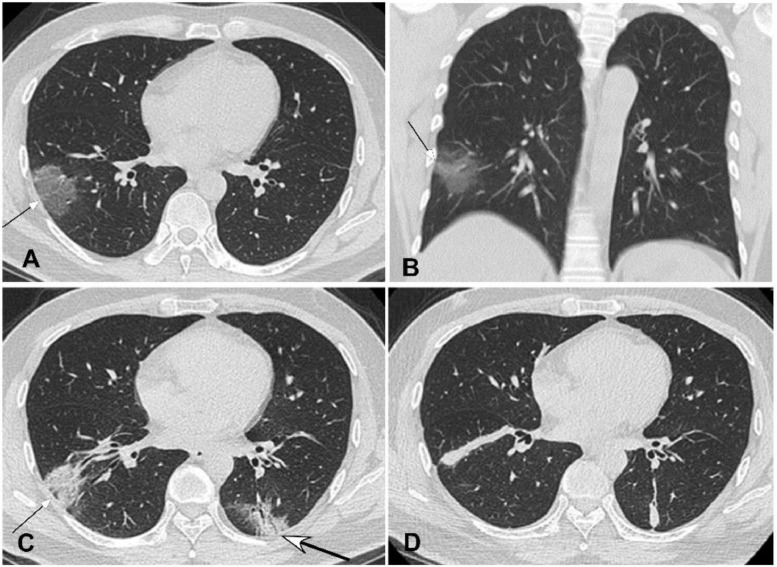
Common novel coronavirus pneumonia in a 46-year-old man with intermittent fever for five days before admission. A&B. Computed tomography pulmonary scan in the axial (A) and coronal (B) plane demonstrated a piece of ground glass opacity (arrow) under the pleura in the right lower lobe. C. Four days after treatment, the extent of lesion (small arrow) was decreased but with increased density, and a new lesion (bigger arrow) appeared in the left lower lobe with air bronchogram inside. D. Eleven days later, the extent of disease in both lungs shrank further and became consolidated with thickened interlobular septa.
Follow-up CT scanning was performed in 30 cases, with 27 cases having repeated CT scanning within one week and three cases over one week. The interval of repeated CT scanning was 3–13 days, with 1–4 CT scanning in total. Nineteen patients showed improved imaging findings at the first CT follow-up, eight patients showed disease progression at the first but improvement at the second CT follow-up, one case had disease progression at the first two CT follow-up but improvement at the fourth follow-up, and the rest two patients had no marked improvement at CT follow-up. The disease was improved between 3 and 13 days. Two mild patients remained negative on CT pulmonary imaging.
Discussion
Patients infected with SARS-CoV-2 may present primarily with low fever, fatigue and dry cough with accompanied symptoms of nasal congestion, runny nose and diarrhea like common cold in some cases.1, 2, 3 , 8, 9, 10, 11 Mild patients may just have low fever and slight weakness without pneumonia, severe patients will have dyspnea and/or hypoxemia, and those with critically severe illness will quickly progress to acute respiratory distress syndrome, septic shock, uncorrectable metabolic acidosis, coagulation dysfunction and even death. Unlike infection with SARS-CoV and H7N9 avian influenza which usually result in high fever at the beginning of infection,12 the initial symptoms of SARS-CoV-2 infection are atypical with only low fever and even a long incubation period, which leads to its strong infectiousness.
Laboratory tests usually reveal at early stages normal or reduced counts of peripheral blood leukocytes and lymphocytes. In some patients, the liver enzyme, lactate dehydrogenase (LDH), muscle enzyme and myoglobin may increase while some severe patients may have increased troponin. Most patients have increased C-reactive protein and elevated erythrocyte sedimentation rate. The virus nucleic acid can be detected in swabs, secretions and sputum from the respiratory tract, blood or excrement. In some patients with negative virus nucleic acid, chest CT scanning may detect abnormality.7 , 13, 14, 15, 16
The primary CT imaging findings are summarized here. Most lesions occur in the peripheral area or under the pleura along the bronchovascular bundles. Multiple locations are involved with occasionally single or double lesions, lower lobes are involved more often than the upper and middle lobes, and the right middle lobe is the least to be infected. The lesion may be patchy, nodular, honeycomb, grid or strips, and the lesion density is mostly uneven with the primary presentation of ground glass opacity accompanied by thickening of interlobular or intralobular septa. The lesion may also present as paving stones with consolidations and formation of fiber stripes. The lesion may be accompanied by air bronchogram but rarely by pleural effusion and enlarged mediastinal nodes. The imaging manifestations of this group are sometimes inconsistent with the clinical manifestations.
Mild NCP has no abnormality in the pulmonary imaging, but these patients still have infectivity and should be properly isolated and treated. Mild patients were significantly younger than the other types of patients, suggesting that children, teenagers and younger patients were mostly mild. Patients with severe (mean 54.7 year) and critically severe (mean 65.7 years) NCP were significantly (P<0.05) older than those with mild and common (44.5 years) NCP. Severe and critically severe NCP involved more commonly 4–5 lobes and most significantly (P<0.05) bilateral lower and upper lobes compared with common NCP. The lesion was primarily located in the peripheral area under the pleura with possible extension towards the pulmonary hilum in big lesions or when the disease is deteriorated. In a short period of time, the lesion may change quickly with occurrence of new lesions in other areas of the lung or improvement at three days after treatment, necessitating repeated CT imaging scan for guiding disease progression and implementing proper treatment.
NCP should be differentiated from other pneumonia caused by other viruses like influenza virus, parainfluenza virus, adenovirus and SARS-CoV or by other microorganisms including Mycoplasma, Chlamydia and bacteria. Bacterial pneumonia presents as small pieces of shadow distributing along the bronchus, which can fuse into a large lesion or a large piece of consolidation. Laboratory tests can show increased count of leukocytes in bacterial pneumonia for differentiation. Other viruses cause pneumonia with large diffused lesions of ground glass opacity in both lungs accompanied with interlobular septa, which may be difficult to differentiate from SARS-CoV-2 pneumonia, however, definite epidemical history is useful for this disease. Virus nucleic acid detection helps determine the diagnosis.
In summary, imaging presentations of NCP are mostly patchy ground glass opacities in the peripheral areas under the pleura with partial consolidation which will be absorbed with formation of fibrotic stripes if improved. The lesion may have quick changes with formation of new lesions in other areas and extend from the peripheral to the central area if deteriorated. Repeated CT scanning is helpful for monitoring disease progression and implementing timely treatment.
Declaration of Competing Interest
None.
Contributor Information
Xiao-Yan Lv, Email: 15001008285@139.com.
Xiao-Ping Yin, Email: yinxiaoping78@sina.com.
Bu-Lang Gao, Email: browngao@163.com.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in wuhan, china. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kui L., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in hubei province. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000744. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, china. JAMA. 2020 doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang X., Rayner S., Luo M.H. Does sars-cov-2 has a longer incubation period than sars and mers? J Med Virol. 2020 doi: 10.1002/jmv.25708. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorusso A., Calistri P., Petrini A., Savini G., Decaro N. Novel coronavirus (sars-cov-2) epidemic: a veterinary perspective. Vet Ital. 2020 doi: 10.12834/VetIt.2173.11599.1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Lin L., Li T.S. [interpretation of "guidelines for the diagnosis and treatment of novel coronavirus (2019-ncov) infection by the national health commission (trial version 5)"] Zhonghua Yi Xue Za Zhi. 2020;100(0):E001. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Pan Y., Guan H. Imaging changes in patients with 2019-ncov. Eur Radiol. 2020 doi: 10.1007/s00330-020-06713-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W., Xian J. The progress of 2019 novel coronavirus (2019-ncov) event in china. J Med Virol. 2020 doi: 10.1002/jmv.25705. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen K., Yang Y., Wang T., Zhao D., Jiang Y., Jin R. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement. World J Pediatr. 2020 doi: 10.1007/s12519-020-00343-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined chinese and western medicine treatment. Biosci Trends. 2020 doi: 10.5582/bst.2020.01030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Wu Y.C., Chen C.S., Chan Y.J. Overview of the 2019 novel coronavirus (2019-ncov): the pathogen of severe specific contagious pneumonia (sscp) J Chin Med Assoc. 2020 doi: 10.1097/JCMA.0000000000000270. [Epub ahead of print] [DOI] [Google Scholar]
- 12.Wong K.T., Antonio G.E., Hui D.S., Lee N., Yuen E.H., Wu A. Thin-section ct of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228:395–400. doi: 10.1148/radiol.2283030541. [DOI] [PubMed] [Google Scholar]
- 13.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. Ct imaging features of 2019 novel coronavirus (2019-ncov) Radiology. 2020 doi: 10.1148/radiol.2020200230. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei J., Li J., Li X., Qi X. Ct imaging of the 2019 novel coronavirus (2019-ncov) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200236. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi H., Han X., Zheng C. Evolution of ct manifestations in a patient recovered from 2019 novel coronavirus (2019-ncov) pneumonia in Wuhan, China. Radiology. 2020 doi: 10.1148/radiol.2020200269. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest ct for typical 2019-ncov pneumonia: relationship to negative rt-pcr testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]