Abstract
Background
An ongoing outbreak of pneumonia associated with the severe acute respiratory coronavirus 2 (SARS-CoV-2) started in December, 2019, in Wuhan, China. Information about critically ill patients with SARS-CoV-2 infection is scarce. We aimed to describe the clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia.
Methods
In this single-centered, retrospective, observational study, we enrolled 52 critically ill adult patients with SARS-CoV-2 pneumonia who were admitted to the intensive care unit (ICU) of Wuhan Jin Yin-tan hospital (Wuhan, China) between late December, 2019, and Jan 26, 2020. Demographic data, symptoms, laboratory values, comorbidities, treatments, and clinical outcomes were all collected. Data were compared between survivors and non-survivors. The primary outcome was 28-day mortality, as of Feb 9, 2020. Secondary outcomes included incidence of SARS-CoV-2-related acute respiratory distress syndrome (ARDS) and the proportion of patients requiring mechanical ventilation.
Findings
Of 710 patients with SARS-CoV-2 pneumonia, 52 critically ill adult patients were included. The mean age of the 52 patients was 59·7 (SD 13·3) years, 35 (67%) were men, 21 (40%) had chronic illness, 51 (98%) had fever. 32 (61·5%) patients had died at 28 days, and the median duration from admission to the intensive care unit (ICU) to death was 7 (IQR 3–11) days for non-survivors. Compared with survivors, non-survivors were older (64·6 years [11·2] vs 51·9 years [12·9]), more likely to develop ARDS (26 [81%] patients vs 9 [45%] patients), and more likely to receive mechanical ventilation (30 [94%] patients vs 7 [35%] patients), either invasively or non-invasively. Most patients had organ function damage, including 35 (67%) with ARDS, 15 (29%) with acute kidney injury, 12 (23%) with cardiac injury, 15 (29%) with liver dysfunction, and one (2%) with pneumothorax. 37 (71%) patients required mechanical ventilation. Hospital-acquired infection occurred in seven (13·5%) patients.
Interpretation
The mortality of critically ill patients with SARS-CoV-2 pneumonia is considerable. The survival time of the non-survivors is likely to be within 1–2 weeks after ICU admission. Older patients (>65 years) with comorbidities and ARDS are at increased risk of death. The severity of SARS-CoV-2 pneumonia poses great strain on critical care resources in hospitals, especially if they are not adequately staffed or resourced.
Funding
None.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia is a newly recognised illness that has spread rapidly throughout Wuhan (Hubei province) to other provinces in China and around the world.1, 2, 3, 4 As of Feb 19, 2020, the total number of patients has risen sharply to 74 283 in the mainland of China, with 2009 (2·7%) deceased. The clinical spectrum of SARS-CoV-2 pneumonia ranges from mild to critically ill cases. Previous studies have only described the general epidemiological findings, clinical presentation, and clinical outcomes of patients of SARS-CoV-2 pneumonia.1, 2, 5 However, specific information characterising critically ill patients remains unknown.
The data on the clinical characteristics and outcomes of critically ill patients with SARS-CoV-2 infection are scarce, but are of paramount importance to reduce mortality. In this study, we investigated critically ill patients with confirmed SARS-CoV-2 pneumonia who were admitted to Wuhan Jin Yin-tan hospital. The baseline SARS-CoV-2-associated morbidity and mortality data from this study will be of considerable value for the early identification of individuals who are at risk of becoming critically ill and who are most likely to benefit from intensive care treatment.
Methods
Study design and participants
This single-centre, retrospective, observational study was done at Wuhan Jin Yin-tan hospital (Wuhan, China), which is a designated hospital to treat patients with SARS-CoV-2 pneumonia. All patients, except infected healh-care workers from Jin Yin-tan hospital, were transferred from other hospitals. We retrospectively analysed patients from Dec 24, 2019, to Jan 26, 2020, who had been diagnosed with SARS-CoV-2 pneumonia, according to WHO interim guidance, and who were critically ill.6 Laboratory confirmation of SARS-CoV-2 infection was performed by the local health authority as previously described.1, 2 Critically ill patients were defined as those admitted to the intensive care unit (ICU) who required mechanical ventilation or had a fraction of inspired oxygen (FiO2) of at least 60% or more.7, 8, 9 Identification of critically ill patients was achieved by reviewing and analysing admission logs and histories from all available electronic medical records and patient care resources. For patients who were alive by Jan 26, 2020, their living status was confirmed on Feb 9, 2020.
Research in context.
Evidence before this study
The novel coronavirus disease 2019 is a disease that has affected populations around the world. We searched PubMed for articles published up to Feb 11, 2020, using the keywords “2019 novel coronavirus”, “2019-nCoV”, “COVID-19”, or “SARS-CoV-2”. We identified eight articles that describe the epidemiological and clinical characteristics of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, none of these studies focused on characterising critically ill patients infected with SARS-CoV-2, who are at increased risk of death.
Added value of this study
We report the clinical courses and clinical outcomes of 52 critically ill patients from 710 laboratory-confirmed cases of SARS-CoV-2. 35 (67%) patients had acute respiratory distress syndrome (ARDS) and 37 (71%) required mechanical ventilation. 32 (61·5%) patients had died at 28 days, and the median duration from intensive care unit (ICU) admission to death was 7 (IQR 3–11) days in non-survivors. Compared with survivors, non-survivors were older (64·6 years [11·2] vs 51·9 years [12·9]), more likely to develop acute respiratory distress (ARDS; 26 [81%] patients vs 9 [45%] patients), and more likely to receive mechanical ventilation (30 [94%] patients vs 7 [35%] patients), either invasively or non-invasively.
Implications of all the available evidence
The mortality of critically ill patients with SARS-CoV-2 pneumonia at 28 days is considerable. The survival time of non-survivors is likely to be within 1–2 weeks after ICU admission. Older patients (>65 years) with comorbidities and ARDS are at increased risk of death. The severity of SARS-CoV-2 pneumonia poses great strain on critical care resources in hospitals, especially if they are not adequately staffed or resourced.
The Ethics Commission of Jin Yin-tan hospital approved this study (KY-2020–06.01). Written informed consent was waived due to the rapid emergence of this infectious disease.
Data collection
We reviewed clinical electronic medical records, nursing records, laboratory findings, and radiological examinations for all patients with laboratory confirmed SARS-CoV-2 infection. The admission data of these patients were collected. Data were evaluated and collected, by using a case record form modified from the standardised International Severe Acute Respiratory and Emerging Infection Consortium case report forms.10 Any missing or uncertain records were collected and clarified through direct communication with involved health-care providers and their families.
We collected data on age, sex, exposure history, chronic medical histories (chronic cardiac disease, chronic pulmonary disease, cerebrovascular disease, chronic neurological disorder, diabetes, malignancy, dementia, malnutrition, and smoking), symptoms from onset to hospital admission (fever, cough, dyspnoea, myalgia, malaise, rhinorrhoea, arthralgia, chest pain, headache, and vomiting), vital signs at ICU admission (heart rate, respiratory rate, blood pressure), laboratory values on admission (haemoglobin concentration, lymphocyte count, platelet count, arterial blood gas analysis, FiO2, partial pressure of oxygen (PaO2), and lactate concentration), coexisted infection, treatment (oxygen therapy, vasoconstrictive agents, antiviral agents, antibacterial agents, corticosteroids, and immunoglobulin), as well as living status. During the outbreak of SARS-CoV-2 infection, the number of critically ill patients exceeded the capacity of ICUs. Therefore, two provisional ICUs were urgently established in Jin Yin-tan hospital and hence most mechanical ventilator settings and recordings were not recorded, except records of positive end-expiratory pressure in some cases. As a routine, electronic medical data were archived onto a local server, from which we retrieved these data.
Outcomes
The primary outcome was 28-day mortality after ICU admission. Secondary outcomes included incidence of SARS-CoV-2-related acute respiratory distress syndrome (ARDS) and the proportion of patients requiring mechanical ventilation. ARDS and shock were defined according to the guidance of WHO for novel coronavirus disease 2019 (COVID-19).6 Acute kidney injury was identified on the basis of serum creatinine.11 Cardiac injury was diagnosed if the serum concentration of hypersensitive cardiac troponin I (hsTNI) was above the upper limit of the reference range (>28 pg/mL), measured in the laboratory of Jin Yin-tan Hospital.
Statistical analysis
The aim of this study is to report the clinical courses and clinical outcomes of critically ill patients being cared for in the hospital during the study period. There were, therefore, no formal hypotheses being implemented to drive the sample size calculation and we included the maximum number of patients who met the inclusion criteria.
We expressed descriptive data as mean (SD) or median (IQR) for continuous variables and number (%) for categorical variables. We assessed differences between survivors and non-survivors using two-sample t test or Wilcoxon rank-sum test depending on parametric or non-parametric data for continuous variables and Fisher's exact test for categorical variables. We used a Kaplan-Meier plot for survival data.
Tests were two-sided with significance set at α less than 0·05. The Stata/IC 15.1 software (StataCorp, College Station, TX, USA) was applied for all analyses.
Results
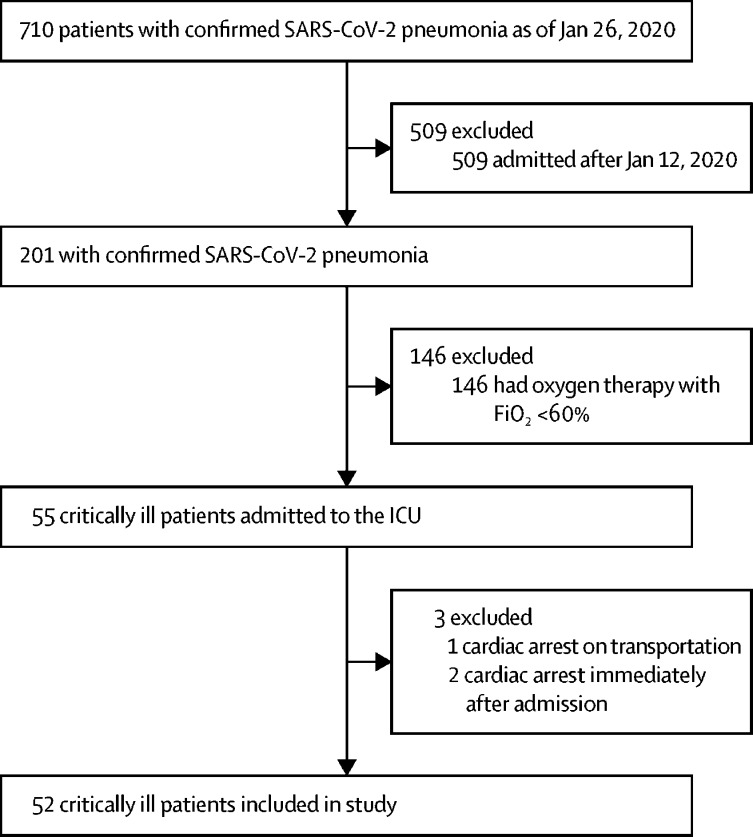
By Jan 26, 2020, 710 patients had been admitted to Wuhan Jin Yin-tan hospital with confirmed SARS-CoV-2 pneumonia, of whom 658 (93%) were considered ineligible, including three patients who had cardiac arrest immediately after admission. 52 (7%) critically ill patients were included in this study (figure 1 ). All patients were residents of Wuhan City and were transferred from other hospitals. The mean age was 59·7 years (SD 13·3), and 27 (52%) were older than 60 years (table 1 ). 35 (67%) patients were men. 17 (33%) patients had a history of exposure to the Huanan seafood market, and 10 (19%) had exposure to patients with confirmed or highly suspected SARS-CoV-2 infection. 21 (40%) patients had chronic diseases, including cerebrovascular diseases in seven (13·5%) patients, all of whom died at 28 days. All patients had bilateral infiltrates on chest x-ray.
Figure 1.
Study flow diagram
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. FiO2=fraction of inspired oxygen.
Table 1.
Demographics and baseline characteristics of patients with severe SARS-CoV-2 pneumonia
| Survivors (n=20) | Non-survivors (n=32) | All patients (n=52) | ||
|---|---|---|---|---|
| Age, years | 51·9 (12·9) | 64·6 (11·2) | 59·7 (13·3) | |
| Age range, years | ||||
| 30–39 | 6 (30%) | 0 | 6 (11·5%) | |
| 40–49 | 3 (15%) | 3 (9%) | 6 (11·5%) | |
| 50–59 | 4 (20%) | 9 (28%) | 13 (25%) | |
| 60–69 | 6 (30%) | 11 (34%) | 17 (33%) | |
| 70–79 | 1 (5%) | 7 (22%) | 8 (15%) | |
| ≥80 | 0 | 2 (6%) | 2 (4%) | |
| Sex | ||||
| Female | 6 (30%) | 11 (34%) | 17 (33%) | |
| Male | 14 (70%) | 21 (66%) | 35 (67%) | |
| Exposure | ||||
| Exposure to Huanan seafood market | 9 (45%) | 8 (25%) | 17 (33%) | |
| Exposure to patients* | 2 (10%) | 8 (25%) | 10 (19%) | |
| Chronic medical illness | 5 (25%) | 16 (50%) | 21 (40%) | |
| Chronic cardiac disease | 2 (10%) | 3 (9%) | 5 (10%) | |
| Chronic pulmonary disease | 2 (10%) | 2 (6%) | 4 (8%) | |
| Cerebrovascular disease | 0 | 7 (22%) | 7 (13·5%) | |
| Diabetes | 2 (10%) | 7 (22%) | 9 (17%) | |
| Malignancy | 1 (5%) | 1 (3%) | 2 (4%) | |
| Dementia | 0 | 1 (3%) | 1 (2%) | |
| Malnutrition | 0 | 1 (3%) | 1 (2%) | |
| Smoking | 2 (10%) | 0 | 2 (4%) | |
Data are n (%) or mean (SD), unless otherwise specified. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Patients who have confirmed SARS-CoV-2 infection or are highly suspected of being infected.
The most common symptoms were fever (98%), cough (77%), and dyspnoea (63·5%; table 2 ). Among 52 critically ill patients, six (11%) did not experienced fever until 2–8 days after the onset of symptoms related to SARS-CoV-2 infection. The median duration from onset of symptoms to radiological confirmation of pneumonia was 5 (IQR 3–7) days. The median duration from onset of symptoms to ICU admission was 9·5 (7·0–12·5) days.
Table 2.
Symptoms, comorbidities, and treatments of patients with severe SARS-CoV-2 pneumonia
| Survivors (n=20) | Non-survivors (n=32) | All patients (n=52) | ||
|---|---|---|---|---|
| Symptoms | ||||
| Fever | 20 (100%) | 31 (97%) | 51 (98%) | |
| Cough | 15 (75%) | 25 (78%) | 40 (77%) | |
| Dyspnoea | 12 (60%) | 21 (66%) | 33 (63·5%) | |
| Myalgia | 2 (10%) | 4 (12·5%) | 6 (11·5%) | |
| Malaise | 4 (20%) | 14 (44%) | 18 (35%) | |
| Rhinorrhoea | 0 | 3 (9%) | 3 (6%) | |
| Arthralgia | 1 (5%) | 0 | 1 (2%) | |
| Chest pain | 1 (5%) | 0 | 1 (2%) | |
| Headache | 1 (5%) | 2 (6%) | 3 (6%) | |
| Vomiting | 1 (5%) | 1 (3%) | 2 (4%) | |
| Comorbidities | ||||
| Acute respiratory distress syndrome | 9 (45%) | 26 (81%) | 35 (67%) | |
| Acute kidney injury | 3 (15%) | 12 (37·5%) | 15 (29%) | |
| Cardiac injury | 3 (15%) | 9 (28%) | 12 (23%) | |
| Liver dysfunction | 6 (30%) | 9 (28%) | 15 (29%) | |
| Hyperglycaemia | 7 (35%) | 11 (34%) | 18 (35%) | |
| Gastrointestinal haemorrhage | 0 | 2 (6%) | 2 (4%) | |
| Pneumothorax | 1 (5%) | 0 | 1 (2%) | |
| Hospital-acquired pneumonia | 4 (20%) | 2 (6%) | 6 (11·5%) | |
| Bacteraemia | 0 | 1 (3%) | 1 (2%) | |
| Urinary tract infection | 0 | 1 (3%) | 1 (2%) | |
| Treatment | ||||
| High flow nasal cannula | 17 (85%) | 16 (50%) | 33 (63·5%) | |
| Mechanical ventilation | 7 (35%) | 30 (94%) | 37 (71%) | |
| Non-invasive | 6 (30%) | 23 (72%) | 29 (56%) | |
| Invasive | 3 (15%) | 19 (59%) | 22 (42%) | |
| Prone position ventilation | 2 (10%) | 4 (12·5%) | 6 (11·5%) | |
| Extracorporeal membrane oxygenation | 1 (5%) | 5 (16%) | 6 (11·5%) | |
| Renal replacement therapy | 1 (5%) | 8 (25%) | 9 (17%) | |
| Vasoconstrictive agents | 2 (10%) | 16 (50%) | 18 (35%) | |
| Antiviral agents | 13 (65%) | 10 (31%) | 23 (44%) | |
| Antibacterial agents | 19 (95%) | 30 (94%) | 49 (94%) | |
| Glucocorticoids | 14 (70%) | 16 (50%) | 30 (58%) | |
| Immunoglobulin | 9 (45%) | 19 (59%) | 28 (54%) | |
Data are n (%). SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
The median Acute Physiology and Chronic Health Evaluation II (APACHE II) score of all patients was 17 (IQR 14–19; table 3 ). Most patients had organ function damage, including 35 (67%) with ARDS, 15 (29%) with acute kidney injury, 12 (23%) with cardiac injury, 15 (29%) with liver dysfunction, and one (2%) with pneumothorax (table 2). The median hsTNI was 161·0 (IQR 41·8–766·1) pg/mL. Hospital-acquired infection was noted in seven (13·5%) patients, including one (2%) patient who had pulmonary and blood stream infection of carbapenem-resistant Klebsiella pneumoniae. Other microorganisms identified from respiratory tract secretions in five (10%) patients included Aspergillus flavus, A fumigatus, extended-spectrum β-Lactamase (ESBL)-positive K pneumonia, ESBL-positive Pseudomonas aeruginosa, and ESBL-negative Serratia marcescens, with each microorganism found in one patient each. Candida albicans was identified in the urine culture of one (2%) patient (table 2).
Table 3.
Differences in intensive care measures and vital signs between survivors and non-survivors of severe SARS-CoV-2 pneumonia
| Survivors (n=20) | Non-survivors (n=32) | |
|---|---|---|
| Duration from onset of symptoms to radiological confirmation of pneumonia, days | 5 (3–9) | 5 (3–7) |
| Duration from onset of symptoms to ICU admission, days | 9 (6–12) | 11 (7–14) |
| Heart rate, beats per min | 89 (20) | 89 (15) |
| Systolic blood pressure, mm Hg | 133 (20) | 140 (21) |
| Ratio of PaO2 to FiO2, mm Hg | 100·0 (66·6–126·7) | 62·5 (52·0–74·1) |
| APACHE II score on day 1 | 14 (12–17) | 18 (16–20) |
| SOFA score on day 1 | 4 (3–4) | 6 (4–8) |
| Haemoglobin concentration, g/L | 127 (20) | 129 (14) |
| Lymphocyte count, × 109/L | 0·74 (0·40) | 0·62 (0·37) |
| Platelet count, × 109/L | 164 (74) | 191 (63) |
| Prothrombin time, s | 10·9 (2·7) | 12·9 (2·9) |
| Total bilirubin concentration, μmol/L | 13·1 (4·3) | 19·5 (11·6) |
| Serum creatinine concentration, μmol/L | 76·3 (27·4) | 80·7 (32·3) |
| Lactate concentration, mmol/L | 1·6 (1·3–1·6) | 1·9 (1·4–3·2) |
Data are median (IQR) or mean (SD). COVID-19=novel coronavirus disease 2019. APACHE II=Acute Physiology and Chronic Health Evaluation II. FiO2=fraction of inspired oxygen. PaO2=partial pressure of oxygen. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. SOFA=Sequential Organ Failure Assessment.
33 (63·5%) patients were treated with high-flow nasal cannula, 37 (71%) with mechanical ventilation, six (11·5%) with prone position ventilation, six (11·5%) with extracorporeal membrane oxygenation (ECMO), nine (17%) with renal replacement therapy, and 18 (35%) with vasoconstrictive agents (table 2). 23 (44%) patients received antiviral agents, 49 (94%) received antibacterial agents, and 30 (58%) patients received glucocorticoids (table 2). Oseltamivir was given to 18 (35%) patients, ganciclovir to 14 (27%), and lopinavir to seven (13·5%).
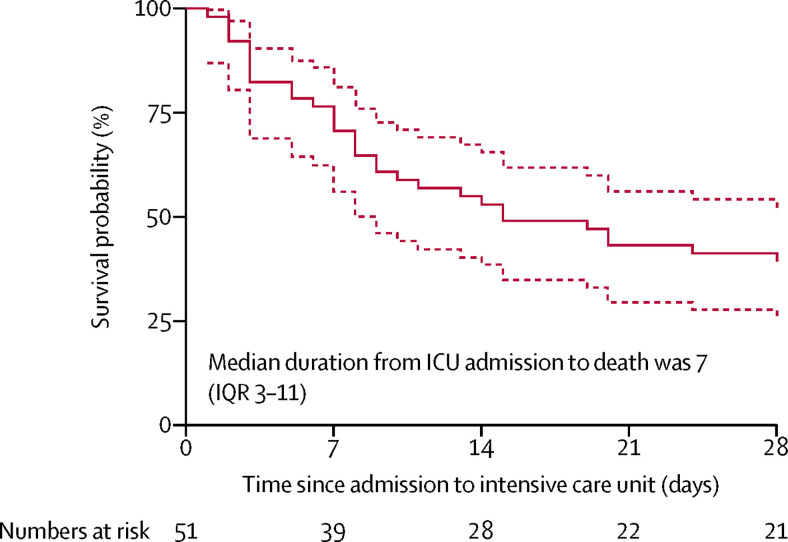
For the primary outcome, among 52 critically ill patients with SARS-CoV-2 infection, 32 (61·5%) patients had died at 28 days, and the median duration from ICU admission to death was 7 (IQR 3–11) days in the non-survivors (figure 2 ). Compared with survivors, non-survivors were more likely to develop ARDS (26 [81%] vs 9 [45%]) and were more likely to receive mechanical ventilation (30 [94%] vs 7 [35%]). 30 (81%) of 37 patients requiring mechanical ventilation had died by 28 days.
Figure 2.
Survival of critically ill patients with SARS-CoV-2 pneumonia
Dashed lines represent 95% CIs. One patient died within 24 h after admission to the intensive care unit (ICU).
Of the 20 patients who survived, eight patients were discharged. Three patients were still on invasive ventilation at 28 days, including one patient who was also on ECMO. One patient was on non-invasive ventilation, two were using high-flow nasal cannula, and six were using common nasal cannula.
Compared with survivors, non-survivors were older (64·6 [SD 11·2] vs 51·9 [12·9]) and were more likely to have chronic medical illnesses (17 (53%) patients vs 4 (20%) patients; table 1). Neither the median duration from onset of symptoms to radiological confirmation of pneumonia or from onset of symptoms to ICU admission were different between survivors and non-survivors (table 3). The ratio of partial pressure of oxygen (PaO2) to FiO2 was significantly lower in non-survivors. Based on APACHE II score and SOFA score at ICU admission, non-survivors were in a more critical condition than survivors. In the cohort, lymphocytopenia occurred in 44 (85%) patients, with no significant difference between the two groups. Compared with survivors, non-survivors were more likely to develop ARDS, and to receive mechanical ventilation, either invasively or non-invasively.
Discussion
We report on 52 critically ill patients with confirmed SARS-CoV-2 infection, characterised by severe hypoxaemia. 32 (61·5%) of critically ill patients had died at 28 days. Of all included patients, 37 (71%) required mechanical ventilation and 35 (67%) had ARDS.
Since no specialised medication to treat SARS-CoV-2 infection has been identified at this time, the mainstay of treatment has been supportive care. Patients are being treated in isolation, and their close contacts are being quarantined. For non-critically ill patients, close follow-up is likely to be sufficient to manage the disease.1, 2, 3 For critically ill patients, however, aggressive treatments and intensive care are needed. To our knowledge, this is the first study to characterise critically ill patients infected by SARS-CoV-2. In three previously published studies of crtically ill patients, the patient numbers were too small to summarise the characteristics and mortality of these patients with SARS-CoV-2 pneumonia.1, 2, 5
Like SARS-CoV and Middle Eastern respiratory syndrome (MERS)-CoV, SARS-CoV-2 is a coronavirus that can be transmitted to humans, and these viruses are all related to high mortality in critically ill patients.12 However, the mortality rate in patients with SARS-CoV-2 infection in our cohort is higher than that previously seen in critically ill patients with SARS. In a cohort of 38 critically ill patients with SARS from 13 hospitals in Canada, 29 (76%) patients required mechanical ventilation, 13 (43%) patients had died at 28 days, and six (16%) patients remained on mechanical ventilation.8 17 (38%) of 45 patients and 14 (26%) of 54 patients who were critically ill with SARS infection were also reported to have died at 28 days in a Singapore cohort13 and a Hong Kong cohort,14 respectively. The mortality rate in our cohort is likely to be higher than that seen in critically ill patients with MERS infection. In a cohort of 12 patients with MERS from two hospitals in Saudi Arabia, seven (58%) patients had died at 90 days.15 Since the follow-up time is shorter in our cohort, we postulate that the mortality rate would be higher after 28 days than that seen in patients with MERS-CoV.
The fundamental pathophysiology of severe viral pneumonia is severe ARDS. Men and people of an older age (>65 years) are more likely to develop ARDS than women or those of a younger age.16 Therefore, it is reasonable that the mortality at 28 days of severe SARS-CoV-2 pneumonia is similar to the mortality of severe ARDS, which is near 50%.17 With a substantial increase in the number of critically ill patients infected by SARS-CoV-2, more provisional ICUs are being established in Wuhan, China. Qualified specialists are coming to Wuhan from other provinces of China and are currently working in these provisional ICUs, fever clinics, and newly constructed hospitals.18 As the clinical capacity to treat patients improves, the mortality of critically ill patients with SARS-CoV-2 pneumonia is expected to decrease.
As mentioned in previous studies, nearly 70% of patients infected by SARS-CoV-2 were men.1, 2 The patients are older in our study than in previous studies.1, 2 We observed that non-survivors were older than survivors. Based on previous studies, evidence suggests that older, male patients are the most susceptible to SARS-CoV-2 infection,2 which is supported by our data. As previously reported, patients with a history of cerebrovascular disease are at increased risk of becoming critically ill or dying if they have SARS-CoV-2 infection.2, 5
In our cohort, fever is the most common symptom in patients with SARS-CoV-2 pneumonia, which is in accordance with previous studies, but not all patients had fever.1, 2, 5 We also found that fever was not detected at the onset of illness in six (11·5%), and that it was in fact detected 2–8 days later. The delay of fever manifestation hinders early identification of patients infected with SARS-CoV-2—if patients are asymptomatic identification of suspected cases is more difficult.19, 20 The median duration from onset of symptoms to radiological confirmation of pneumonia was 5 (3–7) days, meaning that early or repeated radiological examinations are useful in screening patients with SARS-CoV-2 pneumonia.4
As for laboratory tests, lymphocytopenia occurred in more than 80% of critically ill patients in our cohort. Lymphocytopenia is a prominent feature of critically ill patients with SARS-CoV infection because targeted invasion by SARS-CoV viral particles damages the cytoplasmic component of the lymphocyte and causes its destruction.21 Additionally, lymphocytopenia is also common in the critically ill patients with MERS infection, which is the result of apoptosis of lymphocytes.22, 23 Therefore, we postulate that necrosis or apoptosis of lymphocytes also induces lymphocytopenia in critically ill patients with SARS-CoV-2 infection. In a previous study, mainly in non-critical patients infected with SARS-CoV-2, 35% of patients had only mild lymphocytopenia,2 suggesting that the severity of lymphocytopenia reflects the severity of SARS-CoV-2 infection.
Mechanical ventilation is the main supportive treatment for critically ill patients. The PaO2/FiO2 ratio was lower in our patients than in patients admitted to Zhongnan Hospital.5 The substantial difference in PaO2/FiO2 ratio between survivors and non-survivors in our study, indicates this ratio is associated with the severity of illness and thus prognosis. Barotrauma seems less severe in patients with SARS-CoV-2 infection who are being mechanically ventilated than that seen in mechanically ventilated patients with SARS-CoV. In our study, barotrauma occurred in only one (2%) patient, who had been hospitalised for nearly 1 month, and he is currently on a ventilator and receiving ECMO. In patients with SARS, barotrauma occurred in about 25% of patients on mechanical ventilation.14 The lower occurrence of barotrauma in our cohort is probably related to the widely accepted strategy of protective ventilation in mainland China.24 At the same time, prone position and ECMO have been used to treat patients with SARS-CoV-2 pneumonia.
Without solid evidence, nearly half of the patients were given antiviral agents, and more than half were given intravenous glucocorticoids. Patients treated with lopinavir were from an ongoing clinical trial registered on Chinese Clinical Trial Registry (ChiCTR2000029308). Remdesivir was given to the first patients with SARS-CoV-2 pneumonia in the USA.4 Trials on remdesivir are about to recruit both mild to moderate patients (NCT04252664) and severe patients (NCT04257656) infected with SARS-CoV-2. Although, intravenous glucocorticoids were commonly used in patients with severe SARS or MERS pneumonia, their efficacy remains controversial and their use to treat SARS-CoV-2 infection is also controversial.5, 25 An ongoing clinical trial (NCT04244591) might shed some light on the safety and efficacy of these drugs as treatment.
This study has several limitations. First, only 52 critically ill patients were included. However, the population from which they were sampled was much larger than that of the three studies previously published.1, 2, 5 We included all the critically ill patients being cared for in the ICU of Jin Yin-tan hospital who met the inclusion criteria. Due to the exploratory nature of the study, which was not driven by formal hypotheses, the sample size calculation was waived. Instead, we hope that the findings presented here will encourage a larger cohort study or potentially some randomly controlled trials. Second, some specific information from the ICU was missing, such as mechanical ventilation settings. The data on radiographical examination, supportive treatment, living status, and the duration from ICU admission to death, however, are indisputable. Third, this is a retrospective study. The data in this study permit a preliminary assessment of the clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia. Further studies are still needed.
In conclusion, the mortality of critically ill patients with SARS-CoV-2 pneumonia is high. The survival term of the non-survivors is likely to be within 1–2 weeks after ICU admission. Older patients (>65 years) with comorbidities and ARDS are at increased risk of death. The severity of SARS-CoV-2 pneumonia poses great strain to hospital critical care resources, especially if they are not adequately staffed or resourced.
This online publication has been corrected. The corrected version first appeared at thelancet.com/respiratory on February 28, 2020
Data sharing
After publication, the data will be made available to others on reasonable requests to the corresponding author. A proposal with detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability of requests. Additional materials might also be required during the process of evaluation. Deidentified participant data will be provided after approval from the corresponding author and Wuhan Jin Yin-tan Hospital.
Acknowledgments
Acknowledgments
We thank all patients and their families involved in the study.
Contributors
XY, YY, JXu, HS, HL, and YS collected the epidemiological and clinical data. JXi, YWu, LZ, ZY, MF, and TY summarised all data. XY, YY, HL, JXi, YWa, SP, and YS drafted the manuscript. XZ and SY revised the final manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. published online Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. published online Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. published online Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Jan 11, 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 7.Dominguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 8.Fowler RA, Lapinsky SE, Hallett D, et al. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 10.The International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) https://isaric.tghn.org/
- 11.Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group KDIGO clinical practice guideline for acute kidney injury. March 2012. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf
- 12.Parry J. Wuhan: Britons to be evacuated as scientists estimate 44 000 cases of 2019-nCOV in the city. BMJ. 2020;368:m351. doi: 10.1136/bmj.m351. [DOI] [PubMed] [Google Scholar]
- 13.Lew TW, Kwek TK, Tai D, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 14.Gomersall CD, Joynt GM, Lam P, et al. Short-term outcome of critically ill patients with severe acute respiratory syndrome. Intensive Care Med. 2004;30:381–387. doi: 10.1007/s00134-003-2143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 16.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 18.USA TODAY China built a hospital in 10 days to battle coronavirus. https://www.usatoday.com/story/news/world/2020/02/03/coronavirus-photos-show-wuhan-huoshenshan-hospital-built-10-days/4643377002/
- 19.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020 doi: 10.1056/NEJMc2001468. published online Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Health Commission of the People's Republic of China Asymptomatic cases complicate efforts. http://en.nhc.gov.cn/2020-02/01/c_76086.htm
- 21.Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu H, Zhou J, Wong BH, et al. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis. 2016;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu WJ, Zhao M, Liu K, et al. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Yang Y, Gao Z, et al. Practice of diagnosis and management of acute respiratory distress syndrome in mainland China: a cross-sectional study. J Thorac Dis. 2018;10:5394–5404. doi: 10.21037/jtd.2018.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
After publication, the data will be made available to others on reasonable requests to the corresponding author. A proposal with detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability of requests. Additional materials might also be required during the process of evaluation. Deidentified participant data will be provided after approval from the corresponding author and Wuhan Jin Yin-tan Hospital.