Summary
The emergence of SARS-CoV-2 has resulted in >90,000 infections and >3,000 deaths. Coronavirus spike (S) glycoproteins promote entry into cells and are the main target of antibodies. We show that SARS-CoV-2 S uses ACE2 to enter cells and that the receptor-binding domains of SARS-CoV-2 S and SARS-CoV S bind with similar affinities to human ACE2, correlating with the efficient spread of SARS-CoV-2 among humans. We found that the SARS-CoV-2 S glycoprotein harbors a furin cleavage site at the boundary between the S1/S2 subunits, which is processed during biogenesis and sets this virus apart from SARS-CoV and SARS-related CoVs. We determined cryo-EM structures of the SARS-CoV-2 S ectodomain trimer, providing a blueprint for the design of vaccines and inhibitors of viral entry. Finally, we demonstrate that SARS-CoV S murine polyclonal antibodies potently inhibited SARS-CoV-2 S mediated entry into cells, indicating that cross-neutralizing antibodies targeting conserved S epitopes can be elicited upon vaccination.
Keywords: coronavirus, SARS-CoV-2, SARS-CoV, spike glycoprotein, antibodies, neutralizing antibodies, viral receptor, cryo-EM
Graphical Abstract
Highlights
-
•
SARS-CoV-2 uses ACE2 to enter target cells
-
•
SARS-CoV-2 and SARS-CoV bind with similar affinities to ACE2
-
•
Structures of SARS-CoV-2 spike glycoprotein in two conformations
-
•
SARS-CoV polyclonal antibodies inhibit SARS-CoV-2 spike-mediated entry into cells
SARS-CoV-2, a newly emerged pathogen spreading worldwide, binds with high affinity to human ACE2 and uses it as an entry receptor to invade target cells. Cryo-EM structures of the SARS-CoV-2 spike glycoprotein in two distinct conformations, along with inhibition of spike-mediated entry by SARS-CoV polyclonal antibodies, provide a blueprint for the design of vaccines and therapeutics.
Introduction
Three coronaviruses have crossed the species barrier to cause deadly pneumonia in humans since the beginning of the 21st century: severe acute respiratory syndrome coronavirus (SARS-CoV) (Drosten et al., 2003, Ksiazek et al., 2003), Middle-East respiratory syndrome coronavirus (Zaki et al., 2012) (MERS-CoV), and SARS-CoV-2 (Huang et al., 2020, Zhu et al., 2020). SARS-CoV emerged in the Guangdong province of China in 2002 and spread to five continents through air travel routes, infecting 8,098 people and causing 774 deaths. In 2012, MERS-CoV emerged in the Arabian Peninsula, where it remains a major public health concern, and was exported to 27 countries, infecting a total of ∼2,494 individuals and claiming 858 lives. A previously unknown coronavirus, named SARS-CoV-2, was discovered in December 2019 in Wuhan, Hubei province of China and was sequenced and isolated by January 2020 (Zhou et al., 2020, Zhu et al., 2020). SARS-CoV-2 is associated with an ongoing outbreak of atypical pneumonia (Covid-2019) that has affected over 90,000 people and killed more than 3,000 of those affected in >60 countries as of March 3, 2020. On January 30, 2020, the World Health Organization declared the SARS-CoV-2 epidemic a public health emergency of international concern.
MERS-CoV was suggested to originate from bats, but the reservoir host fueling spillover to humans is unequivocally dromedary camels (Haagmans et al., 2014, Memish et al., 2013). Both SARS-CoV and SARS-CoV-2 are closely related and originated in bats, who most likely serve as reservoir host for these two viruses (Ge et al., 2013, Hu et al., 2017, Li et al., 2005b, Yang et al., 2015a, Zhou et al., 2020). Whereas palm civets and racoon dogs have been recognized as intermediate hosts for zoonotic transmission of SARS-CoV between bats and humans (Guan et al., 2003, Kan et al., 2005, Wang et al., 2005), the SARS-CoV-2 intermediate host remains unknown. The recurrent spillovers of coronaviruses in humans along with detection of numerous coronaviruses in bats, including many SARS-related coronaviruses (SARSr-CoVs), suggest that future zoonotic transmission events may continue (Anthony et al., 2017, Ge et al., 2013, Hu et al., 2017, Li et al., 2005b, Menachery et al., 2015, Menachery et al., 2016, Yang et al., 2015a, Zhou et al., 2020). In addition to the highly pathogenic zoonotic pathogens SARS-CoV, MERS-CoV, and SARS-CoV-2, all belonging to the β-coronavirus genus, four low-pathogenicity coronaviruses are endemic in humans: HCoV-OC43, HCoV-HKU1, HCoV-NL63, and HCoV-229E. To date, no therapeutics or vaccines are approved against any human-infecting coronaviruses.
Coronavirus entry into host cells is mediated by the transmembrane spike (S) glycoprotein that forms homotrimers protruding from the viral surface (Tortorici and Veesler, 2019). S comprises two functional subunits responsible for binding to the host cell receptor (S1 subunit) and fusion of the viral and cellular membranes (S2 subunit). For many CoVs, S is cleaved at the boundary between the S1 and S2 subunits, which remain non-covalently bound in the prefusion conformation (Belouzard et al., 2009, Bosch et al., 2003, Burkard et al., 2014, Kirchdoerfer et al., 2016, Millet and Whittaker, 2014, Millet and Whittaker, 2015, Park et al., 2016, Walls et al., 2016a). The distal S1 subunit comprises the receptor-binding domain(s) and contributes to stabilization of the prefusion state of the membrane-anchored S2 subunit that contains the fusion machinery (Gui et al., 2017, Kirchdoerfer et al., 2016, Pallesen et al., 2017, Song et al., 2018, Walls et al., 2016a, Walls et al., 2017b, Yuan et al., 2017). For all CoVs, S is further cleaved by host proteases at the so-called S2′ site located immediately upstream of the fusion peptide (Madu et al., 2009, Millet and Whittaker, 2015). This cleavage has been proposed to activate the protein for membrane fusion via extensive irreversible conformational changes (Belouzard et al., 2009, Heald-Sargent and Gallagher, 2012, Millet and Whittaker, 2014, Millet and Whittaker, 2015, Park et al., 2016, Walls et al., 2017b). As a result, coronavirus entry into susceptible cells is a complex process that requires the concerted action of receptor-binding and proteolytic processing of the S protein to promote virus-cell fusion.
Different coronaviruses use distinct domains within the S1 subunit to recognize a variety of attachment and entry receptors, depending on the viral species. Endemic human coronaviruses OC43 and HKU1 attach via their S domain A (SA) to 5-N-acetyl-9-O-acetyl-sialosides found on glycoproteins and glycolipids at the host cell surface to enable entry into susceptible cells (Hulswit et al., 2019, Tortorici et al., 2019, Vlasak et al., 1988). MERS-CoV S, however, uses domain A to recognize non-acetylated sialoside attachment receptors (Li et al., 2017, Park et al., 2019), which likely promote subsequent binding of domain B (SB) to the entry receptor, dipeptidyl-peptidase 4 (Lu et al., 2013, Raj et al., 2013). SARS-CoV and several SARS-related coronaviruses (SARSr-CoV) interact directly with angiotensin-converting enzyme 2 (ACE2) via SB to enter target cells (Ge et al., 2013, Kirchdoerfer et al., 2018, Li et al., 2005a, Li et al., 2003, Song et al., 2018, Yang et al., 2015a).
As the coronavirus S glycoprotein is surface-exposed and mediates entry into host cells, it is the main target of neutralizing antibodies (Abs) upon infection and the focus of therapeutic and vaccine design. S trimers are extensively decorated with N-linked glycans that are important for proper folding (Rossen et al., 1998) and for modulating accessibility to host proteases and neutralizing Abs (Walls et al., 2016b, Walls et al., 2019, Xiong et al., 2018, Yang et al., 2015b). We previously characterized potent human-neutralizing Abs from rare memory B cells of individuals infected with SARS-CoV (Traggiai et al., 2004) or MERS-CoV (Corti et al., 2015) in complex with SARS-CoV S and MERS-CoV S to provide molecular-level information of the mechanism of competitive inhibition of SB attachment to the host receptor (Walls et al., 2019). The S230 anti-SARS-CoV Ab also acted by functionally mimicking receptor attachment and promoting S fusogenic conformational rearrangements through a ratcheting mechanism that elucidated the unique nature of the coronavirus membrane fusion activation (Walls et al., 2019).
We report here that ACE2 could mediate SARS-CoV-2 S-mediated entry into cells, establishing it as a functional receptor for this newly emerged coronavirus. The SARS-CoV-2 SB engages human ACE2 (hACE2) with comparable affinity to SARS-CoV SB from viral isolates associated with the 2002–2003 epidemic (i.e., binding with high affinity to hACE2). Tight binding to hACE2 could partially explain the efficient transmission of SARS-CoV-2 in humans, as was the case for SARS-CoV. We identified the presence of an unexpected furin cleavage site at the S1/S2 boundary of SARS-CoV-2 S, which is cleaved during biosynthesis—a novel feature setting this virus apart from SARS-CoV and SARSr-CoVs. Abrogation of this cleavage motif moderately affected SARS-CoV-2 S-mediated entry into VeroE6 or BHK cells but may contribute to expand the tropism of this virus, as reported for several highly pathogenic avian influenza viruses and pathogenic Newcastle disease virus (Klenk and Garten, 1994, Steinhauer, 1999). We determined cryoelectron microscopy (cryo-EM) structures of the SARS-CoV-2 S ectodomain trimer and reveal that it adopts multiple SB conformations that are reminiscent of previous reports on both SARS-CoV S and MERS-CoV S. Finally, we show that SARS-CoV S mouse polyclonal sera potently inhibited entry into target cells of SARS-CoV-2 S pseudotyped viruses. Collectively, these results pave the way for designing vaccines eliciting broad protection against SARS-CoV-2, SARS-CoV, and SARSr-CoV.
Results
ACE2 Is an Entry Receptor for SARS-CoV-2
The SARS-CoV-2 S glycoprotein shares 76% amino acid sequence identity with the SARS-CoV S Urbani and 80% identity with bat SARSr-CoV ZXC21 S and ZC45 S glycoprotein. The latter two SARSr-CoV sequences were identified from Rinolophus sinicus (Chinese horseshoe bats), the species from which SARSr-CoV WIV-1 and WIV-16 were isolated (Ge et al., 2013, Yang et al., 2015a). Furthermore, Zhou et al. (2020) recently reported that SARS-CoV-2 is most closely related to the bat SARSr-CoV RaTG13, with which it forms a distinct lineage from other SARSr-CoVs, and that their S glycoproteins share 97% amino acid sequence identity. SARS-CoV recognizes its entry receptor hACE2 at the surface of type II pneumocytes using SB, which shares ∼75% overall amino acid sequence identity with SARS-CoV-2 SB and 50% identity within their receptor-binding motifs (RBMs) (Li et al., 2005a, Li et al., 2003, Li et al., 2005c, Wan et al., 2020). Previous studies also showed that the host proteases cathepsin L and TMPRSS2 prime SARS-CoV S for membrane fusion through cleavage at the S1/S2 and at the S2′ sites (Belouzard et al., 2009, Bosch et al., 2008, Glowacka et al., 2011, Matsuyama et al., 2010, Millet and Whittaker, 2015, Shulla et al., 2011).
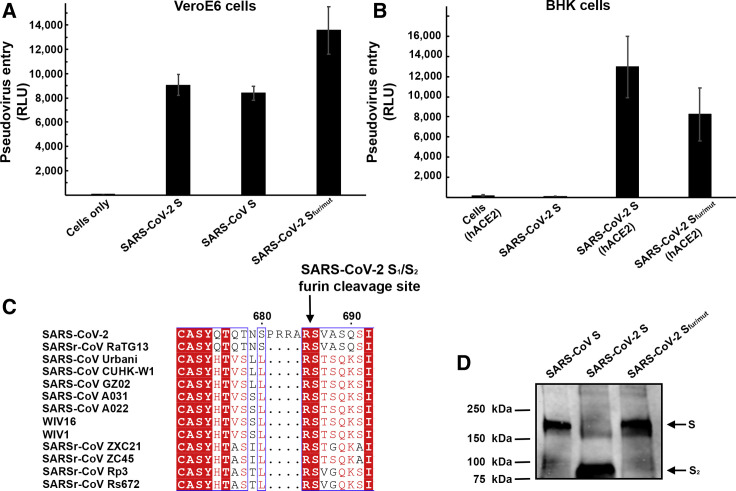
We set out to investigate the functional determinants of S-mediated entry into target cells using a murine leukemia virus (MLV) pseudotyping system (Millet and Whittaker, 2016). To assess the ability of SARS-CoV-2 S to promote entry into target cells, we first compared transduction of SARS-CoV-2 S-MLV and SARS-CoV S-MLV into VeroE6 cells, that are known to express ACE2 and support SARS-CoV replication (Drosten et al., 2003, Ksiazek et al., 2003). Both pseudoviruses entered cells equally well (Figure 1 A), suggesting that SARS-CoV-2 S-MLV could use African green monkey ACE2 as entry receptor. To confirm these results, we evaluated entry into BHK cells and observed that transient transfection with hACE2 rendered them susceptible to transduction with SARS-CoV-2 S-MLV (Figure 1B). These results demonstrate hACE2 is a functional receptor for SARS-CoV-2, in agreement with recently reported findings (Hoffmann et al., 2020, Letko et al., 2020, Zhou et al., 2020).
Figure 1.
ACE2 Is a Functional Receptor for SARS-CoV-2 S
(A) Entry of MLV pseudotyped with SARS-CoV-2 S, SARS-CoV S and SARS-CoV-2 Sfur/mut in VeroE6 cells. Data are represented as mean ± standard deviation of technical triplicates.
(B) Entry of MLV pseudotyped with SARS-CoV-2 S or SARS-CoV-2 Sfur/mut in BHK cells transiently transfected with hACE2. The experiments were carried out with two independent pseudovirus preparations and a representative experiment is shown. Data are represented as mean ± standard deviation of technical triplicates.
(C) Sequence alignment of SARS-CoV-2 S with multiple related SARS-CoV and SARSr-CoV S glycoproteins reveals the introduction of an S1/S2 furin cleavage site in this novel coronavirus. Identical and similar positions are respectively shown with white or red font. The four amino acid residue insertion at SARS-CoV-2 S positions 681-684 is indicated with periods. The entire sequence alignment is presented in Data S1.
(D) Western blot analysis of SARS-CoV S-MLV, SARS-CoV-2 S-MLV, and SARS-CoV-2 Sfur/mut-MLV pseudovirions. See also Data S1.
Sequence analysis of SARS-CoV-2 S reveals the presence of a four amino acid residue insertion at the boundary between the S1 and S2 subunits compared with SARS-CoV S and SARSr-CoV S (Figure 1C). This results in the introduction of a furin cleavage site, a feature conserved among the 144 SARS-CoV-2 isolates sequenced to date but not in the closely related RaTG13 S (Zhou et al., 2020). Using western blot analysis, we observed that SARS-CoV-2 S was virtually entirely processed at the S1/S2 site during biosynthesis in HEK293T cells, presumably by furin in the Golgi compartment (Figure 1D). This observation contrasts with SARS-CoV S, which was incorporated into pseudovirions largely uncleaved (Figure 1D). To study the influence on pseudovirus entry of the SARS-CoV-2 S1/S2 furin cleavage site, we designed an S mutant lacking the four amino acid residue insertion and the furin cleavage site by mutating Q677TNSPRRAR↓SV687 (wild-type SARS-CoV-2 S) to Q677TILR↓SV683 (SARS-CoV-2 Sfur/mut). SARS-CoV-2 Sfur/mut preserves only the conserved arginine residue at position 685 of wild-type SARS-CoV-2 S, thereby mimicking the S1/S2 cleavage site of the related SARSr-CoV S CZX21 (Figure 1D). SARS-CoV-2 Sfur/mut is therefore expected to undergo processing at the S1/S2 site upon encountering a target cell, similar to SARS-CoV S and SARSr-CoV S, i.e., via TMPRSS2 and/or cathepsin L (Belouzard et al., 2009, Bosch et al., 2008, Glowacka et al., 2011, Matsuyama et al., 2010, Millet and Whittaker, 2015, Shulla et al., 2011). As expected, SARS-CoV-2 Sfur/mut-MLV harbored uncleaved S upon budding (Figure 1D). The observed transduction efficiency of VeroE6 cells was higher for SARS-CoV-2 Sfur/mut-MLV than for SARS-CoV-2 S-MLV (Figure 1A), whereas the opposite trend was observed for transduction of hACE2-expressing BHK cells (Figure 1B). These results suggest that S1/S2 cleavage during S biosynthesis was not necessary for S-mediated entry in the conditions of our experiments (Figures 1C and 1D). We speculate that the detection of a polybasic cleavage site in the fusion glycoprotein of SARS-CoV-2 could putatively expand its tropism and/or enhance its transmissibility, compared with SARS-CoV and SARSr-CoV isolates, due to the near-ubiquitous distribution of furin-like proteases and their reported effects on other viruses (Klenk and Garten, 1994, Millet and Whittaker, 2015, Steinhauer, 1999).
SARS-CoV-2 Recognizes hACE2 with Comparable Affinity to SARS-CoV
The binding affinity of SARS-CoV for hACE2 correlates with the overall rate of viral replication in distinct species as well as with transmissibility and disease severity (Guan et al., 2003, Li et al., 2004, Li et al., 2005c, Wan et al., 2020). Indeed, specific SB mutations enabled efficient binding to hACE2 of SARS-CoV isolates from the three phases of the 2002–2003 epidemic, which were associated with marked disease severity (Consortium, 2004, Kan et al., 2005, Li et al., 2005c, Sui et al., 2004). In contrast, SARS-CoV isolates detected during the brief 2003–2004 re-emergence interacted more weakly with hACE2 and had low pathogenicity and transmissibility (Consortium, 2004, Kan et al., 2005, Li et al., 2005c).
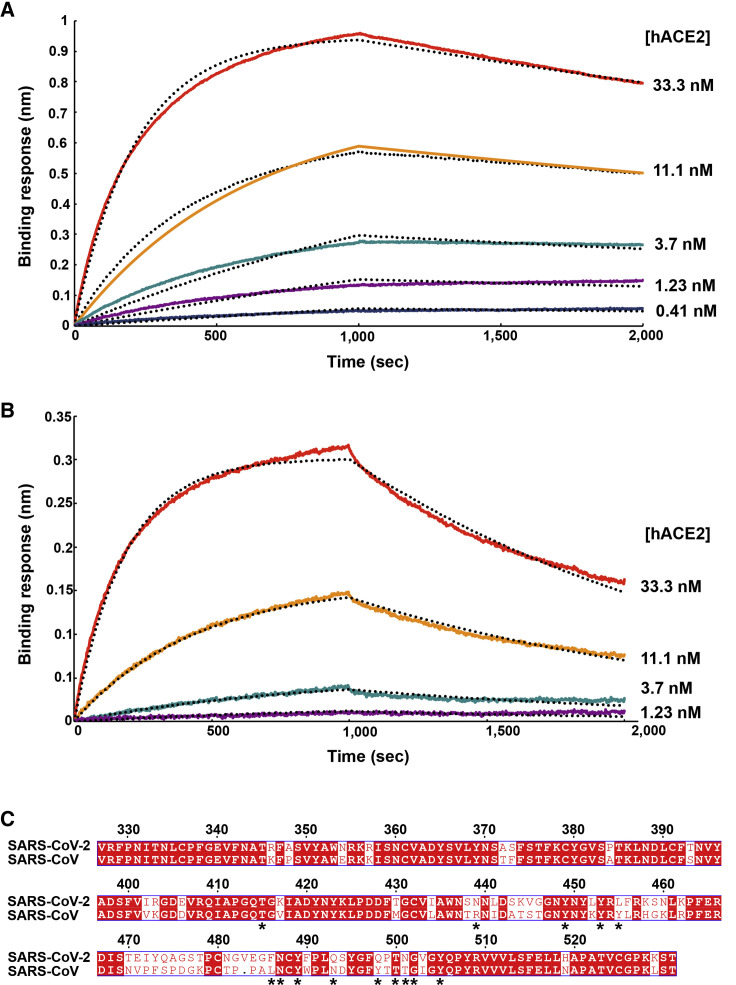
To understand the contribution of receptor interaction to the infectivity of SARS-CoV-2, we characterized engagement of hACE2 by SARS-CoV-2 SB and SARS-CoV SB side-by-side. We used biolayer interferometry to study binding kinetics and affinity of the purified hACE2 ectodomain to SARS-CoV-2 SB and SARS-CoV SB immobilized at the surface of biosensors. We found that hACE2 bound to SARS-CoV-2 SB and SARS-CoV SB with respective equilibrium dissociation constants of 1.2 nM (Figure 2 A) and 5.0 nM (Figure 2B), and comparable kinetic rate constants, although the off-rate, were slightly higher for SARS-CoV SB (Table 1 ). The affinity determined here for SARS-CoV SB binding to hACE2 is in line with prior studies and the tighter apparent interactions formed by SARS-CoV SB compared with the SARS-CoV S1 subunit or the SARS-CoV S trimer (Kirchdoerfer et al., 2018, Li et al., 2005c, Sui et al., 2004, Walls et al., 2019, Wong et al., 2004). Previous structural work identified 14 positions that are key for binding of SARS-CoV SB to hACE2: T402, R426, Y436, Y440, Y442, L472, N473, Y475, N479, Y484, T486, T487, G488, and Y491 (Li et al., 2005a). Analysis of the 144 SARS-CoV-2 genome sequences available from the Global Initiative on Sharing All Influenza Data (GISAID) (Elbe and Buckland-Merrett, 2017) shows that 8 out of these 14 positions are strictly conserved in SARS-CoV-2 SB, whereas the other 6 positions are (semi)conservatively substituted: R426SARS-CoVN439SARS-CoV-2, Y442SARS-CoVL455SARS-CoV-2, L472SARS-CoVF486SARS-CoV-2, N479SARS-CoVQ493SARS-CoV-2, Y484SARS-CoVQ498SARS-CoV-2, and T487SARS-CoVN501SARS-CoV-2 (Figure 2C). The conservation of many key contact residues could explain the similar binding affinities of SARS-CoV-2 SB and SARS-CoV SB for hACE2. No mutations of residues predicted to contact hACE2 have been observed among SARS-CoV-2 S sequences available to date. However, we note that SARSr-CoV ZXC21 and ZC45, which harbor the most closely related S sequences after SARSr-CoV RaTG13, have a deletion in the RBD that could affect binding to hACE2 (between SARS-CoV-2 S residues 473–486 ) (Data S1). Collectively, these results suggest that SARS-CoV-2 is at least as well adapted to the hACE2 ortholog as the 2002–2003 epidemic strains of SARS-CoV, which could explain the efficient transduction efficiency mediated by their respective S glycoproteins (Figures 1A and 1B) and the current rapid SARS-CoV-2 transmission in humans.
Figure 2.
SARS-CoV-2 S Recognizes hACE2 with Comparable Affinity to SARS-CoV S
(A and B) Biolayer interferometry binding analysis of the hACE2 ectodomain to immobilized SARS-CoV-2 SB (A) or SARS-CoV SB (B). The experiments were repeated with different protein preparations and one representative set of curves is shown. Dotted lines correspond to a global fit of the data using a 1:1 binding model.
(C) Sequence alignment of SARS-CoV-2 SB and SARS-CoV SB Urbani (late phase of the 2002–2003 SARS-CoV epidemic). Identical and similar positions are respectively shown with white or red font. The single amino acid insertion at position 483 of the SARS-CoV-2 SB is indicated with a period at the corresponding SARS-CoV SB position. The 14 residues that are key for binding of SARS-CoV SB to hACE2 are labeled with a star. See also Data S1.
Table 1.
Kinetic Analysis of hACE2 Binding to SARS-CoV-2 SB and SARS-CoV SB by Biolayer Interferometry
| SARS-CoV-2 SB | SARS-CoV SB | |
|---|---|---|
| KD (nM) | 1.2 ± 0.1 | 5.0 ± 0.1 |
| kon (M−1.s−1) | 1.4 × 105 (2.3 ± 1.4 × 105) | 1.4 × 105 (1.7 ± 0.7 × 105) |
| koff (s−1) | 1.6 × 10−4 (1.7 ± 0.8 × 10−4) | 7.1x 10−4 (8.7 ± 5.1 × 10−4) |
Values reported represent the global fit to the data shown in Figures 2A and 2B and the averages obtained from five (SARS-CoV-2) or four (SARS-CoV) replicates carried out with different protein preparations are shown in parentheses.
Architecture of the SARS-CoV-2 S Glycoprotein Trimer
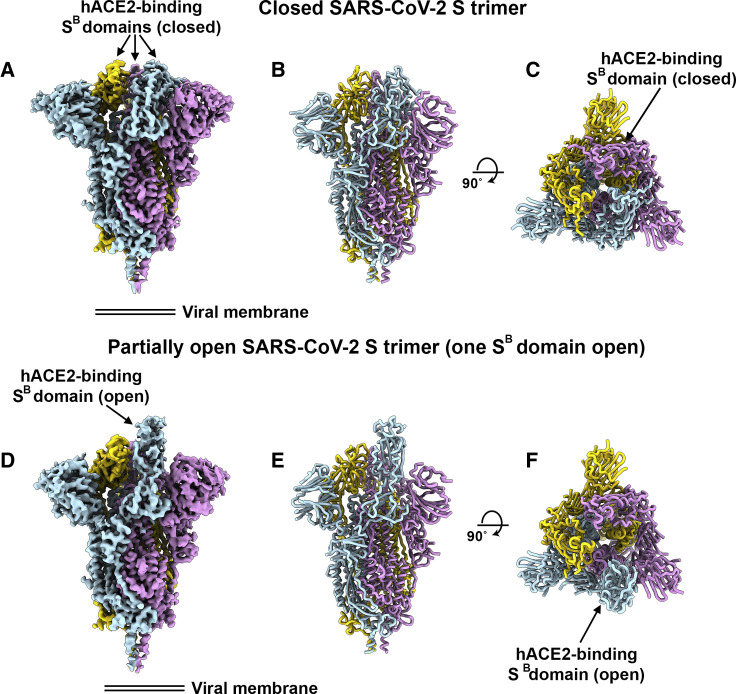
To enable single-particle cryo-EM study of the SARS-CoV-2 S glycoprotein, we designed a prefusion stabilized ectodomain trimer construct with an abrogated furin S1/S2 cleavage site (Tortorici et al., 2019, Walls et al., 2017a, Walls et al., 2016a, Walls et al., 2019), two consecutive proline stabilizing mutations (Kirchdoerfer et al., 2018, Pallesen et al., 2017), and a C-terminal foldon trimerization domain (Miroshnikov et al., 1998). Three-dimensional (3D) classification of the cryo-EM data revealed the presence of multiple conformational states of SARS-CoV-2 S corresponding to distinct organization of the SB domains within the S1 apex. Approximately half of the particle images selected correspond to trimers harboring a single SB domain opened whereas the remaining half was accounted for by closed trimers with the three SB domains closed. A recently determined SARS-CoV-2 S structure also detected trimers with a single SB domain opened but none entirely closed (Wrapp et al., 2020). The observed conformational variability of SB domains is reminiscent of observations made with SARS-CoV S and MERS-CoV S trimers, although we did not detect trimers with two SB domains open and the distribution of particles across the S conformational landscape varies among studies (Gui et al., 2017, Kirchdoerfer et al., 2018, Pallesen et al., 2017, Song et al., 2018, Walls et al., 2019, Yuan et al., 2017).
We determined a reconstruction of the closed SARS-CoV-2 S ectodomain trimer at 2.8 Å resolution (applying 3-fold symmetry) and an asymmetric reconstruction of the trimer with a single SB domain opened at 3.2 Å resolution (Figures 3 A–3H and S1 ; Table S1). The S2 fusion machinery is the best resolved part of the map, whereas the SA and SB domains are less well resolved, presumably because of conformational heterogeneity. The atomic model comprises residues 27-1147, with internal breaks corresponding to flexible regions (including part of the RBM), and lacks the C-terminal most segment (including the heptad repeat 2) that is not visible in the map, as is the case for all S structures determined to date. Overall, the SARS-CoV-2 S ectodomain is a 160-Å-long trimer with a triangular cross-section, resembling the closely related SARS-CoV S structure (Figures S2 A–S2F) (Gui et al., 2017, Kirchdoerfer et al., 2018, Song et al., 2018, Walls et al., 2019, Yuan et al., 2017).
Figure 3.
Cryo-EM Structures of the SARS-CoV-2 S Glycoprotein
(A) Closed SARS-CoV-2 S trimer unsharpened cryo-EM map.
(B and C) Two orthogonal views from the side (B) and top (C) of the atomic model of the closed SARS-CoV-2 S trimer.
(D) Partially open SARS-CoV-2 S trimer unsharpened cryo-EM map (one SB domain is open).
(E-F) Two orthogonal views from the side (E) and top (F) of the atomic model of the closed SARS-CoV-2 S trimer. The glycans were omitted for clarity. See also Figures S1 and S2.
Figure S1.
Cryo-EM Data Processing and Validation, Related to Figures 3, 4, and 5
A-B. Representative electron micrograph (A) and class averages (B) of SARS-CoV-2 S embedded in vitreous ice. Scale bar: 100nm. C. Gold-standard Fourier shell correlation curves for the closed (blue) and partially open trimers (red). The 0.143 cutoff is indicated by horizontal dashed lines. D-E. Local resolution map calculated using cryoSPARC for the closed (D) and partially open (E) reconstructions.
Figure S2.
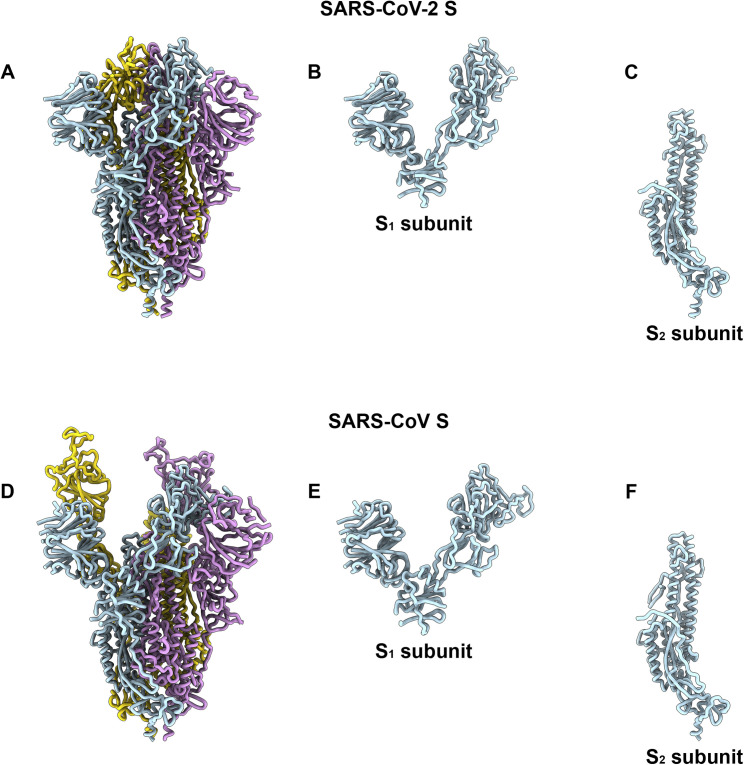
Comparison of the SARS-CoV-2 and SARS-CoV S Structures, Related to Figures 3, 4, and 5
Ribbon diagrams of the SARS-CoV-2 S (A) and SARS-CoV S (PDB 6NB6, D) ectodomain cryoEM structures. The SARS-CoV-2 (B) and SARS-CoV (E) S1 subunits. The SARS-CoV-2 (C) and SARS-CoV (F) S2 subunits.
As is the case for other β-coronavirus S glycoproteins, including SARS-CoV S (Gui et al., 2017, Kirchdoerfer et al., 2018, Pallesen et al., 2017, Park et al., 2019, Song et al., 2018, Tortorici et al., 2019, Walls et al., 2016a, Walls et al., 2016b, Walls et al., 2019, Yuan et al., 2017), the S1 subunit has a V-shaped architecture (Figures S2B–S2E). In the closed S trimer, the three hACE2-recognition motifs, whose location was inferred based on the crystal structure of SARS-CoV SB in complex with hACE2 (Li et al., 2005a), are buried at the interface between protomers. As a result, SARS-CoV-2 SB opening is expected to be necessary for interacting with ACE2 at the host cell surface and initiating the conformational changes leading to cleavage of the S2′ site, membrane fusion and viral entry (Gui et al., 2017, Kirchdoerfer et al., 2018, Millet and Whittaker, 2014, Pallesen et al., 2017, Park et al., 2016, Song et al., 2018, Walls et al., 2019, Yuan et al., 2017).
As the SARS-CoV-2 and SARS-CoV S2 subunits share 88% sequence identity, they are structurally conserved and can be superimposed with 1.2 Å root-mean-square deviation (rmsd) over 417 aligned Cα positions (Figures S2E and S2F). Only the most N-terminal part of the fusion peptide is resolved in the map, as was the case for previously determined SARS-CoV S structures. The sequence and conformational conservation of the fusion peptide region observed across SARS-CoV-2 S and SARS-CoV S suggests that Abs targeting this functionally important motif might cross-react and neutralize the two viruses as well as related coronaviruses.
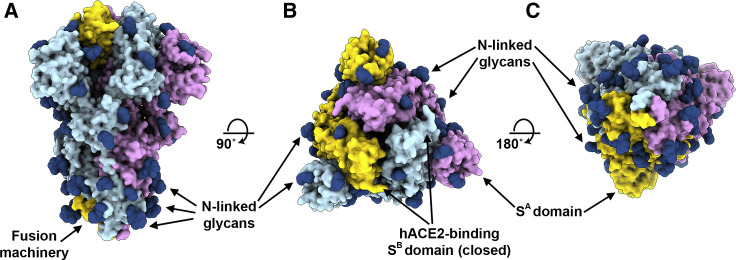
We previously showed that coronavirus S glycoproteins are densely decorated by heterogeneous N-linked glycans protruding from the trimer surface (Walls et al., 2016b, Walls et al., 2019, Xiong et al., 2018). These oligosaccharides participate in S folding (Rossen et al., 1998), affect priming by host proteases (Yang et al., 2015b), and might modulate antibody recognition (Pallesen et al., 2017, Walls et al., 2019). SARS-CoV-2 S comprise 22 N-linked glycosylation sequons per protomer and oligosaccharides are resolved in the cryo-EM map for 16 of these sites (Figure 4 ). By comparison, SARS-CoV S possesses 23 N-linked glycosylation sequons per protomer, and we previously experimentally confirmed that at least 19 of them are glycosylated (Walls et al., 2019). 20 out of 22 SARS-CoV-2 S N-linked glycosylation sequons are conserved in SARS-CoV S (Table 2 ). Specifically, 9 out of 13 glycans in the S1 subunit and all 9 glycans in the S2 subunit are conserved among SARS-CoV-2 S and SARS-CoV S. Furthermore, S2 N-linked glycosylation sequons are mostly conserved across SARSr-CoV S glycoproteins, suggesting that accessibility of the fusion machinery to Abs will be comparable among these viruses (Table 2 and Data S1).
Figure 4.
Organization of the SARS-CoV-2 S N-Linked Glycans
(A–C) Ribbon diagrams of the SARS-CoV-2 S closed structure rendered as a surface with glycans resolved in the cryo-EM map rendered as dark blue spheres. See also Table 2 and Data S1.
Table 2.
Conservation of N-Linked Glycosylation Sequons in SARS-CoV-2 S and SARS-CoV S
| SARS-CoV-2 S | SARS-CoV S |
|---|---|
| N17LT | T21FD |
| P25PA | N29YT |
| N61VT | N65VT |
| H69VS | N73HT |
| N74GT | . . . |
| S112KT | N109KS |
| N121NA | N118NS |
| N122AT | N119ST |
| N149KS | T146QT |
| N165CT | N158CT |
| N234IT | N227IT |
| N282GT | N269GT |
| N331IT | N318IT |
| N343AT | N330AT |
| N370SA | N357ST |
| N603TS | N589AS |
| N616CT | N602CT |
| N657NS | D643TS |
| N709NS | N691NT |
| N717FT | N699FS |
| N801FS | N783FS |
| N1074FT | N1056FT |
| N1098GT | N1080GT |
| N1134NT | N1116NT |
| N1158HT | N1140HT |
| N1173AS | N1155AS |
| N1194ES | N1176ES |
Italic font indicates the absence of a glycosylation sequon and deletions are indicated with periods. Glycans observed in the SARS-CoV-2 S cryo-EM map are underlined. See also Data S1.
SARS-CoV S Elicits Neutralizing Abs Against SARS-CoV-2 S
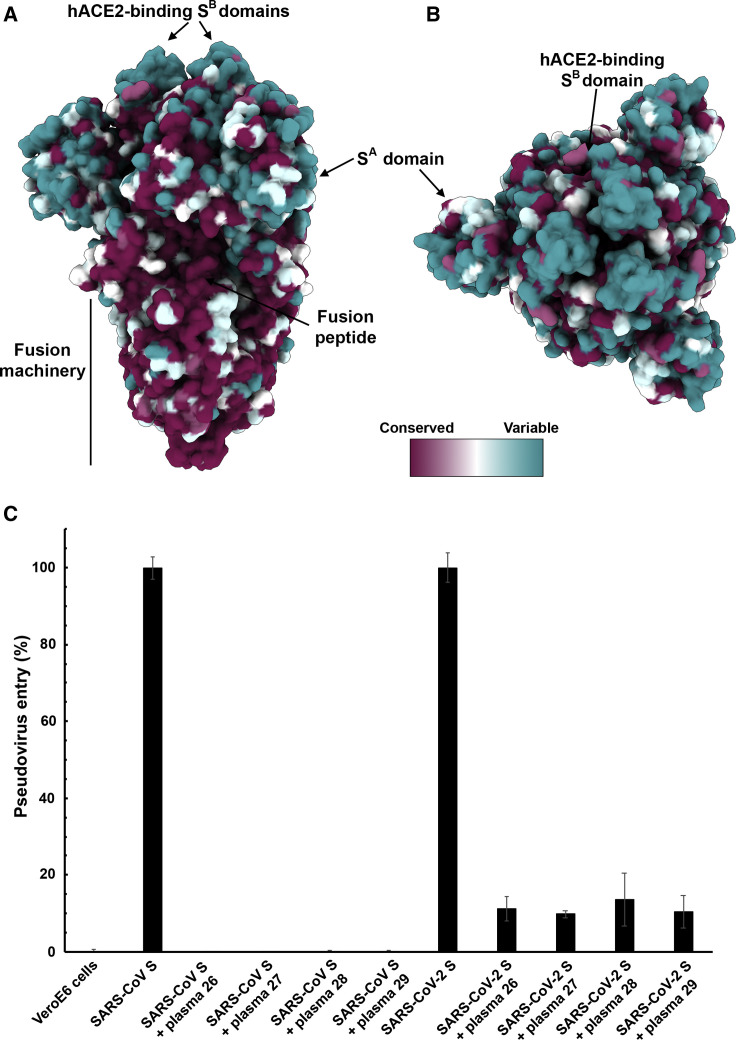
Mapping of the sequence conservation across multiple S sequences from the sarbecovirus subgenus underscores that the S2 fusion machinery is more conserved than the S1 subunit with the highest divergence found within SA and SB (Figures 5A and 5B). These observations are in line with (1) the fact that some, but not all, of these viruses use ACE2 as entry receptor (Ge et al., 2013, Ren et al., 2008, Yang et al., 2015a); and (2) that the S1 subunit is more exposed at the viral surface than the fusion machinery and is likely to be subject to a more stringent selection pressure from the immune system.
Figure 5.
SARS-CoV S Elicits Antibodies Neutralizing SARS-CoV-2 S-Mediated Entry into Host Cells
(A and B) Sequence conservation of sarbecovirus S glycoproteins plotted on the SARS-CoV-2 S structure viewed from the side (A) and top (B). The sequence alignment was generated using 48 SARS-CoV-2 S sequences obtained from GISAID in addition to the sequences listed in Data S1.
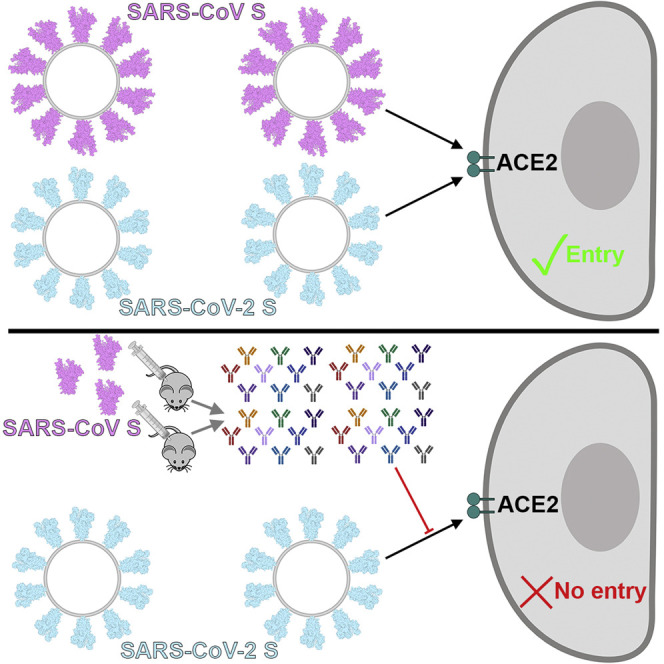
(C) Entry of SARS-CoV-2 S-MLV and SARS-CoV S-MLV is potently inhibited by four SARS-CoV S mouse polyclonal immune plasma.
Based on these observations, we hypothesized that exposure to one of the two viruses could elicit cross-reactive and potentially neutralizing Abs against the other virus. We therefore investigated the ability of plasma from four mice immunized with a stabilized SARS-CoV S to inhibit SARS-CoV-2 S- and SARS-CoV S-mediated entry into target cells. All sera tested completely inhibited transduction of SARS-CoV S-MLV and reduced SARS-CoV-2 S-MLV transduction to ∼10% of control in VeroE6 cells (Figure 5C). The elicitation of a heterotypic response blocking SARS-CoV-2 S-mediated entry into host cells concurs with the sequence and structural conservation of SARS-CoV-2 S and SARS-CoV S along with their comparable glycans shields and suggests that immunity against one virus of the sarbecovirus subgenus can potentially provide protection against related viruses.
Discussion
Receptor recognition is the first step of viral infection and is a key determinant of host cell and tissue tropism. Enhanced binding affinity between SARS-CoV S and hACE2 was proposed to correlate with increased virus transmissibility and disease severity in humans (Li et al., 2005c). Indeed, SARS-CoV isolates from the three phases of the 2002–2003 epidemic were more efficiently transmitted among humans and more pathogenic than the isolates associated with the 2003–2004 re-emergence that caused only a few cases, in line with their binding affinities for hACE2 (Consortium, 2004, Kan et al., 2005, Li et al., 2005c). Moreover, the ability to engage ACE2 from different animal species appears to reflect host susceptibility to SARS-CoV infection and facilitated the jump of the virus from animals to humans (Li, 2008, Li et al., 2004). We report here that SARS-CoV-2 uses hACE2 as an entry receptor and recognizes it with a similar affinity to the 2002–2003 SARS-CoV isolates, which suggests it can spread efficiently in humans, in agreement with the numerous SARS-CoV-2 human-to-human transmission events reported to date.
Besides binding to host cell receptors, priming of the S glycoprotein by host proteases through cleavage at the S1/S2 and the S2′ sites is another crucial factor modulating tropism and pathogenicity (Millet and Whittaker, 2015). For instance, entry of the MERS-CoV-related bat coronavirus HKU4 into human cells required addition of exogenous trypsin, indicating that S proteolytic activation of this bat virus did not occur in human cells (despite its ability to recognize human DPP4) (Wang et al., 2014, Yang et al., 2014). Subsequent work suggested that a glycan present near the S1/S2 boundary accounted for the lack of proteolytic priming of HKU4 S and that its removal enhanced pseudovirus entry in human cells (Yang et al., 2015b). The presence of a polybasic cleavage site, that can be processed by furin-like proteases, is a signature of several highly pathogenic avian influenza viruses and pathogenic Newcastle disease virus (Klenk and Garten, 1994, Steinhauer, 1999). Strikingly, SARS-CoV-2 S harbors a furin cleavage site at the S1/S2 boundary, which is processed during biosynthesis. The presence of a furin cleavage site sets SARS-CoV-2 S apart from SARS-CoV S (and SARSr-CoV S) that possesses a monobasic S1/S2 cleavage site processed upon entry of target cells (Belouzard et al., 2009, Bosch et al., 2008, Glowacka et al., 2011, Matsuyama et al., 2010, Millet and Whittaker, 2015, Shulla et al., 2011). We speculate that the almost ubiquitous expression of furin-like proteases could participate in expanding SARS-CoV-2 cell and tissue tropism, relative to SARS-CoV, as well as increasing its transmissibility and/or altering its pathogenicity.
We previously suggested that coronaviruses use conformational masking and glycan shielding to limit recognition by the immune response of infected hosts (Walls et al., 2016b, Walls et al., 2019, Xiong et al., 2018). Similarly to SARS-CoV S and MERS-CoV S (Gui et al., 2017, Kirchdoerfer et al., 2018, Pallesen et al., 2017, Song et al., 2018, Walls et al., 2019, Yuan et al., 2017), we found that the SARS-CoV-2 S trimer exists in multiple, distinct conformational states resulting from SB opening at the trimer apex. These structural changes are necessary for receptor engagement of these three viruses and lead to initiation of fusogenic conformational changes (Song et al., 2018, Walls et al., 2017b, Walls et al., 2019). In contrast, only closed S trimers have been detected for the four other human-infecting coronaviruses: HCoV-NL63 (Walls et al., 2016b), HCoV-OC43 (Tortorici et al., 2019), HCoV-HKU1 (Kirchdoerfer et al., 2016), and HCoV-229E (Li et al., 2019). As HCoV-NL63 and HCoV-229E are known to engage protein receptors through SB (Wong et al., 2017, Wu et al., 2009), trimer opening is also expected to occur to expose their RBMs that are otherwise buried at the interface between protomers in the closed S trimers (Li et al., 2019, Walls et al., 2016b). Regardless of the nature of the receptor and the location of the receptor-binding domains, removal of the trimeric S1 crown is expected to be necessary for all coronaviruses to allow the large-scale S2 conformational changes leading to fusion of the viral and host membranes (Walls et al., 2017b). Collectively, these data underscore that S glycoprotein trimers found in highly pathogenic human coronaviruses appear to exist in partially opened states, while they remain largely closed in human coronaviruses associated with common colds. Based on the aforementioned data correlating the binding affinity of SARS-CoV for hACE2 with the rate of transmissibility, viral replication in distinct species, and disease severity (Guan et al., 2003, Li et al., 2004, Li et al., 2005c, Wan et al., 2020), we hypothesize that the most pathogenic coronaviruses will exhibit S glycoprotein trimers spontaneously sampling closed and open conformations, as is the case for SARS-CoV-2, SARS-CoV and MERS-CoV.
The striking structural similarity and sequence conservation among the SARS-CoV-2 S and SARS-CoV S glycoproteins emphasize the close relationship between these two viruses that recognize hACE2 to enter target cells. This resemblance is further strengthened by our finding that SARS-CoV S elicited polyclonal Ab responses, potently neutralizing SARS-CoV-2 S-mediated entry into cells. We surmise most of these Abs target the highly conserved S2 subunit (including the fusion peptide region) based on its structural similarity across SARS-CoV-2 and SARS-CoV, the lack of cross-reactivity of several SB-directed Abs (Tian et al., 2020, Wrapp et al., 2020), and previous reports showing that sera from SARS-CoV-infected individuals target this region (Zhang et al., 2004). We note that most SARS-CoV neutralizing Abs isolated to date target the SB domain and that several of them recognize the RBM and prevent receptor engagement (Hwang et al., 2006, Rockx et al., 2008, Rockx et al., 2010, Traggiai et al., 2004, Walls et al., 2019). As the SARS-CoV-2 and SARS-CoV SB domains share 75% amino acid sequence identity, future work will be necessary to evaluate whether any of these Abs neutralize the newly emerged coronavirus. These findings also indicate that it might be difficult to distinguish exposure to SARS-CoV-2 from other SARSr-CoVs in serological studies using S ectodomain trimers and that specific assays will need to be designed. Our results provide a structural framework to identify conserved and accessible epitopes across S glycoproteins that will support ongoing vaccine design efforts. Finally, elicitation of diverse, polyclonal Ab responses might prove key in light of the diversity of viruses circulating in animal reservoirs and in preventing the possible emergence of viral neutralization escape mutants.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| AlexaFluor680-conjugated AffiniPure Goat Anti Human IgG | JacksonImmuno | Cat# 109-625-098 Lot# 139337 |
| Biological Samples | ||
| 57BL/6J Mice | Jackson Laboratory | Cat# 000664 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Thrombin | EMD Millipore | Ref# 605195 Lot# 2946006 |
| One-GloEX | Promega | Cat# E8110 |
| 293-Free Transfection Reagent | Millipore Sigma | Cat# 72181 |
| Lipofectamine-2000 Transfection Reagent | ThermoFisher | Cat# 11668500 |
| Adjuvant | Sigma-Aldrich | Cat# S6322 |
| Deposited Data | ||
| SARS-CoV-2 S CryoEM maps | This Paper | EMD: 21452, EMD: 21457 |
| SARS-CoV-2 S atomic models | This Paper | PDB: 6VXX, PDB: 6VYB |
| Experimental Models: Cell Lines | ||
| Freestyle HEK293 | ThermoFisher | Cat# R79007 |
| HEK293T/17 adherent | Millet and Whittaker, 2016 | ATCC CRL-11268 |
| Vero(C1008)E6 adherent | ECACC General Collection | Cat# 85020206 |
| Baby Hamster Kidney (BHK) | ATCC | Cat# CCL-10 Lot# 70009857 |
| Recombinant DNA | ||
| pCMV-SARS-CoV-2 S 2P furin cleavage mutation | Genscript | N/A |
| pcD5 - ACE2 | Raj et al., 2013 | N/A |
| pcDNA3.1- SARS-CoV-2 SB | Genscript | N/A |
| pcDNA3.1- SARS-CoV SB | This paper | N/A |
| pcDNA3.1(-) –Full-length wild-type SARS-CoV-2 S | Genscript | N/A |
| pcDNA3.1(-) –Full-length wild-type SARS-CoV S | Millet and Whittaker, 2016 | N/A |
| pCMV-murine leukemia virus gag-pol | Millet and Whittaker, 2016 | N/A |
| pTG-Luciferase | Millet and Whittaker, 2016 | N/A |
| Software and Algorithms | ||
| UCSF Chimera | Goddard et al., 2007 | https://www.rbvi.ucsf.edu/chimera/ |
| UCSF ChimeraX | Goddard et al., 2018 | https://www.rbvi.ucsf.edu/chimerax/ |
| Coot | Emsley et al., 2010 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| Relion | Scheres and Chen, 2012, Zivanov et al., 2019 | https://www2.mrc-lmb.cam.ac.uk/relion/index.php?title=Main_Page |
| Rosetta | Frenz et al., 2019, Wang et al., 2016 | https://www.rosettacommons.org/software |
| Leginon | Suloway et al., 2005 | http://emg.nysbc.org/redmine/projects/leginon/wiki/Leginon_Homepage |
| WARP | Tegunov and Cramer, 2019 | https://github.com/cramerlab/warp |
| CryoSPARC | Punjani et al., 2017 | https://cryosparc.com |
| MolProbity | Chen et al., 2010 | http://molprobity.biochem.duke.edu/ |
| Other | ||
| Octet Red | ForteBio, Pall LLC | https://www.fortebio.com/products/label-free-bli-detection/8-channel-octet-systems |
| Anti-Penta-HIS (HIS1K) | ForteBio, Pall LLC | Part# 18-5120 Lot# 1910224A |
| HisTALON Superflow cartridge | Takara | Cat# 635683 Lot# 1509573A |
| HiTrap Protein A HP | GE Life Sciences | Cat# 29-0485-76 |
| Superose 6 Increase 10/300 | GE Life Sciences | Cat# 29-0915-96 Lot# 10226998 |
| Lacey Formvar/Carbon 400 mesh, Cu Grids | Ted Pella | Prod# 01885-F Lot# 020218 |
Lead Contact and Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact David Veesler (dveesler@uw.edu).
The unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
The number of replicates carried out for each experiment is described in the figure/table legends.
Experimental Model and Subject Details
Cell lines
HEK293F is a female human embryonic kidney cell line transformed and adapted to grow in suspension (Life Technologies). HEK293F cells were grown in 293FreeStyle expression medium (Life Technologies), cultured at 37°C with 8% CO2 and 130 rpm. HEK293T/17 is a female human embryonic kidney cell line (ATCC). VeroE6 is a female kidney epithelial cell from African green monkey. BHK is a kidney cell line from baby hamsters. All adherent cells were cultured at 37°C with 8% CO2 in flasks with DMEM + 10% FBS + 1% penicillin-streptomycin. Cells lines were not tested for mycoplasma contamination nor authenticated.
Method Details
Transient expression of SARS-CoV-2 and SARS-CoV SB
The SARS-CoV SB construct was cloned from a SARS-CoV S ectodomain (Walls et al., 2019) synthesized by GeneArt (ThermoFisher Scientific) into a modified pOPING vector with an N-terminal mu-phosphatase signal peptide and a C-terminal hexa-histidine tag (G-HHHHHH). The boundaries of the construct are N-terminal 306RVVPSG311 and C-terminal 571LDISP5975. The SARS-CoV-2 SB construct was synthesized by GenScript into pcDNA3.1- with an N-terminal mu-phosphatase signal peptide and a C-terminal hexa-histidine tag (G-HHHHHH). The boundaries of the construct are N-terminal 328RFPN331 and C-terminal 530STNL533. Both constructs were produced in 500mL HEK293F cells grown in suspension using FreeStyle 293 expression medium (Life technologies) at 37°C in a humidified 8% CO2 incubator rotating at 130 rpm. The cultures were transfected using 293-Free transfection reagent (Millipore) with cells grown to a density of 1 million cells per mL and cultivated for 3–4 days. The supernatants were harvested and cells resuspended for another 3–4 days, yielding two harvests. Proteins were purified from clarified supernatants using a 5mL Cobalt affinity column (Takara), concentrated and flash frozen in a buffer containing 20 mM Tris pH 8.0 and 300 mM NaCl prior to analysis. SDS-PAGE was run to check purity.
Transient expression of hACE2
Expression and purification of human angiotensin-converting enzyme ectodomain (ACE2, residues 1–614) fused to the Fc region of human IgG (hFc) was performed as previously described (Walls et al., 2019) and cleavage of the Fc fragment was carried out with thrombin. Briefly, hACE2 was produced in 500mL HEK293F cells grown in suspension using FreeStyle 293 expression medium (Life technologies) at 37°C in a humidified 8% CO2 incubator rotating at 130 rpm. The cultures were transfected using 293-Free transfection reagent (Millipore) with cells grown to a density of 1 million cells per mL and cultivated for 4 days. The supernatants were harvested and cells resuspended for another four days, yielding two harvests. Clarified supernatants were concentrated using Vivaflow tangential filtration cassettes (Sartorius, 10-kDa cut-off) before affinity purification using a Protein A column (GE LifeSciences) followed by gel filtration chromatography using a Superdex 200 10/300 GL column (GE Life Sciences) equilibrated in 20 mM Tris-HCl, pH 8.0, 100 mM NaCl. The Fc tag was removed by thrombin cleavage in a reaction mixture containing 3 mg of recombinant ACE2-FC ectodomain and 10 μg of thrombin in 20 mMTris-HCl pH8.0, 150 mM NaCl and 2.5 mM CaCl2.The reaction mixture was incubated at 25°C overnight and re-loaded in a Protein A column to remove uncleaved protein and the Fc tag. The cleaved protein was further purified by gel filtration using a Superdex 200 column 10/300 GL (GE Life Sciences) equilibrated in a buffer containing 20 mM Tris pH 8.0 and 100 mM NaCl. The purified protein was quantified using absorption at 280 nm and concentrated to approximately 1 mg/mL·
Pseudovirus production
MLV-based SARS-CoV S, SARS-CoV-2 S, and SARS-CoV-2 Sfur/mut pseudotypes were prepared as previously described (Millet and Whittaker, 2016). HEK293T cells were co-transfected using Lipofectamine 2000 (Life Technologies) with an S encoding-plasmid, an MLV Gag-Pol packaging construct and the MLV transfer vector encoding a luciferase reporter, according to the manufacturer’s instructions. Cells were incubated for 5 h at 37°C with transfection medium. Cells were then washed with DMEM two times and then DMEM containing 10% FBS was added for 60 h. The supernatants were then harvested and filtered through 0.45-mm membranes, concentrated with a 30kDa membrane for 10 min at 3,000 rpm and then frozen at −80°C.
Western Blotting
SARS-CoV S, SARS-CoV-2 S, and SARS-CoV-2 Sfur/mut pseudovirions were thawed and 4X SDS loading buffer was added prior to boiling for 10 min at 95°C. Samples were run on a 4%–20% gradient Tris-Glycine Gel (BioRad) and transferred to a PVDF membrane. An anti-S2 SARS-CoV S monoclonal primary antibody (1:150 dilution) and an Alexa Fluor 680-conjugated goat anti-human or mouse secondary antibody (1:30,000 and 1:15,000 dilution respectively, Jackson Laboratory) were used for Western-blotting. A LI-COR processor was used to develop images.
Pseudovirus entry assays
VeroE6 and BHK cells were cultured in 10% FBS, 1% PenStrep DMEM. BHK or VeroE6 cells were plated into 12 well plates at a density of 0.3 × 106 for 16 h. BHK cells were either not transfected or transfected with 0.8 μg ACE2 per well using standard lipofectamine2000 (Life Technologies) protocols and incubated for another 16 h. 20uL of concentrated pseudovirus was added to the wells after washing three times with DMEM. After 2-3 h, 20% FBS and 2% PenStrep containing DMEM was added to the cells for 48 h. Following the 48-h infection, One-Glo-EX (Promega) was added to the cells in equivalent culturing volume and incubated in the dark for 10 min prior to reading on a Varioskan LUX plate reader (ThermoFisher). For entry inhibition assays, 1:100 diluted plasma was incubated with an equal amount of pseudovirus for 30 min at room temperature prior to infection with normalized amounts of pseudovirus added to the cells. Measurements were done in triplicate and relative luciferase units (RLU) were plotted.
Biolayer interferometry
Assays were performed on an Octet Red (ForteBio) instrument at 30°C with shaking at 1,000 RPM. Anti-penta His biosensors were hydrated in water for 30 min prior to a 1 min incubation in 10X kinetics buffer (undiluted). Either SARS-CoV-2 SB or SARS-CoV SB were loaded at 10-20 μg/mL in 10X Kinetics Buffer for 200-900 s prior to baseline equilibration for 180 s in 10X kinetics buffer. Association of ACE2 in 10X kinetics buffer at various concentrations in a two-fold dilution series from 20nM to 2.5nM was carried out for 1,000 s prior to dissociation for 1,000 s. The data were baseline subtracted prior to fitting performed using a 1:1 binding model and the ForteBio data analysis software. Mean kon, koff values were determined with a global fit applied to all data. The experiments were done with two separate purification batches of SARS-CoV SB, SARS-CoV-2-SB, and ACE2 each in duplicate and the average is reported.
Protein expression and purification
The SARS-CoV-2 2P S (GenBank: YP_009724390.1) ectodomain was produced in 500mL HEK293F cells grown in suspension using FreeStyle 293 expression medium (Life technologies) at 37°C in a humidified 8% CO2 incubator rotating at 130 rpm The culture was transfected using 293fectin (ThermoFisher Scientific) with cells grown to a density of 1 million cells per mL and cultivated for three days. The supernatant was harvested and cells resuspended for another three days, yielding two harvests. Clarified supernatants were purified using a 5mL Cobalt affinity column (Takara). Purified protein was filtered or concentrated and flash frozen in a buffer containing 20 mM Tris pH 8.0 and 150 mM NaCl prior to cryoEM analysis.
CryoEM sample preparation and data collection
3 μL of 0.16 mg/mL SARS-CoV-2 S was loaded onto a freshly glow discharged (30 s at 20 mA) lacey carbon grid with a thin layer of evaporated continuous carbon prior to plunge freezing using a vitrobot MarkIV (ThermoFisher Scientific) using a blot force of −1 and 2.5 s blot time at 100% humidity and 25°C. Data were acquired using the Leginon software (Suloway et al., 2005) to control an FEI Titan Krios transmission electron microscope operated at 300 kV and equipped with a Gatan K2 Summit direct detector and Gatan Quantum GIF energy filter, operated in zero-loss mode with a slit width of 20 eV. Automated data collection was carried out using Leginon at a nominal magnification of 130,000x with a pixel size of 0.525 Å. The dose rate was adjusted to 8 counts/pixel/s, and each movie was acquired in super-resolution mode fractionated in 50 frames of 200 ms. 4,500 micrographs were collected in a single session with a defocus range comprised between 1.0 and 2.5 μm.
Cryo-EM data processing
Movie frame alignment, estimation of the microscope contrast-transfer function parameters, particle picking and extraction were carried out using Warp (Tegunov and Cramer, 2019). Particle images were extracted with a box size of 800 binned to 400 yielding a pixel size of 1.05 Å. For each dataset two rounds of reference-free 2D classification were performed using cryoSPARC (Punjani et al., 2017) to select well-defined particle images. Subsequently, two rounds of 3D classification with 50 iterations each (angular sampling 7.5° for 25 iterations and 1.8° with local search for 25 iterations) were carried out using Relion (Zivanov et al., 2018) without imposing symmetry to separate distinct SARS-CoV-2 S conformations. 3D refinements were carried out using non-uniform refinement along with per-particle defocus refinement in cryoSPARC (Punjani et al., 2017) using an ab initio model generated using cryoSPARC. Particle images were subjected to Bayesian polishing (Zivanov et al., 2019) before performing another round of non-uniform refinement in cryoSPARC (Punjani et al., 2017) followed by per-particle defocus refinement and again non-uniform refinement. Local resolution estimation, filtering and sharpening was carried out using CryoSPARC. Reported resolutions are based on the gold-standard Fourier shell correlation (FSC) of 0.143 criterion and Fourier shell correlation curves were corrected for the effects of soft masking by high-resolution noise substitution (Scheres and Chen, 2012).
CryoEM model building and analysis
UCSF Chimera (Goddard et al., 2007) and Coot were used to fit atomic models (PDB 6NB6) into the cryoEM map. The model was subsequently manually rebuilt using Coot and the C3-symmetrized map (Brown et al., 2015, Emsley et al., 2010). This model was then used to build features specific of the C1-map. N-linked glycans were hand-built into the density where visible and the models were refined and relaxed using Rosetta (Wang et al., 2016). Glycan refinement relied on a dedicated Rosetta protocol, which uses physically realistic geometries based on prior knowledge of saccharide chemical properties (Frenz et al., 2019), and was aided by using both sharpened and unsharpened maps. Models were analyzed using MolProbity (Chen et al., 2010), EMringer (Barad et al., 2015), Phenix (Liebschner et al., 2019) and privateer (Agirre et al., 2015) to validate the stereochemistry of both the protein and glycan components (Table S1). Figures were generated using UCSF ChimeraX (Goddard et al., 2018) and consurf (Ashkenazy et al., 2016).
Immunizations with SARS-CoV S
57BL/6J mice were obtained from the Jackson Laboratory and maintained at the Comparative Medicine Facility at the Fred Hutchinson Cancer Research Center. After collecting a pre-bleed, mice were immunized with 5μg of SARS-CoV 2P S ectodomain trimer (this construct was previously described (Walls et al., 2019)) formulated in 50μl of PBS and 50μl of Sigma Adjuvant System subcutaneously in the base of the tail at weeks 0, 4, and 12 and bleeds were collected 2 weeks after each immunization. Plasma from immunized animals was heat inactivated at 56°C for 1 h and then stored at 4°C until use. All experiments were conducted at the Fred Hutchinson Cancer Research Center according to approved Institutional Animal Care and Use Committee protocols.
Quantification and Statistical Analysis
Western-blots and biolayer interferometry assays were performed multiple times with at least two independent preparations of each sample.
No methods were used to determine whether the data met assumptions of the statistical approach.
Data and Code Availability
The cryoEM maps and atomic models have been deposited at the Electron Microscopy Data Bank and the Protein Data Bank with accession codes EMD: 21452 and PDB: 6VXX (closed SARS-CoV-2 S), as well as EMD: 21457 and PDB: 6VYB (SARS-CoV-2 S with one SB open). A list of software used in this study can be found in the Key Resources Table.
Acknowledgments
This study was supported by the National Institute of General Medical Sciences (R01GM120553 to D.V.), the National Institute of Allergy and Infectious Diseases (HHSN272201700059C to D.V.), a Pew Biomedical Scholars Award (D.V.), an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund (D.V.), the University of Washington Arnold and Mabel Beckman cryoEM center, the Washington Research Foundation, and the Pasteur Institute (M.A.T.). We are grateful to Lynda Stuart for sharing the full-length hACE2 plasmid, to Gary Whittaker for the MLV pseudotyping system, and to Ning Zheng for providing access to the Octet device.
Author Contributions
A.C.W., Y.-J.P., A.T.M., and D.V. designed the experiments. A.C.W. and M.A.T. expressed and purified the proteins. A.C.W. carried out binding assays and pseudovirus entry assays. Y.-J.P. prepared samples for cryo-EM and collected the data. Y.-J.P. and D.V. processed the data and built and refined the atomic models. A.W. and A.T.M. immunized mice. A.C.W., Y.-J.P., and D.V. analyzed the data and prepared the manuscript with input from all authors.
Declaration of Interests
The authors declare no competing financial interests.
Published: March 9, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.cell.2020.02.058.
Supplemental Information
The following sequences were used: SARS-CoV-2 (YP_009724390.1), SARSr-CoV RaTG13 (QHR63300.2), SARS-CoV Urbani (AAP13441.1), SARS-CoV CUHK-W1 (AAP13567.1), SARS-CoV GZ02 (AAS00003.1), SARS-CoV A031 (AAV97988.1), SARS-CoV A022 (AAV91631.1), WIV-16 (ALK02457.1), WIV-1 (AGZ48828.1), SARSr-CoV ZXC21 (AVP78042.1), SARSr-CoV ZC45 (AVP78031.1), SARSr-CoV Rp3 (Q3I5J5.1), SARSr-CoV Rs672 (ACU31032.1).
References
- Agirre J., Iglesias-Fernández J., Rovira C., Davies G.J., Wilson K.S., Cowtan K.D. Privateer: software for the conformational validation of carbohydrate structures. Nat. Struct. Mol. Biol. 2015;22:833–834. doi: 10.1038/nsmb.3115. [DOI] [PubMed] [Google Scholar]
- Anthony S.J., Gilardi K., Menachery V.D., Goldstein T., Ssebide B., Mbabazi R., Navarrete-Macias I., Liang E., Wells H., Hicks A. Further Evidence for Bats as the Evolutionary Source of Middle East Respiratory Syndrome Coronavirus. MBio. 2017;8:e00373-17. doi: 10.1128/mBio.00373-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazy H., Abadi S., Martz E., Chay O., Mayrose I., Pupko T., Ben-Tal N. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44:W344-50. doi: 10.1093/nar/gkw408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad B.A., Echols N., Wang R.Y., Cheng Y., DiMaio F., Adams P.D., Fraser J.S. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods. 2015;12:943–946. doi: 10.1038/nmeth.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., Bartelink W., Rottier P.J. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 2008;82:8887–8890. doi: 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A., Long F., Nicholls R.A., Toots J., Emsley P., Murshudov G. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr. D Biol. Crystallogr. 2015;71:136–153. doi: 10.1107/S1399004714021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., Pelkmans L., Rottier P.J., Bosch B.J., de Haan C.A. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10:e1004502. doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen V.B., Arendall W.B., 3rd, Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., Murray L.W., Richardson J.S., Richardson D.C. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, C.S.M.E.; Chinese SARS Molecular Epidemiology Consortium Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- Corti D., Zhao J., Pedotti M., Simonelli L., Agnihothram S., Fett C., Fernandez-Rodriguez B., Foglierini M., Agatic G., Vanzetta F. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc. Natl. Acad. Sci. USA. 2015;112:10473–10478. doi: 10.1073/pnas.1510199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob Chall. 2017;1:33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenz B., Rämisch S., Borst A.J., Walls A.C., Adolf-Bryfogle J., Schief W.R., Veesler D., DiMaio F. Automatically fixing errors in glycoprotein structures with Rosetta. Structure. 2019;27:134–139.e3. doi: 10.1016/j.str.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Müller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard T.D., Huang C.C., Ferrin T.E. Visualizing density maps with UCSF Chimera. J. Struct. Biol. 2007;157:281–287. doi: 10.1016/j.jsb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Goddard T.D., Huang C.C., Meng E.C., Pettersen E.F., Couch G.S., Morris J.H., Ferrin T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Gui M., Song W., Zhou H., Xu J., Chen S., Xiang Y., Wang X. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 2017;27:119–129. doi: 10.1038/cr.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B.L., Al Dhahiry S.H., Reusken C.B., Raj V.S., Galiano M., Myers R., Godeke G.J., Jonges M., Farag E., Diab A. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect. Dis. 2014;14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald-Sargent T., Gallagher T. Ready, set, fuse! The coronavirus spike protein and acquisition of fusion competence. Viruses. 2012;4:557–580. doi: 10.3390/v4040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Krüger N., Müller M., Drosten C., Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 2020 doi: 10.1101/2020.01.31.929042. [DOI] [Google Scholar]
- Hu B., Zeng L.P., Yang X.L., Ge X.Y., Zhang W., Li B., Xie J.Z., Shen X.R., Zhang Y.Z., Wang N. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13:e1006698. doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Lancet; China: 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulswit R.J.G., Lang Y., Bakkers M.J.G., Li W., Li Z., Schouten A., Ophorst B., van Kuppeveld F.J.M., Boons G.J., Bosch B.J. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc. Natl. Acad. Sci. USA. 2019;116:2681–2690. doi: 10.1073/pnas.1809667116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W.C., Lin Y., Santelli E., Sui J., Jaroszewski L., Stec B., Farzan M., Marasco W.A., Liddington R.C. Structural basis of neutralization by a human anti-severe acute respiratory syndrome spike protein antibody, 80R. J. Biol. Chem. 2006;281:34610–34616. doi: 10.1074/jbc.M603275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan B., Wang M., Jing H., Xu H., Jiang X., Yan M., Liang W., Zheng H., Wan K., Liu Q. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J. Virol. 2005;79:11892–11900. doi: 10.1128/JVI.79.18.11892-11900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R.N., Wang N., Pallesen J., Wrapp D., Turner H.L., Cottrell C.A., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci. Rep. 2018;8:15701. doi: 10.1038/s41598-018-34171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H.D., Garten W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 1994;2:39–43. doi: 10.1016/0966-842x(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0688-y. Published online February 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structural analysis of major species barriers between humans and palm civets for severe acute respiratory syndrome coronavirus infections. J. Virol. 2008;82:6984–6991. doi: 10.1128/JVI.00442-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Greenough T.C., Moore M.J., Vasilieva N., Somasundaran M., Sullivan J.L., Farzan M., Choe H. Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. J. Virol. 2004;78:11429–11433. doi: 10.1128/JVI.78.20.11429-11433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.K., Huang I.C., Xu K., Vasilieva N. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Hulswit R.J.G., Widjaja I., Raj V.S., McBride R., Peng W., Widagdo W., Tortorici M.A., van Dieren B., Lang Y. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc. Natl. Acad. Sci. USA. 2017;114:E8508–E8517. doi: 10.1073/pnas.1712592114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Tomlinson A.C., Wong A.H., Zhou D., Desforges M., Talbot P.J., Benlekbir S., Rubinstein J.L., Rini J.M. The human coronavirus HCoV-229E S-protein structure and receptor binding. eLife. 2019;8:e51230. doi: 10.7554/eLife.51230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebschner D., Afonine P.V., Baker M.L., Bunkóczi G., Chen V.B., Croll T.I., Hintze B., Hung L.W., Jain S., McCoy A.J. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 2019;75:861–877. doi: 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madu I.G., Roth S.L., Belouzard S., Whittaker G.R. Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide. J. Virol. 2009;83:7411–7421. doi: 10.1128/JVI.00079-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Mishra N., Olival K.J., Fagbo S.F., Kapoor V., Epstein J.H., Alhakeem R., Durosinloun A., Al Asmari M., Islam A. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menachery V.D., Yount B.L., Jr., Debbink K., Agnihothram S., Gralinski L.E., Plante J.A., Graham R.L., Scobey T., Ge X.Y., Donaldson E.F. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 2015;21:1508–1513. doi: 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menachery V.D., Yount B.L., Jr., Sims A.C., Debbink K., Agnihothram S.S., Gralinski L.E., Graham R.L., Scobey T., Plante J.A., Royal S.R. SARS-like WIV1-CoV poised for human emergence. Proc. Natl. Acad. Sci. USA. 2016;113:3048–3053. doi: 10.1073/pnas.1517719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Murine Leukemia Virus (MLV)-based Coronavirus Spike-pseudotyped Particle Production and Infection. Bio Protoc. 2016;6:e2035. doi: 10.21769/BioProtoc.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroshnikov K.A., Marusich E.I., Cerritelli M.E., Cheng N., Hyde C.C., Steven A.C., Mesyanzhinov V.V. Engineering trimeric fibrous proteins based on bacteriophage T4 adhesins. Protein Eng. 1998;11:329–332. doi: 10.1093/protein/11.4.329. [DOI] [PubMed] [Google Scholar]
- Pallesen J., Wang N., Corbett K.S., Wrapp D., Kirchdoerfer R.N., Turner H.L., Cottrell C.A., Becker M.M., Wang L., Shi W. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E., Li K., Barlan A., Fehr A.R., Perlman S., McCray P.B., Jr., Gallagher T. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc. Natl. Acad. Sci. USA. 2016;113:12262–12267. doi: 10.1073/pnas.1608147113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.J., Walls A.C., Wang Z., Sauer M.M., Li W., Tortorici M.A., Bosch B.J., DiMaio F., Veesler D. Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat. Struct. Mol. Biol. 2019;26:1151–1157. doi: 10.1038/s41594-019-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punjani A., Rubinstein J.L., Fleet D.J., Brubaker M.A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R., Muth D., Demmers J.A., Zaki A., Fouchier R.A. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W., Qu X., Li W., Han Z., Yu M., Zhou P., Zhang S.Y., Wang L.F., Deng H., Shi Z. Difference in receptor usage between severe acute respiratory syndrome (SARS) coronavirus and SARS-like coronavirus of bat origin. J. Virol. 2008;82:1899–1907. doi: 10.1128/JVI.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Corti D., Donaldson E., Sheahan T., Stadler K., Lanzavecchia A., Baric R. Structural basis for potent cross-neutralizing human monoclonal antibody protection against lethal human and zoonotic severe acute respiratory syndrome coronavirus challenge. J. Virol. 2008;82:3220–3235. doi: 10.1128/JVI.02377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Donaldson E., Frieman M., Sheahan T., Corti D., Lanzavecchia A., Baric R.S. Escape from human monoclonal antibody neutralization affects in vitro and in vivo fitness of severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2010;201:946–955. doi: 10.1086/651022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen J.W., de Beer R., Godeke G.J., Raamsman M.J., Horzinek M.C., Vennema H., Rottier P.J. The viral spike protein is not involved in the polarized sorting of coronaviruses in epithelial cells. J. Virol. 1998;72:497–503. doi: 10.1128/jvi.72.1.497-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres S.H., Chen S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods. 2012;9:853–854. doi: 10.1038/nmeth.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Gui M., Wang X., Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14:e1007236. doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer D.A. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology. 1999;258:1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K., Moore M.J., Tallarico A.S., Olurinde M., Choe H. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. USA. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suloway C., Pulokas J., Fellmann D., Cheng A., Guerra F., Quispe J., Stagg S., Potter C.S., Carragher B. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Tegunov D., Cramer P. Real-time cryo-electron microscopy data preprocessing with Warp. Nat. Methods. 2019;16:1146–1152. doi: 10.1038/s41592-019-0580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici M.A., Walls A.C., Lang Y., Wang C., Li Z., Koerhuis D., Boons G.J., Bosch B.J., Rey F.A., de Groot R.J., Veesler D. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R., Murphy B.R., Rappuoli R., Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Spaan W., Palese P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc. Natl. Acad. Sci. USA. 1988;85:4526–4529. doi: 10.1073/pnas.85.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Tortorici M.A., Bosch B.J., Frenz B., Rottier P.J.M., DiMaio F., Rey F.A., Veesler D. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531:114–117. doi: 10.1038/nature16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Tortorici M.A., Frenz B., Snijder J., Li W., Rey F.A., DiMaio F., Bosch B.J., Veesler D. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat. Struct. Mol. Biol. 2016;23:899–905. doi: 10.1038/nsmb.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A., Tortorici M.A., Bosch B.J., Frenz B., Rottier P.J., DiMaio F., Rey F.A., Veesler D. Crucial steps in the structure determination of a coronavirus spike glycoprotein using cryo-electron microscopy. Protein Sci. 2017;26:113–121. doi: 10.1002/pro.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Tortorici M.A., Snijder J., Xiong X., Bosch B.-J., Rey F.A., Veesler D. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc. Natl. Acad. Sci. USA. 2017;114:11157–11162. doi: 10.1073/pnas.1708727114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Xiong X., Park Y.J., Tortorici M.A., Snijder J., Quispe J., Cameroni E., Gopal R., Dai M., Lanzavecchia A. Unexpected Receptor Functional Mimicry Elucidates Activation of Coronavirus Fusion. Cell. 2019;176:1026–1039. doi: 10.1016/j.cell.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS. J. Virol. 2020 doi: 10.1128/JVI.00127-20. Published online January 29, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Yan M., Xu H., Liang W., Kan B., Zheng B., Chen H., Zheng H., Xu Y., Zhang E. SARS-CoV infection in a restaurant from palm civet. Emerg. Infect. Dis. 2005;11:1860–1865. doi: 10.3201/eid1112.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Qi J., Yuan Y., Xuan Y., Han P., Wan Y., Ji W., Li Y., Wu Y., Wang J. Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26. Cell Host Microbe. 2014;16:328–337. doi: 10.1016/j.chom.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.Y., Song Y., Barad B.A., Cheng Y., Fraser J.S., DiMaio F. Automated structure refinement of macromolecular assemblies from cryo-EM maps using Rosetta. eLife. 2016;5:e17219. doi: 10.7554/eLife.17219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A.H.M., Tomlinson A.C.A., Zhou D., Satkunarajah M., Chen K., Sharon C., Desforges M., Talbot P.J., Rini J.M. Receptor-binding loops in alphacoronavirus adaptation and evolution. Nat. Commun. 2017;8:1735. doi: 10.1038/s41467-017-01706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020 doi: 10.1126/science.abb2507. Published online February 19, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Li W., Peng G., Li F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc. Natl. Acad. Sci. USA. 2009;106:19970–19974. doi: 10.1073/pnas.0908837106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Tortorici M.A., Snijder J., Yoshioka C., Walls A.C., Li W., McGuire A.T., Rey F.A., Bosch B.J., Veesler D. Glycan shield and fusion activation of a deltacoronavirus spike glycoprotein fine-tuned for enteric infections. J. Virol. 2018;92:e01628-17. doi: 10.1128/JVI.01628-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Du L., Liu C., Wang L., Ma C., Tang J., Baric R.S., Jiang S., Li F. Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc. Natl. Acad. Sci. USA. 2014;111:12516–12521. doi: 10.1073/pnas.1405889111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.L., Hu B., Wang B., Wang M.N., Zhang Q., Zhang W., Wu L.J., Ge X.Y., Zhang Y.Z., Daszak P. Isolation and Characterization of a Novel Bat Coronavirus Closely Related to the Direct Progenitor of Severe Acute Respiratory Syndrome Coronavirus. J. Virol. 2015;90:3253–3256. doi: 10.1128/JVI.02582-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Liu C., Du L., Jiang S., Shi Z., Baric R.S., Li F. Two Mutations Were Critical for Bat-to-Human Transmission of Middle East Respiratory Syndrome Coronavirus. J. Virol. 2015;89:9119–9123. doi: 10.1128/JVI.01279-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Cao D., Zhang Y., Ma J., Qi J., Wang Q., Lu G., Wu Y., Yan J., Shi Y. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat. Commun. 2017;8:15092. doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang G., Li J., Nie Y., Shi X., Lian G., Wang W., Yin X., Zhao Y., Qu X. Identification of an antigenic determinant on the S2 domain of the severe acute respiratory syndrome coronavirus spike glycoprotein capable of inducing neutralizing antibodies. J. Virol. 2004;78:6938–6945. doi: 10.1128/JVI.78.13.6938-6945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. Published online February 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivanov J., Nakane T., Forsberg B.O., Kimanius D., Hagen W.J., Lindahl E., Scheres S.H. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 2018;7:e42166. doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivanov J., Nakane T., Scheres S.H.W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ. 2019;6:5–17. doi: 10.1107/S205225251801463X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following sequences were used: SARS-CoV-2 (YP_009724390.1), SARSr-CoV RaTG13 (QHR63300.2), SARS-CoV Urbani (AAP13441.1), SARS-CoV CUHK-W1 (AAP13567.1), SARS-CoV GZ02 (AAS00003.1), SARS-CoV A031 (AAV97988.1), SARS-CoV A022 (AAV91631.1), WIV-16 (ALK02457.1), WIV-1 (AGZ48828.1), SARSr-CoV ZXC21 (AVP78042.1), SARSr-CoV ZC45 (AVP78031.1), SARSr-CoV Rp3 (Q3I5J5.1), SARSr-CoV Rs672 (ACU31032.1).
Data Availability Statement
The cryoEM maps and atomic models have been deposited at the Electron Microscopy Data Bank and the Protein Data Bank with accession codes EMD: 21452 and PDB: 6VXX (closed SARS-CoV-2 S), as well as EMD: 21457 and PDB: 6VYB (SARS-CoV-2 S with one SB open). A list of software used in this study can be found in the Key Resources Table.