Abstract
MERS-coronavirus infection is currently responsible for considerable morbidity and mortality in Saudi Arabia. Understanding its burden, as an emerging infectious disease, is vital for devising appropriate control strategies. In this study, the burden of MERS-CoV was estimated over 31 months period from June 6, 2012 to January 5, 2015. The total number of patients was 835; 528 (63.2%) patients were male, 771 (92.3%) patients were ≥25 years of age, and 210 (25.1%) patients were healthcare workers. A total of 751 (89.9%) patients required hospitalization. The median duration between onset of illness and hospitalization was 2 days (interquartile range, 0–5). The median length of hospital stay was 14 days (IQR, 6–27). The overall case fatality rate was 43.1%. Basic reproductive number was 0.9. Being Saudi, non-healthcare workers, and age ≥65 years were significantly associated with higher mortality. In conclusion, MERS-CoV infection caused a substantial health burden in Saudi Arabia.
Keywords: MERS-Coronavirus, Saudi Arabia, Burden, Case fatality rate, Health care workers
Introduction
In 2012, a novel viral infection causing severe acute respiratory illness in humans was identified in Saudi Arabia. The virus, now known as Middle East respiratory syndrome coronavirus (MERS-CoV), was subsequently reported from 27 other countries in in the Middle East, North Africa, Asia, Europe and the United States of America [1]. The largest outbreaks were reported from Saudi Arabia, United Arab Emirates, and the Republic of Korea. As of July 2019, WHO reported a total of 2458 laboratory-confirmed cases worldwide including at least 848 deaths since April 2012 [1]. Among all cases reported worldwide, 2067 (84%) cases were reported from Saudi Arabia [1,2].
Patients with MERS-CoV infection usually present with acute respiratory signs and symptoms including fever, cough, headache, myalgia, and sometimes nausea, vomiting, or diarrhea. The respiratory illness often evolves into shortness of breath and severe respiratory illness. Disease severity varies widely from asymptomatic cases to fatal outcomes. It is believed that most infected persons do not show symptoms as indicated by a seroprevalence survey that estimated the number of seropositive people in Saudi Arabia to be nearly 45,000 persons [3]. The case fatality rate among clinical cases has been estimated to be 40% [4]. Although the high case fatality of the disease and the frequent occurrence of outbreaks provoked calls to develop a vaccine [5], several obstacles were encountered including lack of an animal model and the high cost of vaccine development [6].
MERS-CoV causes considerable morbidity and mortality with a substantial healthcare cost. Understanding the burden of this emerging infectious disease is vital for devising control strategies. Previous studies described the epidemiology of hospital outbreaks [7,8], community outbreaks [9], as well as regional or time-specific cases [[10], [11], [12], [13]]. The objective of this study was to estimate the burden of MERS-CoV over a 31 months-period following its first identification in 2012 including the nation-wide MERS-CoV epidemic that occurred in Saudi Arabia in 2014 up to January 2015.
Methods
This study analyzed the data of all MERS-CoV cases that confirmed by Real time PCR by Ministry of Health’s surveillance program from June 6, 2012 to January 5, 2015.
This surveillance was nationwide under the supervision of Ministry of Health to ensure that the case definition was followed as per national MERS-CoV guidelines.
Ethical approval was obtained from the Institutional review board of Jeddah Health directorate (IRB approval number A00223).
Basic information for the confirmed cases that was collected included age, sex, occupation, date of onset, hospitalization, duration of hospital stay for hospitalized patients, and mortality. Duration between onset of illness and admission to hospital, case fatality rate, secondary attack rate, and basic reproduction number were calculated.
Descriptive epidemiology was performed on demographic data, and analytic epidemiology was performed to assess any difference in case fatality rate among health care workers and others. Statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL). Categorical variables were presented as frequency and percentages. Age was presented as mean and standard deviation (SD). Length of stay in hospital and duration between onset of illness and admission were presented as median and interquartile range (IQR).
The basic reproduction number or ratio (R0), defined as the expected number of secondary cases produced by a typical infected individual early in an epidemic in an otherwise uninfected and completely susceptible population [14], was calculated using the following formula:
| R0 = (infection/contact) × (contact/time) × (time/infection). |
| = (835/19) × (19/7) × (7/835) = 0.9. |
Multiple logistic regressions analysis was performed to adjust for confounding factors and identify the factors associated with case fatality. All variable with p value < 0.1 in the bivariate analysis were included in the regression model. This was presented as odds ratio (OR) with confidence interval (CI). The level of significance was set at p ≤ 0.05.
Results
The total number of reported cases during the study period was 835 cases. Table 1 shows the demographic characteristics of all laboratory-confirmed MERS-CoV cases. Males were more commonly affected than females (63.2% versus 36.8%). The vast majority of patients were between 25-54 years (52%) or ≥65 years (23.4%) of age. HCWs comprised 25.1% of the cases. Health Care workers were younger than the other patients (mean age 38.9 ± 11.6 vs 52.7 ± 20.3 years, respectively, p value < 0.001). Saudis comprised 63.2% of the cases. Although MERS-CoV cases were reported from virtually all regions of the country, about two thirds of the cases were reported from Riyadh (29.9%) and Jeddah (28.7%).
Table 1.
Demographic characteristics of laboratory-confirmed MERS-CoV cases – June 1st, 2012–January 5th, 2015 (n = 835).
| Characteristics | No. (%) |
|---|---|
| Age | |
| <15 | 28 (3.3) |
| 15–<25 | 36 (4.3) |
| 25–<55 | 434 (52.0) |
| 55–<65 | 142 (17.0) |
| ≥65 | 195 (23.4) |
| Sex | |
| Male | 528(63.2) |
| Female | 307 (36.8) |
| Nationality | |
| Saudi | 545 (65.3) |
| Non Saudi | 290 (34.7) |
| Region | |
| Jeddah | 240 (28.7) |
| Riyadh | 250(29.9) |
| Others | 345 (41.4) |
| Job | |
| Health care workers | 210 (25.1) |
| Non-health care workers | 625 (74.9) |
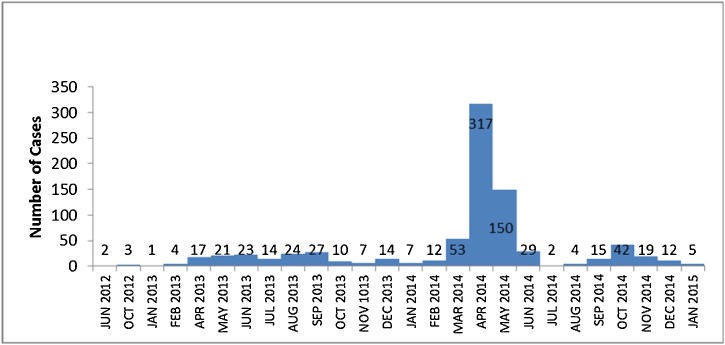
After the first 2 cases of MERS-CoV infection reported in 2012, the number of reported cases ranged between 1-27 cases per month. This rate continued till March 2014 when 53 cases were reported in that month followed by a more dramatic increase in the number of cases in the following month (April 2014) when the number of cases reached up to 317 cases in one month. Emergency investigations and control measures coordinated by the Ministry of Health led to a sharp decline in the number of reported cases to 150 cases in May 2014 and further down to 29 cases in June 2014. Since then, the number of reported cases returned back to its previous rate (Fig. 1 ). The basic reproductive number was 0.9.
Fig. 1.
Epidemic curve of MERS-CoV cases by month and outcome based on date of onset for symptomatic and date of diagnosis for asymptomatic patients in Saudi Arabia from June 1st, 2012–January 5th, 2015 (total: 835 cases).
Hospitalization associated with the MERS-CoV was estimated to be 2 per 100,000 population. The median duration between the onset of clinical symptoms and presentation to hospitals was 2 days with an interquartile range (IQR) of 0–5 days. HCWs presented to hospitals earlier than non−HCWs (2 versus 3 days, respectively, p = 0.001). The median length of hospital stay was 14 days (Table 2 ). The length of stay among non−HCWs was significantly more than that among HCWs (15 versus 12 days, respectively, p = 0.023).
Table 2.
Length of stay and duration between onset of symptom and admission among health care workers (HCWs) and non-health care workers (Non−HCWs).
| Characteristics | All cases (n = 574) |
HCWs (n = 178) |
Non-HCWs (n = 396) |
p-Value** | |||
|---|---|---|---|---|---|---|---|
| Median | IQR* | Median | IQR* | Median | IQR* | ||
| Length of stay | 14 | 6–27 | 12 | 6–21 | 15 | 6–31 | 0.023 |
| Duration between onset of symptom and admission | 2 | 0–5 | 2 | 0–5 | 3 | 1–6 | 0.001 |
Inter-quartile range.
Man–Whitney test.
Table 3 shows the case fatality rate by year from 2012 to 2014. The overall case fatality rate was 43.2%. The highest case fatality rate was observed in the first 2 years (60% for 2012 and 51.5% for 2013) which decreased to 36.9% in 2014 when more cases were reported during the outbreak in 2014. Table 4 shows the case fatality rate by age groups and age-specific mortality rate per 100,000 population.
Table 3.
Number of cases and case fatality rate of laboratory-confirmed MERS-CoV cases by year.
| Year | Number of cases | Deaths | Case fatality rate |
|---|---|---|---|
| 2012 | 5 | 3 | 60 |
| 2013 | 160 | 84 | 51.5 |
| 2014 | 670 | 274 | 36.9 |
Table 4.
Case fatality rate by age groups and age specific mortality rate per 100,000 population of patients with laboratory-confirmed MERS-CoV infection from June 1st, 2012 to January 5th, 2015 (n: 835 cases).
| Age (years) | Number of patients | Deaths | Case fatality rate | Age-specific mortality rate/100,000 population |
|---|---|---|---|---|
| <15 | 28 | 7 | 25.0 | 0.33 |
| 15–<25 | 36 | 8 | 22.2 | 0.64 |
| 25–<55 | 434 | 116 | 26.7 | 3.3 |
| 55–<65 | 142 | 76 | 53.5 | 10.8 |
| ≥65 | 195 | 154 | 79.0 | 21.1 |
| All | 835 | 361 | 43.2 | 1.24 |
Table 5 shows the results of the multivariable logistic regression analysis for patients’ characteristics associated with mortality.
Table 5.
Factors associated with mortality among MERS-CoV cases.
| Characteristic | Total number of cases | Number of deaths (%) | Odds Ratio (95% CI) | p Value |
|---|---|---|---|---|
| Age | ||||
| <15 | 28 | 7 (25) | Reference | |
| 15–<25 | 36 | 8 (22.5) | 2.7 (0.6–12) | 0.2 |
| 25–<55 | 434 | 116 (26.7) | 4 (1.1–14.1) | 0.03 |
| 55–<65 | 142 | 76 (53.5) | 10.3 (2.8–37.9) | <0.001 |
| ≥65 | 195 | 154 (79) | 35.6 (9.6–132.3) | <0.001 |
| Sex | ||||
| Female | 307 | 107 (34.9) | Reference | |
| Male | 528 | 253 (47.9) | 1.4 (0.9–2) | 0.1 |
| Nationality | ||||
| Non-Saudi | 290 | 70 (24.1) | Reference | |
| Saudi | 545 | 290 (53.2) | 1.6 (1–2.6) | 0.07 |
| Region | ||||
| Jeddah | 240 | 101 (42.1) | Reference | |
| Riyadh | 250 | 108 (43.2) | 0.7 (0.4–1.2) | 0.2 |
| Others | 345 | 151 (43.8) | 0.8 (0.5–1.3) | 0.4 |
| Job | ||||
| Healthcare workers | 210 | 18 (8.6) | Reference | |
| Non- healthcare workers | 625 | 342 (54.7) | 5.4 (2.9–10.1) | <0.001 |
Each factor is adjusted for all other factors in the table.
Discussion
The emergence of MRES-CoV is a big challenge for the Saudi Arabian health care system. Since the Middle East is a center for tourism and business activities, traveling to this region provides opportunities to acquire and spread MERS-CoV beyond its boundaries. Saudi Arabia, as a destination of millions of pilgrims for the Hajj season every year and for Omra year-round, is particularly under an enormous challenge to protect not only its own citizens and residents but also the entire world’s population as a whole from this emerging infectious disease.
Emergency investigations and evidence-based control measures for the MERS-CoV epidemic that occurred in April and May 2014 in Saudi Arabia seems to have led to a sharp decline of cases down to the baseline level [15,16].
Jeddah and Riyadh were the hardest-hit cities and Saudi citizens comprised two thirds of the cases. Health care workers comprised quarter of the reported cases. This led to acute shortage of staff in health care facilities particularly in Jeddah and Riyadh as a result of sick leaves and fear from going to the work place (Personal communication, Ministry of Health).
Recent studies showed that the primary source of MERS-CoV infection to humans is dromedary camels [17,18]. Humans primarily acquire the virus from camels either directly through direct contact with infected camels’ respiratory secretions or indirectly through contact with people who have had contact with infected camels’ respiratory secretions [18]. These primary infections may in sequence lead to secondary human-to-human transmission through close contact with infected human-respiratory secretions usually in the healthcare [11,12,16,[19], [20], [21], [22]] or, less commonly, the household settings [[23], [24], [25], [26]]. Community-based transmission outside the household settings is rare [12,27]. Factors that cause amplification of MERS-CoV infection in the healthcare settings include suboptimal adherence to standard infection control practice and respiratory etiquette, failure to perform triage to segregate patients with acute respiratory illness particularly in the Emergency Departments and Dialysis units, overcrowding of patients, and delayed diagnosis because of atypical presentation often mimicking heart failure or acute dengue [16,[19], [20], [21], [22],28].
The basic reproduction number or ratio (R0) is used to measure the transmission potential of a disease. It is thought of as the number of secondary infections produced by a typical case of an infection in a population that is totally susceptible. When R0 is <1, the infection will die out in the long run, whereas, if R0 is > 1, the infection will be able to spread in a population. Generally, the larger the value of R0, the harder it is to control the epidemic. The basic reproductive number in our study was 0.9 which indicated that each case would on average lead to less than one additional case and that transmission of this infection will eventually stop if there was no external re-infection. The continually reported MERS-CoV cases with occasional clusters observed in Saudi Arabia despite the low R0 is likely due to repeated external re-introduction of the virus from camels.
The increase of community-acquired cases of MERS-CoV infection noted in the period between March-May in Saudi Arabia may correspond to a seasonal factor in community-based transmission likely linked to exposure to camels [29]. This possible seasonal increase of human MERS-CoV cases corresponds to the end of the calving season (December–February) for camels in Saudi Arabia [30,31]. It also corresponds to the weaning season when camels are weaned of milk at 12–16 months of age. A recent prospective study showed that MERS-CoV detection by RT-PCR in dromedary camels was more common in the period between November and January, corresponding to the camels’ calving season [32]. Several studies also showed that MERS-CoV RNA shedding is more common in juvenile than in adult camels [[31], [32], [33], [34]]. Therefore, juvenile camels younger than 2 years of age are likely to be an important source of new MERS-CoV infections in camels and humans leading to higher incidence of human infection during the period between March and May [31,33,34].
The overall CFR was 43.2%. The highest rate was among cases ≥65 years (79%) of age, while the lowest rate was among cases 15–25 years (22.2%) of age. There was an exponential increase in CFR after age 55. Patients ≥65 years of age had the highest risk of mortality with OR of 35.6 (95% CI = 9.6–132.3). Males had 1.4 times higher case fatality compared to females but this was not statistically significant. A higher risk of mortality was also noticed among Saudi cases compared to non-Saudis (OR = 1.6; 95% CI = 1.0–2.6). Non−HCW had significantly higher mortality rate compared to HCWs (OR = 5.4 95% CI = 2.9–10.1). Multiple logistic regression showed that being Saudi, non-healthcare workers, and ≥65 years of age were significantly associated with higher CFR. Unlike the related SARS coronavirus, the MERS-CoV is associated with a relatively higher CFR (>30%) and noticeable age and sex differences among confirmed cases [12]. The male to female ratio among confirmed cases of MRES-CoV is reported to be 2:1, with highest rates of morbidity and mortality occurring among patients ≥50 years of age [12]. The significantly lower mortality rate observed among HCWs in comparison with non−HCWs is likely due to earlier presentation (2 vs 3 days, respectively), younger age (38.9 vs 52.7 years, respectively), and lower risk of having comorbidities.
We acknowledge two limitations to our study including that persons who develop mild symptoms will likely lead to an underestimation of the diseases burden and an overestimation of the severity. The surveillance data did not indicate whether patients were admitted to the ICU or not. Therefore, we were unable to identify the burden of MERS CoV on the ICU admissions.
In conclusion, MERS-CoV infection caused a substantial health burden in Saudi Arabia due to high morbidity, mortality, and hospitalization rate, long hospital stay, and shortage of staff. Enhancing MERS-CoV data-sharing and analysis for better understanding of the epidemiology of this novel virus would help in devising effective preventive strategies to reduce the burden of this viral infection on the health system.
Funding
No funding sources.
Competing interests
None declared.
References
- 1.World Health Organization . 2019. Middle East respiratory syndrome coronavirus (MERS-CoV)http://www.who.int/emergencies/mers-cov/en/ (Accessed 24 August 2019) [Google Scholar]
- 2.Ministry of Health . 2019. Command and control center.https://www.moh.gov.sa/en/CCC/Pages/default.aspx (Accessed 24 August 2019) [Google Scholar]
- 3.Müller M.A., Meyer B., Corman V.M., Al-Masri M., Turkestani A., Ritz D. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15:559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majumder M.S., Rivers C., Lofgren E., Fisman D. Estimation of MERS-coronavirus reproductive number and case fatality rate for the spring 2014 Saudi Arabia Outbreak: insights from publicly available data. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.98d2f8f3382d84f390736cd5f5fe133c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang N., Jiang S., Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev Vaccines. 2014;13:761–774. doi: 10.1586/14760584.2014.912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaneri A.B., Johnson R.F., Wada J., Bollinger L., Jahrling P.B., Kuhn J.H. Middle East respiratory syndrome: obstacles and prospects for vaccine development. Expert Rev Vaccines. 2015;14:949–962. doi: 10.1586/14760584.2015.1036033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A.T. Hospital outbreak of middle east respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Abdallat M.M., Payne D.C., Alqasrawi S., Rha B., Tohme R.A., Abedi G.R. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memish Z.A., Cotten M., Watson S.J., Kellam P., Zumla A., Alhakeem R.F. Community case clusters of Middle East respiratory syndrome coronavirus in Hafr Al-Batin, Kingdom of Saudi Arabia: a descriptive genomic study. Int J Infect Dis. 2014;23:63–68. doi: 10.1016/j.ijid.2014.03.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alghamdi I.G., Hussain I.I., Almalki S.S., Alghamdi M.S., Alghamdi M.M., El-Sheemy M.A. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drosten C., Muth D., Corman V.M., Hussain R., Al Masri M., HajOmar W. An observational, laboratory-based study of outbreaks of Middle East respiratory syndrome coronavirus in Jeddah and Riyadh, kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60:369–377. doi: 10.1093/cid/ciu812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oboho I.K., Tomczyk S.M., Al-Asmari A.M., Banjar A.A., Al-Mugti H., Aloraini M.S. 2014 MERS-CoV outbreak in Jeddah — a link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser C., Donnelly C.A., Cauchemez S., Hanage W.P., Van Kerkhove M.D., Hollingsworth T.D. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madani T.A. Case definition and management of patients with MERS coronavirus in Saudi Arabia. Lancet Infect Dis. 2014;14:911–913. doi: 10.1016/S1473-3099(14)70918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastings D.L., Tokars J.I., Abdel Aziz I.Z.A.M., Alkhaldi K.Z., Bensadek A.T., Alraddadi B.M. Outbreak of middle east respiratory syndrome at tertiary care hospital, Jeddah, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:794–801. doi: 10.3201/eid2205.151797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 18.Alraddadi B.M., Watson J.T., Almarashi A., Abedi G.R., Turkistani A., Sadran M. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi J.Y. An outbreak of Middle East respiratory syndrome coronavirus infection in South Korea, 2015. Yonsei Med J. 2015;56:1174–1176. doi: 10.3349/ymj.2015.56.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ki M. 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health. 2015;37 doi: 10.4178/epih/e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S.S., Wong N.S. Probable transmission chains of Middle East respiratory syndrome coronavirus and the multiple generations of secondary infection in South Korea. Int J Infect Dis. 2015;38:65–67. doi: 10.1016/j.ijid.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan A., Farooqui A., Guan Y., Kelvin D.J. Lessons to learn from MERS-CoV outbreak in South Korea. J Infect Dev Ctries. 2015;9:543–546. doi: 10.3855/jidc.7278. [DOI] [PubMed] [Google Scholar]
- 23.Memish Z.A., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A.A., Stephens G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 24.Abroug F., Slim A., Ouanes-Besbes L., Hadj Kacem M.-A., Dachraoui F., Ouanes I. Family cluster of Middle East respiratory syndrome coronavirus infections, Tunisia, 2013. Emerg Infect Dis. 2014;20:1527–1530. doi: 10.3201/eid2009.140378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omrani A.S., Matin M.A., Haddad Q., Al-Nakhli D., Memish Z.A., Albarrak A.M. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17:e668–72. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drosten C., Meyer B., Müller M.A., Corman V.M., Al-Masri M., Hossain R. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371:828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization . 2019. Middle East respiratory syndrome coronavirus (MERS-CoV): summary of current situation, literature update and risk assessment–as of 5 February 2015.http://www.who.int/csr/disease/coronavirus_infections/mers-5-february-2015.pdf?ua=1 (Accessed 15 April 2016) [Google Scholar]
- 28.Madani T.A., Althaqafi A.O., Alraddadi B.M. Infection prevention and control guidelines for patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Saudi Med J. 2014;35:897–913. [PubMed] [Google Scholar]
- 29.Sprenger M., Coulombier D. Middle East respiratory syndrome coronavirus - two years into the epidemic. Euro Surveill Bull Eur Sur Les Mal Transm = Eur Commun Dis Bull. 2014;19:20783. doi: 10.2807/1560-7917.es2014.19.16.20783. [DOI] [PubMed] [Google Scholar]
- 30.Almutairi S.E., Boujenane I., Musaad A., Awad-Acharari F. Non-genetic factors influencing reproductive traits and calving weight in Saudi camels. Trop Anim Health Prod. 2010;42:1087–1092. doi: 10.1007/s11250-010-9529-y. [DOI] [PubMed] [Google Scholar]
- 31.Hemida M.G., Chu D.K.W., Poon L.L.M., RAPM Perera, Alhammadi M.A., Ng H.-Y. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis. 2014;20:1231–1234. doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalafalla A.I., Lu X., Al-Mubarak A.I.A., Dalab A.H.S., Al-Busadah K.A.S., Erdman D.D. MERS-CoV in upper respiratory tract and lungs of dromedary camels, Saudi Arabia, 2013-2014. Emerg Infect Dis. 2015;21:1153–1158. doi: 10.3201/eid2107.150070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5:e00884–14. doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wernery U., Corman V.M., Wong E.Y.M., Tsang A.K.L., Muth D., Lau S.K.P. Acute middle East respiratory syndrome coronavirus infection in livestock Dromedaries, Dubai, 2014. Emerg Infect Dis. 2015;21:1019–1022. doi: 10.3201/eid2106.150038. [DOI] [PMC free article] [PubMed] [Google Scholar]