Abstract
Objectives
The aim of the present study was to prevent cross-infection in the operating room during emergency procedures for patients with confirmed or suspected 2019 novel coronavirus (2019-nCoV) by following anesthesia management protocols, and to document clinical- and anesthesia-related characteristics of these patients.
Design
This was a retrospective, multicenter clinical study.
Setting
This study used a multicenter dataset from 4 hospitals in Wuhan, China.
Participants
Patients and health care providers with confirmed or suspected 2019-nCoV from January 23 to 31, 2020, at the Wuhan Union Hospital, the Wuhan Children's Hospital, The Central Hospital of Wuhan, and the Wuhan Fourth Hospital in Wuhan, China.
Interventions
Anesthetic management and infection control guidelines for emergency procedures for patients with suspected 2019-nCoV were drafted and applied in 4 hospitals in Wuhan.
Measurements and Main Results
Cross-infection in the operating rooms of the 4 hospitals was effectively reduced by implementing the new measures and procedures. The majority of patients with laboratory-confirmed 2019-nCoV infection or suspected infection were female (23 [62%] of 37), and the mean age was 41.0 years old (standard deviation 19.6; range 4-78). 10 (27%) patients had chronic medical illnesses, including 4 (11%) with diabetes, 8 (22%) with hypertension, and 8 (22%) with digestive system disease. Twenty-five (68%) patients presented with lymphopenia, and 23 (62%) patients exhibited multiple mottling and ground-glass opacity on computed tomography scanning.
Conclusions
The present study indicates that COVID 19–specific guidelines for emergency procedures for patients with confirmed or suspected 2019-nCoV may effectively prevent cross-infection in the operating room. Most patients with confirmed or suspected COVID 19 presented with fever and dry cough and demonstrated bilateral multiple mottling and ground-glass opacity on chest computed tomography scans.
Key Words: infection control, cross-infection, occupational health, 2019 novel coronavirus, viral pneumonia, COVID 19, 2019 nCoV
A NOVEL, ongoing outbreak of pneumonia was reported in Wuhan city, Hubei province, China, in December 2019,1, 2, 3 and this virus has caused significant concerns internationally. Isolation of a novel coronavirus (CoV) responsible for the COVID 19 outbreak—a novel beta-coronavirus—in patients from Wuhan subsequently was completed by Chinese scientists, and it was named 2019 novel coronavirus (2019-nCoV).4 As of March 8, 2020, a total of 80,735 laboratory-confirmed 2019-nCoV infections had been reported in China, with 3,119 deaths and 5,111 of the cases being classified as severe.5
By January 2, 2020, Huang et al.2 had reported clinical features of the first 41 laboratory-confirmed patients with 2019-nCoV infections in Wuhan. Clinical manifestations are very similar to those of severe acute respiratory syndrome (SARS)-CoV, including dyspnea, which developed in 22 (55%) of 40 patients. Furthermore, patients with severe 2019-nCoV developed acute respiratory distress syndrome and required intensive care unit admission, oxygen therapy, and endotracheal intubation.
Concurrently, human-to-human transmission was confirmed by Fuk-Woo Chan et al.,6 and infections in hospital staff caring for patients with 2019-nCoV were reported.7 Fifteen hospital staff members in Wuhan Union Hospital, some of whom were working in the same ward, were confirmed as being infected with 2019-nCoV,4 although the possible role of so-called super-spreaders remains to be clarified.
Considering that many patients with SARS and Middle East respiratory syndrome were infected in the hospital,8 precautions need to be taken to prevent nosocomial spread of this new 2019-nCoV virus, in particular considering the evidence of efficient humantohuman transmission of 2019-nCoV.6 , 7 , 9 Furthermore, cases of COVID 19 now have been reported in the United States10 and other countries throughout the world. The patient with the first documented case in the United States presented with pneumonia and showed nonspecific signs and symptoms of mild illness, and this type of presentation undoubtedly increases the difficulty of early protective preparation for operating room personnel. In an effort to contain the spread of COVID 19 in health care settings in China, the Department of Anesthesiology at the Wuhan Union Hospital drafted a guideline for 2019-nCoV–related prevention and control that includes emergency intubation, preoperative evaluation, and infection control in the operating room. The present article discusses that guideline and describes clinical features and anesthesia-related characteristics of patients with confirmed or suspected 2019-nCoV (COVID 19) presenting for emergency intubation and surgical anesthesia.
The aim of the present study is to help prevent cross-infection in the operating room by implementing strict anesthesia management and infection control procedures while caring for patients with confirmed or suspected COVID 19. Moreover, the clinical features and anesthesia-related characteristics may provide additional insights into 2019-nCoV from the perspective of anesthesiology.
Materials and Methods
Patients
For this retrospective, multicenter clinical study, patients with confirmed or suspected COVID 19 from January 23 to 31, 2020, at the Wuhan Union Hospital, the Wuhan Children's Hospital, The Central Hospital of Wuhan, and the Wuhan Fourth Hospital in Wuhan, China, were recruited. All study methods were conducted following the relevant regulations and guidelines of the institutional ethics committee of Tongji Medical College, Huazhong University of Science and Technology.
Data Collection and Procedures Draft
The electronic medical records, anesthesia records, preoperative evaluation records, nursing records, laboratory findings, chest x-rays, and computed tomography (CT) examinations for all patients were reviewed. Clinical outcomes were followed-up to January 31, 2020. American Society of Anesthesiologists (ASA) physical status classification and the Mallampati score were determined by skilled anesthesiologists. All data were collected and checked by 2 independent investigators (S.Z. and K.L.). The protocols of anesthesia management for emergency procedures in patients with confirmed or suspected 2019-nCoV infection were drafted by an expert panel from the Department of Anesthesiology at Wuhan Union Hospital.
Outcomes
The outcomes for the present study were demographics; key clinical features (eg, fever, cough); anesthesia-related clinical characteristics (eg, ASA classification, Mallampati score, anesthetic methods); key laboratory findings (eg, lymphocyte count); and chest x-ray and CT findings for all patients.
Statistical Analysis
Data are expressed as mean (standard deviation) and categorical variables as count (%). For laboratory results, the authors also assessed whether the measurements were outside the normal range. All statistical analysis was performed with GraphPad Prism, version 8 (Graph-Pad Software Inc, San Diego, CA) and SPSS software, version 25 for Mac (IBM Corp, Armonk, NY).
Results
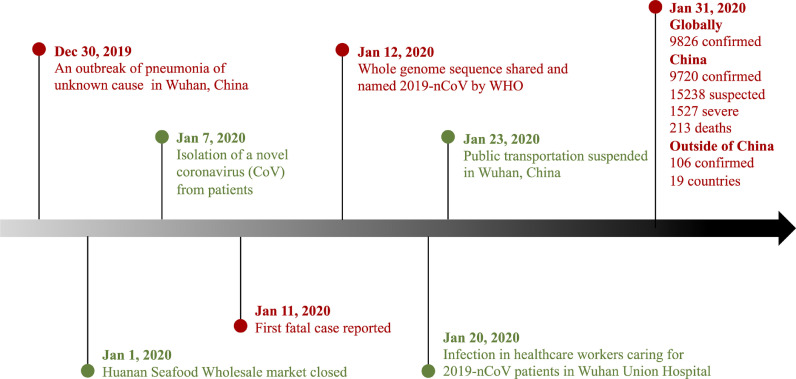
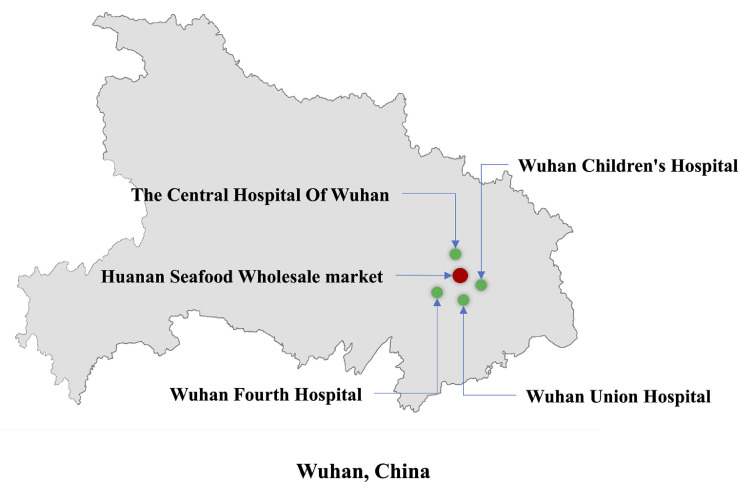
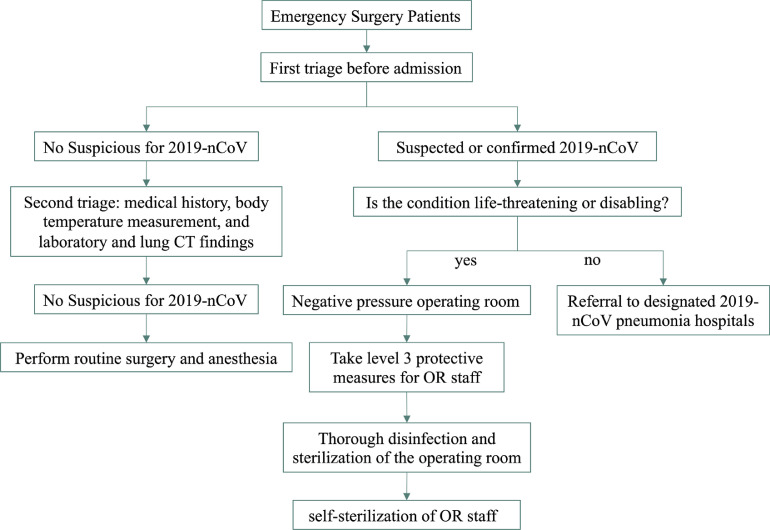
The date of January 23, 2020, for starting the present study was chosen because it is the same date the Chinese Lunar New Year begins and when Wuhan began suspending public transportation (Fig 1 ); after that time, the major hospitals in Wuhan suspended all elective procedures. The 4 hospitals selected for the study were the closest hospitals in proximity to the Huanan Seafood Wholesale Market (1-4 km away), which is the suspected origin of 2019-nCoV outbreak (Fig 2 ). Anesthesiologists in Wuhan have made great efforts to control this epidemic (Fig 3 ). As previously mentioned, anesthetic management and infection control guidelines for emergency procedures in patients with confirmed or suspected 2019-nCoV were drafted by an expert panel from the Department of Anesthesiology at Wuhan Union Hospital (Fig 4 ); these procedures are detailed in the Supplementary Material as follows: (1) anesthesiologists’ procedures for emergency intubation of patients with confirmed or suspected 2019-nCoV infection, (2) precautions for 2019-nCoV infection in outpatient evaluation center, and (3) precautions for 2019-nCoV infection control in the operating rooms. After adopting the aforementioned procedures, the 4 hospitals in the study completed 321 cases of emergency surgical anesthesia, including for 37 patients who were laboratory-confirmed as having or were suspected of having 2019-nCoV. In the present study, 3 health care providers were infected with 2019-nCoV in the clinic setting from January 23 to 31, 2020.
Fig 1.
Timeline of the early stages of the 2019 novel coronavirus outbreak. 2019-nCoV, 2019 novel coronavirus.
Fig 2.
Geographical map of the 4 hospitals included in the study and the Huanan Seafood Wholesale market in Wuhan, China. ASA, American Society of Anesthesiologists.
Fig 3.
Photo of health care providers, including anesthesiologist, surgeons, and nurses, performing an emergency surgery.
Fig 4.
Flow chart of anesthesia and infection control management guidelines for emergency procedures in patients with confirmed or suspected 2019 novel coronavirus. 2019-nCoV, 2019 novel coronavirus; OR, operating room.
The present retrospective, multicenter clinical study comprised 37 patients (5 confirmed and 32 suspected 2019nCoV cases). Most patients were female (23 of 37), with a mean age of 41.0 years old (standard deviation 19.6; range 4-78). 10 (27%) patients had chronic medical illnesses, including 4 (11%) with diabetes, 8 (22%) with hypertension, 8 (22%) with digestive system disease, 3 (8%) with nervous system disease, and 8 (22%) with respiratory system disease (Table 1 ). On admission or before anesthesia, many patients presented with fever (16 [43%]), cough (12 [32%]), or sputum production (2 [5%]) (see Table 1). Twenty-five (68%) patients demonstrated lymphopenia (lymphocyte count <1.1 × 109/L [normal range 1.1-3.2]) (see Table 1). According to chest xray and CT findings, abnormalities in chest images were detected in 30 (81%) patients. In particular, 10 (27%) patients exhibited bilateral pneumonia, and 15 (41%) patients exhibited unilateral pneumonia (Table 2 ). Twenty-three (62%) patients exhibited multiple mottling and ground-glass opacity (see Table 1).
Table 1.
Demographics and Key Clinical and Laboratory Features of 37 Patients With Confirmed or Suspected 2019 Novel Coronavirus Infection
| Patients (n = 37) | |
|---|---|
| Characteristics | |
| Age, y | |
| Mean (SD) | 41.0 (19.6) |
| Range | 4-78 |
| ≤39 | 21 (57%) |
| 40-59 | 7 (19%) |
| 60-69 | 6 (16%) |
| ≥70 | 3 (8%) |
| Sex | |
| Female | 23 (62%) |
| Male | 14 (38%) |
| Chronic medical illness | 10 (27%) |
| Diabetes | 4 (11%) |
| Hypertension | 8 (22%) |
| Digestive system disease | 8 (22%) |
| Nervous system disease | 3 (8%) |
| Respiratory system disease | 8 (22%) |
| Signs and symptoms | |
| Fever | |
| <37.3°C | 21 (57%) |
| 37.3-38.0°C | 11 (30%) |
| 38.1-39.0°C | 5 (13%) |
| >39.0°C | 0 (0%) |
| Cough | 12 (32%) |
| Sputum production | 2 (5%) |
| Dyspnea | 0 |
| Laboratory findings | |
| Lymphocyte count (× 109/L) | |
| <1.1 | 25 (68%) |
| ≥1.1 | 12 (32%) |
| Chest x-ray and CT findings | |
| Unilateral pneumonia | 10 (27%) |
| Bilateral pneumonia | 15 (41%) |
| Multiple mottling and ground-glass opacity | 23 (62%) |
NOTE. Data are presented as number (%), n (%), and mean (standard deviation).
Abbreviations: CT, computed tomography; SD, standard deviation.
Table 2.
Anesthesia-Related Clinical Characteristics and Clinical Outcomes of 37 Patients With Confirmed or Suspected 2019 Novel Coronavirus Infection
| Patients (n = 37) | |
|---|---|
| ASA classification | |
| I or II | 21 (57%) |
| III | 10 (27%) |
| IV | 5 (13%) |
| V | 1 (3%) |
| Mallampati score | |
| I | 8 (22%) |
| II | 28 (75%) |
| III | 0 (0%) |
| IV | 1 (3%) |
| Type of surgery | |
| Abdominal surgery | 10 (27%) |
| Cardiovascular surgery | 2 (5%) |
| Orthopedic surgery | 6 (16%) |
| Obstetrics and gynecological surgery | 11 (30%) |
| Neurosurgery | 2 (5%) |
| Other | 6 (16%) |
| Anesthetic methods | |
| General anesthesia | 26 (70%) |
| Spinal anesthesia | 11 (30%) |
| Duration of anesthesia (h) | |
| ≤1 | 6 (16%) |
| 1-3 | 22 (60%) |
| 3-5 | 6 (16%) |
| ≥5 | 3 (8%) |
| Clinical outcome | |
| Hospitalization | 27 (73%) |
| Discharge | 10 (27%) |
| Death | 0 |
NOTE. Data are presented as number (%) and n (%).
Abbreviation: ASA, American Society of Anesthesiologists.
All patients underwent surgery under anesthesia, including 26 (70%) patients with general anesthesia and 11 (30%) patients with spinal anesthesia (see Table 2). Types of surgery included abdominal surgery (10 [27%]), cardiovascular surgery (2 (5%]), orthopedic surgery (6 [16%]), obstetric and gynecological surgery (11 [30%]), neurosurgery (2 [5%]), and other types of surgery (6 [16%]) (see Table 2). Most patients (21 [57%]) were healthy or had well-controlled medical conditions (ASA physical status classification I and II). Only 1 (3%) patient with acute peritonitis was determined as ASA V (see Table 2). Thirty-six (97%) patients were found to have a Mallampati score of I/II, and 1 was classified as having a Mallampati score of IV on airway evaluations (see Table 2).
For anesthesia duration, 28 (76%) patients underwent a short surgical anesthesia time (≤3 h), and 9 (24%) patients underwent a longer surgical anesthesia time (>3 h). Finally, as of January 31, 2020, 10 (27%) of the 37 patients had been discharged, 27 (73%) patients still were hospitalized, and no patient had died (see Table 2).
Discussion
For the present study, the authors drafted and published anesthesia management and infection control guidelines for emergency procedures in patients with confirmed or suspected COVID 19 to prevent nosocomial infection. In operating rooms in which infection control procedures are rigorously applied, the risk for staff to contract 2019-nCoV from patient contact is low, despite long exposure times. In addition, for the present study, the anesthesia-related characteristics of patients with confirmed or suspected COVID 19 in the 4 most affected hospitals in Wuhan, China, were examined.
On January 7, 2020, a novel coronavirus was identified by the Chinese Center for Disease Control and Prevention and subsequently named COVID-19 (also named as 2019nCoV earlier) by the World Health Organization.4 , 5 Coronaviruses, such as SARS11 , 12 and Middle East respiratory syndrome,13 , 14 mainly cause respiratory tract infections in humans. In the present study, most patients presented with fever, dry cough, and bilateral multiple mottling and ground-glass opacity on chest CT scans. These data are consistent with recent reports.2 , 6 , 9
Transmission rates are unknown for 2019nCoV; however, there is strong evidence of efficient humantohuman transmission.6 , 7 , 9 The number of infections is increasing quickly. By January 30, 2020, 9,692 laboratory-confirmed 2019-nCoV infections had been reported in China, with 213 fatal cases.5 In particular, at the same time, 15 health care workers had been reported to be infected by 2019nCoV, 14 of whom were assumed to have been infected by caring for 2019-nCoV patients. The overall infection rate of health care workers at the time of the present study was unknown. However, precautions and related management procedures need to be implemented immediately to prevent any additional infections. This has challenged health care workers to use effective infection control procedures and measures. Anesthesiologists, as specialists in airway management, generally perform endotracheal intubation in severe cases of hypoxia and provide emergency anesthesia for surgery in these cases. This undoubtedly increases the exposure risk for anesthesiologists because of the frequent exposure to patients’ respiratory secretions and blood. Therefore, 2019-nCoV–specific anesthesia guidelines need to be developed and used to prevent nosocomial spread of the virus.
Some of the infected patients in the present study had no obvious symptoms initially, including slight fever or even no fever, which is consistent with other recent reports,2 , 9 , 10 and infectious characteristics became apparent during the incubation period (1-14 d medical observation period).7 Transmission of 2019-nCoV probably occurs by means of large droplets and contact and less so by means of aerosols and fomites, and exposure to oral and respiratory secretions at the time of endotracheal intubation puts anesthesiologists at high risk for infection. Therefore, all anesthesiologists who may be in contact with patients with confirmed or suspected 2019-nCoV must take level 3 protective measures, which are detailed in the Supplementary Materials. The World Health Organization also has released important information that has prompted health care workers caring for patients infected with 2019-nCoV to reexamine the precautionary procedures of infection control.4
Furthermore, diagnosis before the patient enters the operating room is important in the effort to prevent cross-infection. Body temperature, laboratory findings (especially lymphocyte count), and chest x-ray and CT findings (especially multiple mottling and ground-glass opacity) should be confirmed before a patient enters the operating room. If the patient has a fever of unknown cause, the examination results show pulmonary infection or low oxygen saturation of unknown cause (< 90%), and the surgery is not an emergency case, anesthesiologists should communicate to the patient, family, and the surgeon that the surgery should be suspended. Meanwhile, during emergency surgical cases, close attention should be paid to the patient's heart rate, blood pressure, and oxygen saturation, especially during anesthesia induction and the procedure.
To prevent cross-infection in the operating room, single use of all anesthetic equipment, utensils, and drugs for each patient must be guaranteed. Anesthetic devices in contact with the respiratory tract, such as video laryngoscope lenses, plastic respiratory pipes, filters, respiratory balloons, suction tubes, and sputum suction tubes, should be discarded after single use. Furthermore, thorough disinfection and sterilization of the operating room should be conducted at the end of each day that surgery has been performed or immediately after surgery of patients with confirmed or suspected 2019-nCoV cases. This sterilization includes routine disinfection of the anesthesia machine, whole operating room ultraviolet radiation, disinfectant spray, and mopping. All these procedures should be inspected by the infection control team for backtracking purposes.
In addition, health care workers who routinely are exposed to viral respiratory infections in the hospital may transmit infection to others;15 therefore, the presence of potentially contaminated clothing in the operating room by health care workers must be addressed. Thus, transmission-preventive protocols and disinfection measures should be followed properly before health care workers enter the operating room after they have been involved with intubation, consultation, or postoperative patient transportation of patients with confirmed or suspected 2019-nCoV infection. Hence, the guidelines forbid any potentially contaminated clothing in the operating room.
If health care workers develop symptoms of 2019-nCoV infection, such as fever, cough, soreness, and feebleness, after contact with suspected or confirmed cases, evaluations such as blood test, C-reactive protein, and pulmonary imaging should be acquired in a timely fashion. Moreover, they also should immediately report them to the hospital and isolate themselves at home. Their vital signs and health conditions should be monitored closely, and they should receive proper diagnosis and treatment if their symptoms get worse.
Conclusions
Despite the series of precautionary measures suggested by the authors’ institutional guidelines, it still is difficult to consider all possible problems encountered in clinical practice. Questions concerning best practice remain. A low infection rate in the operating rooms of 4 hospitals in close proximity to the suspected outbreak was demonstrated after health care staff followed strict guidelines of infection control procedures. The authors believe that these protective measures are effective when rigorously applied and are responsible for the relatively low infection rate among the health care workers.
Public health measures and precautions in health care settings are critical in controlling 2019-nCoV. The present study may help prevent cross-infection in the operating room by means of implementing anesthesia and infection control management procedures for emergency procedures in patients with confirmed or suspected COVID 19. Implementation of similar measures might be important and, it is hoped, successful in reducing the nosocomial transmission of 2019-nCoV in the perioperative setting.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
This work was supported by the National Natural Science Foundation of China (No. 81571075) and the National Key Research and Development Project (No. 2018YFC2001802).
Supplementary material associated with this article can be found in the online version at doi:10.1053/j.jvca.2020.02.039.
Appendix. Supplementary materials
References
- 1.December I. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Coronavirus disease (COVID-19) outbreak. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed January 30, 2020.
- 5.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/index.html. Accessed January 30, 2020.
- 6.Fuk-Woo Chan J., Yuan S., Kok K.-H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;6736:1–10. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Wit E., Van Doremalen N., Falzarano D. SARS and MERS: Recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ksiazek T.G., Erdman D., Goldsmith C.S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 12.Drosten C., Gunther S., Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 13.de Groot R.J., Baker S.C., Baric R.S. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaki A.M., van Boheemen S., Bestebroer T.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 15.Radonovich L.J., Simberkoff M.S., Bessesen M.T. N95 respirators vs medical masks for preventing influenza among health care personnel: A randomized clinical trial. JAMA. 2019;322:824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.