Highlights
-
•
The COVID-19 outbreak highlights the need for early diagnosis, isolation and treatment
-
•
The sensitivity of the CT was 97.2%, while the sensitivity of initial rRT-PCR was 83.3%.
-
•
Patients with typical CT findings but negative rRT-PCR results should be isolated.
Abbreviations: GGOs, ground-glass opacities; CT, computed tomographic; HRCT, high-resolution CT; COVID-19, Coronavirus Disease 19; HCT, hematocrit; GLU, glucose; WHO, World Health Organization; ARDS, acute respiratory distress syndrome; rRT-PCR, real-time reverse-transcriptase-polymerase chain reaction; MERS, Middle East Respiratory Syndrome
Keywords: Severe Acute Respiratory Syndrome, Coronavirus, Pneumonia, Tomography, X-Ray Computed
Abstract
Purpose
To evaluate the diagnostic value of computed tomography (CT) and real-time reverse-transcriptase-polymerase chain reaction (rRT-PCR) for COVID-19 pneumonia.
Methods
This retrospective study included all patients with COVID-19 pneumonia suspicion, who were examined by both CT and rRT-PCR at initial presentation. The sensitivities of both tests were then compared. For patients with a final confirmed diagnosis, clinical and laboratory data, in addition to CT imaging findings were evaluated.
Results
A total of 36 patients were finally diagnosed with COVID-19 pneumonia. Thirty-five patients had abnormal CT findings at presentation, whereas one patient had a normal CT. Using rRT-PCR, 30 patients were tested positive, with 6 cases initially missed. Amongst these 6 patients, 3 became positive in the second rRT-PCR assay(after 2 days, 2 days and 3 days respectively), and the other 3 became positive only in the third round of rRT-PCR tests(after 5 days, 6 days and 8 days respectively). At presentation, CT sensitivity was therefore 97.2%, whereas the sensitivity of initial rRT-PCR was only 83.3%.
Conclusion
rRT-PCR may produce initial false negative results. We suggest that patients with typical CT findings but negative rRT-PCR results should be isolated, and rRT-PCR should be repeated to avoid misdiagnosis.
1. Introduction
Since December 2019, multiple cases of pneumonia of unknown cause have emerged in Wuhan, China. Through unbiased sequencing of patient samples, a previously unknown β-cyclotron virus was discovered. A novel coronavirus was isolated from human airway epithelial cells and termed SARS CoV2, responsible for Coronavirus Disease (COVID)-19. Like MERS-CoV and SARS-CoV, COVID-19 is the 7th member of the coronavirus family which infects humans [1]. The source of infection is wild animals, possibly rhinolophus sinicus. Importantly, the virus can be transmitted from human to human. At present, COVID-19 has been mainly breaking out in Wuhan, and by February 8th, 2020, a total of 34627 cases had been confirmed, of which 732 cases had died. These numbers are still increasing.
Previous studies have shown that the vast majority of patients with COVID-19 had a history of exposure to the epidemic area of Wuhan. These patients had clinical symptoms including fever and cough. Imaging plays an important role in the diagnosis and evaluation of the disease [2,3]. Final diagnosis relies on real-time reverse-transcriptase-polymerase chain reaction (rRT-PCR) positivity for the presence of coronavirus [4,5]. Because of the strong infectivity of COVID-19, rapid and accurate diagnostic methods are urgently required to identify, isolate and treat the patients as soon as possible, which could reduce mortality rates and the risk of public contamination. However, rRT-PCR results often require 5 to 6 hours, whereas CT examinations results can be obtained much faster. Additionally, it remains unclear whether rRT-PCR is the gold standard, and whether false-positive or false-negative results are common.
This retrospective study included patients with confirmed COVID-19 pneumonia diagnosed in Yichang Yiling Hospital. In these patients, we compared the sensitivity of CT imaging and rRT-PCR testing at presentation.
2. Materials and Methods
2.1. Participants
This study was approved by the Ethics Committee of the Yichang Yiling Hospital. Signed informed consent was exempted due to the retrospective nature of the study. Inclusion criteria were as follows: (a) Patients with a fever of > 38℃ and COVID-19 pneumonia suspicion (b) who underwent both thin-section CT of the chest and rRT-PCR examinations. Exclusion criteria: Patients transferred to another hospital or lost to follow-up.
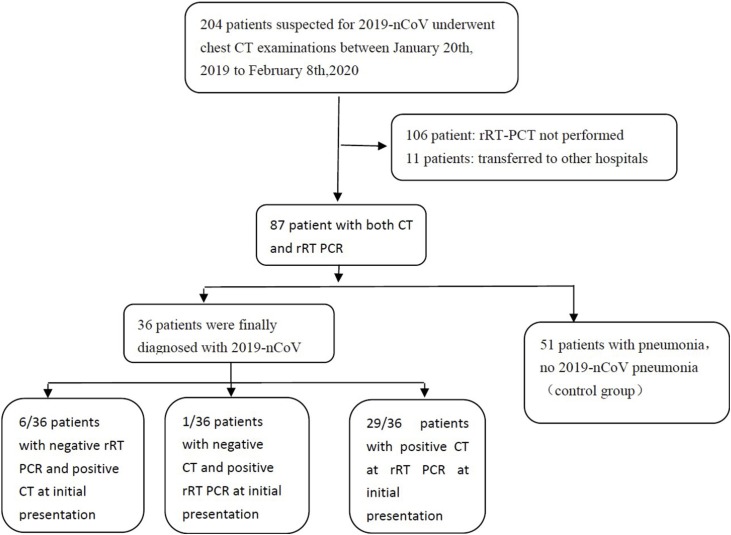
From January 20th, 2019 to February 8th, 2020, a total of 204 patients suspected for COVID-19 underwent chest CT examinations. Of the patients, 106 were not tested using rRT-PCR. Eleven other patients were transferred to other hospitals and were also excluded. The remaining 87 patients underwent both CT and rRT-PCR in our hospital. The gold standard for a final diagnosis was positivity of first or repeated rRT-PCR tests.. Amongst the 87 included cases, 36 patients were finally diagnosed with COVID-19 pneumonia. The other 51 patients without COVID-19 pneumonia served as the control group (Fig. 1 ).
Fig. 1.
Flowchart for patient inclusion.
2.2. Image acquisition
CT examinations were performed on a 64-section scanner (Brilliance CT, Phlilips Healthcare). The scanning parameters were as follows: 120 kV; 250 mAs: ; rotation time, 0.35 second; pitch, 1.5. Images were reconstructed with a 2 mm slice thickness, using a high frequency reconstruction algorithm. Acquisitions were performed during a deep inspiration breath-hold, without contrast administration.
Two radiologists (X.Z. and C.L., with 10 and 15 years of experience in chest imaging, respectively) retrospectively reviewed all chest CT images. In cases of disagreement, a consensus was reached. CT evaluations included the lobar location and pattern of the lesion. In addition, the outer 1/3 of the lung field was defined as a peripheral distribution, whereas the remainder was defined as a central distribution. In terms of pattern, ground-glass opacity (GGO) was defined as a modest increase in lung attenuation on lung window CT images, not obscuring the pulmonary vessels. Consolidation was defined as high-density patchy opacities, inside which air bronchogram(s) could be observed. Lymphadenopathy was defined as a lymph node >1.0 cm in short-axis diameter.
2.3. Statistical analysis
SPSS17.0 software (Chicago, IL) was used for statistical analysis. Quantitative data are expressed as mean ± standard divisions (SD), and compared through the analysis of variances or independent sample t-tests. Qualitative data were compared using a chi-square test. P < 0.05 was considered statistically significant.
3. Results
3.1. Clinical and laboratory findings
Demographic and clinical characteristics of the 87 patients are shown in Table 1 .
Table 1.
Demographic and Clinical Characteristics of the 87 patients suspected with COVID-19 pneumonia (x ± s)
| Variable | COVID-19 (N = 36) |
Control group (N = 51) |
P |
|---|---|---|---|
| Gender | |||
| Female | 16 | 25 | 0.674 |
| Male | 20 | 26 | |
| Age(year) | 44.8 ± 18.2 | 47.1 ± 18.8 | 0.597 |
| Exposure History | 33(91.7%) | 29(56.8%) | 0.000 |
| Duration of fever (days) | 2.6 ± 1.7 | 3.2 ± 1.6 | 0.781 |
| leukocyte count (normal or decreased) |
33(91.7%) | 21(41.2%) | 0.000 |
| lymphocytes (decreased) | 23(63.8%) | 12(23.5%) | 0.000 |
| fasting glucose (increased) | 17(47.2%) | 14(27.5%) | 0.058 |
Exposure History was defined as having been to Wuhan within 2 weeks or having been exposed to infected patients. Normal leukocyte counts: (4.0-10.0)×109/L, normal percentage of lymphocytes: 20%-50%, normal fasting glucose level: 3.9-6.1 mmol/L.
There were no significant differences in terms of gender, age, and time from fever to visit between the COVID-19 pneumonia group and control group. The exposure history of the COVID-19 pneumonia group exceeded that of the control group (P < 0.05). Regarding laboratory examinations, the proportions of normal or decreased leukocyte counts and decreased lymphocytes in the COVID-19 group were higher than those of the control group (P < 0.05). Additionally, the percentage of increased fasting glucose was higher in the infected vs. control group (47.2% vs 27.5%, P = 0.058).
In the 36 patients with confimed COVID-19, clinical symptoms were as follows: fever (36/36, 100%), cough (27/36, 75.0%), myalgia or fatigue (14/36, 38.9%), nausea or diarrhea (6/36, 16.6%).
3.2. CT Imaging findings
The distribution of the lesions in the 36 patients with confirmed COVID-19 pneumonia was as follows: right lower lobe (26/36, 72.2%), left lower lobe (24/36, 66.7%),left upper lobe (20/36, 55.6%), right middle lobe (20/36, 55.6%) and right upper lobe (19/36, 52.7%), The distribution patterns were as follows: peripheral distribution (26/36, 72.2%) and central distribution (10/36, 27.8%). Except for 11 patients (11/36, 30.6%) with a single lesion, the majority of patients (25/36, 69.4%) had more multiple CT abnormalities. Compared to the control group, peripheral distribution was more common in the COVID-19 pneumonia group (P < 0.05), with a random distribution in all lobes (Fig. 2 ). In patients without COVID-19 pneumonia, lower lobes distribution was more common than upper lungs location (Table 2 ).
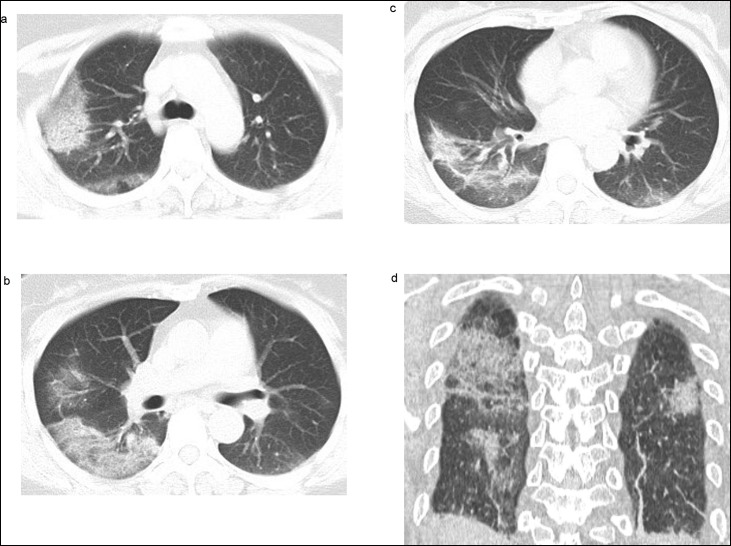
Fig. 2.
A 45-year-old male patient with COVID-19 pneumonia showed patchy consolidations and ground glass opacities in both lungs. These were mainly distributed peripherally, with a random distribution pattern.
Table 2.
CT Imaging findings in the 87 patients suspected with COVID-19 pneumonia.
| Group | COVID-19 pneumonia | Control group | P |
|---|---|---|---|
| (n=36) | (n=51) | ||
| Distribution of the lesions | |||
| left upper lobe | 20/36 (55.6%) | 17/51 (33.3%) | 0.039 |
| left lower lobe | 24/36 (66.7%) | 35/51 (68.6%) | 0.847 |
| right upper lobe | 19/36 (52.7%) | 19/51 (37.3%) | 0.151 |
| right middle lobe | 20/36 (55.6%) | 26/51 (50.9%) | 0.674 |
| right lower lobe | 26/36 (72.2%) | 33/51 (64.7%) | 0.460 |
| Peripheral/central | 26: 10 (2.6 : 1) | 24: 26 (0.92 : 1) | 0.025 |
| multiple/single | 25: 11 (2.27 : 1) | 31: 20 (1.55 : 1) | 0.406 |
| Pattern of the lesions | |||
| GGO | 11/36 (30.6%) | 8/51 (15.7%) | 0.098 |
| Consolidation | 6/36 (16.7%) | 22/51 (43.1%) | 0.001 |
| GGO with consolidation | 19/36 (52.7%) | 21/51 (41.2%) | 0.285 |
| Lymphadenopathy | 1/36 (2.78%) | 4/51 (7.84%) | 0.317 |
| pleural effusion | 2/36 (5.56%) | 7/51 (13.73%) | 0.218 |
The occurrence of GGO or GGO with consolidation was more frequent in the COVID-19 pneumonia group, whereas the occurrence of consolidation was more common in the non-COVID-19 pneumonia group (P < 0.05). Only one patient(2.78%)had lymphadenopathy and two patients(5.56%)had pleural effusion in the COVID-19 pneumonia group.
3.3. Comparison of the diagnostic efficacy between CT and rRT-PCR
A total of 36 cases were finally diagnosed with COVID-19 pneumonia. Thirty-five patients had abnormal CT findings at presentation, and only one patient had a normal thoracic CT. Using rRT-PCR, 30 cases showed positivity, with 6 cases initially missed. Amongst these 6 missed cases, 3 had a positive result in the second rRT-PCR test(after 2 days, 2 days and 3 days respectively), and the other 3 were positive in the third round of rRT-PCR assessments(after 5 days, 6 days and 8 days respectively) (Fig. 3 ). Therefore, sensitivity of CT examinations was 97.2% at presentation, whereas first round rRT-PCR sensitivity was 84.6%.
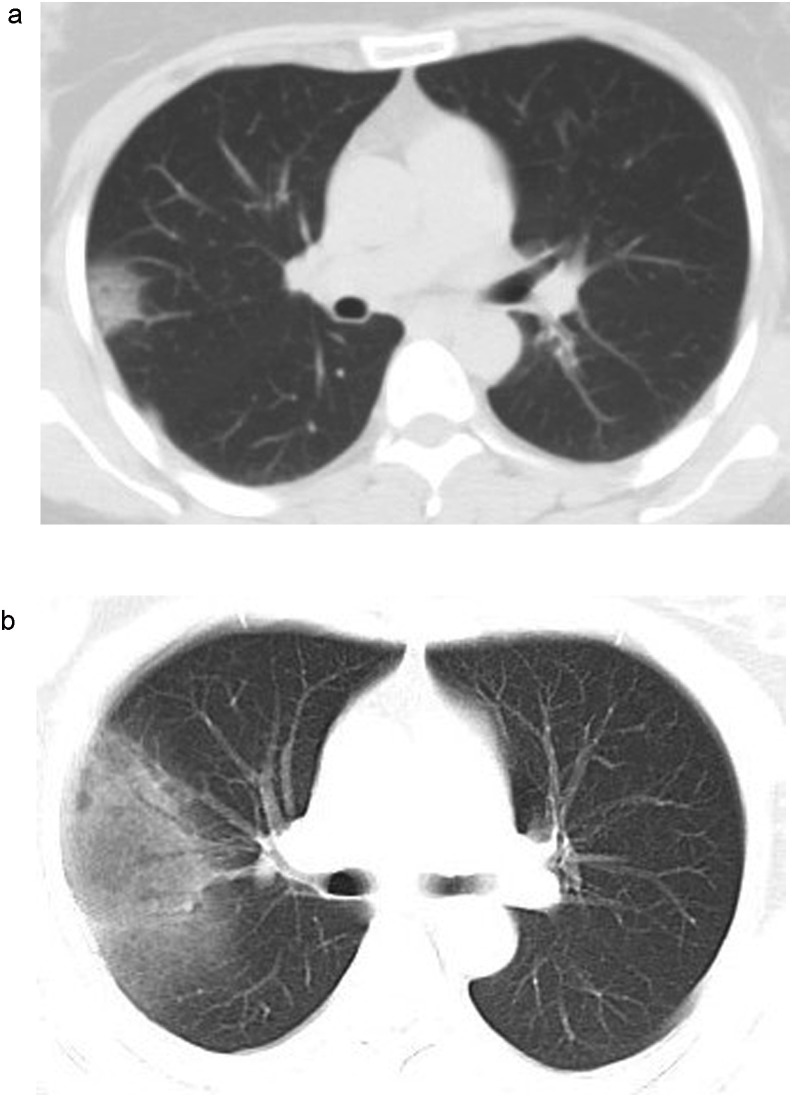
Fig. 3.
A 41-year-old female patient presented with a fever for 3 days. CT examination showed ground glass opacities in the upper lobe of right lung (A). rRT-PCR results on the same day were negative. Re-examinations with CT 2 days later showed that CT abnormalities had expanded and increased (B). Second round rRT-PCR remained negative. Upon another repeat rRT-PCR the next day, the patient was confirmed as virus positive.
4. Discussion
COVID-19 pneumonia broke out in Wuhan. On January 30th, 2020, the pneumonia epidemic caused by a novel coronavirus was issued as a public health emergency of international concern by the WHO [6,7]. The source of the infection was a novel coronavirus(SARS CoV2), with symptomatic and asymptomatic infections reported. To-date, respiratory droplets and direct contact have been identified as the main transmissions routes. Aerosol and digestive tract transmission remain to be confirmed. The incubation period of the disease is generally 3-7 days, but no longer than 14 days [5,[8], [9], [10], [11], [12], [13], [14], [15]]. Due to its strong infectivity profile, early diagnosis and treatment are crucial, otherwise human mediated disease spread can seriously endanger public health.
Our retrospective analysis showed that the sensitivity of initial CT was 97.2%, whereas initial rRT-PCR sensitivity was 83.3%, with 6 initially missed cases. This may be related to sample collection as pharyngeal oral and nasal sampling are easier collection methods, whereas lower respiratory tract sampling is relatively difficult to perform, with medical staff susceptible to get infected [16]. The sensitivity of the rRT-PCR kit can also contribute to false negatives. Chung reported that 3 patients diagnosed with COVID-19 pneumonia showed normal CT findings [2]. In this study, only one patient was observed with positive rRT-PCR but negative CT. Considering that the results of rRT-PCR may be false-negative, and the relatively long assay time, we recommend that the patients with typical imaging findings should be isolated and rRT-PCR repeated to avoid misdiagnosis.
For the COVID-19 pneumonia group in this study, most patients had a clear contact history with the epidemic area. In these patients, the total number of leukocytes was normal or decreased, similar to previous reported in the literature[10,[17], [18], [19]]. Interestingly, we found that more patients with increased blood glucose levels were observed in the COVID-19 pneumonia group as compared to the control group (47.2% vs 27.5%), although the difference was not statistically significant (P = 0.058). It remains unclear how many patients in this group will finally be diagnosed with diabetes, but those with high blood fasting glucose level might be more sensitive to COVID-19 pneumonia, which requires further confirmation. The typical imaging features of COVID-19 pneumonia consist in single or multiple patchy consolidations or GGO in both lungs. In this study, GGO with consolidation was the commonest abnormality. The distribution of the lesions was predominantly peripheral, seen in 72.2% of patients. Pleural effusion and lymphadenopathy were rarely observed, consistent with previous studies [[20], [21], [22]].
This study had some limitations. Firstly, due to the outbreak of COVID-19 pneumonia in this area, the supply of nucleic acid detection kits was limited, and the rRT-PCR examinations were only performed in patients with fever and positive CT tests. Additionally, the sample size of this study was small, and the cases lacked follow-up due to time constraints. Larger sample sizes are therefore required for further verification.
In summary, CT examinations appear sensitive virus detection, whereas rRT-PCR may produce false- negative results. We therefore recommend that patients with positive imaging findings but negative rRT-PCR results should be isolated and rRT-PCR repeated to avoid misdiagnosis.
Author contributions
Guarantors of integrity of entire study, B. Fan, C. Long, H. Xu; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; experimental studies, B. Fan, C. Long, H. Xu, Q. Shen, X. Zhang; statistical analysis, C. Wang, B Ceng, Z Li; and manuscript editing, all authors.
Declaration of Competing Interest
All authors declare that they have no conflict of interest.
Funding
None.
Acknowledgement
N/A.
References
- 1.Zhu N., Zhang D., Wang W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung M., Bernheim A., Mei X. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020 doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan Y., Guan H. Imaging changes in patients with 2019-nCov. Eur Radiol. 2020 doi: 10.1007/s00330-020-06713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin E.J., Baden L.R., Morrissey S., Campion E.W. Medical Journals and the 2019-nCoV Outbreak. N Engl J Med. 2020 doi: 10.1056/NEJMe2001329. [DOI] [PubMed] [Google Scholar]
- 6.Lancet T. Emerging understandings of 2019-nCoV. Lancet. 2020;395(10221):311. doi: 10.1016/S0140-6736(20)30186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu H., Stratton C.W., Tang Y.W. Outbreak of Pneumonia of Unknown Etiology in Wuhan China: the Mystery and the Miracle. J Med Virol. 2020 doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gralinski L.E., Menachery V.D. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12(2) doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duarte R., Furtado I., Sousa L., Carvalho C.F.A. The 2019 Novel Coronavirus (2019-nCoV): Novel Virus, Old Challenges. Acta Med Port. 2020 doi: 10.20344/amp.13547. [DOI] [PubMed] [Google Scholar]
- 10.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin X., Gong Z., Xiao Z. Novel Coronavirus Pneumonia Outbreak in 2019: Computed Tomographic Findings in Two Cases. Korean J Radiol. 2020 doi: 10.3348/kjr.2020.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlos W.G., Dela CCS Cao B, Pasnick S., Jamil S. Novel Wuhan (2019-nCoV) Coronavirus. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 13.Liu W., Morse J.S., Lalonde T., Xu S. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. Chembiochem. 2020 doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Team E.E. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Euro Surveill. 2020 doi: 10.2807/1560-7917.ES.2020.25.5.200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Team E.E. Note from the editors: novel coronavirus (2019-nCoV) Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian H.Y. 2019-nCoV: new challenges from coronavirus. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54(0):E001. doi: 10.3760/cma.j.issn.0253-9624.2020.0001. [DOI] [PubMed] [Google Scholar]
- 17.Li X., Zai J., Wang X., Li Y. Potential of large’ first generation’ human-to-human transmission of 2019-nCoV. J Med Virol. 2020 doi: 10.1002/jmv.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao S., Lin Q., Ran J. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N., Wang L., Deng X. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020 doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei J., Li J., Li X., Qi X. CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanne J.P. Chest CT Findings in 2019 Novel Coronavirus (2019-nCoV) Infections from Wuhan, China: Key Points for the Radiologist. Radiology. 2020 doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song F., Shi N., Shan F. Emerging Coronavirus 2019-nCoV Pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]