Abstract
We reported two cases with community-acquired pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) who returned from Wuhan, China in January, 2020. The reported cases highlight non-specific clinical presentations of 2019 novel coronavirus disease (COVID-19) as well as the importance of rapid laboratory-based diagnosis.
Keywords: COVID-19, SARS-CoV-2, Pneumonia, Emerging infectious diseases, Zoonosis
Introduction
After 17 years, physicians in Taiwan face another novel coronavirus outbreak originating in China.1 With the past experience of severe acute respiratory syndrome (SARS),2 , 3 we respond quickly this time. However, a new pathogen inevitably raises new challenges.4 Clinical data and experience sharing may contribute to the control of emerging infectious diseases.5 Here we present two cases of 2019 novel coronavirus disease (COVID-19).
Case reports
Case 1
A 74 year-old female visitor from Wuhan City, China presented to the hospital with fever, malaise, and poor appetite. She reported no underlying medical conditions. There was no chillness, cough, rhinorrhea, sore throat, myalgia, chest discomfort, dyspnea, abdominal pain, or diarrhea. Physical examination disclosed body temperature of 38.1 °C, blood pressure of 129/68 mm Hg, heart rate of 79 beats per minute, respiratory rate of 18 breaths per minute. Chest radiography (CXR) revealed mild increased infiltration over bilateral lower lung field. Peripheral-blood white-cell count was 3770 per cubic millimeter (with 62.3% neutrophils and 32.1% lymphocytes). Nasopharyngeal swab was positive for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by real-time reverse-transcriptase polymerase chain reaction (rRT-PCR) assay performed by the Centers for Diseases Control in Taiwan (Taiwan CDC).
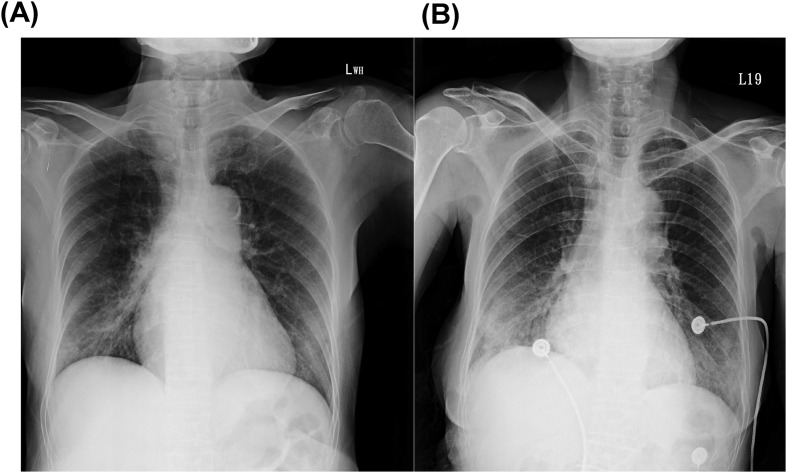
On day 6 in hospital, the patient remained febrile, malaise and poor appetite. Follow-up CXR revealed increasing opacity at right middle and lower lung fields (Fig. 1 A). Levofloxacin was initiated. On hospital day 12, after a 6-day course of levofloxacin, her fever abated with improved appetite and physical activity. She became free of symptoms afterward.
Figure 1.
Chest radiographs of two patients returned from Wuhan, China, with pneumonia caused by SARS-CoV-2 in middle Taiwan. (A) Case 1: increasing opacity at right middle and lower lung fields at hospital day 6. (B) Case 2: patchy consolidation over bilateral lower lung fields of at hospital day 6.
Case 2
A 73 year-old previously health female visitor returning from Wuhan City 3 days ago presented to the hospital with dry cough, fever, malaise and poor appetite. She denied chillness, rhinorrhea, sore throat, chest discomfort, myalgia, dyspnea, abdominal pain, or diarrhea. Her body temperature was 38.7 °C with blood pressure of 117/47 mm Hg, heart rate of 82 beats per minute, respiratory rate of 18 breaths per minute. CXR demonstrated non-specific mild increased infiltration over bilateral lower lung field. Peripheral-blood white-cell count was 3420 per cubic millimeter (with 69.3% neutrophils and 26.9% lymphocytes). Nasopharyngeal swab was positive for SARS-CoV-2 by rRT-PCR assay reported from Taiwan CDC.
On day 6 in hospital, the patient remained febrile, malaise and poor appetite. She reported worsening of cough. Follow-up CXR revealed patchy consolidation over bilateral lower lung field (Fig. 1B). Parenteral cefepime and oral clarithromycin therapy were initiated. On day 9, she was afebrile with improved general condition. Antimicrobial therapy was shifted to oral moxifloxacin. She remained free of symptoms afterward.
Discussion
The nonspecific presentations of these two cases are consistent with early reports of COVID-19 from China.6 , 7 Fever remains the most common complain. Some cases didn't have cough, and upper respiratory tract infections (URI) symptoms such as rhinorrhea and sore throat were rare. Similar clinical manifestations have been reported in SARS,8 since URI symptoms were uncommon and cough was not always present in SARS patients. Although routine laboratory testing was not diagnostic, certain patterns of laboratory abnormalities were observed in COVID-19. Leukopenia, lymphopenia, anemia, elevation of liver enzymes and lactate dehydrogenase, have been reported in different series.6 , 7 Also, a similar observation has been made in SARS.8
The clinical utility of CXR in the early diagnosis of COVID-19 is questionable. In this report, initial CXR of both cases was non-diagnostic, and more evident radiological abnormalities were detected on day 6. Similar findings were reported in the first case of COVID-19 in the United States, and pulmonary patch/consolidation was not detected by CXR until day 5 in hospital (day 9 of illness).9 Similarly, in a case series of SARS patients from the Amoy Gardens housing estate, 29.3% (22/75) cases had normal CXR on admission, however four of 22 cases developed acute respiratory distress syndrome (ARDS) afterward.8 In general, 80% (60/75) of cases experienced radiological worsening at a mean of 7.4 days.8 Both unifocal and bilateral lung infiltration could be observed in our report. Of 99 COVID-19 cases in China, 25% presented with unilateral pneumonia and 75% presented with bilateral pneumonia.7
As discussed above, COVID-19 cannot be reliably distinguished by clinical, radiologic, or laboratory criteria from other causes of pneumonia. Moreover, in the clinical setting of community transmission, exposure or travel history alone would be not useful to identify the risk population of COVID-19 cases. Hence, laboratory-based diagnosis is critical. At the present time, RT-PCR assays were most widely used to detect SARS-CoV-2. Current data are insufficient to determine their sensitivity and specificity for SARS-CoV-2. A previous study demonstrated SARS virus could be detected in approximately one-third of patients during early phase of the illness, and most frequently during the second week of illness.8 We believe that the diagnostic performance of molecular testing improves during the decades. However, more data are needed to address the issue.
Another challenge is the surge of clinical needs of laboratory testing. It is anticipated that there will be an overwhelming demand for the supply chain of testing reagents and laboratory equipment, and the availability of well trained and experienced staff during the outbreak. There are several alternative diagnostic strategies under development. Feng et al. proposed a CRISPR-based technique for the diagnosis of COVID-19 (https://www.broadinstitute.org/files/publications/special/COVID-19%20detection%20(updated).pdf). After RNA extraction, the test result could be read using a dipstick in less than an hour. RNA-based metagenomic sequencing is another promising approach, and has been used to diagnose COVID-19 in the first cluster related to the Huanan Seafood Market in China.10
Currently, clinical management and treatment of COVID-19 is largely supportive. In the cases of pneumonia, our diagnostic capacity could be hindered by the nature of isolation units. For example, for the first COVID-19 case in the United States, only point-of-care laboratory testing was permitted initially.9 The current rate of co-infection and distribution of coexisting organisms in the cases of COVID-19 remain undefined. Of note, current evidence suggests that co-infection is not uncommon in the patients with community-acquired pneumonia.11 In a prospective study of 2259 patients with pneumonia and specimens submitted for comprehensive bacterial and viral testing, etiologic agents were detected in only 38% cases. Among these cases, the percentages of co-infections were associated with the severity and geographic regions.11 Clinical guideline for the treatment of community-acquired pneumonia in Taiwan shall be considered for these cases.12
In conclusion, we reported the clinical features of two patients with COVID-19 in Taiwan, and highlight the nonspecific nature of clinical presentations of COVID-19 and the importance of laboratory-based diagnosis.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Contributor Information
Zhi-Yuan Shi, Email: zyshi@vghtc.gov.tw.
Po-Yu Liu, Email: pyliu@vghtc.gov.tw.
References
- 1.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome Composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020 Feb 7 doi: 10.1016/j.chom.2020.02.001. pii: S1931-3128(20)30072-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yen M.Y., Chiu A.W., Schwartz J., King C.C., Lin Y.E., Chang S.C. From SARS in 2003 to H1N1 in 2009: lessons learned from Taiwan in preparation for the next pandemic. J Hosp Infect. 2014;87:185–193. doi: 10.1016/j.jhin.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsueh P.R., Hsiao C.H., Yeh S.H., Wang W.K., Chen P.J., Wang J.T. Microbiologic characteristics, serologic responses, and clinical manifestations in severe acute respiratory syndrome, Taiwan. Emerg Infect Dis. 2003;9:1163–1167. doi: 10.3201/eid0909.030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee P.I., Hsueh P.R. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J Microbiol Immunol Infect. 2020;53:365–367. doi: 10.1016/j.jmii.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020 Feb 17 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Jan 24 doi: 10.1016/S0140-6736(20)30183-5. pii: S0140-6736(20)30183-30185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Jan 30 doi: 10.1016/S0140-6736(20)30211-7. pii: S0140-6736(20)30211-30217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 Jan 31 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020 Feb 3 doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M. Community-acquired pneumonia requiring hospitalization among U.S. Adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou C.C., Shen C.F., Chen S.J., Chen H.M., Wang Y.C., Chang W.S. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J Microbiol Immunol Infect. 2019;52:172–199. doi: 10.1016/j.jmii.2018.11.004. [DOI] [PubMed] [Google Scholar]