The outbreak of the COVID-19 pandemia in 2019/2020 has stimulated research on clinical laboratory findings in this condition, as evidenced by two recent papers in this journal [1], [2]. We know that angiotensin-converting enzyme is involved in corona virus infection. Pathogenic corona viruses (severe acute respiratory syndrome corona virus [SARS-CoV] and SARS-CoV-2) bind to their target cells through angiotensin-converting enzyme 2 (ACE2), [3], [4]. Not only has ACE2 facilitated the invasion of SARS virus for rapid replication, but also ACE2 is depleted from the cell membrane and therefore the damaging effects of Ang II are enhanced, resulting in acute deterioration of lung tissues [5]. The angiotensin-converting 1 (ACE1) enzyme is characterized by a genetic deletion/insertion (D/I) polymorphism in intron 16, which is associated with alterations in circulating and tissue concentrations of ACE. The D allele is associated with a reduced expression of ACE2. Although ACE2 and ACE share only 42% of amino acid identity, they both act as carboxypeptidases to cleave amino acids from the peptides’ carboxyl terminal [6].
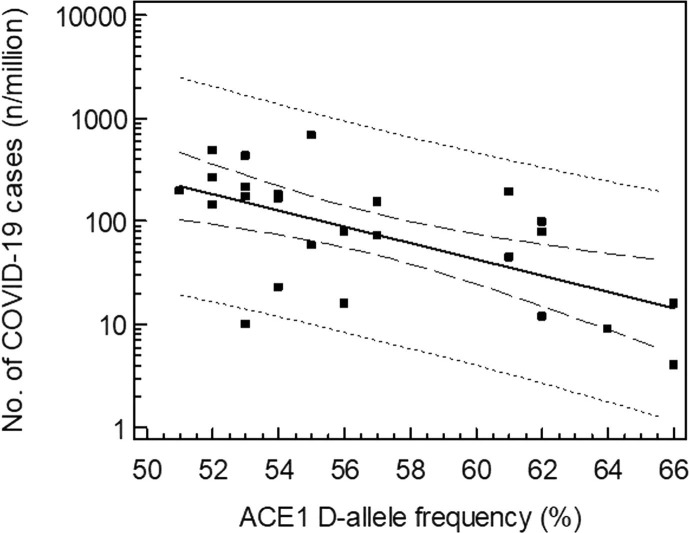
As this D/I polymorphism shows an important geographical variation [7], we postulated that the variability in D/I genotype distribution might partly explain the variable prevalence of the COVID-19 infection amongst continental European countries. Therefore, we compared the D-allele frequency of the ACE1 gene as obtained in 25 different European countries with the prevalence and mortality of COVID-19, as calculated on March 20, 2020 by Johns Hopkins [8]. Fig. 1 shows the regression data. Data from Austria, Belgium, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Israel, Italy, Lithuania, Moldova, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, Switzerland, and Turkey were included in the analysis. The log transformed prevalence of COVID-19 infections inversely correlates with the ACE D allele frequency: log (prevalence; number of cases/106 inhabitants) = 6.358–0.079 (D-allele frequency, %), r2 = 0.378; p = 0.001. About 38% of the variability of the prevalence can be explained by the relative frequency of the ACE1 D-allele. Similarly, a significant correlation could be noted between COVID-19 caused mortality (Spearman r = −0.510, p = 0.01) and the prevalence of the ACE1 D-allele. It should also be noted that the two Asian countries which were initially severely hit by the virus, China and Korea, are also characterized by low D allele frequencies.
Fig. 1.
Prevalence of COVID 19 in 25 European countries (on March 20, 2020 vs. ACE1 D-allele frequency (%): log (prevalence; no. of cases/106 inhabitants) = 6.358–0.079 (D-allele frequency, %), r2 = 0.378; p = 0.001.
These data suggest that ACE1 D/I polymorphism may be regarded as a confounder in the spread of COVID-19 and the outcome of the infection in various European populations. These findings are in agreement with the role of ACE in pulmonary infections caused by corona viruses [4]. The ACE D/I genotype may affect the clinical course of the infection.
References
- 1.Lippi G., Plebani M., Michael Henry B. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta. 2020 doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chim. Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020 doi: 10.1126/science.abb2762. pii: eabb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006;6:271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong M.K.S. Angiotensin converting enzymes, subchapter 29D. In: Takei Y., Ando H., Tsutsui K., editors. Handbook of Hormones. Comparative Endocrinology for Basic and Clinical Research. Elsevier; 2016. pp. 263–265. e29D-1-e29D-4. [Google Scholar]
- 6.Donoghue M., Hsieh F., Baronas E., Godbout K. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 7.Saab Y.B., Gard P., Overall A. The geographic distribution of the ACE II genotype: a novel finding. Genet. Res. 2007;89:259–267. doi: 10.1017/S0016672307009019. [DOI] [PubMed] [Google Scholar]
- 8.www.worldometers.info/coronavirus/countries. Assessed March 20, 2020.