Abstract
Oligonucleotide-based drugs have received considerable attention for their capacity to modulate gene expression very specifically and as a consequence they have found applications in the treatment of many human acquired or genetic diseases. Clinical translation has been often hampered by poor biodistribution, however. Cell-penetrating peptides (CPPs) appear as a possibility to increase the cellular delivery of non-permeant biomolecules such as nucleic acids. This review focuses on CPP-delivery of several classes of oligonucleotides (ONs), namely antisense oligonucleotides, splice switching oligonucleotides (SSOs) and siRNAs. Two main strategies have been used to transport ONs with CPPs: covalent conjugation (which is more appropriate for charge-neutral ON analogues) and non-covalent complexation (which has been used for siRNA delivery essentially). Chemical synthesis, mechanisms of cellular internalization and various applications will be reviewed. A comprehensive coverage of the enormous amount of published data was not possible. Instead, emphasis has been put on strategies that have proven to be effective in animal models of important human diseases and on examples taken from the authors' own expertise.
Keywords: Cell penetrating peptides, Delivery, Antisense oligonucleotides, Splice switching oligonucleotides, siRNAs
Graphical abstract
1. Introduction
In recent years there has been a dramatic re-evaluation of how genes are regulated and of gene expression modalities. Two major sets of discoveries centre on the key roles of non-coding RNAs, and in particular those involved in the RNA interference (RNAi) pathway, as well as the mosaic structure of eukaryotic genes.
The antisense ON strategy was proposed more than two decades ago for the artificial regulation of eukaryotic gene expression in cultured cells via the hybridization of short ONs to mRNA targets through the pioneering work of Stephenson and Zamecnik [1], [2]. The immense potential of this strategy, which in principle only requires knowledge of the target mRNA sequence, was quickly realized within both academic and industrial laboratories. Interestingly, “Mother Nature” has also exploited this potential, as convincingly demonstrated in bacteria [3] and later on in eukaryotes [4] by their ability to capitalize on such potent biochemical and genetic tools. The best demonstration that the antisense concept is exploited came from the discovery that antisense genes are able to control transcription in bacteria (and with less experimental evidence in eukaryotic cells) and finely tune the expression of target genes. However, these natural antisense RNAs turned out to be long and highly structured, and attempts to use this knowledge in the design of synthetic antisense genes proved disappointing. Most studies so far in the antisense field have instead focussed on short single-stranded DNA mimics, the hybridization of which allows recruitment of the cellular enzyme RNase H and as a consequence leads to the destruction of the RNA target [5].
Somewhat unexpectedly, eukaryotic coding genes are transcribed as immature mRNA precursors, the splicing of which by the complex nuclear machinery leads to intron removal. It became rapidly realized that a major outcome is the possibility of exon re-assortment and that this is the case for the majority of human genes. Importantly in terms of potential clinical translation, several human diseases are associated with dysfunction of the splicing machinery (as in β-thalassemia) or with the preferential use of one splicing event rather than another. Intervention with the process of exon selection using low molecular weight drugs has turned out to be difficult and the most promising strategy was proposed instead by Ryszard Kole and colleagues as detailed in Section 2 of this issue. In contrast to the “classical” antisense ON strategy, splice switching oligonucleotides (SSOs) are designed to prevent (or promote) the insertion of exons through high affinity binding at obligatory splicing sequences in the nuclear pre-mRNA (for example donor or acceptor splice sites) and therefore RNase H-incompetent ON analogues must be used [6].
The discovery of RNA interference by Fire et al. has also revolutionized our concepts of gene expression regulation [7]. The current detailed knowledge of RNAi processing and recognition by the RNA-induced silencing complex (RISC) has led to the possibility of rational design and synthesis of artificial siRNAs able to recognize any mRNA on the sole basis of their sequences. As for RNase H-competent antisense ON, sequence-specific recognition of the RNA target leads to its degradation by the RISC-associated nuclease. Further, several hundred human genes have now been identified to code for short stem-loop structures, known as micro RNAs (miRNAs), which are processed to allow targeting of the 3′-UTRs of mRNAs and many such mRNA targets have been identified [8], [9], [10]. Astonishingly, miRNAs do not need to hybridize over their entire sequence to promote the down-regulation of an mRNA target [11]. As a consequence, a single miRNA is able to regulate the expression of a complex set of mRNAs, which are often related in terms of cellular function. Although still incomplete, ongoing studies show that specific sets of miRNAs control most cellular functions and that dysregulation of their expression is associated with many human diseases. Regulation of the levels of certain miRNAs or interference with their binding to mRNA targets are both now heavily explored strategies.
Synthetic antisense ONs, SSOs, siRNAs and miRNAs have become routine and invaluable tools to dissect cellular functions and they can be efficiently transfected into most laboratory cell lines using commercially available reagents such as cationic lipid formulations. However, their systemic in vivo administration has been plagued by toxicity and by low efficiency in the presence of serum proteins. Therapeutic developments centred on the use of naked ONs have thus met with only limited successes. Indeed, more than two decades of therapeutic developments have only led to three FDA approved drugs, with two used for easier-to-manage topical ocular applications (Fomivirsen as an antisense treatment for ocular cytomegalovirus infections in immunocompromised patients [12] and Ranibizumab, an aptamer for the treatment of macular degeneration [13]). Of more promise is the recent approval in the US (but not yet in Europe) of Mipomersen [14], an antisense ON for the treatment of homozygous familial hypercholesterolemia, which capitalizes on the accumulation of phosphorothioate (PS) ONs in the liver after systemic administration. Encouraging small scale clinical data have also been reported in the treatment of Duchenne muscular dystrophy with 2′-O-methyl phosphorothioate (2′-OMePS) ONs (Drisapersen [15], [16], [17]) and phosphorodiamidate morpholino oligonucleotides (PMO, Eteplirsen [18], [19], [20]), as will be extensively discussed later in Section 4 of this chapter.
Despite these successes, it is generally agreed that degradation in biological fluids, passage across cellular barriers and intracellular trafficking are all limiting factors in nucleic acids-based therapies [21]. Extensive searches for ON chemical modifications have improved their metabolic stabilities significantly as well as their affinities for RNA targets, and have to some extent reduced off-target effects. No ON chemical modification has significantly improved cellular uptake or tissue targeting, however.
The design of efficient and non-toxic delivery vectors for ON-based drugs has therefore become a major concern in both academic and industrial laboratories. Among the many proposed tools for delivery, cell penetrating peptides (CPPs) have appeared as an easy to implement strategy and have led to encouraging data at least in murine models of human diseases, as will be reviewed later in this chapter.
This review does not pretend to be exhaustive and will be restricted (1) to the CPP delivery of nucleic acids-based drugs and mainly to SSOs and siRNAs and (2) to delivery strategies which have turned efficient in animal models of human diseases.
2. Historical background of CPPs as delivery vectors for nucleic acids and their limitations
The harnessing of cationic peptides to deliver drugs across biological membranes was first attempted by Ryser and his colleagues [22]. They demonstrated that an anticancer drug could be delivered as a poly-l-lysine (PLL) conjugate in drug resistant cells in vitro and in vivo in mouse models [23]. Likewise, the chemical conjugation of antisense ONs to PLL led to the generation of a potent antiviral activity in several in vitro models of viral infections [24]. Unfortunately, conjugates were poorly characterized in view of the polydispersed character of commercial PLL preparations and, more importantly, they led to acute cytotoxicity upon systemic administration in mice.
It was discovered serendipitously by virologists that much shorter stretches of cationic peptides could promote the cellular uptake of macromolecules. For example, it was found that the full size purified HIV-1 Tat protein trans-activates the HIV-1 LTR promoter when incubated with cells [25]. More astonishingly, this same Tat protein was able as a conjugate to promote the cellular internalization of the non-permeable protein β-galactosidase and this paved the way for the use of Tat as a delivery vector for biomolecules. Along the same lines, the purified Antennapedia protein from drosophila was able to exert its transcriptional activity when incubated with nerve cells and this property was ascribed to a short peptide named Penetratin [26]. Dissection of the Tat protein did also show that cell penetration was due to a small, basic amino acids-rich peptide known as the Tat peptide [27]. Initial studies of the cellular trafficking of both Penetratin and Tat suggested an unusual non-receptor dependent mechanism of direct translocation across the plasma membrane. Although challenged later on as detailed in Section 7, this mechanism and the possibility to use such so called cell-penetrating peptides (CPPs) as non-viral delivery vectors for biomolecules, fostered a very large interest. Many CPPs were rapidly discovered and proposed as vectors for the transport of various drugs across biological membranes, starting from low molecular mass drugs to very large molecular entities such as nanoparticles.
Most CPPs were designed for the transport of a chemically conjugated cargo (see Table 1 ). Since the chemical conjugation and purification of negatively charged ONs with the most popular cationic CPPs has turned out to be difficult, most applications have concerned charge-neutral ON analogues such as Peptide Nucleic Acids (PNAs) and PMO (see Section 3). The most advanced studies have involved the use of these conjugates for RNA splicing regulation.
Table 1.
Selection of oligonucleotides and peptide–oligonucleotide conjugates used in vivo.
| Peptide | ON/ON analogue | Strategy | Reference |
|---|---|---|---|
| w/o | LNA | – | [28] |
| w/o | LNA | – | [11] |
| F-3 | 2′-OMe/DNA/PS gapmer | CL | [29] |
| Pip2a | PNA | CL | [30] |
| B-peptide | PMO | CL | [31] |
| B-MSP | PMO | CL | [32] |
| Pip5e | PMO | CL | [33] |
| PKKKRKV | PNA | CL | [34] |
| Penetratin | PNA | CL | [35] |
| Lys4 | PNA | CL | [36], [37] |
| SPACE | siRNA | CL | [38] |
| Tat-DRBD | siRNA | CL | [39] |
| (RXR)4 | PMO | CL | [40], [41], [42], [43], [44] |
| (RFF)3RXB | PMO | CL | [45] |
| (KFF)3K | PNA | CL | [46] |
| R9F2 | PMO | CL | [47] |
| T-cell-derived CPP | PMO | CL | [48] |
| Pep-3 | PNA | CF | [49] |
| PEGPep-3 | PNA | CF | [49] |
| MPG-8 | siRNA | CF | [50] |
| MPG-8-Chol | siRNA | CF | [50] |
| PepFect6 | siRNA | CF | [51] |
| P5RHH | siRNA | CF | [52] |
| R15 | siRNA | CF | [53] |
| Chol-R9 | siRNA | CF | [54] |
Footnotes: w/o, without CPP; LNA, locked nucleic acid; ON, oligonucleotide; PMO, phosphorodiamidate morpholino oligonucleotide; PNA, peptide nucleic acid; CL, covalently linked; CF, complex formation; Pip, PNA internalization peptide; DRBD, Domain-dsRNA Binding Domain; Chol, cholesterol.
Early work described the use of Penetratin for the delivery of conjugated PS ONs [55] and of Transportan (another popular CPP) for PNA transport [56]. Unexpectedly, in a well-characterized HeLa 705 cell assay with a positive read-out (Fig. 1 ), splicing redirection using PNA or PMO oligomers conjugated to various standard CPPs (Tat, Penetratin or oligo-arginines) was not achieved in our research groups [57].
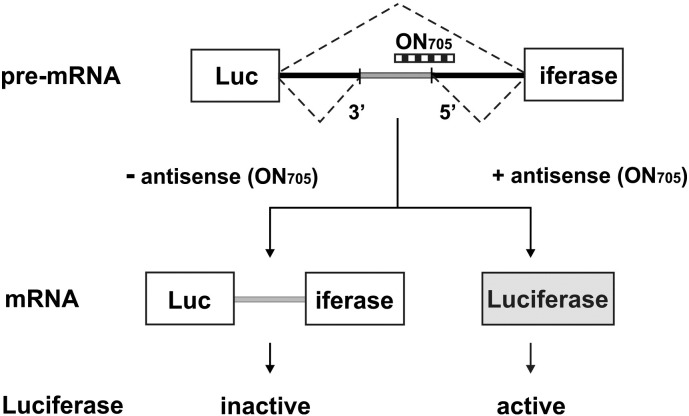
Fig. 1.
Outline of the HeLa splicing redirection assay. Note that antisense ON (705) needs a delivery method for HeLa cell entry.
HeLa pLuc 705 cells were stably transfected with a construction in which the coding sequence of the luciferase gene is interrupted by a mutated intron 2 of the human β-globin gene. This mutation creates a 5′splice site and activates a 3′splice site. Masking of the 5′splice site by a RNase H-incompetent antisense ON (705) restores the production of functional luciferase mRNA and protein. This assay was provided by Kole and colleagues [58] and allows a reliable and easy to implement comparative evaluation of ON analogues and ON-delivery vectors. Intronic point mutations in a β-thalassemia globin gene activate cryptic splice sites leading to the aberrant splicing of this intron and as a consequence to a non-functional protein. Masking of the mutated site with a steric-block ON re-orients the splicing machinery toward complete removal of the intron and leads to the production of a correctly spliced mRNA. This mutated intron has been introduced into the coding region of a reporter luciferase gene and the construction has been stably transfected in HeLa cells, which are available from ATCC (ATCC® CCL-2™). Luciferase expression can be easily monitored enzymatically or by PCR. This assay is advantageous in providing a positive read-out with a low background and a large dynamic range. It has been adopted by many laboratories in the field thus allowing easy comparisons.
Extensive studies of their cellular trafficking revealed that these CPP–ON conjugates were efficiently taken up by cells but remained stuck in endocytic vesicles. In keeping with this hypothesis, further incubation with chloroquine (an endosomolytic drug) or with saponin (a membrane-permeabilizing agent) allowed by-pass of this restriction [57]. Similar conclusions were reached using the same well-characterized assay by the Nielsen group [59].
Understanding the mechanisms of cell uptake using CPPs via comparison of literature data has proved extremely difficult, since the behaviours of CPPs seem to be strongly influenced by experimental conditions. Among relevant factors are CPP sequence, type of cargo, concentration (which seems to be crucial to foster endocytosis or direct translocation) and cell type [60].
The first data showing strong activity in the HeLa 705 splicing redirection assay were obtained with a derivative of oligo-arginines in which the spatial distribution of guanidinium side chains was optimized by the use of a non-natural aminohexanoic acid spacer [61]. Similarly, the addition of 6 arginine residues on the N-terminus of Penetratin also resulted in strong activity in this HeLa 705 assay for PNA conjugates [57]. This led to the development of several arginine-rich peptides as PMO conjugates for use in muscle cells and in vivo mouse models of DMD as outlined in Section 4.
In addition to covalent conjugation, some CPPs have been designed for use as complexes, particularly for siRNA delivery (Table 1). This is because, as previously alluded to, chemical conjugation and purification of ONs and siRNAs with cationic CPPs has been difficult to achieve. For example, in the case of ONs, the Heitz and Divita group described a new class of such CPPs, with MPG as the lead compound, which can be complexed with negatively charged ONs essentially through electrostatic interactions. This and other complexation peptides are detailed in Section 6.
3. Chemical synthesis of CPP–ON conjugates
3.1. Negatively charged oligonucleotides and siRNA
Since practically all CPPs have a high cationic charge (due to Lys and Arg residues), it has proved extremely hard technically for covalent conjugation of most CPPs to standard negatively charged (phosphodiester or phosphorothioate) ONs because of aggregation or precipitation that can occur. It was possible for thiol linker-functionalised ONs (for example mixmers of 2′-O-methyl/LNA steric blocking ONs) to be conjugated with cysteine-functionalised classic CPPs such as Tat and Penetratin to give disulphide linkages, if the conjugations were carried out in the presence of a denaturing agent such as formamide [62]. In this way, it was found that fluorescent versions of such conjugates were taken up much better into endosomal compartments of model HeLa cells than unconjugated versions, but release into the nucleus to generate steric block RNA targeting activity proved not to be achievable [62]. A disulphide linkage is also not thought to be compatible with sufficient conjugate stability using systemic delivery, although lung delivery by disulphide-linked Penetratin and Tat conjugated siRNA has been attempted [63].
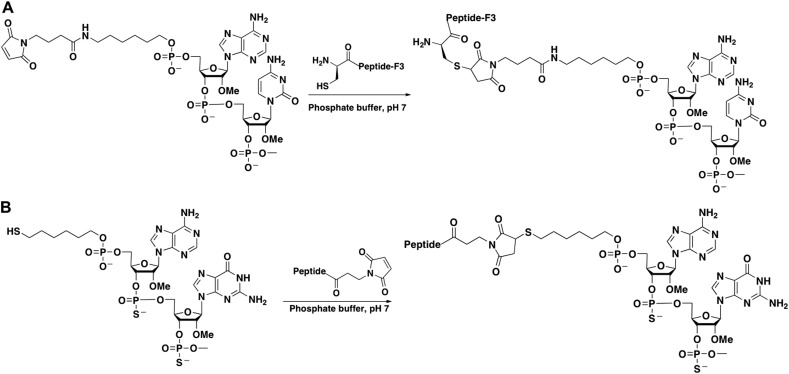
Thus the few rare examples of peptide-conjugated negatively charged ONs used in vivo have generally utilised a thiol-maleimide linkage between ON and peptide (Fig. 2 ). In one case the ON was 5′-functionalised during synthesis with an aminohexyl linker and the resulting amino group reacted with a bifunctional N-(gamma-maleimidobutyrloxy)-succinimide (GMBS) reagent to give a maleimide derivative. The maleimide group was subsequently reacted with a Cys-containing peptide, a cationic CPP known as F3, to furnish the desired conjugate (Fig. 2A) [29]. Surprisingly, chain aggregation was not reported in this conjugation reaction.
Fig. 2.
Schemes showing methods of conjugation of negatively charged ONs to peptides via thiol-maleimide linkage (A and B).
Alternatively and more conveniently, 7-mer non-cationic peptides discovered by a phage display technique were functionalised at the N-terminus by reaction with maleimidopropionic acid at the final stage of peptide synthesis and after purification were conjugated to a 2′-O-Me phosphorothioate ON synthesized with a 5′-thiohexyl linker (Fig. 2B). Such conjugates were designed for enhanced uptake in muscle and heart due to use of homing peptides selected by a phage display method [64]. Non-cationic homing peptides are likely to be explored further for delivery of ONs to specific tissue types. Indeed there was a recent report of a phage-selected SPACE peptide conjugated covalently to siRNA showing enhanced skin penetration [38]. One might also expect increasing use of conjugations using the modern “Click” chemistry, such as via the copper-catalysed reaction between an azido functionality and an alkyne group, where each of the peptide and ON contains a clickable functionality [65] or where the ON contains a 2′-O-propyargyl functionality [66].
3.2. Peptide nucleic acid (PNA)
Despite the large body of literature on the use of peptide-conjugated PNAs in cell culture [67] there are surprisingly few examples of their use in vivo. In the case of short peptide attachments to PNA, these can be assembled directly on the N or C-terminus of the PNA chain during solid-phase synthesis without needing any specific conjugation step. The amide couplings of protected amino acids are identical to that of protected PNA monomers as long as a compatible protecting group scheme is used. In the standard syntheses of PNA, it has been common to add a few Lys residues in any case, particularly to enhance the aqueous solubility of the PNA. Thus the first report of PNA activity involved merely use of (Lys)4 N-terminally functionalised PNA (the Lys residues effectively acting as a CPP) in an in vivo splicing assay using a green fluorescent protein reporter that was up-regulated through redirection of splicing by the PNA [68].
Further, in vivo results were obtained using (Lys)8 derivatives of PNA, synthesized in the same continuous way on the N-terminus of the PNA [69]. The results showed that an (Lys)8–PNA conjugate was rapidly cleared from the circulation but distributed relatively broadly in a mouse with highest concentrations reached in liver, kidney, and spleen. Only very low amounts were detected in lungs, heart, skeletal muscle and testes, however. In vivo analysis was extended to an amphipathic Lys and Leu-containing d-peptide and another rich in Arg and homo-Arg as PNA conjugates, again synthesized continuously on solid phase [70]. Neither showed splicing redirection in liver and only the Lys/Leu peptide–PNA conjugate showed activity in kidney, but both showed significant activity in adipose tissue. However, the lack of potency compared to RNase-H activating ONs and their perceived toxicity profile at high doses led to abandonment of peptide–PNA conjugates as a drug modality for Isis Pharmaceuticals.
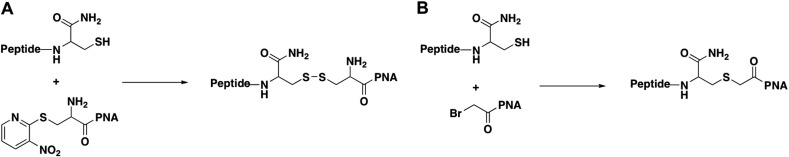
A continuously synthesized nuclear localization signal (NLS) peptide (PKKKRKV) attached to a PNA was also reported to be active in a severe combined immunodeficiency (SCID) mouse model in intronic targeting of c-myc in Burkitt's lymphoma-aimed therapy [34]. Further, a Penetratin peptide was synthesized continuously at the C-terminus of a triplex-forming PNA to target chromosomal DNA for genetic modification of haematopoietic progenitor cells in mice [35]. Promising microRNA knockdown results were obtained also when an antisense Lys–PNA–(Lys)3 derivative was tested in mouse spleen for targeting microRNA-155 [36]. Very recently an anti-microRNA 155 PNA disulphide-conjugated (Fig. 3A) to a tumour-targeting pHLIP peptide has been shown to have potent activity in a mouse model of lymphoma [71]. The only other example of a peptide–PNA active in vivo that was not synthesized by continuous solid-phase assembly was an Arg-rich CPP Pip2a (and related Pip2b) conjugated through a thioether bridge by reaction of a C-terminal Cys residue on the peptide to an N-terminal bromoacetyl group on the PNA (Fig. 3B) [30]. The conjugates were active by intramuscular injection into the mdx mouse, a model of Duchenne muscular dystrophy (DMD), but later higher activity was found in vivo when using PMO rather than PNA as the ON material [33].
Fig. 3.
Schemes showing peptide–PNA conjugation through a disulphide linkage (A) and peptide–PNA conjugation through a thioether linkage (B).
Despite results of mixed fortunes in vivo for CPP conjugates of PNA, these materials deserve further investigation especially in the case of tissues that are harder to reach with other types of ON. A new method of parallel synthesis of arrays of CPP–PNA conjugates (known as SELPEPCON) could prove useful for pre-screening of suitable drug candidates in cell assays or for example by intramuscular delivery
3.3. Phosphorodiamidate morpholino oligomers (PMO)
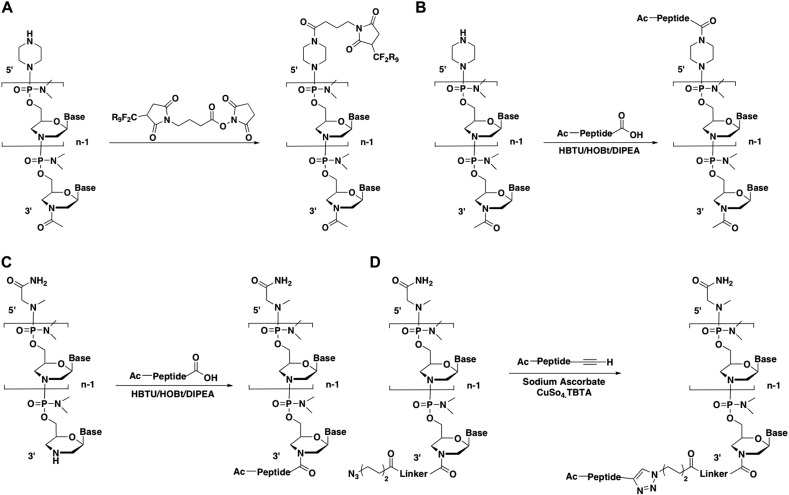
By far the majority of successfully in vivo used CPP conjugates have utilised PMO as ON cargo. The application of CPP–PMO to muscular dystrophies is outlined in Section 4. Regarding Peptide–PMO (P–PMO) synthesis, suffice it to say that it has not been possible to date to use continuous synthesis methods for P–PMO conjugates, since synthesis has been restricted to commercial PMO suppliers. Methods of conjugation are therefore limited to what is available commercially regarding functionalised PMO or instead must employ un-functionalised PMO.
Practically all CPPs used with PMO to date have been Arg-rich starting from the initial observations of Moulton and colleagues [72]. Early work involved use of a bi-functional cross-linker to couple a 5′-piperazino PMO through a maleimide linkage to the peptide (Fig. 4A). Later the 5′-piperazine was directly conjugated through an amide linkage using C-terminally activated peptides (Fig. 4B) [61]. 5′-conjugation can also be carried out with commercially available amino-link functionalised PMO by direct coupling to the C-terminal carboxylic acid of the peptide through an amide link [30], [33]. More generally now, the secondary amine of the 3′-morpholino group is coupled directly to the C-terminal carboxylic acid of the PMO via an amide link (Fig. 4C) [73], [74]. For direct amide conjugation, it is not necessary for Arg-containing peptides to be chemically protected. However, Lys-containing CPPs require a protecting group on the epsilon amino group removable after conjugation.
Fig. 4.
Schemes showing methods of conjugation of peptides to PMO.
Direct 3′-amide conjugation of un-functionalised PMO also allows for peptides to be conjugated to the PMO containing for example alkyne functionalities to allow for azide Click coupling of a fluorescent label to the P–PMO [75]. In addition Click chemistry conjugation features in an adaptation for P–PMO of the SELPEPCON method of parallel conjugate synthesis (Fig. 4D) [76]. Combinations of compatible conjugation techniques such as use of Click chemistries are likely to dominate future P–PMO synthesis methods.
4. Development of CPP–SSO conjugates for the treatment of muscular dystrophies
The term muscular dystrophy describes a large group of hereditary diseases characterized by progressive weakness and degeneration of skeletal muscle [77]. A variety of gene therapies are being developed for this clinically and genetically heterogeneous group of disorders, including antisense ON mediated splice modulation (SSO). Recent advances have placed Duchenne muscular dystrophy (DMD) at the forefront of developments in SSO therapy. In order to overcome the remaining obstacles to the success of this approach, peptide-conjugated SSOs have been extensively investigated in animal models of DMD.
DMD is the most common subtype of muscular dystrophy in the UK [Online Mendelian Inheritance in Man (OMIM [78]) database reference: 310200] affecting one in 3500 new born boys. This severe, X-linked recessive disease results from mutations in the DMD gene [79]. The disorder is characterized by progressive muscle degeneration and wasting, along with the emergence of respiratory and cardiac complications, ultimately leading to premature death [80]. The majority of mutations underlying DMD are genomic deletions, encompassing multiple exons thereby producing a premature truncation of the open reading frame and resulting in the absence of the dystrophin protein. Dystrophin is an integral component of the dystrophin associated protein complex (DAPC), forming a crucial connection between the intracellular actin-cytoskeleton and the extracellular matrix. Exon skipping therapy utilises SSOs to target specific regions of the DMD transcript, inducing the exclusion of individual exons, leading to the restoration of aberrant reading frames and resulting in the production of an internally deleted, yet largely functional, dystrophin protein [8].
Following development in animal models, two AO chemistries have undergone proof-of-concept studies and repeat systemic dose-escalation studies, which have now been completed in the clinical trial setting [15], [17], [18], [19], [81]. Systemic administration of both PMO and 2′OMePS SSOs have yielded specific exon exclusion and partial restoration of dystrophin protein in peripheral muscle of DMD boys. Despite the undoubted potential of exon skipping ON therapy for DMD, the successful application of this approach is currently limited by the relatively inefficient targeting of skeletal muscle, as well as by the inadequate targeting of SSOs to other affected tissues such as the heart [82].
As such, cationic CPPs, which may be co-administered by conjugation with SSOs, have shown dramatic improvement in delivery and induce high levels of dystrophin correction in muscle at comparatively low doses. The majority of work has been performed in the mdx DMD mouse model, which is the most widely utilised model in the DMD field. The dystrophic murine phenotype arises as a result of a spontaneous mutation in exon 23 which encodes for a premature termination site, thus preventing the production of dystrophin protein [83], [84]. The targeted exclusion of exon 23 in this mouse models the therapeutic strategy in patients by restoring the production of dystrophin protein.
The majority of CPPs utilised in the DMD field (Table 1, Table 2 ) may be covalently linked to PMO [31], [85]. However, another class of non-covalent peptides from the Transportan family has been engineered to deliver anionic SSOs such as 2′OMePS, as detailed in Section 6 [86]. A number of modifications of stearylated TP10 [87] gave rise to CPPs capable of inducing high splice correction in vitro in murine H2k mdx myotubes (PepFect6; [51] and PepFect14; [88]) (Table 1). It should be noted that the in vivo efficacies of these CPPs have yet to be assessed.
Table 2.
The derivation of Pip series peptides and their sequences.
| Name | Peptide sequence | Reference | |
|---|---|---|---|
| Parent peptide | Penetratin | RQIKIWFQNRRMKWK | [26], [97] |
| First generation CPP | R6-Pen | RRRRRRR QIKIWFQNRRMKWKKGG | [98] |
| Second generation CPP | Pip1 | RXRRXRRXR-IKILFQN-RRMKWKK | [30] |
| Pip2a | RXRRXRRXR-IdKILFQN-dRRMKWHKB | ||
| Pip2b | RXRRXRRXR-IHILFQN-dRRMKWHKB | ||
| Third generation CPP | Pip5e | RXRRBRRXR-ILFQY-RXRBRXRB | [33] |
| Pip5f | RXRRBRRXR-ILFQY-RXRXRXRB | ||
| Pip5h | RXRRXR-ILFQY-RXRRXR | ||
| Pip5j | RBRRXRRBR-ILFQY-RBRXRBRB | ||
| Pip5k | RBRRXRRBR-ILFQY-RXRBRXRB | ||
| Pip5l | RBRRXRRBR-ILFQY-RXRRXRB | ||
| Pip5m | RBRRXRRBR-ILFQY-RXRBRXB | ||
| Pip5n | RXRRBRRXR-ILFQY-RXRRXRB | ||
| Pip5o | RXRRBRRXR-ILFQY-RXRBRXB | ||
| Fourth generation CPP | Pip6a | RXRRBRRXR-YQFLI-RXRBRXRB | [73] |
| Pip6b | RXRRBRRXR-IQFLI-RXRBRXRB | ||
| Pip6c | RXRRBRRXR-QFLI-RXRBRXRB | ||
| Pip6d | RXRRBRRXR-QFL-RXRBRXRB | ||
| Pip6e | RXRRBRRX-YRFLI-RXRBRXRB | ||
| Pip6f | RXRRBRRXR-FQILY-RXRBRXRB | ||
| Pip6g | RXRRBRRX-YRFRLI-XRBRXRB | ||
| Pip6h | RXRRBRRX-ILFRY-RXRBRXRB |
Notes: B — beta-alanine, X — amino hexanoic acid, dK/dR — amino acid as d-isomer.
The covalent class of CPPs comprises 3 subtypes, specifically oligo-arginine derivatives, phage peptides, and Penetratin derivatives. Oligo-arginines spaced by 6-aminohexanoic acid and/or β-alanine exhibit high splicing efficiency and serum stability in vitro [89], [90], [91]. The (RXR)4 peptide was the first CPP conjugate to be administered in the mdx mouse at a range of doses, time-intervals and via different delivery routes. Generally, a single intravenous administration induced high dystrophin exon skipping in skeletal muscle, diaphragm and, for the first time, in heart [92]. Another arginine-rich peptide, (RXRRBR)2 peptide (B-peptide), identified from a screen using the EGFP-654 splicing reporter mouse model [74], also gave rise to impressive exon skipping notably in the heart, when delivered using higher doses and via the retro-orbital route [85]. Improvements in cardiac function such as resistance to dobutamine stress testing and improvements in end systolic volume and end diastolic volume were also observed.
The identification of phage motifs from phage display-libraries allows the specific homing of a target tissue. As such, peptides with preferential binding to muscle and cardiac tissue were identified specifically for the treatment of DMD. These include muscle specific peptide (MSP) which exhibits enhanced in vivo muscle binding capacity [93], a 12-mer peptide (M12) which revealed better splicing efficiency over MSP [94], and a 7-mer peptide (P4) which exhibited a moderate improvement in splicing activity over naked SSO [64]. The beneficial tissue targeting attributes of phage peptides may be further enhanced when combined with the delivery efficiency of CPPs, to develop a chimeric peptide. This was demonstrated when MSP was coupled to the B-peptide to determine the combined efficacy in mdx mice [32], [95]. The specific orientation of these peptides was crucial to dystrophin splicing activity, and when coupled in the configuration ‘B-MSP-PMO’ revealed a 2–5 fold improvement in skeletal muscle restoration compared to B-PMO [96]. However, no improvement in dystrophin restoration was observed in cardiac muscle.
More recently, the PNA/PMO internalization peptide (Pip) series was derived from the parent Penetratin peptide [26], [97]. Subsequent modifications including the addition of 6 arginine residues to the N-terminus (R6-Penetratin) [98], the addition of a C-terminal cysteine residue, and the utilisation of disulphide conjugation methods [30] fashioned this group of peptides into the established ‘Pip’ sequence conformation, consisting of a central hydrophobic core flanked on either side by arginine-rich sequences. Additional in vivo screening of Pip–PMO compounds was carried out to identify optimal CPPs for dystrophin splicing and correction in mdx mice. In the Pip5-series (Pip5e-o), the core sequence, ILFQY, was retained and there was alteration in the composition and length of the flanking regions [33]. The number of arginine residues ranged between 8 and 10, and the number and placement of 6-aminohexanoic acid (X) and ß-alanine (B) spacer residues also varied in the flanking regions (Table 2). Pip5e–, Pip5j–, Pip5l–, and Pip5n–PMO resulted in the greatest number of dystrophin positive fibres following intramuscular administration (tibialis anterior muscle). Of these highly efficient CPPs, Pip5e–PMO induced the highest levels of exon skipping and dystrophin restoration body wide including in the heart, following a single 25 mg/kg intravenous administration. When directly compared to B-PMO, Pip5e–PMO was shown to restore considerably greater dystrophin protein levels in the heart (intravenous administration comparison).
As B–PMO and Pip5e–PMO comprised similar arginine sequence and content, it was deduced that the core region of Pip5e- (ILFQY) was responsible for the splicing activity in heart. Therefore, the Pip6- series was designed with identical flanking regions to Pip5e and the core sequence was altered (Table 2). Pip6a–, Pip6b– and Pip6f–PMO, which maintained a 5 amino acid core, exhibited the greatest dystrophin splicing activity in heart over the previous lead candidate, Pip5e–PMO [73] (Fig. 5 ). Peptides such as Pip6c- and Pip6d-PMO, with a shortened core, resulted in a substantial reduction in efficacy. As cardiac and respiratory complications are the leading causes of death amongst DMD patients, the ability of Pip6–PMO to restore cardiac function is vital. These compounds demonstrated their restorative ability in a long term, low dose administration study (10 mg/kg over 3 month time-course) in which 30% dystrophin protein was restored in heart and the onset of cardiomyopathy was prevented in an exercised mdx mouse model (unpublished data). Liver and kidney toxicity has been assessed 2 weeks after the administration of Pip–PMO conjugates in mdx mice with no sign of toxicity [33], [99]. Further toxicology studies are currently being pursued however have not been published yet. Of course, continuous optimisations to dose, pharmacokinetics and toxicity are underway which will facilitate the progression of this class of CPPs to clinical trial.
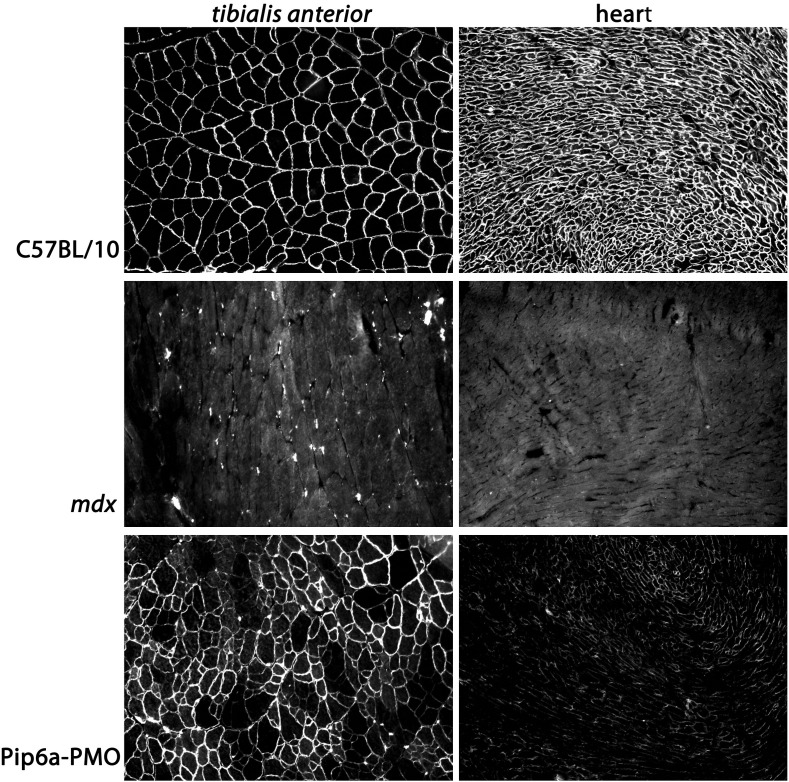
Fig. 5.
Immunohistochemical staining of dystrophin following Pip6a–PMO treatment. Dystrophin staining in the tibialis anterior and heart of C57BL/10, untreated mdx and Pip6a–PMO treated mdx mice. The treated cohort received a single, intravenous 12.5 mg/kg dose, and tissues were harvested 2 weeks later.
The development of CPP–ON conjugates for the treatment of DMD has served as a foundation for the treatment of other diseases facing similar antisense ON delivery challenges. Antisense ONs have been used to modulate RNA processing in the triplet repeat disorder myotonic dystrophy [OMIM: 160900, 602668]. This degenerative disease arises from the expansion of pathogenic microsatellite repeats within noncoding regions of either the dystrophia myotonica-protein kinase (DMPK) or CCHC-type zinc finger, nucleic acid binding protein (CNBP) gene loci [99], [100]. A variety of antisense ON strategies have been used to combat the effect of these toxic RNA expansions, although currently the most promising is the use of steric block ONs [101]. Using this approach, ONs were designed to bind to the DMPK repeat regions, therefore preventing the detrimental sequestering of RNA-binding proteins to within the expansion. Due to the multisystemic nature of myotonic dystrophy, a CPP conjugated antisense ON strategy has recently been tested in a mouse model to induce body wide distribution [102].
Spinal muscular atrophy [OMIM: 253300, 253400, 253550, 271150] is a neuromuscular disorder that results from the loss of motor neurons and skeletal muscle atrophy. This autosomal recessive disease is caused by mutations in the survival of motor neuron 1 (SMN1) gene. The related SMN2 gene undergoes alternative splicing due to a single nucleotide polymorphism, and therefore lacks exon 7 in the majority of transcripts, resulting in low protein expression. In order to functionally compensate for the loss of SMN1 protein in patients, SSOs have been used to promote the inclusion of exon 7 in SMN2 transcripts, and therefore increase the production of SMN2 protein [8]. Substantial headway has been made with naked SSOs in animal models of SMA [103] and clinical trials are ongoing. However, the effective delivery of antisense ONs to motor neurons in the CNS constitutes a major challenge, which may be combatted by CPP conjugation.
As described, multiple diseases may benefit from antisense ON therapy and indeed some have reached clinical trial and demonstrated significant therapeutic potential. These successes may be further amassed with the utility of CPPs, which is a rapidly evolving field of research. Whilst studies pertaining to toxicity and bio-distribution are underway, it is anticipated that CPP–ON therapies will reach clinical trial in the near future.
5. Antiviral and antibacterial applications of CPP–ONs
5.1. Antiviral applications
Amongst antiviral applications, CPP–PNAs were designed several years ago for targeting the HIV-1 trans-activation responsive element TAR [62], [104]. Reassuringly, a Tat–PNA was found to be non-toxic in mice at high (300 mg/kg) doses [105]. However, no further in vivo antiviral studies of CPP–PNA have been published since. By contrast, CPP–PMO antiviral applications have been plentiful for a range of viruses, such as Dengue, Cocksackie, Ebola and Marburg viruses [106].
Only a few in vivo studies of CPP-directed antiviral delivery have been published, all of these involving Arg-rich CPPs, notably (RXR)4XB or R9F2, as conjugates of PMO (Table 1). For example, (RXR)4XB-PMO blocked viral replication of Human respiratory syncytial virus (RSV) expression in BALB/c mice, where the PMO sequence was targeted to the start of the coding region [40]. There was also a reduction in lung viral titres seen when mice were treated 3 h after infection, but not 8 h after infection. This suggested that the PMO must be present in the cell soon after infection if it is to have an impact on virus production.
Promising results were obtained in pre- and post-infection (RXR)4XB–PMO treatment of 3-week old piglets infected with porcine reproductive and respiratory syndrome virus (PRRSV) both in reducing viremia and interstitial pneumonia [41]. An (RXR)4XB–PMO conjugate, where the PMO was targeted to the 5′-terminus of the genomic RNA strand, was found to be substantially more active than naked PMO in reducing viral titers in livers of mice infected with murine hepatitis virus (MHV), a coronavirus, and protected mice from tissue-associated liver damage [42]. CPP–PMO treatment also prolonged survival in two lethal challenge models. However in the case of high dose viral challenge and delayed treatment, the CPP–PMO was not protective and there was some evidence of toxicity in the diseased mice. AG 129 mice treated by intra-peritoneal injection with (RXR)4XB–PMO targeted to the 5′-terminus or the 3′-cyclization sequence (CS) regions of the dengue 2 virus (DENV-2) pre-infection (but not post-infection) were able to increase the average survival time to up to 8 days, but viral rebound was seen eventually [107]. Similarly, dosing of (RXR)4XB–PMO targeted either to the 5′-terminus or the 3′-CS region of West Nile Virus (WNV) RNA partially protected mice from viral challenge but higher doses caused toxicity [108]. Unconjugated PMO had no efficacy.
Although the CPP–PMO approach is very promising in principle for targeting viral RNA, an alternative antiviral approach involves use of PMO analogues with cationic piperazine groups within the PMO. Such “second generation” PMOs, known as PMOplus™, have now generally supplanted CPP–PMO in antiviral applications and these have been reviewed [109], [110].
5.2. Antibacterial applications
CPP–PNA was suggested more than 10 years ago for targeting specific essential bacterial genes [111] and subsequently both CPP–PNA and CPP–PMO have been studied extensively in bacterial cell culture [112], but surprisingly few experiments showing in vivo efficacy have been published. The first proof of principle in vivo was shown in 2005 where a single intravenous injection of a (KFF)3K–PNA targeted to acpP (an essential gene) RNA administered into Escherichia coli K-12-infected BALB/c mice 30 min after bacterial challenge reduced bacterial blood titres and enhanced survival of the infected mice [46]. Similar results were obtained for the Arg-rich (RFF)3RXB–PMO conjugate targeted to the same acpP gene when dosed in mice 12 h after infection with E. coli W3110 [45]. The effect on potency of varying the peptide sequence was investigated in this model and (RXR)4XB was found to be the most potent of various Arg-rich CPPs studied in mice [44].
More recently, CPP–PNA targeting rpoD, a gene that encodes an RNA polymerase primary σ70 subunit essential for bacterial growth, showed broad inhibition in multidrug-resistant Escherichia coli, Salmonella enterica, Klebsiella pneumoniae, and Shigella flexneri both in cells and in vivo, with (RXR)4XB CPP as a PNA conjugate having higher activity in general than (KFF)3K CPP [113].
(RFF)3R-PMO targeted to acpP or gyrA genes was found to be highly effective to increase mouse survival time when mice were challenged with the deadly Ames strain of Bacillus anthracis (the causative agent of anthrax) [114], suggesting that such treatment of anthrax-infected humans might one day become possible. However, just like in the case of pathogenic viruses, cationic backbone-containing PMO (PMOplus™) although not achieving the same efficiency as CPP–PMO [43], may prove to have a more favourable toxicity profile for therapeutic development.
6. CPP:ON non-covalent complexes and their applications
6.1. CPPs used for the non-covalent strategy
As briefly mentioned above, the non-covalent strategy is based on short peptides which are able to form complexes with cargoes without requiring any cross-linking or chemical modifications [115], [116]. Most of the CPPs used in the non-covalent approach have an amphipathic character which enables a combination of electrostatic and hydrophobic interactions with the cargo. The amphipathicity may arise from either the primary structure or the secondary structure. Primary amphipathic peptides can be defined as the sequential assembly of hydrophobic residues with hydrophilic residues, whereas secondary amphipathic peptides are generated by the conformational state that allows distribution of hydrophobic and hydrophilic residues on opposite sides of the molecule [117]. Though originally based on amphipathic peptides, the non-covalent approach has been extended to peptides and peptidic analogues that are able to self-assemble with ONs to form stable CPP:ON complexes [118] and several of them have been reported to improve ON delivery into mammalian cells [116], [118].
The non-covalent approach was originally developed for the delivery of large molecules and several peptides able to bind and condense DNA such as GALA, KALA, JTS1 have been used to improve gene delivery [119], [120]. In 1997, the Divita and Heitz group designed the MPG peptide for the delivery of single and double stranded short ONs [86] and the strategy was then extended to proteins and peptides by the development of Pep-1 [121]. Since MPG, numerous CPP:ON complexes have been developed for the delivery of different types of ONs including AO, SSO and siRNA.
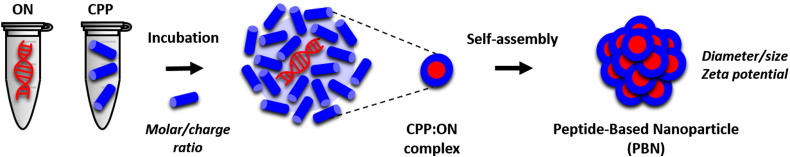
The main advantage of the non-covalent strategy over covalent conjugation lies in its simplicity and the protection that CPP:ON complexes confer to the ON from digestion by nucleases [118]. Compared to a chemical CPP–ON conjugate, the non-covalent complexes usually involve a one-step process consisting of a simple mixing of both partners, CPP and ON [115] (Fig. 6 ).
Fig. 6.
CPP:ON non-covalent complexes and the peptide-based nanoparticle (PBN) strategy.
CPP:ON complexes do not require any chemical cleavage, prevent steric hindrance between peptide and ON, favour a better release of the ON inside the targeted cells and facilitate modifications to increase specificity for the ON and/or the target [118]. With regard to the mechanism of internalization, there is no consensus point of view for the cell entry of non-covalent complexes (see Section 7). However, as these complexes rely on electrostatic and hydrophobic interactions between positively charged CPPs and negatively charged ONs, their cellular uptake mechanism could be also controlled by structural polymorphism of peptides, particle stability and the nature of complexes/membrane interactions [122]. Interestingly, some CPP:ON non-covalent complexes were shown to enter cells through a direct translocation process [123]. Most non-covalent CPPs enable a wide range of chemical modifications and combinations and the modular nature of both peptides and resulting CPP:ONs particles also allows new developments.
6.2. Formulation of Peptide-Based Nanoparticles (PBNs)
The first developments of MPG were based on peptide/ON interactions and investigations of CPP:ON affinity by fluorescence spectrometry to measure the dissociation constant (Kd) as well as the optimal molar ratio and/or charge ratio to obtain stable complexes [86], [124]. A molar ratio (MR) of 7 peptides per ON and a charged-corrected (N/P) ratio of 2 positively charged amino acids (N) for a phosphate group (P) were estimated [125]. Similar approaches were used for other non-covalent CPPs such as Pep-3 for HypNA–pPNA [49], CADY, C6 and MPEG-PCL-CH2R4H2C for siRNA condensation [126], [127], [128], [129]. Fluorescence assays were also combined to circular dichroism (CD) analyses to monitor conformational changes that might occur in the presence of the ON and during CPP:ON complex formation [125], [128], [129], [130]. Although both fluorescence and CD investigations suggest interactions and formation of CPP:ON complexes, the use of gel shift assays has been generalized to demonstrate the formation of complexes [124], [131]. However, none gives a clear characterization of CPP:ON particles in terms of colloidal properties (size, charge and shape). Studies on colloidal properties of CPP:ON complexes have therefore been witnessed over recent years. Methods used for other nanomedicines were applied in order to characterize the size, surface charge and morphology of these CPP:ON complexes. In 2008, Law et al. were the first to report the hydrodynamic diameter and the Zeta potential of R9:siRNA non-covalent complexes [132]. After UV–visible absorbance and CD investigations suggesting R9/siRNA interactions, size and Zeta potential were measured for several charge ratios (+/−) and revealed that R9 forms particles with siRNA, with a hydrodynamic diameter of ~ 1 μm at siRNA saturation [132]. Zeta potential increased with the hydrodynamic diameter of particles, supporting the positive contribution of the guanidinium group of R9 in the surface charge. Size and Zeta potential have to be estimated in parallel since, according to DVLO theory for colloidal systems, low Zeta potential suggest low electrostatic repulsion which induce aggregation of particles whereas high absolute value of Zeta potential are indicative of stable particles suspension [133].
In this light, investigations of size and charge were generalized to other CPP:ON complexes, for different types of ONs (siRNA, SSO, and AS-ON). However, these parameters might be very different according to the CPP, the nature of the ON or the formulation protocol [131]. For example, Kim et al. identified small R15:siRNA nanoparticles with a 224 nm diameter and a + 7 mV Zeta potential for a charge-corrected N/P ratio of 3 in PBS [53] whilst CADY:siRNA nanoparticles have a size of 156 nm and surface charge of + 50 mV whatever the MR (MR20 to MR80) in 5 mM NaCl [134]. CADY:siRNA nanoparticles have a polydispersity index (PDI) of 0.37, suggesting homogeneous and unimodal nanoparticles [134]. For other CPP:ON complexes, the size and the biological activity clearly vary with the MR [50]. Size and charge can also depend on the environment which is important since nanoparticles stability and homogeneity might vary according to the nature of the buffer, as described for C6M1:siRNA particles [130]. In addition physiological conditions such as serum addition can influence colloidal properties. PepFect6:siRNA complexes form homogenous unimodal nanoparticles of 70–100 nm (PDI ~ 0.1–0.2), which remained stable in water, whilst the presence of serum proteins induces larger particles of 125–200 nm with a wider distribution [51]. CPP:ON complexes can also be stabilized by specific excipients, such as lactose [88], 5% mannitol [135] or albumin [52].
Since the shape of nanoparticles also influences the bloodstream circulation half-life [136], the morphology of PBN was studied by electronic microscopy and atomic force microscopy (AFM) and most studies pointed to globular or spherical nano-objects [129], [134], [137]. In fine colloidal characterization suggested a redefinition of the CPP:ON complexes, and with regard to chemical modifications that were included in the design of non-covalent CPP as well as the discrepancy in the formulation of complexes, the more appropriate term of “Peptide-Based-Nanoparticles” (PBNs) was proposed [116], [122]. In this light, formulation of PBN clearly became the key point to consider for further developments, especially with regard to in vivo administration.
As PBNs aim for in vivo perspectives, they have to be improved for membrane permeation and cell targeting abilities, whilst being biocompatible and biodegradable, with minimal cytotoxicity and inflammatory response (see Section 7.5).
6.3. In vivo applications of PBN
Few PBN have been successfully used for the in vivo delivery of antisense ONs, SSOs or siRNA [49], [50], [51], [54], [127], [138]. With regard to siRNA, the first reports on PBN-mediated delivery of siRNA in vivo involved MPG-ΔNLS peptide and a cholesterol oligo-d-arginine (Chol-R9) [54], [138]. MPG-ΔNLS was used for the in vivo delivery of siRNA targeting Oct-4 into mouse blastocytes. Oct-3/4 (POU5f1) is one of the earliest transcription factors expressed in the embryo and both the pluripotency and the fate of ES cells depend upon a tight control of Oct-3/4 expression. MPG-ΔNLS-based nanoparticles induced a siRNA-mediated inhibition of up regulation of Oct-3/4 in ES cells which prevents their specification toward the mesoderm and their differentiation into cardiomyocytes [138]. Similarly, injection of PBN in the inner cell mass of blastocysts impairs cardiogenesis in early embryos [138]. Chol-R9 was applied for the delivery of siRNA targeting VEGF (siVGEF) in CT-26 cells xenografted tumour. Intratumoural administration of Chol-R9:siVGEF complexes induced a significant inhibition of tumour growth associated with a pronounced decrease in VEGF level in the tumour, suggesting a synergistic effect between the d-amino acids of the CPP and the cholesterol moiety [54].
In a similar approach, MPG-ΔNLS was modified in a shortened (MPG-8) and cholesterol-functionalized (MPG-8-Chol) peptide analogue. MPG-8-mediated delivery of siRNA targeting cyclin B1 induces a significant down regulation of both protein and mRNA levels and a systemic administration of MPG-8-based nanoparticles targeting cyclin B1 prevented tumour growth in a xenografted tumour mouse model [50]. Intravenous injections of 0.25 mg/kg reduced tumour growth for 60% by day 50, and administration of 0.5 mg/kg PBN entirely abolished tumour growth [50]. In addition formulation of a mix of MPG-8 and MPG-8-Chol (15%) in PBN revealed that cholesterol increases the biodistribution of siRNA in the tumour by maintaining siRNA in the plasma [50]. Cytokine levels in the plasma were quantified in order to assess the ability of MPG-8:siRNA and MPG-8/MPG-8-Chol:siRNA formulations to induce innate immune response in vivo. No increase in cytokine level was observed 6 h after injection of any formulation, suggesting the lack of immune response induction [50].
In a similar approach, PepFect6 was tested for in vivo RNAi silencing. The hypoxanthine phosphoribosyltransferase 1 (HPRT1) gene was targeted due to the long cellular half-life of the protein (~ 48 h) and the minimal impact on the viability of the transfected cell. Systemic administration of PepFect6:siRNA nanoparticles (1 mg/kg) induced a significant down regulation (> 60%) of the HPRT1 mRNA level in liver, kidney, and lung, without induction of immune response [51]. In addition, PepFect6-based nanoparticles were also used for RNAi-mediated silencing of luciferase (Luc-siRNA) in mice expressing luciferase in the liver and intravenous injection of PepFect6:luc-siRNA (1 mg/kg) displayed decrease of bioluminescence for 2 weeks, reaching 75% gene silencing whilst naked Luc-siRNA was unable to induce a RNAi response [51]. Likewise, Chol-R9, R15:siRNA nanoparticles were formulated for siRNA-mediated knockdown of human epidermal growth factor receptor 2 (HER-2) in human ovary adenocarcinoma cells (SK-OV-3). A reduction of HER-2 expression was associated with the inhibition of SK-OV-3 xenograft tumour growth [139]. Intratumoural injection of R15:siRNA nanoparticles, every 3 days at a dose of 4 μg siRNA per mouse, significantly reduced tumour growth without significant toxicity [53].
Furthermore, different strategies were investigated to stabilize the nanoparticles and to increase blood circulation such as exemplified by albumin- or polyethylene glycol (PEG)-coated nanoparticles. For example, Hou and co-workers have shown that albumin-coated formulation of p5RHH exhibits remarkable transfection efficiency attributable to pH triggered nanoparticle disassembly [52]. Furthermore, Tanaka and co-workers have demonstrated that intravenous injection of MPEG-PCL-CH2R4H2C/siRNA complexes had a significantly higher anti-tumour effect in sarcoma-bearing mice [127].
Most non-covalent CPPs tested in vivo have been formulated for siRNA delivery. However the non-covalent strategy is also intended to deliver other types of ONs, such AS-ONs or SSOs, in vivo. In this light, Pep-3 was formulated with the antisense cyclin B1 HypNA-pPNA and the resulting PBN were evaluated on PC3 xenografted mice [49]. PBN were administrated through intratumoural or intravenous injection every 3 days, and the biological effect of the antisense HyPNA-pPNA was evaluated by monitoring tumour growth during 2 weeks after injection. Intratumoural administration of PBN induced a 50% inhibition of tumour growth at a 1 mg ON dose and more than 92% at a 5 mg dose, whereas intravenous injection only reduced tumour growth by 20% at a 10 mg dose. Consequently in order to improve the in vivo stability of PBN, small amounts of PEGylated-Pep-3 were included in formulation [49]. Intravenous administration of 10 mg of ON with PBN containing 20% of PEGylated-Pep-3 significantly improved PBN stability and inhibited tumour growth by more than 90%, which was 4–5-fold more efficient than un-PEGylated-Pep-3 [49]. These data emphasize the potential of non-covalent CPP for in vivo AS-ON delivery and the importance of PEGylation on nanoparticle stability.
In addition to the physicochemical properties of PBN, some specific characteristics of formulations have to be addressed. Parameters such as affinity, molar or charge ratios, excipients, size and colloidal properties have a strong influence on biological interactions and minor changes can have a major impact on drug delivery efficacy [136]. Thus, as in the FDA draft guidance for liposomal products, the physicochemical properties and specifications should include: morphology, net charge, particle size of PBN, spectroscopic data, light scattering index, in vitro release, content of peptide-engaged in PBN versus free peptide, biodegradation of the PBN.
7. CPP/ON mechanisms of cellular internalization
One of the most challenging questions concerning CPPs is the mechanism by which these peptides enter cells. Early studies using fluorescent dyes linked to CPPs concluded that internalization was energy- and temperature-independent. However, this was later found to be an experimental artefact due to strong CPP adherence to the cell membrane leading to fluorescence overestimation and/or to fixation protocols using methanol allowing the CPP–dye complex to enter cells [140]. Recent studies have suggested that CPP uptake takes place by both endocytosis and energy-independent translocation, with the balance between these two pathways influenced by factors such as CPP sequence [141], temperature and CPP concentration [142], [143], [144], [145]. It is now admitted that both biophysical methods, cell biology assays and biological end-points to assess activity need to be used to dissect the cell import mechanism.
7.1. First contacts with the cell membrane
Molecules approaching a cell first encounter a layer of oligosaccharides — the endothelial glycocalyx which is a network of membrane-bound proteoglycans and glycoproteins, covering the endothelium luminally [146]. Proteoglycans in particular carry large O-linked oligosaccharides consisting of highly negatively charged repeating disaccharide units, the glycosaminoglycans (GAGs), such as heparan sulphates (HS). GAGs are involved in the cellular binding and uptake of several viruses [147], [148] as well as cationic CPPs and polycationic nanoparticles [123], [149].
Oligo-arginines bind to HS with affinities in the upper nanomolar range suggesting that GAGs may act as CPP receptors (reviewed in [150]). For the interaction of cationic CPPs with GAGs, the number of positive charges (number of arginines) was shown critical [151]. As examples, TP10–PNA and Penetratin–PNA conjugates are mainly internalized via macropinocytosis after initial HS interaction on the cell surface [152], [153]. Beside the important role of the positive charges, Sagan and co-workers also show a strong positive correlation between the number of tryptophan residues, GAG binding and cell uptake [154].
Likewise, CADY:siRNA complexes interact with HSPGs, which probably allow the binding and accumulation of the particles at the cell surface as demonstrated by electromobility gel shift assay and nanoparticle dissociation [128].
7.2. Internalization via direct translocation
Several models to explain a direct membrane translocation of CPPs have been proposed, such as the formation of inverted micelles, pore formation (toroidal or barrel slave), the carpet model and the sinking-raft model. These models have been initially proposed for the uptake of membrane-active peptides (reviewed in [155], [156], [157]) and then adapted for CPPs, such as oligo-arginines [158], [159] or Transportan [56].
Likewise, the amphipathic MPG and CADY peptides are internalized via direct membrane translocation. On the basis of physico-chemical investigations (e.g., circular dichroism, Fourier transform infrared and electrophysiological measurements on model membranes), two very similar models have been proposed for MPG:siRNA and CADY:siRNA PBNs based on the formation of transient pore-like structures [125], [160]. A partial conformational change takes place upon MPG complexation with nucleic acids, and an increase in β-sheet content upon association with the cell membrane [125]. CADY:siRNA PBNs were characterized in more details (reviewed in [160]). CADY-mediated cellular uptake of siRNA is extremely rapid [126]. Moreover, internalization and biological activity of CADY:siRNA occurred even at 4 °C and under inhibition of mitochondrial oxidative phosphorylation revealing an energy-independent mechanism [123]. Fluorescence microscopy revealed that neither Transferrin, nor Rab5 co-localized with CADY:siRNA nanoparticles whereas co-localization with Lysotracker was observed to some extent [123]. These data further support an uptake mechanism largely independent of classical endocytosis with a degradation of the nanoparticles via the lysosomal pathway.
7.3. Internalization via endocytotic pathways
Apart for the few cases described in Section 7.2, the present consensus is that cell uptake occurs mainly by endocytosis for arginine-rich CPPs at least at low concentrations [140]. Whether clathrin-coated pit endocytosis, macropinocytosis, or another endocytic route is used is still a matter of debate and the route might differ with cell type, nature of the payload, or concentration [161], [162].
The internalization mechanism was mainly characterized using the HeLa splicing redirection assay (Fig. 1) by correlating cellular uptake and biological efficiency of CPP–PNA or CPP–PMO conjugates. Several conjugates have been analysed in detail such as (Lys)8-PNA-Lys [163], (RXR)4-PMO [61] or Pip2b-PNA [30]. Cell uptake is energy-dependent and leads to sequestration of conjugates in cytoplasmic vesicles after an endocytic mechanism of internalization.
The limitations provided by the endosome sequestration of the payload are well illustrated by the two following sets of data.
PepFect3, a stearylated version of Transportan [87], is able to complex SSOs and these PepFect3:SSO PBNs are rather efficient in the HeLa splicing-redirection assay. However, a significant part of the complexes still remains entrapped in endosomes and can be released upon chloroquine treatment [88]. Based on these results, endosomolytic trifluoromethylquinoline (QN) moieties were grafted on the PepFect3 CPP leading to PepFect6 with largely improved cytoplasmic delivery of the transfected ON and an increased splicing redirection activity. Likewise, PepFect6 was efficient to transfect siRNAs and to promote gene silencing at low concentrations at variance to the PepFect3 formulation [51].
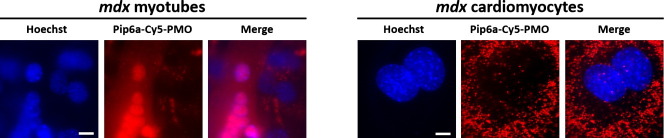
Along the same line and more recently, we could relate the high efficiency of Pip6a–PMO in H2k mdx skeletal muscles with an efficient release from endocytic vesicles after internalization via caveolae-dependent endocytosis. At variance, this same conjugate is internalized by clathrin-dependent endocytosis in primary mdx cardiomyocytes, is less efficiently released from endocytic vesicles and has a lower exon skipping activity [164].
7.4. Methods to analyse the mechanism of cellular internalization
Several strategies can be used to study the cellular internalization of CPP–ON conjugates or CPP:ON complexes [160], [165].
-
1.
Biophysical characterization of CPP/ON using circular dichroism, infra-red spectroscopy, dynamic light scattering or by insertion studies into phospholipid layers.
-
2.
Inhibition of a specific endocytic pathway and assessment of its effect on CPP/ON internalization and biological activity.
-
3.
Co-localization microscopy studies of the association of CPP/ON with fluorescently labelled endocytic markers
The association of CPPs with membranes induces the modification of several physical properties, such as the surface pressure of monolayer (Langmuir Blodgett) and the secondary structure of the peptide (FT-IR, CD, etc.). Deformation of the lipid bilayer due to hydrophobic and hydrophilic interactions between the components as well as the peptide localization in the membrane can be also assessed using NMR, X-ray diffraction, coupled plasmon waveguide resonance, EPR or FRET [166].
Assessing endosomal escape directly has turned difficult and can only be monitored using artificial models such as liposomal leakage assays. Different protocols were developed using large unilamellar vesicles (LUVs) with entrapped dyes such as calcein [167], carboxyfluorescein [168] or ANTS (fluorescent dye)/DPX (quencher) [169], [170].
In cellulo, clathrin-mediated endocytosis can be predominantly characterized by the use of transferrin. Its uptake in cells can indeed be inhibited by a clathrin-specific siRNA or via cell transfection cells with a mutant form of dynamin [171]. However, dynamin is now known to regulate other endocytic and membrane trafficking pathways. Alternatively pharmacological inhibitors, such as chlorpromazine or dynasore (a dynamin inhibitor), can be used to inhibit this pathway even if these reagents cause significant cell toxicity and have to be used over short incubation times [18], [19], [20]. Potassium depletion or hypertonic medium incubation is also commonly used to investigate clathrin-mediated endocytosis [21], [22].
Caveolae are invaginations of the plasma membrane that are localized in lipid rafts. A number of ligands, such as cholera toxin B, SV40 virus and albumin have been shown to be internalized more or less specifically via caveolae. The glycosphingolipid analogue lactosylceramide (LacCer), a fluorescent probe, was also shown to be internalized via this route and may represent a more selective marker for this pathway [172]. A range of pharmacological inhibitors of this pathway have been described and, in the main, they are agents that deplete cholesterol synthesis (lovastatin), agents that rapidly extract cholesterol from lipid rafts (methyl-ß-cyclodextrin), and other cholesterol-interacting molecules such as the antibiotics nystatin and filipin.
Dowdy, Futaki and their colleagues have shown that Tat and other arginine-rich CPPs induce a ubiquitous form of fluid-phase endocytosis termed micropinocytosis [174], [173]. For example, the uptake of octa-arginine (R8) peptide by HeLa cells was significantly suppressed by the macropinocytosis inhibitor ethylisopropylamiloride (EIPA) and the F-actin polymerization inhibitor cytochalasin D, suggesting a role for macropinocytosis in the uptake of the peptide.
7.5. Examples of CPP modifications to improve ON delivery
As mentioned above, CPPs are taken up primarily by endocytic pathways, and in order to promote endosomal escape and increase the transfection efficiency of CPPs, many different strategies have been used. We have mentioned the insertion of endosomolytic moieties in Section 7.3 and additional strategies are described below.
Since endosomes have a low pH, researchers have incorporated histidine residues or appended oligo-histidine tails in the CPP sequence, aiming at capitalizing on a “proton sponge” effect (see review [174]). Due to its protonation at pH 6.0, the imidazole ring of histidine (which is a weak base) counterbalances the accumulation of protons generated by a specific ATPase inside acidic vesicles, neutralizes the lumen of endocytic vesicles and increases their osmolarity. As a consequence, the endosomal vesicles swell and their content is delivered into the cytosol. This has been exploited with success for the Tat-CPP [175], for microsphere coated with ornithine and histidine repeats (O10H6) and for self-assembling nano-constructs of amphiphilic copolymers (see review [176]).
Mason and co-workers have focused on amphipathic α-helical peptides incorporating pH sensitive residues [177]. The histidine residues in the LAH4 peptide are uncharged at neutral pH but when the pH of the endosomal lumen drops, the side chains become protonated, large numbers of peptides are released from the complex and adopt a conformation and alignment in the membrane that induces membrane disorder [178], [179].
Other groups have focused on the addition of cholesterol or on fatty acid modifications. For example, Chol-R9 improves siRNA delivery in a mouse model bearing a subcutaneous tumour [54]. Tat-PNA-mediated splice correction is increased by up to two orders of magnitude when conjugated with decanoic acid [180]. Stearylation of CPPs represents another strategy to increase endosome escape and as a consequence to increase the transfection efficiency of siRNA [181] and phosphorothioate 2′-OMe RNA [182] as already detailed in Section 6.
For in vivo applications, the most frequently modification involves the grafting of a polyethylene glycol (PEG) moiety to prevent rapid clearance. As reported for Pep-3-mediated delivery of antisense-cyclin B1-charged-PNA which blocks tumour growth in vivo upon intratumoural and intravenous injection, PEGylation of Pep-3 significantly improves complex stability and efficiency [49].
In the context of clinical translation, it will be crucial to improve the tissue specificity of CPPs through their functionalization. The screening by in vivo phage display has enabled the identification of numerous peptides that home specifically to various organs under normal or pathological conditions [183]. For example the five residues homing peptides CREKA, which was identified by in vivo screening of phage-displayed peptide libraries in tumour-bearing MMTV-PyMT transgenic breast cancer mice [184], has been combined to the pVEC peptide to yield a CPP with tumour homing specificity [185].
8. Conclusions
Despite their huge potential, the clinical use of nucleic acids-based drugs has been limited by their poor biodistribution. Cell penetrating peptides have therefore been considered as a possible strategy to improve passage across biological barriers and intracellular delivery as reviewed extensively in this chapter.
Both covalent conjugates with neutral antisense ON mimics and non-covalent complexes with either charged antisense ONs or siRNAs have been engineered. Several such constructs have undergone extensive evaluation in animal models (mainly in mice) of both acquired (e.g., viral infections or cancers, in particular) and genetic (e.g., Duchenne muscular dystrophy) human diseases. CPP-delivery has been convincingly shown to increase significantly the efficiency of these nucleic acids cargoes. Clinical trials have however not yet been started to our knowledge. Extensive studies of biodistribution and of possible toxic effects have in particular to be completed.
Despite many studies, mechanisms responsible for the extravasation and for the cellular trafficking of these CPP–drug conjugates or complexes are still poorly understood. Direct translocation across cell membranes appears to be operational in some instances. In most cases however, cell internalization occurs through endocytosis and escape from endocytic vesicles limits biological efficiency. Likewise, CPP delivery does not occur with a similar efficiency in all tissues after systemic administration. Understanding better these limitations will obviously help in engineering of new CPP generations with a superior potential to target various tissues (and pathological conditions) and to deliver their payload within the appropriate cellular compartment. Efforts in these directions have already been started as described in this chapter but much remains to be done.
Acknowledgements
LO'D was supported by a grant from the French muscular dystrophy association AFM (programme number 14784). The work in the laboratory of MJG was supported by the Medical Research Council (MRC programme number U105178803). BL and MJAW were supported by the AFM grant “Advances in oligonucleotide-mediated exon skipping for DMD and related disorders”. Work in the laboratory of PB and SD was partly funded by the FEDER (Fonds Européen de Développement Regional)/La Région Languedoc-Roussillon, by the Agence Nationale de la Recherche (ANR) and by the Centre National de la Recherche Scientifique (CNRS).
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Oligonucleotide Therapeutics”.
References
- 1.Stephenson M.L., Zamecnik P.C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc. Natl. Acad. Sci. U. S. A. 1978;75:285–288. doi: 10.1073/pnas.75.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamecnik P.C., Stephenson M.L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl. Acad. Sci. U. S. A. 1978;75:280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Storz G., Vogel J., Wassarman K.M. Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell. 2011;43:880–891. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y., Vogelstein B., Velculescu V.E., Papadopoulos N., Kinzler K.W. The antisense transcriptomes of human cells. Science. 2008;322:1855–1857. doi: 10.1126/science.1163853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahu N.K., Shilakari G., Nayak A., Kohli D.V. Antisense technology: a selective tool for gene expression regulation and gene targeting. Curr. Pharm. Biotechnol. 2007;8:291–304. doi: 10.2174/138920107782109985. [DOI] [PubMed] [Google Scholar]
- 6.Bauman J., Jearawiriyapaisarn N., Kole R. Therapeutic potential of splice-switching oligonucleotides. Oligonucleotides. 2009;19:1–13. doi: 10.1089/oli.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 8.Muntoni F., Wood M.J.A. Targeting RNA to treat neuromuscular disease. Nat. Rev. Drug Discov. 2011;10:621–637. doi: 10.1038/nrd3459. [DOI] [PubMed] [Google Scholar]
- 9.El Andaloussi S.A., Hammond S.M., Mäger I., Wood M.J.A. Use of cell-penetrating-peptides in oligonucleotide splice switching therapy. Curr. Gene Ther. 2012;12:161–178. doi: 10.2174/156652312800840612. [DOI] [PubMed] [Google Scholar]
- 10.Sibley C.R., Wood M.J.A. The miRNA pathway in neurological and skeletal muscle disease: implications for pathogenesis and therapy. J. Mol. Med. 2011;89:1065–1077. doi: 10.1007/s00109-011-0781-z. [DOI] [PubMed] [Google Scholar]
- 11.Obad S., dos Santos C.O., Petri A., Heidenblad M., Broom O., Ruse C. Silencing of microRNA families by seed-targeting tiny LNAs. Nat. Genet. 2011;43:371–378. doi: 10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geary R.S., Henry S.P., Grillone L.R. Fomivirsen: clinical pharmacology and potential drug interactions. Clin. Pharmacokinet. 2002;41:255–260. doi: 10.2165/00003088-200241040-00002. [DOI] [PubMed] [Google Scholar]
- 13.Ueta T., Noda Y., Toyama T., Yamaguchi T., Amano S. Systemic Vascular Safety of Ranibizumab for Age-related Macular Degeneration: Systematic Review and Meta-analysis of Randomized Trials. Ophthalmology. 2014 doi: 10.1016/j.ophtha.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Gelsinger C., Steinhagen-Thiessen E., Kassner U. Therapeutic potential of mipomersen in the management of familial hypercholesterolaemia. Drugs. 2012;72:1445–1455. doi: 10.2165/11635060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.van Deutekom J.C., Janson A.A., Ginjaar I.B., Frankhuizen W.S., Aartsma-Rus A., Bremmer-Bout M. Local dystrophin restoration with antisense oligonucleotide PRO051. N. Engl. J. Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 16.Flanigan K.M., Voit T., Rosales X.Q., Servais L., Kraus J.E., Wardell C. Pharmacokinetics and safety of single doses of drisapersen in non-ambulant subjects with Duchenne muscular dystrophy: results of a double-blind randomized clinical trial. Neuromuscul. Disord. 2014;24:16–24. doi: 10.1016/j.nmd.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goemans N.M., Tulinius M., van den Akker J.T., Burm B.E., Ekhart P.F., Heuvelmans N. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N. Engl. J. Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 18.Cirak S., Arechavala-Gomeza V., Guglieri M., Feng L., Torelli S., Anthony K. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinali M., Arechavala-Gomeza V., Feng L., Cirak S., Hunt D., Adkin C. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendell J.R., Rodino-Klapac L.R., Sahenk Z., Roush K., Bird L., Lowes L.P. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 2013;74:637–647. doi: 10.1002/ana.23982. [DOI] [PubMed] [Google Scholar]
- 21.Juliano R.L., Ming X., Carver K., Laing B. Cellular uptake and intracellular trafficking of oligonucleotides: implications for oligonucleotide pharmacology. Nucleic Acid Ther. 2014;24:101–113. doi: 10.1089/nat.2013.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryser H.J., Shen W.C. Conjugation of methotrexate to poly(l-lysine) increases drug transport and overcomes drug resistance in cultured cells. Proc. Natl. Acad. Sci. U. S. A. 1978;75:3867–3870. doi: 10.1073/pnas.75.8.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persiani S., Ballou B., Shen W.C., Ryser H.J., Reiland J.M., Hakala T.R. In vivo antitumor effect of methotrexate conjugated to a monoclonal IgM antibody specific for stage-specific embryonic antigen-1, on MH-15 mouse teratocarcinoma. Cancer Immunol. Immunother. 1989;29:167–170. doi: 10.1007/BF00199991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Degols G., Devaux C., Lebleu B. Oligonucleotide-poly(l-lysine)–heparin complexes: potent sequence-specific inhibitors of HIV-1 infection. Bioconjug. Chem. 1994;5:8–13. doi: 10.1021/bc00025a002. [DOI] [PubMed] [Google Scholar]
- 25.Roebuck K.A., Rabbi M.F., Kagnoff M.F. HIV-1 Tat protein can transactivate a heterologous TATAA element independent of viral promoter sequences and the trans-activation response element. AIDS (London, England) 1997;11:139–146. doi: 10.1097/00002030-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Derossi D., Joliot A.H., Chassaing G., Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 27.Vivès E., Brodin P., Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. ( http://www.jbc.org/content/272/25/16010.short (accessed December 19, 2013)) [DOI] [PubMed] [Google Scholar]
- 28.Elmén J., Lindow M., Schütz S., Lawrence M., Petri A., Obad S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 29.Henke E., Perk J., Vider J., de Candia P., Chin Y., Solit D.B. Peptide-conjugated antisense oligonucleotides for targeted inhibition of a transcriptional regulator in vivo. Nat. Biotechnol. 2008;26:91–100. doi: 10.1038/nbt1366. [DOI] [PubMed] [Google Scholar]
- 30.Ivanova G.D., Arzumanov A., Abes R., Yin H., Wood M.J.A., Lebleu B. Improved cell-penetrating peptide–PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acids Res. 2008;36:6418–6428. doi: 10.1093/nar/gkn671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin H., Lu Q., Wood M. Effective exon skipping and restoration of dystrophin expression by peptide nucleic acid antisense oligonucleotides in mdx mice. Mol. Ther. 2008;16:38–45. doi: 10.1038/sj.mt.6300329. [DOI] [PubMed] [Google Scholar]
- 32.Yin H., Moulton H.M., Betts C., Seow Y., Boutilier J., Iverson P.L. A fusion peptide directs enhanced systemic dystrophin exon skipping and functional restoration in dystrophin-deficient mdx mice. Hum. Mol. Genet. 2009;18:4405–4414. doi: 10.1093/hmg/ddp395. [DOI] [PubMed] [Google Scholar]
- 33.Yin H., Saleh A.F., Betts C., Camelliti P., Seow Y., Ashraf S. Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice. Mol. Ther. 2011;19:1295–1303. doi: 10.1038/mt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boffa L.C., Cutrona G., Cilli M., Matis S., Damonte G., Mariani M.R. Inhibition of Burkitt's lymphoma cells growth in SCID mice by a PNA specific for a regulatory sequence of the translocated c-myc. Cancer Gene Ther. 2007;14:220–226. doi: 10.1038/sj.cgt.7701002. [DOI] [PubMed] [Google Scholar]
- 35.Rogers F.A., Lin S.S., Hegan D.C., Krause D.S., Glazer P.M. Targeted gene modification of hematopoietic progenitor cells in mice following systemic administration of a PNA–peptide conjugate. Mol. Ther. 2012;20:109–118. doi: 10.1038/mt.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fabani M.M., Abreu-Goodger C., Williams D., Lyons P.A., Torres A.G., Smith K.G.C. Efficient inhibition of miR-155 function in vivo by peptide nucleic acids. Nucleic Acids Res. 2010;38:4466–4475. doi: 10.1093/nar/gkq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres A.G., Fabani M.M., Vigorito E., Williams D., Al-Obaidi N., Wojciechowski F. Chemical structure requirements and cellular targeting of microRNA-122 by peptide nucleic acids anti-miRs. Nucleic Acids Res. 2012;40:2152–2167. doi: 10.1093/nar/gkr885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu T., Mitragotri S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc. Natl. Acad. Sci. U. S. A. 2011;108:15816–15821. doi: 10.1073/pnas.1016152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michiue H., Eguchi A., Scadeng M., Dowdy S.F. Induction of in vivo synthetic lethal RNAi responses to treat glioblastoma. Cancer Biol. Ther. 2009;8:2306–2313. doi: 10.4161/cbt.8.23.10271. [DOI] [PubMed] [Google Scholar]
- 40.Lai S.-H., Stein D.A., Guerrero-Plata A., Liao S.-L., Ivanciuc T., Hong C. Inhibition of respiratory syncytial virus infections with morpholino oligomers in cell cultures and in mice. Mol. Ther. 2008;16:1120–1128. doi: 10.1038/mt.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Opriessnig T., Patel D., Wang R., Halbur P.G., Meng X.-J., Stein D.A. Inhibition of porcine reproductive and respiratory syndrome virus infection in piglets by a peptide-conjugated morpholino oligomer. Antivir. Res. 2011;91:36–42. doi: 10.1016/j.antiviral.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Burrer R., Neuman B.W., Ting J.P.C., Stein D.A., Moulton H.M., Iversen P.L. Antiviral effects of antisense morpholino oligomers in murine coronavirus infection models. J. Virol. 2007;81:5637–5648. doi: 10.1128/JVI.02360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellbye B.L., Weller D.D., Hassinger J.N., Reeves M.D., Lovejoy C.E., Iversen P.L. Cationic phosphorodiamidate morpholino oligomers efficiently prevent growth of Escherichia coli in vitro and in vivo. J. Antimicrob. Chemother. 2010;65:98–106. doi: 10.1093/jac/dkp392. [DOI] [PubMed] [Google Scholar]
- 44.Mellbye B.L., Puckett S.E., Tilley L.D., Iversen P.L., Geller B.L. Variations in amino acid composition of antisense peptide-phosphorodiamidate morpholino oligomer affect potency against Escherichia coli in vitro and in vivo. Antimicrob. Agents Chemother. 2009;53:525–530. doi: 10.1128/AAC.00917-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilley L.D., Mellbye B.L., Puckett S.E., Iversen P.L., Geller B.L. Antisense peptide-phosphorodiamidate morpholino oligomer conjugate: dose–response in mice infected with Escherichia coli. J. Antimicrob. Chemother. 2007;59:66–73. doi: 10.1093/jac/dkl444. [DOI] [PubMed] [Google Scholar]
- 46.Tan X.-X., Actor J.K., Chen Y. Peptide nucleic acid antisense oligomer as a therapeutic strategy against bacterial infection: proof of principle using mouse intraperitoneal infection. Antimicrob. Agents Chemother. 2005;49:3203–3207. doi: 10.1128/AAC.49.8.3203-3207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson M.H., Stein D.A., Kroeker A.D., Hatlevig S.A., Iversen P.L., Moulton H.M. Arginine-rich peptide conjugation to morpholino oligomers: effects on antisense activity and specificity. Bioconjug. Chem. 2005;16:959–966. doi: 10.1021/bc0501045. [DOI] [PubMed] [Google Scholar]
- 48.Wesolowski D., Tae H.S., Gandotra N., Llopis P., Shen N., Altman S. Basic peptide-morpholino oligomer conjugate that is very effective in killing bacteria by gene-specific and nonspecific modes. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16582–16587. doi: 10.1073/pnas.1112561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris M.C., Gros E., Aldrian-Herrada G., Choob M., Archdeacon J., Heitz F. A non-covalent peptide-based carrier for in vivo delivery of DNA mimics. Nucleic Acids Res. 2007;35:e49. doi: 10.1093/nar/gkm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crombez L., Morris M.C., Dufort S., Aldrian-Herrada G., Nguyen Q., Mc Master G. Targeting cyclin B1 through peptide-based delivery of siRNA prevents tumour growth. Nucleic Acids Res. 2009;37:4559–4569. doi: 10.1093/nar/gkp451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andaloussi S.E.L., Lehto T., Mäger I., Rosenthal-Aizman K., Oprea I.I., Simonson O.E. Design of a peptide-based vector, PepFect6, for efficient delivery of siRNA in cell culture and systemically in vivo. Nucleic Acids Res. 2011;39:3972–3987. doi: 10.1093/nar/gkq1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou K.K., Pan H., Ratner L., Schlesinger P.H., Wickline S.A. Mechanisms of nanoparticle-mediated siRNA transfection by melittin-derived peptides. ACS Nano. 2013;7:8605–8615. doi: 10.1021/nn403311c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S.W., Kim N.Y., Choi Y.B., Park S.H., Yang J.M., Shin S. RNA interference in vitro and in vivo using an arginine peptide/siRNA complex system. J. Control. Release. 2010;143:335–343. doi: 10.1016/j.jconrel.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Kim W.J., Christensen L.V., Jo S., Yockman J.W., Jeong J.H., Kim Y.-H. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Mol. Ther. 2006;14:343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 55.Allinquant B., Hantraye P., Mailleux P., Moya K., Bouillot C., Prochiantz A. Downregulation of amyloid precursor protein inhibits neurite outgrowth in vitro. J. Cell Biol. 1995;128:919–927. doi: 10.1083/jcb.128.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pooga M., Hällbrink M., Zorko M., Langel U. Cell penetration by transportan. FASEB J. 1998;12:67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- 57.Abes S., Turner J.J., Ivanova G.D., Owen D., Williams D., Arzumanov A. Efficient splicing correction by PNA conjugation to an R6-Penetratin delivery peptide. Nucleic Acids Res. 2007;35:4495–4502. doi: 10.1093/nar/gkm418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang S.H., Cho M.J., Kole R. Up-regulation of luciferase gene expression with antisense oligonucleotides: implications and applications in functional assay development. Biochemistry (Mosc) 1998;37:6235–6239. doi: 10.1021/bi980300h. [DOI] [PubMed] [Google Scholar]
- 59.Shiraishi T., Pankratova S., Nielsen P.E. Calcium ions effectively enhance the effect of antisense peptide nucleic acids conjugated to cationic tat and oligoarginine peptides. Chem. Biol. 2005;12:923–929. doi: 10.1016/j.chembiol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 60.Abes R., Arzumanov A.A., Moulton H.M., Abes S., Ivanova G.D., Iversen P.L. Cell-penetrating-peptide-based delivery of oligonucleotides: an overview. Biochem. Soc. Trans. 2007;35:775–779. doi: 10.1042/BST0350775. [DOI] [PubMed] [Google Scholar]
- 61.Abes S., Moulton H.M., Clair P., Prevot P., Youngblood D.S., Wu R.P. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J. Control. Release. 2006;116:304–313. doi: 10.1016/j.jconrel.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Turner J.J., Williams D., Owen D., Gait M.J. Disulfide conjugation of peptides to oligonucleotides and their analogs. In: Beaucage Serge., editor. 2006. (Curr. Protoc. Nucleic Acid Chem.). (Chapter 4 Unit 4.28) [DOI] [PubMed] [Google Scholar]
- 63.Moschos S.A., Jones S.W., Perry M.M., Williams A.E., Erjefalt J.S., Turner J.J. Lung delivery studies using siRNA conjugated to TAT(48–60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjug. Chem. 2007;18:1450–1459. doi: 10.1021/bc070077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jirka S.M.G., Heemskerk H., Tanganyika-de Winter C.L., Muilwijk D., Pang K.H., de Visser P.C. Peptide conjugation of 2′-O-methyl phosphorothioate antisense oligonucleotides enhances cardiac uptake and exon skipping in mdx mice. Nucleic Acid Ther. 2014;24:25–36. doi: 10.1089/nat.2013.0448. [DOI] [PubMed] [Google Scholar]
- 65.Gogoi K., Mane M.V., Kunte S.S., Kumar V.A. A versatile method for the preparation of conjugates of peptides with DNA/PNA/analog by employing chemo-selective click reaction in water. Nucleic Acids Res. 2007;35:e139. doi: 10.1093/nar/gkm935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh I., Heaney F. Metal free, “click and click–click” conjugation of ribonucleosides and 2′-OMe oligoribonucleotides on the solid phase. Org. Biomol. Chem. 2010;8:451–456. doi: 10.1039/b918463e. [DOI] [PubMed] [Google Scholar]
- 67.Said Hassane F., Saleh A.F., Abes R., Gait M.J., Lebleu B. Cell penetrating peptides: overview and applications to the delivery of oligonucleotides. Cell. Mol. Life Sci. 2009;67:715–726. doi: 10.1007/s00018-009-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sazani P., Gemignani F., Kang S.-H., Maier M.A., Manoharan M., Persmark M. Systemically delivered antisense oligomers upregulate gene expression in mouse tissues. Nat. Biotechnol. 2002;20:1228–1233. doi: 10.1038/nbt759. [DOI] [PubMed] [Google Scholar]
- 69.Albertshofer K., Siwkowski A.M., Wancewicz E.V., Esau C.C., Watanabe T., Nishihara K.C. Structure–activity relationship study on a simple cationic peptide motif for cellular delivery of antisense peptide nucleic acid. J. Med. Chem. 2005;48:6741–6749. doi: 10.1021/jm050490b. [DOI] [PubMed] [Google Scholar]
- 70.Wancewicz E.V., Maier M.A., Siwkowski A.M., Albertshofer K., Winger T.M., Berdeja A. Peptide nucleic acids conjugated to short basic peptides show improved pharmacokinetics and antisense activity in adipose tissue. J. Med. Chem. 2010;53:3919–3926. doi: 10.1021/jm901489k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng C.J., Bahal R., Babar I.A., Pincus Z., Barrera F., Liu C. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–110. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moulton H.M., Nelson M.H., Hatlevig S.A., Reddy M.T., Iversen P.L. Cellular uptake of antisense morpholino oligomers conjugated to arginine-rich peptides. Bioconjug. Chem. 2004;15:290–299. doi: 10.1021/bc034221g. [DOI] [PubMed] [Google Scholar]
- 73.Betts C., Saleh A.F., Arzumanov A.A., Hammond S.M., Godfrey C., Coursindel T. Pip6–PMO, A New Generation of Peptide-oligonucleotide Conjugates With Improved Cardiac Exon Skipping Activity for DMD Treatment. Mol. Ther.-Nucleic Acids. 2012;1:e38. doi: 10.1038/mtna.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jearawiriyapaisarn N., Moulton H.M., Buckley B., Roberts J., Sazani P., Fucharoen S. Sustained Dystrophin Expression Induced by Peptide-conjugated Morpholino Oligomers in the Muscles of mdx Mice. Mol. Ther. 2008;16:1624–1629. doi: 10.1038/mt.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shabanpoor F., Gait M.J. Development of a general methodology for labelling peptide-morpholino oligonucleotide conjugates using alkyne-azide click chemistry. Chem. Commun. (Camb.) 2013;49:10260–10262. doi: 10.1039/c3cc46067c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O'Donovan L., Okamoto I., Arzumanov A.A., Williams D.L., Deuss P., Gait M.J. Parallel Synthesis of Cell-Penetrating Peptide Conjugates of PMO Toward Exon Skipping Enhancement in Duchenne Muscular Dystrophy. Nucleic Acid Ther. 2015;25:1–10. doi: 10.1089/nat.2014.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davies K.E., Nowak K.J. Molecular mechanisms of muscular dystrophies: old and new players. Nat. Rev. Mol. Cell Biol. 2006;7:762–773. doi: 10.1038/nrm2024. [DOI] [PubMed] [Google Scholar]
- 78.Hamosh A., Scott A.F., Amberger J., Valle D., McKusick V.A. Online Mendelian Inheritance in Man (OMIM) Hum. Mutat. 2000;15:57–61. doi: 10.1002/(SICI)1098-1004(200001)15:1<57::AID-HUMU12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 79.Birnkrant D.J., Bushby K.M.D., Amin R.S., Bach J.R., Benditt J.O., Eagle M. The respiratory management of patients with duchenne muscular dystrophy: a DMD care considerations working group specialty article. Pediatr. Pulmonol. 2010;45:739–748. doi: 10.1002/ppul.21254. [DOI] [PubMed] [Google Scholar]
- 80.Manzur A.Y., Kinali M., Muntoni F. Update on the management of Duchenne muscular dystrophy. Arch. Dis. Child. 2008;93:986–990. doi: 10.1136/adc.2007.118141. [DOI] [PubMed] [Google Scholar]
- 81.Lu Q.L., Mann C.J., Lou F., Bou-Gharios G., Morris G.E., Xue S. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat. Med. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- 82.Alter J., Lou F., Rabinowitz A., Yin H., Rosenfeld J., Wilton S.D. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat. Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- 83.Bulfield G., Siller W.G., Wight P.A., Moore K.J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. U. S. A. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sicinski P., Geng Y., Ryder-Cook A.S., Barnard E.A., Darlison M.G., Barnard P.J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 85.Wu B., Moulton H.M., Iversen P.L., Jiang J., Li J., Li J. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14814–14819. doi: 10.1073/pnas.0805676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morris M.C., Vidal P., Chaloin L., Heitz F., Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997;25:2730–2736. doi: 10.1093/nar/25.14.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mäe M., El Andaloussi S., Lundin P., Oskolkov N., Johansson H.J., Guterstam P. A stearylated CPP for delivery of splice correcting oligonucleotides using a non-covalent co-incubation strategy. J. Control. Release. 2009;134:221–227. doi: 10.1016/j.jconrel.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 88.Ezzat K., Andaloussi S.E.L., Zaghloul E.M., Lehto T., Lindberg S., Moreno P.M.D. PepFect 14, a novel cell-penetrating peptide for oligonucleotide delivery in solution and as solid formulation. Nucleic Acids Res. 2011;39:5284–5298. doi: 10.1093/nar/gkr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mitchell D.J., Kim D.T., Steinman L., Fathman C.G., Rothbard J.B. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000;56:318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 90.Wu R.P., Youngblood D.S., Hassinger J.N., Lovejoy C.E., Nelson M.H., Iversen P.L. Cell-penetrating peptides as transporters for morpholino oligomers: effects of amino acid composition on intracellular delivery and cytotoxicity. Nucleic Acids Res. 2007;35:5182–5191. doi: 10.1093/nar/gkm478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Youngblood D.S., Hatlevig S.A., Hassinger J.N., Iversen P.L., Moulton H.M. Stability of cell-penetrating peptide-morpholino oligomer conjugates in human serum and in cells. Bioconjug. Chem. 2007;18:50–60. doi: 10.1021/bc060138s. [DOI] [PubMed] [Google Scholar]
- 92.Yin H., Moulton H.M., Seow Y., Boyd C., Boutilier J., Iverson P. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum. Mol. Genet. 2008;17:3909–3918. doi: 10.1093/hmg/ddn293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samoylova T.I., Smith B.F. Elucidation of muscle-binding peptides by phage display screening. Muscle Nerve. 1999;22:460–466. doi: 10.1002/(sici)1097-4598(199904)22:4<460::aid-mus6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 94.Gao X., Zhao J., Han G., Zhang Y., Dong X., Cao L. Effective dystrophin restoration by a novel muscle-homing peptide -morpholino conjugate in dystrophin-deficient mdx mice. Mol. Ther. 2014 doi: 10.1038/mt.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yin H., Moulton H.M., Betts C., Merritt T., Seow Y., Ashraf S. Functional rescue of dystrophin-deficient mdx mice by a chimeric peptide-PMO. Mol. Ther. 2010;18:1822–1829. doi: 10.1038/mt.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin H., Boisguerin P., Moulton H.M., Betts C., Seow Y., Boutilier J. Context Dependent Effects of Chimeric Peptide Morpholino Conjugates Contribute to Dystrophin Exon-skipping Efficiency. Mol. Ther.-Nucleic Acids. 2013;2:e124. doi: 10.1038/mtna.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perez F., Joliot A., Bloch-Gallego E., Zahraoui A., Triller A., Prochiantz A. Antennapedia homeobox as a signal for the cellular internalization and nuclear addressing of a small exogenous peptide. J. Cell Sci. 1992;102(Pt 4):717–722. doi: 10.1242/jcs.102.4.717. [DOI] [PubMed] [Google Scholar]
- 98.Turner J.J., Ivanova G.D., Verbeure B., Williams D., Arzumanov A.A., Abes S. Cell-penetrating peptide conjugates of peptide nucleic acids (PNA) as inhibitors of HIV-1 Tat-dependent trans-activation in cells. Nucleic Acids Res. 2005;33:6837–6849. doi: 10.1093/nar/gki991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Day J.W., Ricker K., Jacobsen J.F., Rasmussen L.J., Dick K.A., Kress W. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology. 2003;60:657–664. doi: 10.1212/01.wnl.0000054481.84978.f9. [DOI] [PubMed] [Google Scholar]
- 100.Jiang H., Mankodi A., Swanson M.S., Moxley R.T., Thornton C.A. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum. Mol. Genet. 2004;13:3079–3088. doi: 10.1093/hmg/ddh327. [DOI] [PubMed] [Google Scholar]
- 101.Wheeler T.M., Sobczak K., Lueck J.D., Osborne R.J., Lin X., Dirksen R.T. Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science. 2009;325:336–339. doi: 10.1126/science.1173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leger A.J., Mosquea L.M., Clayton N.P., Wu I.-H., Weeden T., Nelson C.A. Systemic delivery of a Peptide-linked morpholino oligonucleotide neutralizes mutant RNA toxicity in a mouse model of myotonic dystrophy. Nucleic Acid Ther. 2013;23:109–117. doi: 10.1089/nat.2012.0404. [DOI] [PubMed] [Google Scholar]
- 103.Passini M.A., Bu J., Richards A.M., Kinnecom C., Sardi S.P., Stanek L.M. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001777. (72ra18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tripathi S., Chaubey B., Ganguly S., Harris D., Casale R.A., Pandey V.N. Anti-HIV-1 activity of anti-TAR polyamide nucleic acid conjugated with various membrane transducing peptides. Nucleic Acids Res. 2005;33:4345–4356. doi: 10.1093/nar/gki743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chaubey B., Tripathi S., Pandey V.N. Single acute-dose and repeat-doses toxicity of anti-HIV-1 PNA TAR-penetratin conjugate after intraperitoneal administration to mice. Oligonucleotides. 2008;18:9–20. doi: 10.1089/oli.2007.0088. [DOI] [PubMed] [Google Scholar]
- 106.Moulton H.M., Moulton J.D. Antisense morpholino oligomers and their peptide conjugates. In: Kurreck J., editor. Ther. Oligonucleotides. Royal Society of Chemistry; Cambridge: 2008. pp. 43–79. [Google Scholar]
- 107.Stein D.A., Huang C.Y.-H., Silengo S., Amantana A., Crumley S., Blouch R.E. Treatment of AG129 mice with antisense morpholino oligomers increases survival time following challenge with dengue 2 virus. J. Antimicrob. Chemother. 2008;62:555–565. doi: 10.1093/jac/dkn221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Deas T.S., Bennett C.J., Jones S.A., Tilgner M., Ren P., Behr M.J. In vitro resistance selection and in vivo efficacy of morpholino oligomers against West Nile virus. Antimicrob. Agents Chemother. 2007;51:2470–2482. doi: 10.1128/AAC.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iversen P.L., Warren T.K., Wells J.B., Garza N.L., Mourich D.V., Welch L.S. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012;4:2806–2830. doi: 10.3390/v4112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Warren T.K., Shurtleff A.C., Bavari S. Advanced morpholino oligomers: a novel approach to antiviral therapy. Antivir. Res. 2012;94:80–88. doi: 10.1016/j.antiviral.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Good L., Awasthi S.K., Dryselius R., Larsson O., Nielsen P.E. Bactericidal antisense effects of peptide–PNA conjugates. Nat. Biotechnol. 2001;19:360–364. doi: 10.1038/86753. [DOI] [PubMed] [Google Scholar]
- 112.Järver P., Coursindel T., Andaloussi S.E., Godfrey C., Wood M.J., Gait M.J. Peptide-mediated Cell and In Vivo Delivery of Antisense Oligonucleotides and siRNA. Mol. Ther.-Nucleic Acids. 2012;1:e27. doi: 10.1038/mtna.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bai H., You Y., Yan H., Meng J., Xue X., Hou Z. Antisense inhibition of gene expression and growth in gram-negative bacteria by cell-penetrating peptide conjugates of peptide nucleic acids targeted to rpoD gene. Biomaterials. 2012;33:659–667. doi: 10.1016/j.biomaterials.2011.09.075. [DOI] [PubMed] [Google Scholar]
- 114.Panchal R.G., Geller B.L., Mellbye B., Lane D., Iversen P.L., Bavari S. Peptide conjugated phosphorodiamidate morpholino oligomers increase survival of mice challenged with Ames Bacillus anthracis. Nucleic Acid Ther. 2012;22:316–322. doi: 10.1089/nat.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heitz F., Morris M.C., Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crombez L., Morris M.C., Deshayes S., Heitz F., Divita G. Peptide-based nanoparticle for ex vivo and in vivo drug delivery. Curr. Pharm. Des. 2008;14:3656–3665. doi: 10.2174/138161208786898842. [DOI] [PubMed] [Google Scholar]
- 117.Deshayes S., Morris M.C., Divita G., Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell. Mol. Life Sci. 2005;62:1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Margus H., Padari K., Pooga M. Cell-penetrating peptides as versatile vehicles for oligonucleotide delivery. Mol. Ther. 2012;20:525–533. doi: 10.1038/mt.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gottschalk S., Sparrow J.T., Hauer J., Mims M.P., Leland F.E., Woo S.L. A novel DNA–peptide complex for efficient gene transfer and expression in mammalian cells. Gene Ther. 1996;3:448–457. [PubMed] [Google Scholar]
- 120.Wyman T.B., Nicol F., Zelphati O., Scaria P.V., Plank C., Szoka F.C. Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry (Mosc) 1997;36:3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 121.Morris M.C., Depollier J., Mery J., Heitz F., Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 122.Deshayes S., Konate K., Aldrian G., Crombez L., Heitz F., Divita G. Structural polymorphism of non-covalent peptide-based delivery systems: highway to cellular uptake. Biochim. Biophys. Acta. 2010;1798:2304–2314. doi: 10.1016/j.bbamem.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 123.Rydström A., Deshayes S., Konate K., Crombez L., Padari K., Boukhaddaoui H. Direct translocation as major cellular uptake for CADY self-assembling peptide-based nanoparticles. PLoS One. 2011;6:e25924. doi: 10.1371/journal.pone.0025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morris M.C., Chaloin L., Méry J., Heitz F., Divita G. A novel potent strategy for gene delivery using a single peptide vector as a carrier. Nucleic Acids Res. 1999;27:3510–3517. doi: 10.1093/nar/27.17.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Deshayes S., Gerbalchaloin S., Morris M., Aldrianherrada G., Charnet P., Divita G. On the mechanism of non-endosomial peptide-mediated cellular delivery of nucleic acids. Biochim. Biophys. Acta, Biomembr. 2004;1667:141–147. doi: 10.1016/j.bbamem.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 126.Crombez L., Aldrian-Herrada G., Konate K., Nguyen Q.N., McMaster G.K., Brasseur R. A new potent secondary amphipathic cell-penetrating peptide for siRNA delivery into mammalian cells. Mol. Ther. 2009;17:95–103. doi: 10.1038/mt.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanaka K., Kanazawa T., Horiuchi S., Ando T., Sugawara K., Takashima Y. Cytoplasm-responsive nanocarriers conjugated with a functional cell-penetrating peptide for systemic siRNA delivery. Int. J. Pharm. 2013;455:40–47. doi: 10.1016/j.ijpharm.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 128.Konate K., Crombez L., Deshayes S., Decaffmeyer M., Thomas A., Brasseur R. Insight into the cellular uptake mechanism of a secondary amphipathic cell-penetrating peptide for siRNA delivery. Biochemistry (Mosc) 2010;49:3393–3402. doi: 10.1021/bi901791x. [DOI] [PubMed] [Google Scholar]
- 129.Jafari M., Xu W., Naahidi S., Chen B., Chen P. A new amphipathic, amino-acid-pairing (AAP) peptide as siRNA delivery carrier: physicochemical characterization and in vitro uptake. J. Phys. Chem. B. 2012;116:13183–13191. doi: 10.1021/jp3072553. [DOI] [PubMed] [Google Scholar]
- 130.Jafari M., Xu W., Pan R., Sweeting C.M., Karunaratne D.N., Chen P. Serum stability and physicochemical characterization of a novel amphipathic peptide C6M1 for siRNA delivery. PLoS One. 2014;9:e97797. doi: 10.1371/journal.pone.0097797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Asbeck A.H., Beyerle A., McNeill H., Bovee-Geurts P.H.M., Lindberg S., Verdurmen W.P.R. Molecular parameters of siRNA–cell penetrating peptide nanocomplexes for efficient cellular delivery. ACS Nano. 2013;7:3797–3807. doi: 10.1021/nn305754c. [DOI] [PubMed] [Google Scholar]
- 132.Law M., Jafari M., Chen P. Physicochemical characterization of siRNA–peptide complexes. Biotechnol. Prog. 2008;24:957–963. doi: 10.1002/btpr.13. [DOI] [PubMed] [Google Scholar]
- 133.Hunter R.J. Academic Press; London; San Diego: 1989. Zeta Potential in Colloid Science: Principles and Applications. [Google Scholar]
- 134.Deshayes S., Konate K., Rydström A., Crombez L., Godefroy C., Milhiet P.-E. Self-assembling peptide-based nanoparticles for siRNA delivery in primary cell lines. Small. 2012;8:2184–2188. doi: 10.1002/smll.201102413. [DOI] [PubMed] [Google Scholar]
- 135.Ezzat K., Zaghloul E.M., El Andaloussi S., Lehto T., El-Sayed R., Magdy T. Solid formulation of cell-penetrating peptide nanocomplexes with siRNA and their stability in simulated gastric conditions. J. Control. Release. 2012;162:1–8. doi: 10.1016/j.jconrel.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Petros R.A., DeSimone J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 137.Hou K.K., Pan H., Lanza G.M., Wickline S.A. Melittin derived peptides for nanoparticle based siRNA transfection. Biomaterials. 2013;34:3110–3119. doi: 10.1016/j.biomaterials.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zeineddine D., Papadimou E., Chebli K., Gineste M., Liu J., Grey C. Oct-3/4 dose dependently regulates specification of embryonic stem cells toward a cardiac lineage and early heart development. Dev. Cell. 2006;11:535–546. doi: 10.1016/j.devcel.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 139.Urban-Klein B., Werth S., Abuharbeid S., Czubayko F., Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 140.Richard J.P., Melikov K., Vives E., Ramos C., Verbeure B., Gait M.J. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 141.Müller J., Triebus J., Kretzschmar I., Volkmer R., Boisguerin P. The agony of choice: how to find a suitable CPP for cargo delivery. J. Pept. Sci. 2012;18:293–301. doi: 10.1002/psc.2396. [DOI] [PubMed] [Google Scholar]
- 142.Jiao C.-Y., Delaroche D., Burlina F., Alves I.D., Chassaing G., Sagan S. Translocation and Endocytosis for Cell-penetrating Peptide Internalization. J. Biol. Chem. 2009;284:33957–33965. doi: 10.1074/jbc.M109.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guterstam P., Madani F., Hirose H., Takeuchi T., Futaki S., El Andaloussi S. Elucidating cell-penetrating peptide mechanisms of action for membrane interaction, cellular uptake, and translocation utilizing the hydrophobic counter-anion pyrenebutyrate. Biochim. Biophys. Acta. 2009;1788:2509–2517. doi: 10.1016/j.bbamem.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 144.Tünnemann G., Ter-Avetisyan G., Martin R.M., Stöckl M., Herrmann A., Cardoso M.C. Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. J. Pept. Sci. 2008;14:469–476. doi: 10.1002/psc.968. [DOI] [PubMed] [Google Scholar]
- 145.Jones A.T., Sayers E.J. Cell entry of cell penetrating peptides: tales of tails wagging dogs. J. Control. Release. 2012;161:582–591. doi: 10.1016/j.jconrel.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 146.Reitsma S., Slaaf D.W., Vink H., van Zandvoort M.A.M.J., oude Egbrink M.G.A. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. - Eur. J. Physiol. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Day P.M., Lowy D.R., Schiller J.T. Heparan sulfate-independent cell binding and infection with furin-precleaved papillomavirus capsids. J. Virol. 2008;82:12565–12568. doi: 10.1128/JVI.01631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van der Schaar H.M., Rust M.J., Chen C., van der Ende-Metselaar H., Wilschut J., Zhuang X. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 2008;4:e1000244. doi: 10.1371/journal.ppat.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Christianson H.C., Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014;35:51–55. doi: 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 150.Favretto M.E., Wallbrecher R., Schmidt S., van de Putte R., Brock R. Glycosaminoglycans in the cellular uptake of drug delivery vectors — bystanders or active players? J. Control. Release. 2014;180:81–90. doi: 10.1016/j.jconrel.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 151.Nakase I., Tadokoro A., Kawabata N., Takeuchi T., Katoh H., Hiramoto K. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry (Mosc) 2007;46:492–501. doi: 10.1021/bi0612824. [DOI] [PubMed] [Google Scholar]
- 152.Andaloussi S.E., Guterstam P., Langel Ü. Assessing the delivery efficacy and internalization route of cell-penetrating peptides. Nat. Protoc. 2007;2:2043–2047. doi: 10.1038/nprot.2007.302. [DOI] [PubMed] [Google Scholar]
- 153.El-Andaloussi S., Johansson H.J., Lundberg P., Langel U. Induction of splice correction by cell-penetrating peptide nucleic acids. J. Gene Med. 2006;8:1262–1273. doi: 10.1002/jgm.950. [DOI] [PubMed] [Google Scholar]
- 154.Bechara C., Pallerla M., Zaltsman Y., Burlina F., Alves I.D., Lequin O. Tryptophan within basic peptide sequences triggers glycosaminoglycan-dependent endocytosis. FASEB J. 2013;27:738–749. doi: 10.1096/fj.12-216176. [DOI] [PubMed] [Google Scholar]
- 155.Tréhin R., Merkle H.P. Chances and pitfalls of cell penetrating peptides for cellular drug delivery. Eur. J. Pharm. Biopharm. 2004;58:209–223. doi: 10.1016/j.ejpb.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 156.Lindsay M.A. Peptide-mediated cell delivery: application in protein target validation. Curr. Opin. Pharmacol. 2002;2:587–594. doi: 10.1016/s1471-4892(02)00199-6. [DOI] [PubMed] [Google Scholar]
- 157.Fischer R., Fotin-Mleczek M., Hufnagel H., Brock R. Break on through to the other side-biophysics and cell biology shed light on cell-penetrating peptides. ChemBioChem. 2005;6:2126–2142. doi: 10.1002/cbic.200500044. [DOI] [PubMed] [Google Scholar]
- 158.Futaki S. Arginine-rich peptides: potential for intracellular delivery of macromolecules and the mystery of the translocation mechanisms. Int. J. Pharm. 2002;245:1–7. doi: 10.1016/s0378-5173(02)00337-x. [DOI] [PubMed] [Google Scholar]
- 159.Wender P.A., Mitchell D.J., Pattabiraman K., Pelkey E.T., Steinman L., Rothbard J.B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Konate K., Rydstrom A., Divita G., Deshayes S. Everything You Always Wanted to Know About CADY-Mediated siRNA Delivery (But Afraid to Ask) Curr. Pharm. Des. 2013;19:2869–2877. doi: 10.2174/1381612811319160004. ( http://www.ingentaconnect.com/content/ben/cpd/2013/00000019/00000016/art00004 (accessed December 19, 2013)) [DOI] [PubMed] [Google Scholar]
- 161.Mueller J., Kretzschmar I., Volkmer R., Boisguerin P. Comparison of cellular uptake using 22 CPPs in 4 different cell lines. Bioconjug. Chem. 2008;19:2363–2374. doi: 10.1021/bc800194e. [DOI] [PubMed] [Google Scholar]
- 162.Wadia J.S., Stan R.V., Dowdy S.F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 163.Abes S., Williams D., Prevot P., Thierry A., Gait M.J., Lebleu B. Endosome trapping limits the efficiency of splicing correction by PNA–oligolysine conjugates. J. Control. Release. 2006;110:595–604. doi: 10.1016/j.jconrel.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 164.Lehto T., Castillo Alvarez A., Gauck S., Gait M.J., Coursindel T., Wood M.J.A. Cellular trafficking determines the exon skipping activity of Pip6a–PMO in mdx skeletal and cardiac muscle cells. Nucleic Acids Res. 2014;42:3207–3217. doi: 10.1093/nar/gkt1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Deshayes S., Konate K., Aldrian G., Heitz F., Divita G. Interactions of amphipathic CPPs with model membranes. Methods Mol. Biol. 2011;683:41–56. doi: 10.1007/978-1-60761-919-2_4. [DOI] [PubMed] [Google Scholar]
- 166.Langel U., editor. Cell-Penetrating Peptides. CRC Press; Boca Raton: 2007. [Google Scholar]
- 167.Magzoub M., Oglecka K., Pramanik A., Göran Eriksson L.E., Gräslund A. Membrane perturbation effects of peptides derived from the N-termini of unprocessed prion proteins. Biochim. Biophys. Acta. 2005;1716:126–136. doi: 10.1016/j.bbamem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 168.Fuchs S.M., Raines R.T. Pathway for polyarginine entry into mammalian cells. Biochemistry (Mosc) 2004;43:2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abes R., Moulton H.M., Clair P., Yang S.-T., Abes S., Melikov K. Delivery of steric block morpholino oligomers by (R-X-R)4 peptides: structure–activity studies. Nucleic Acids Res. 2008;36:6343–6354. doi: 10.1093/nar/gkn541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hassane F.S., Abes R., El Andaloussi S., Lehto T., Sillard R., Langel U. Insights into the cellular trafficking of splice redirecting oligonucleotides complexed with chemically modified cell-penetrating peptides. J. Control. Release. 2011;153:163–172. doi: 10.1016/j.jconrel.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 171.Cleal K., He L., Watson P.D., Jones A.T. Endocytosis, intracellular traffic and fate of cell penetrating peptide based conjugates and nanoparticles. Curr. Pharm. Des. 2013;19:2878–2894. doi: 10.2174/13816128113199990297. [DOI] [PubMed] [Google Scholar]
- 172.Al Soraj M., He L., Peynshaert K., Cousaert J., Vercauteren D., Braeckmans K. siRNA and pharmacological inhibition of endocytic pathways to characterize the differential role of macropinocytosis and the actin cytoskeleton on cellular uptake of dextran and cationic cell penetrating peptides octaarginine (R8) and HIV-Tat. J. Control. Release. 2012;161:132–141. doi: 10.1016/j.jconrel.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 173.Nakase I., Niwa M., Takeuchi T., Sonomura K., Kawabata N., Koike Y. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol. Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 174.Midoux P., Pichon C., Yaouanc J.-J., Jaffrès P.-A. Chemical vectors for gene delivery: a current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br. J. Pharmacol. 2009;157:166–178. doi: 10.1111/j.1476-5381.2009.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lo S.L., Wang S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials. 2008;29:2408–2414. doi: 10.1016/j.biomaterials.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 176.Jhaveri A.M., Torchilin V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014;5:77. doi: 10.3389/fphar.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kichler A., Mason A.J., Bechinger B. Cationic amphipathic histidine-rich peptides for gene delivery. Biochim. Biophys. Acta. 2006;1758:301–307. doi: 10.1016/j.bbamem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 178.Lam J.K.W., Liang W., Lan Y., Chaudhuri P., Chow M.Y.T., Witt K. Effective endogenous gene silencing mediated by pH responsive peptides proceeds via multiple pathways. J. Control. Release. 2012;158:293–303. doi: 10.1016/j.jconrel.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Langlet-Bertin B., Leborgne C., Scherman D., Bechinger B., Mason A.J., Kichler A. Design and evaluation of histidine-rich amphipathic peptides for siRNA delivery. Pharm. Res. 2010;27:1426–1436. doi: 10.1007/s11095-010-0138-2. [DOI] [PubMed] [Google Scholar]
- 180.Koppelhus U., Shiraishi T., Zachar V., Pankratova S., Nielsen P.E. Improved cellular activity of antisense peptide nucleic acids by conjugation to a cationic peptide-lipid (CatLip) domain. Bioconjug. Chem. 2008;19:1526–1534. doi: 10.1021/bc800068h. [DOI] [PubMed] [Google Scholar]
- 181.Nakamura Y., Kogure K., Futaki S., Harashima H. Octaarginine-modified multifunctional envelope-type nano device for siRNA. J. Control. Release. 2007;119:360–367. doi: 10.1016/j.jconrel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 182.Lehto T., Abes R., Oskolkov N., Suhorutsenko J., Copolovici D.-M., Mäger I. Delivery of nucleic acids with a stearylated (RxR)4 peptide using a non-covalent co-incubation strategy. J. Control. Release. 2010;141:42–51. doi: 10.1016/j.jconrel.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 183.Laakkonen P., Vuorinen K. Homing peptides as targeted delivery vehicles. Integr. Biol. 2010;2:326. doi: 10.1039/c0ib00013b. [DOI] [PubMed] [Google Scholar]
- 184.Simberg D., Duza T., Park J.H., Essler M., Pilch J., Zhang L. Biomimetic amplification of nanoparticle homing to tumors. Proc. Natl. Acad. Sci. U. S. A. 2007;104:932–936. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mäe M., Myrberg H., El-Andaloussi S., Langel Ü. Design of a Tumor Homing Cell-Penetrating Peptide for Drug Delivery. Int. J. Pept. Res. Ther. 2009;15:11–15. [Google Scholar]