Abstract
Background
Coronavirus 229E (HCoV-229E), one of the causes of the common cold, exacerbates chronic obstructive pulmonary disease (COPD) and bronchial asthma. Long-acting muscarinic antagonists and β2-agonists and inhaled corticosteroids inhibit the exacerbation of COPD and bronchial asthma caused by infection with viruses, including HCoV-229E. However, the effects of these drugs on HCoV-229E replication and infection-induced inflammation in the human airway are unknown.
Methods
Primary human nasal (HNE) and tracheal (HTE) epithelial cell cultures were infected with HCoV-229E.
Results
Pretreatment of HNE and HTE cells with glycopyrronium or formoterol decreased viral RNA levels and/or titers, the expression of the HCoV-229E receptor CD13, the number and fluorescence intensity of acidic endosomes where HCoV-229E RNA enters the cytoplasm, and the infection-induced production of cytokines, including IL-6, IL-8, and IFN-β. Treatment of the cells with the CD13 inhibitor 2′2′-dipyridyl decreased viral titers. Pretreatment of the cells with a combination of three drugs (glycopyrronium, formoterol, and budesonide) exerted additive inhibitory effects on viral titers and cytokine production. Pretreatment of HNE cells with glycopyrronium or formoterol reduced the susceptibility to infection, and pretreatment with the three drugs inhibited activation of nuclear factor-kappa B p50 and p65 proteins. Pretreatment with formoterol increased cAMP levels and treatment with cAMP decreased viral titers, CD13 expression, and the fluorescence intensity of acidic endosomes.
Conclusions
These findings suggest that glycopyrronium, formoterol, and a combination of glycopyrronium, formoterol, and budesonide inhibit HCoV-229E replication partly by inhibiting receptor expression and/or endosomal function and that these drugs modulate infection-induced inflammation in the airway.
Keywords: Airway epithelial cells, HCoV-229E, CD13, Long-acting β2 agonist, Long-acting muscarinic antagonist
Abbreviations: ASL, airway surface liquid; BUD, budesonide; CAPE, caffeic acid phenethyl ester; DMSO, dimethyl sulfoxide; ELISA, enzyme-linked immunosorbent assay; FRM, formoterol; GFB, a combination of glycopyrronium; formoterol., and budesonide; GLP, glycopyrronium; COPD, chronic obstructive pulmonary disease; ICI, ICI 118,551; IL, interleukin; HCoV, human coronavirus; HNE, human nasal epithelial; HTE, human tracheal epithelial; ICS, inhaled corticosteroid; IFN, interferon; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LLC-MK, rhesus monkey kidney epithelial; NF-κB, nuclear factor-kappa B; RT-PCR, real-time quantitative reverse transcription polymerase chain reaction; TCID, tissue culture infective dose
1. Introduction
Human coronaviruses (HCoV)-229E and HCoV-OC43 cause the common cold [1,2] and exacerbate chronic obstructive pulmonary disease (COPD) and bronchial asthma [3,4].
HCoV-229E binds to the aminopeptidase N receptor (CD13) [5] and enters cells via endosomal and cell-surface pathways using proteases [6,7]. HCoV-229E can replicate in an airway epithelial cell line [8]; however, HCoV-229E replication in primary human airway epithelial cell cultures has not been studied.
Long-acting muscarinic antagonists (LAMAs), long-acting β2-agonists (LABAs), and inhaled corticosteroids (ICSs) prevent COPD and bronchial asthma exacerbation [9,10] induced by viral infection, including infection with HCoV-229E [3,4]. We reported that the LAMA tiotropium, the LABA formoterol, and the ICS budesonide reduce rhinovirus replication by reducing receptor expression and/or the number of acidic endosomes where rhinoviral RNA enters the cytoplasm [11,12]. Acidic endosomes are also critical for the entry of HCoV-229E [7]; however, the effects of these drugs on HCoV-229E replication are unknown.
The clinical benefits of these drugs may be related to various effects, including the inhibition of viral infection-induced cytokine production, bronchospasm, and mucus hypersecretion [13]. However, the effects of these drugs on HCoV-229E infection-induced cytokine production in airway cells are unclear.
In the present study, we examined the inhibitory effects of the LAMA glycopyrronium, the LABA formoterol, and the ICS budesonide on HCoV-229E replication in primary human nasal epithelial (HNE) and human tracheal epithelial (HTE) cells cultured on filter membranes with air-interface methods, which exhibited a higher differentiation than those cultured in immersion conditions [14]. We also examined the effects of these drugs on CD13 expression and acidic endosomes to study the mechanisms underlying their effects.
2. Materials and methods
2.1. HNE and HTE cells
Nasal polyps were obtained from 50 subjects with chronic rhinosinusitis (age; 55 ± 2 y) undergoing endoscopic surgery. Tracheal samples were obtained from 10 patients after death (age; 69 ± 4 years). The sex, reason for surgery, cause of death, presence of allergic rhinitis, bronchial asthma, or COPD, as well as the drugs used for treatment, are shown in Table 1 . A total of 21, 11, 18, 18, and 0 subjects were treated with nasal corticosteroids, oral corticosteroids, ICSs, LABAs, or LAMAs, respectively. One tracheal sample donor had COPD. This study was approved by the Tohoku University Ethics Committee (IRB numbers: 2018-1-15 and 2018-1-16).
Table 1.
Characteristics of the subjects.
| HNE cell donors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No1) | Sex | Reason for Surgery | Allergic Rhinitis, Asthma, or COPD | Drugs | No1) | Sex | Reason for Surgery | Allergic Rhinitis, Asthma, or COPD | Drugs |
| 1 | F | CRS | BA | OS, LABA | 26 | F | CRS | ND | NT |
| 2 | M | CRS | AR | NS | 27 | M | CRS | BA, AR | NT |
| 3 | F | CRS | AR | NT | 28 | M | CRS | BA | NS, OS, ICS, LABA |
| 4 | M | CRS | ND | NT | 29 | M | CRS | BA, AR | OS |
| 5 | F | CRS | BA | NS, OS, ICS, LABA | 30 | M | CRS | BA | NS, ICS, LABA |
| 6 | F | CRS | BA | NS, ICS, LABA | 31 | M | CRS | BA, AR | NS, OS, ICS, LABA |
| 7 | M | CRS | BA | NS, ICS | 32 | F | CRS | ND | NT |
| 8 | M | CRS | BA, AR | NS, OS, ICS, LABA | 33 | M | CRS | BA, AR | OS |
| 9 | F | CRS | ND | NT | 34 | M | CRS | BA | NS, OS, ICS, LABA |
| 10 | M | CRS | ND | NT | 35 | M | CRS | BA | ICS, LABA |
| 11 | F | CRS | ND | NT | 36 | M | CRS | ND | NT |
| 12 | M | CRS | ND | NS | 37 | F | CRS | BA | OS, ICS, LABA |
| 13 | F | CRS | ND | NT | 38 | M | CRS | ND | NT |
| 14 | M | CRS | BA | NS | 39 | M | CRS | ND | NT |
| 15 | M | CRS | BA | NS, ICS, LABA | 40 | M | CRS | ND | NS |
| 16 | F | CRS | AR | OS | 41 | M | CRS | ND | NT |
| 17 | M | CRS | ND | NS | 42 | F | CRS | BA | OS, ICS, LABA |
| 18 | M | CRS | ND | NT | 43 | M | CRS | BA | NS, ICS, LABA |
| 19 | M | CRS | BA | ICS, LABA | 44 | M | CRS | ND | NS |
| 20 | M | CRS | BA, AR | ICS, LABA | 45 | M | CRS | ND | NS |
| 21 | F | CRS | BA, AR | NS | 46 | M | CRS | ND | NT |
| 22 | M | CRS | ND | NT | 47 | F | CRS | BA, AR | ICS, LABA |
| 23 | M | CRS | ND | NT | 48 | M | CRS | ND | NS |
| 24 | F | CRS | BA | ICS, LABA | 49 | M | CRS | ND | NS |
| 25 |
M |
CRS |
ND |
NT |
50 |
M |
CRS |
AR |
NS |
| HTE cell donors | |||||||||
| No1) |
Sex |
Cause of death |
Allergic Rhinitis, Asthma, or COPD |
Drugs |
No1) |
Sex |
Cause of death |
Allergic Rhinitis, Asthma, or COPD |
Drugs |
| 1 | F | TC | ND | NT | 6 | F | MSA | ND | NT |
| 2 | F | ML | ND | NT | 7 | F | Sepsis | ND | NT |
| 3 | M | GC | ND | NT | 8 | F | LF, PN | ND | NT |
| 4 | M | AP | ND | NT | 9 | M | DIC | ND | NT |
| 5 | F | PC | ND | NT | 10 | M | EC | COPD | NT |
AP: acute pancreatitis, AR: allergic rhinitis, BA: bronchial asthma, COPD: chronic obstructive pulmonary disease, CRS: chronic rhinosinusitis, DIC: disseminated intravascular coagulation, EC: endocrine cancer, GC: gastric cancer, ICS: inhaled corticosteroid, LABA: long-acting β2-agonist, LF: lung fibrosis, MSA: multiple system atrophy, ML: malignant lymphoma, NS: nasal corticosteroid, OS: oral corticosteroid, PC: pancreatic cancer, PN: pneumonia, TC: thyroid cancer.
ND, not determined; the presence of allergic rhinitis, bronchial asthma, and COPD were assessed but not found.
NT, not treated; patients were not treated with oral or nasal corticosteroids, long-acting β2-agonists, or long-acting muscarinic antagonists.
No1): subject number.
Cells were cultured on filter membranes (Transwell, Corning, ME, USA) [14], in 24-well plates (Becton Dickinson, NJ, USA), or on coverslips in Petri-dishes [15].
2.2. Viral stocks
A stock of clinically isolated HCoV-229E [2] was prepared by infecting Rhesus monkey kidney epithelial (LLC-MK2) cells.
2.3. Detection and titration of viruses
HCoV-229E in the airway surface liquids (ASLs) was detected and titrated using LLC-MK2 cells and endpoint methods [15,16].
2.4. Viral infection
HNE or HTE cells were infected with HCoV-229E at a multiplicity of infection of 0.08 or 0.8 for 90 min, according to the methods described in a previous report using rhinovirus [15], and were subsequently cultured at 33 °C.
2.5. Collection of airway surface liquid and basolateral supernatant for analysis
ASL was collected by rinsing the apical surfaces of HNE or HTE cells with 200 μL of fresh medium before infection and at 24 h, 48 h, 72 h, or 120 h after infection. The collected medium was used to examine the HCoV-229E titers or the levels of CD13, cyclic AMP (cAMP), interleukin (IL)-6, IL-8, and interferons (IFNs) [17]. The basolateral supernatant was collected and replaced with fresh medium [17] and was used to measure the cAMP and IFN levels.
2.6. Treatment with drugs
The cells were pretreated with drugs (100 nM) starting at 72 h before infection and lasting until the end of the experimental period [15]. To determine the concentration-dependent effects of the drugs, the cells were pretreated with the drugs at concentrations ranging from 10−9 M to 10−6 M.
Uninfected cells were pretreated with the drugs (100 nM, 72 h) to examine their effect on CD13 expression, acidic endosomes, and nuclear factor-kappa B (NF-κB) activation.
The drugs were obtained from AstraZeneca PLC (Cambridge, UK).
2.7. Effects of drugs on susceptibility to HCoV-229E infection
The cells were pretreated with drugs (100 nM, 72 h) before infection. The susceptibility to HCoV-229E infection was evaluated as previously described [15].
2.8. Quantification of HCoV-229E RNA and mRNA levels of CD13 and interferons
The levels of HCoV-229E RNA and mRNA of CD13, IFN-β, IFN-λ1, or IFN-γ were determined using real-time quantitative reverse transcription (RT)-PCR using TaqMan® Gene Expression Master Mix (Applied Biosystems, CA, USA) or TB Green Premix Ex Taq™ (Takara, Shiga, Japan), as previously described [[18], [19], [20], [21], [22]].
2.9. Measurement of CD13, cAMP, and cytokine production and NF-κB activation
The levels of CD13, cAMP, IL-6, and IL-8 were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Boster Bio, CA, USA), a cyclic AMP selective enzyme immunoassay (EIA) kit (Cayman Chemicals, MI, USA), a solid phase chemiluminescent ELISA kit (QuantinGlo®; ELISA, R&D Systems, MN, USA), and an IL-8 EIA kit (Invitrogen, CA, USA). The levels of IFN-β, IFN-λ1, or IFN-γ were measured using a Human IFN-β Quantikine ELISA Kit, a Human IL-29/IFN-λ1 DuoSet ELISA kit (R&D Systems), and a Quantikine Human IFN-γ Immunoassay kit.
The presence of translocated p50 and p65 in nuclear extracts was assessed using a TransAM NFκB Family Kit (Active Motif), as previously described [15].
2.10. Measurement of changes in acidic endosomes
The distribution and fluorescence intensity of acidic endosomes in the cells were measured with LysoSensor DND-189 dye (Molecular Probes, OR, USA) using live-cell imaging [15]. The fluorescence intensity was calculated using a fluorescence image analyzer system (Lumina Vision®; Mitani, Fukui, Japan).
2.11. Statistical analysis
The results are expressed as the mean ± standard error of the mean (SEM). Statistical analysis was performed using two-way repeated measures analysis of variance (ANOVA). The subsequent post-hoc analyses were performed using Bonferroni's method. Student's t-test was used for comparisons between two groups. Values of p < 0.05 were indicated a significant difference. In all experiments, n refers to the number of donors (nasal tissue or trachea) from whom the epithelial cells were obtained. All analyses were performed using SPSS version 20 (IBM Japan, Tokyo, Japan).
3. Results
3.1. Effects on HCoV-229E replication in HNE cells
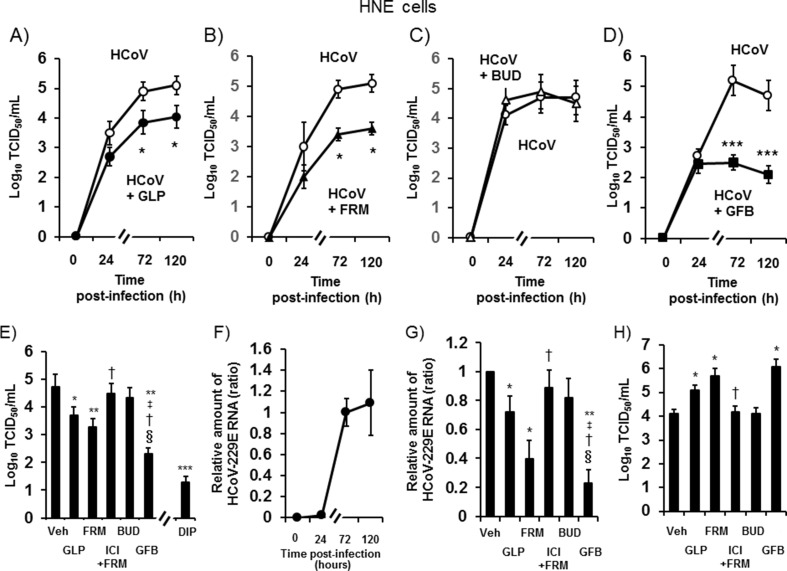
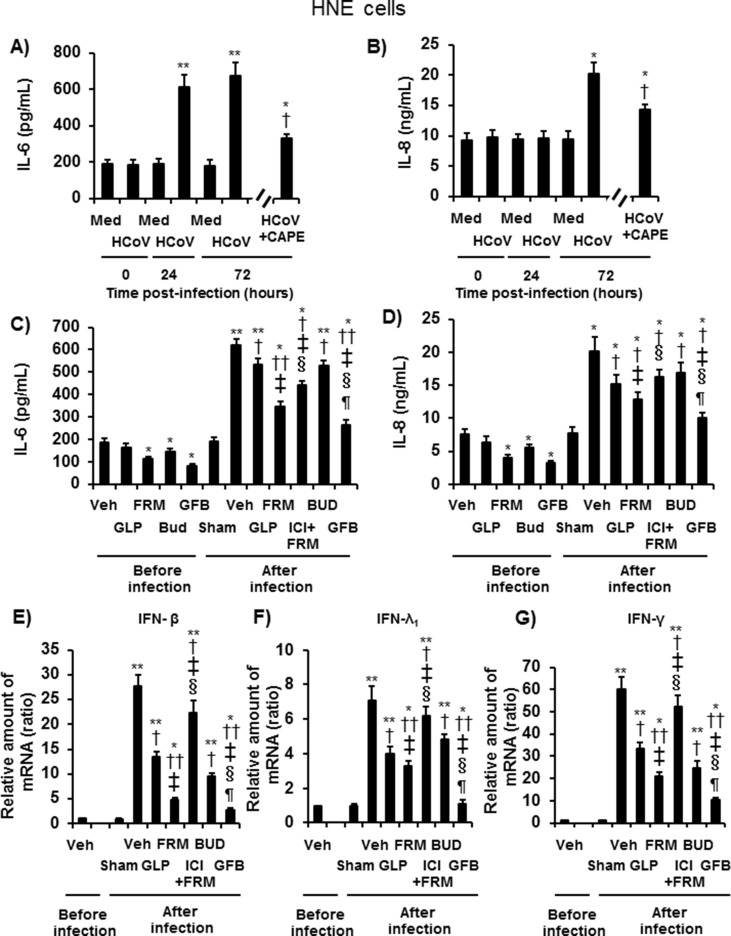
HCoV-229E was detected in the ASL 24 h after infection and increased between 24 h and 72 h (p < 0.05 by ANOVA), Peak titers were observed between 24 h and 120 h (Fig. 1 A–1D).
Fig. 1.
A–D: The time course of HCoV-229E release into ASL from HNE cells pretreated with glycopyrronium (HCoV + GLP) (A, closed circles), formoterol (HCoV + FRM) (B, closed triangles), budesonide (HCoV + BUD) (C, open triangles), a combination of glycopyrronium, formoterol and budesonide (HCoV + GFB) (D, closed squares), or vehicle (HCoV) (open circles, 0.001% dimethyl sulfoxide: DMSO) at different times after viral infection. The viral titers are expressed as the log10 TCID50 (50% tissue culture infective dose)/mL. E: Viral titers in ASL collected between 24 h and 72 h after infection of HNE cells pretreated with glycopyrronium (GLP), formoterol (FRM), the selective β2-adrenergic receptor antagonist ICI 118,551 (1 μM) plus formoterol (100 nM) (ICI + FRM), budesonide (BUD), a combination of these three drugs (GFB), the CD13 inhibitor 2′2′-dipyridyl (2.5 mM) (DIP), or vehicle (Veh). The cells were pretreated with drugs starting at 72 h before infection and lasting until the end of the experiments. The cells were pretreated with 2′2′-dipyridyl starting at 1 h before infection. F: The time course of HCoV-229E RNA replication in HNE cells measured at different times after infection. G: HCoV-229E RNA replication in HNE cells pretreated with glycopyrronium (GLP), formoterol (FRM), ICI 118,551 plus formoterol (ICI + FRM), budesonide (BUD), a combination of the three drugs (GFB), or vehicle (Veh) at 72 h after infection. H: The minimum dose of virus necessary to cause infection in HNE cells treated with glycopyrronium (GLP), formoterol (FRM), ICI 118,551 plus formoterol (ICI + FRM), budesonide (BUD), a combination of the three drugs (GFB), or vehicle (Veh). A-H: The concentrations of glycopyrronium, formoterol, and budesonide were 100 nM. The results are presented as the mean ± SEM of five (A-D, F–H) or seven (E) subjects. Significant differences compared with cells pretreated with vehicle are indicated by *p < 0.05, **p < 0.01, and ***p < 0.001. Significant differences compared with cells treated with glycopyrronium, formoterol, and budesonide are indicated by ‡p < 0.05, †p < 0.05, and §p < 0.05, respectively.
Pretreatment with glycopyrronium (100 nM), formoterol (100 nM), and a combination of glycopyrronium, formoterol, and budesonide (GFB; 100 nM each drug) decreased the viral titers (Fig. 1A, 1B, 1D and 1E); however, treatment with budesonide alone (100 nM) did not decrease the viral titers (Fig. 1C and E).
The viral titers in the GFB-pretreated cells were lower than those in cells treated with glycopyrronium, formoterol, or budesonide alone (Fig. 1E).
The selective β2-adrenergic receptor antagonist ICI 118,551 (ICI, Sigma-Aldrich, MO, USA) [23] reversed the inhibitory effects of formoterol on viral titers, and the CD13 inhibitor 2′2′-dipyridyl [5] reduced the viral titers (Fig. 1E).
The peak titers in cells from asthmatic patients (5.11 ± 0.28 log10 TCID50/mL, n = 18; TCID: tissue culture infective dose), which were defined according to the 2017 Global Initiative for Asthma guidelines [10], or the titers from allergic rhinitis patients (4.80 ± 0.37 log10 TCID50/mL, n = 10), tended to be higher than those from patients without asthma and allergic rhinitis (4.36 ± 0.61 log10 TCID50/mL, n = 12). However, these differences were not significant (p > 0.10).
3.2. Effects on viral RNA expression
HCoV-229E RNA expression was consistent in HNE cells starting at 24 h after infection, increasing between 24 h and 72 h after infection, and was consistent again at 120 h (Fig. 1F).
Pretreatment with glycopyrronium, formoterol, and GFB decreased the HCoV-229E RNA levels at 72 h after infection, and ICI reversed the effects of formoterol (Fig. 1G). The HCoV-229E RNA levels in GFB-pretreated cells were lower than those in cells treated with glycopyrronium, formoterol, or budesonide alone (Fig. 1G).
3.3. Effects on susceptibility to viral infection
When the viral release was measured in ASL collected 72 h after infection, the minimum dose of HCoV-229E necessary to infect HNE cells pretreated with glycopyrronium, formoterol, or GFB was higher than the dose needed to infect the vehicle-treated cells. ICI reversed the effects of formoterol (Fig. 1H).
3.4. Concentration-dependent effects on HCoV-229E replication
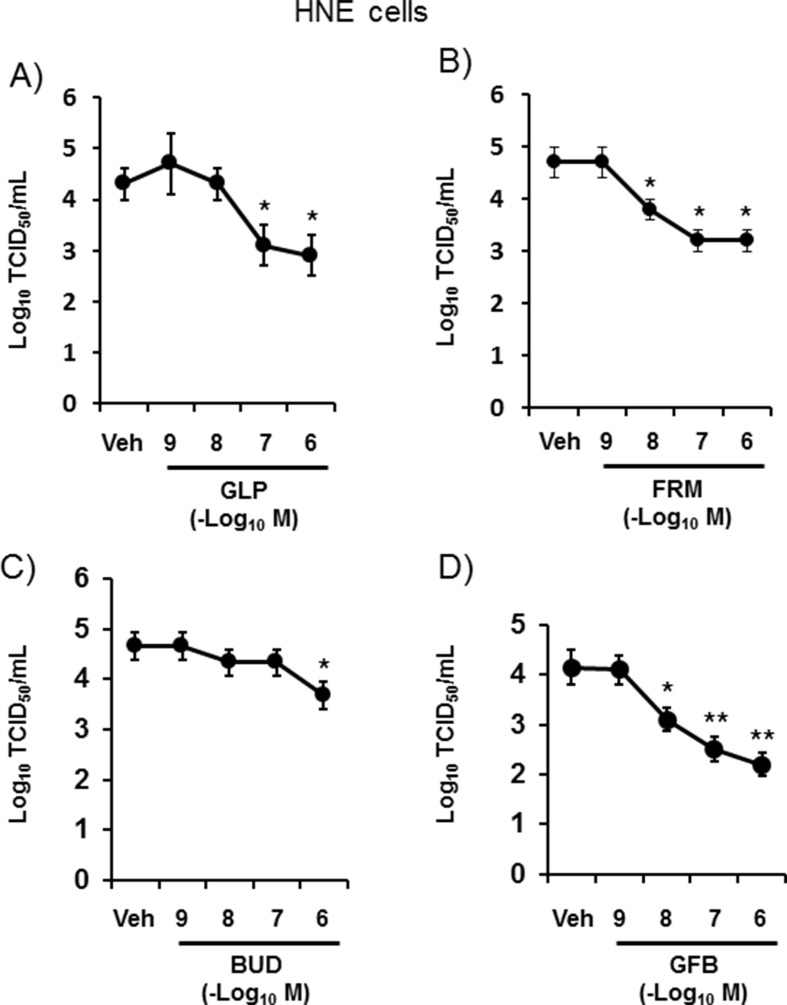
Pretreatment of HNE cells with glycopyrronium at a concentration of 100 nM or higher, with formoterol at 10 nM or higher, and with GFB at 10 nM or higher reduced the HCoV-229E titers in a concentration-dependent manner (Fig. 2 ). Pretreatment with budesonide at concentrations less than 1 μM did not reduce the HCoV-229 E titers.
Fig. 2.
Concentration-dependent effects of pretreatment with glycopyrronium (A) (GLP), formoterol (B) (FRM), budesonide (C) (BUD), a combination of these three drugs (D) (GFB), or vehicle (Veh) on HCoV-229E release into ASL collected between 24 h and 72 h after infection. The results are presented as the mean ± SEM of five subjects. Significant differences compared with cells pretreated with vehicle are indicated by *p < 0.05 and **p < 0.01.
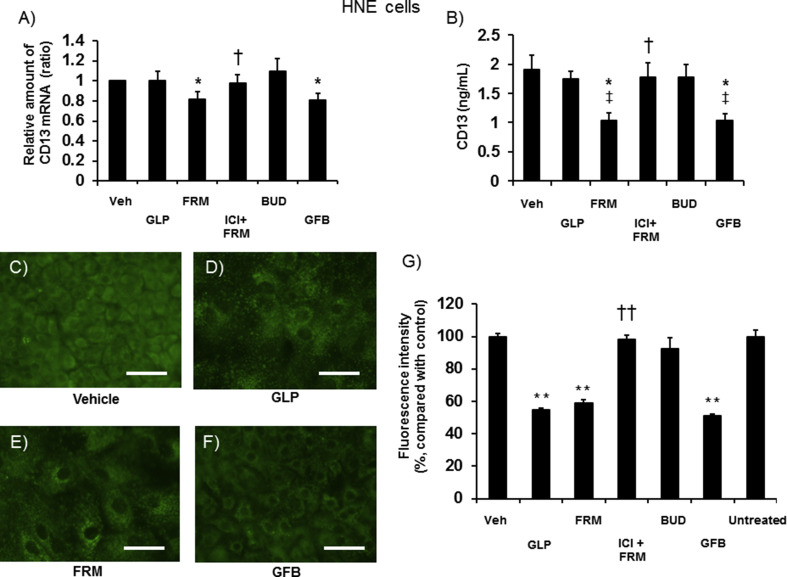
3.5. Effects on CD13 expression
Uninfected HNE cells expressed a significant amount of CD13 mRNA. CD13 protein was detected in the ASL (Fig. 3 A and B).
Fig. 3.
A and B: CD13 mRNA (A) and protein concentrations in the ASL (B) of uninfected HNE cells pretreated with glycopyrronium (GLP), formoterol (FRM), ICI 118,551 (1 μM) plus formoterol (100 nM) (ICI + FRM), budesonide (BUD), a combination of these three drugs (GFB), or vehicle (Veh) for 72 h. C–F: Distribution of acidic endosomes exhibiting green fluorescence in HNE cells at 72 h after pretreatment with glycopyrronium (GLP) (D), formoterol (FRM) (E), a combination of the three drugs (GFB) (F), or vehicle (C). Data are representative of four different experiments. Scale bar = 100 μm. G: The fluorescence intensity of acidic endosomes in HNE cells at 72 h after pretreatment with glycopyrronium (GLP), formoterol (FRM), ICI 118,551 plus formoterol (ICI + FRM), budesonide (BUD), a combination of the three drugs (GFB), or vehicle (Veh), and in untreated cells (Untreated). The mean value of the fluorescence intensity of the vehicle-treated cells was set to 100%. A–G: The concentrations of glycopyrronium, formoterol, and budesonide were 100 nM. The results are presented as the mean ± SEM of five (A and B) or eight (G) subjects. Significant differences compared with cells pretreated with vehicle are indicated by *p < 0.05 and **p < 0.01 (A, B, and G). Significant differences compared with cells pretreated with formoterol are indicated by †p < 0.05 and ††p < 0.01 (A, B, and G). Significant differences compared with cells pretreated with glycopyrronium are indicated by ‡p < 0.05 (B).
The mRNA and protein levels of CD13 in cells treated with formoterol or GFB were lower than those in vehicle-treated cells (Fig. 3A and B). Treatment with glycopyrronium or budesonide alone did not affect CD13 expression.
3.6. Effects on the acidification of endosomes
The treatment of HNE cells with glycopyrronium, formoterol, and GFB reduced the number and fluorescence intensity of acidic endosomes (Fig. 3C–3G). ICI reversed the effects of formoterol (Fig. 3G).
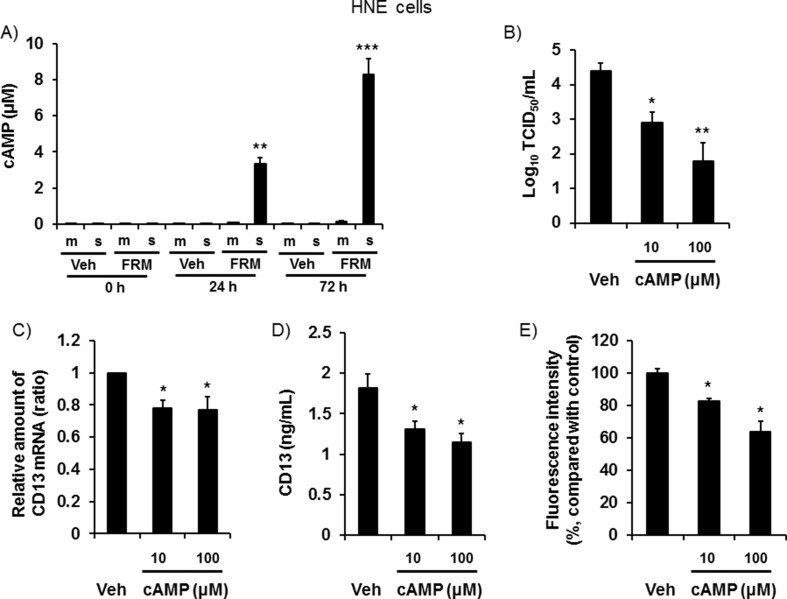
3.7. Effects of formoterol on cAMP production and the effects of cAMP on viral replication
The cAMP levels in basolateral supernatants increased to approximately 8 μM at 72 h after treatment with formoterol (Fig. 4 A). Furthermore, pretreatment with cAMP (10 μM and 100 μM) reduced the viral titers, CD13 mRNA and protein levels, as well as the fluorescence intensity, of the acidic endosomes (Fig. 4B–4E).
Fig. 4.
A: Time course of the cAMP concentration in ASL (m) or basolateral supernatant (s) of uninfected HNE cells treated with formoterol (FRM, 100 nM) or vehicle (Veh) collected before and at 24 h and 72 h after pretreatment. B: Viral titers in ASL from HNE cell cultures pretreated with cAMP (10 μM or 100 μM) or vehicle (Veh) collected between 24 h and 72 h after infection. C and D: The CD13 mRNA (C) and protein concentrations in the ASL (D) of uninfected HNE cells pretreated with cAMP (10 μM or 100 μM) or vehicle (Veh) for 72 h. E: The fluorescence intensity of acidic endosomes in HNE cells at 72 h after pretreatment with cAMP (10 μM or 100 μM) or vehicle (Veh). The mean value of the fluorescence intensity of the vehicle-treated cells was set to 100%. A–E: The results are presented as the mean ± SEM of four (A, C-E) or five (B) subjects. Significant differences compared with cells pretreated with vehicle are indicated by *p < 0.05, **p < 0.01, and ***p < 0.001.
3.8. Effects on cytokine production
The levels of IL-6 and IL-8 in the ASL of uninfected HNE cells cultured for 24 h or 72 h did not differ from those at time 0 (Fig. 5 A and B). HCoV-229E infection increased IL-6 levels in cells cultured for 24 and 72 h and IL-8 levels in cells cultured for 72 h (Fig. 5A and B). The NF-κB inhibitor caffeic acid phenethyl ester (CAPE) (25 μg/mL) (Calbiochem, CA, USA) [24] reduced the levels of IL-6 and IL-8 after infection (Fig. 5A and B).
Fig. 5.
A and B: Time course of the release of IL-6 (A) and IL-8 (B) into the ASL of HNE cells before (time 0) and after infection with HCoV-229E in the presence or absence of the NF-κB inhibitor CAPE or sham treatment (medium) (Med). HNE cells were pretreated with CAPE (25 μg/mL) starting at 2 h before infection and lasting until the end of the experiments. The results are reported as the mean ± SEM of cells from five different subjects. Significant differences compared with sham (medium)-infected cells are indicated by *p < 0.05 and **p < 0.01. Significant differences compared with cells infected with HCoV-229E alone (HCoV) at 72 h after infection are indicated by †p < 0.05. C and D: The release of IL-6 (C) and IL-8 (D) into the ASL of HNE cells pretreated with glycopyrronium (GLP), formoterol (FRM), budesonide (BUD), a combination of the three drugs (GFB), ICI 118,551 plus formoterol (ICI + FRM), or vehicle collected before and between 24 h and 72 h after infection with HCoV-229E or after sham infection (Sham). E–G: The mRNA expression of IFN-β (E), IFN-λ1 (F), or IFN-γ (G) in HNE cells pretreated with glycopyrronium (GLP), formoterol (FRM), budesonide (BUD), a combination of the three drugs (GFB), ICI plus formoterol (ICI + FRM), or vehicle (Veh) collected before and at 72 h after infection with HCoV-229E or sham infection (Sham). C–G: The results are reported as the mean ± SEM of cells from five different subjects. Significant differences compared with the values from cells treated with vehicle (Veh) before infection are indicated by *p < 0.05 and **p < 0.01. Significant differences compared with the values from cells infected with HCoV-229E alone in the presence of vehicle (Veh) are indicated by †p < 0.05 and ††p < 0.01. Significant differences compared with the values from cells pretreated with glycopyrronium, formoterol, and budesonide after infection are indicated by ‡p < 0.05, §p < 0.05, and ¶p < 0.05, respectively.
Before infection, pretreatment with formoterol, budesonide, or GFB, but not glycopyrronium, reduced the baseline secretion of IL-6 and IL-8 compared with that in the vehicle-treated cells (Fig. 5C and D).
Pretreatment with glycopyrronium, formoterol, budesonide, and GFB reduced the infection-induced secretion of IL-6 and IL-8 compared with that of vehicle-treated cells (Fig. 5C and D). The IL-6 and IL-8 concentrations in GFB-treated cells were lower than those in cells treated with glycopyrronium, formoterol, or budesonide alone (Fig. 5C and D).
The mRNA levels of IFN-β, IFN-λ1, and IFN-γ in HNE cells increased after infection. Pretreatment with glycopyrronium, formoterol, budesonide, and GFB decreased these levels (Fig. 5E–G). In contrast, the concentrations of IFN-β, IFN-λ1, and IFN-γ in ASL and the basolateral supernatants were below the detection limits (7.8 pg/mL for IFN-β, 62.5 pg/mL for IFN-λ1, and 15.6 pg/mL for IFN-γ) before and after infection.
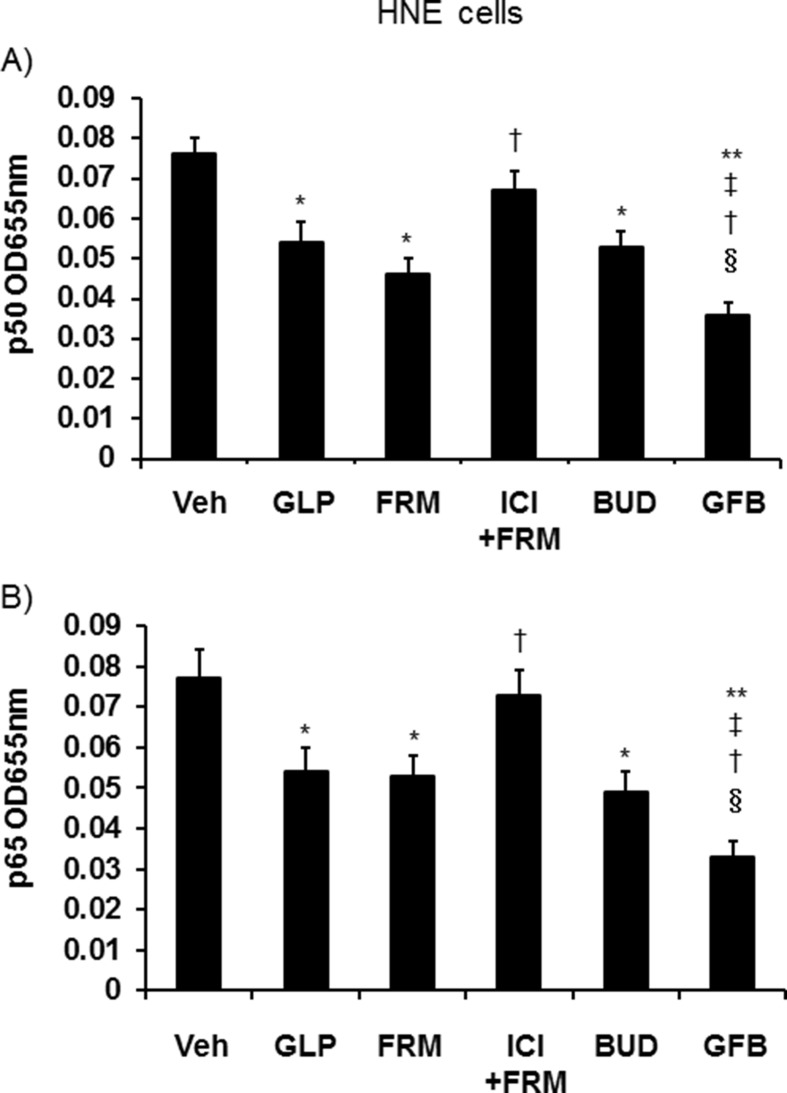
3.9. Effects on NF-κB
Glycopyrronium, formoterol, and budesonide treatment of uninfected HNE cells reduced the amounts of NF-κB p50 and p65 in the nuclear extracts compared with those in the vehicle-treated cells. The effects of GFB were additive (Fig. 6 ).
Fig. 6.
Levels of p50 (A) and p65 (B) in nuclear extracts of uninfected HNE cells treated with glycopyrronium (GLP), formoterol (FRM), ICI 118,551 plus formoterol (ICI + FRM), budesonide (BUD), and a combination of glycopyrronium, formoterol, and budesonide (GFB), or vehicle (Veh) for 72 h. HNE cells were cultured in 24-well dishes. The results are expressed as the OD and the mean ± SEM of five samples. Significant differences compared with the values from vehicle-treated cells (Veh) are indicated by *p < 0.05 and **p < 0.01. Significant differences compared with the values from cells treated with glycopyrronium, formoterol, and budesonide are indicated by ‡p < 0.05, †p < 0.05, and §p < 0.05, respectively.
3.10. Effects on HTE cells
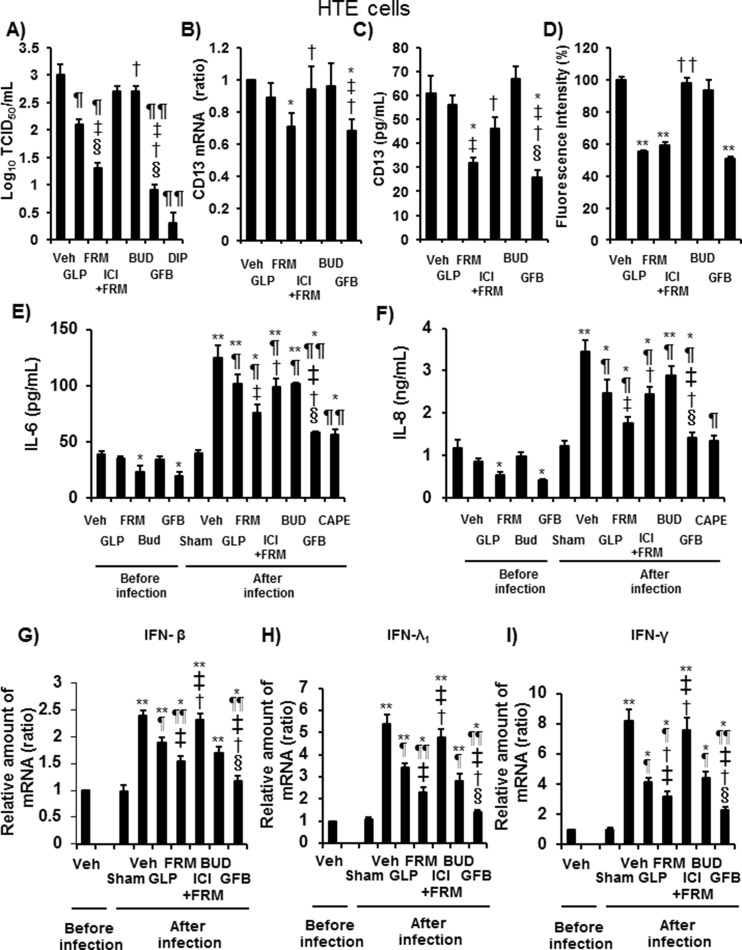
We also confirmed the effects of the drugs on HCoV-229E replication in HTE cells. Pretreatment with glycopyrronium (100 nM), formoterol (100 nM), or GFB (100 nM) and 2′2′-dipyridyl (2.5 mM) decreased the HCoV-229E titers. Moreover, the viral titers of the GFB-treated cells were lower than those of cells pretreated with glycopyrronium, formoterol, or budesonide alone (all 100 nM) (Fig. 7 A).
Fig. 7.
A: The viral titers in ASL collected between 24 h and 72 h after the infection of HTE cells pretreated with glycopyrronium (GLP), formoterol (FRM), the selective β2-adrenergic receptor antagonist ICI 118,551 (1 μM) plus formoterol (100 nM) (ICI + FRM), budesonide (BUD), a combination of these three drugs (GFB), the CD13 inhibitor 2′2′-dipyridyl (2.5 mM) (DIP), or vehicle (Veh). The cells were pretreated with drugs starting at 72 h before infection and lasting until the end of the experiments. The cells were pretreated with 2′2′-dipyridyl starting at 1 h before infection. B–D: CD13 mRNA (B) and protein concentrations in the ASL (C) of uninfected HTE cells and the fluorescence intensity of acidic endosomes in HTE cells (D) after pretreatment with glycopyrronium (GLP), formoterol (FRM), ICI 118,551 plus formoterol (ICI + FRM), budesonide (BUD), a combination of these three drugs (GFB), or vehicle (Veh) for 72 h. The mean value of the fluorescence intensity of the vehicle-treated cells was set to 100%. E and F: The release of IL-6 (E) and IL-8 (F) into the ASL of HTE cells pretreated with glycopyrronium (GLP), formoterol (FRM), ICI 118,551 plus formoterol (ICI + FRM), budesonide (BUD), a combination of three drugs (GFB), or vehicle (Veh) collected before and between 24 h and 72 h after infection with HCoV-229E or sham infection (Sham). G–I: The mRNA expression of IFN-β (G), IFN-λ1 (H), or IFN-γ (I) in HTE cells pretreated with glycopyrronium (GLP), formoterol (FRM), ICI plus formoterol (ICI + FRM), budesonide (BUD), a combination of the three drugs (GFB), or vehicle (Veh) extracted before and at 72 h after infection with HCoV-229E or after sham infection (Sham). A–I: The concentrations of glycopyrronium, formoterol, and budesonide were 100 nM. The results are reported as the mean ± SEM for cells of five (A-C, E-I) or seven (D) different subjects. A: Significant differences compared with the values of HTE cells after infection that were treated with vehicle, glycopyrronium, formoterol, or budesonide are indicated by ¶p < 0.05 and ¶¶p < 0.01, ‡p < 0.05, †p < 0.05, or §p < 0.01, respectively. B–D: Significant differences compared with the values of uninfected HTE cells treated with vehicle, glycopyrronium, formoterol, or budesonide are indicated by *p < 0.05 and **p < 0.05, ‡p < 0.05, †p < 0.05 and ††p < 0.01, or §p < 0.01, respectively. E–I: Significant differences compared with the values from uninfected vehicle-treated HTE cells are indicated by *p < 0.05 and **p < 0.01. Significant differences compared with the values of HTE cells after infection treated with vehicle, glycopyrronium, formoterol, or budesonide are indicated by ¶p < 0.05 and ¶¶p < 0.01, ‡p < 0.05, †p < 0.05, or §p < 0.01, respectively.
The mRNA and ASL protein levels of CD13 in uninfected HTE cells pretreated with formoterol or GFB were lower than those in vehicle-treated cells (Fig. 7B and C).
Pretreatment with glycopyrronium, formoterol, or GFB decreased the fluorescence intensity of the acidic endosomes compared with that of vehicle-treated cells (Fig. 7D).
Before infection, pretreatment with formoterol or GFB reduced the baseline secretion of IL-6 and IL-8 compared with that of vehicle-treated cells (Fig. 7E and F).
HCoV-229E infection increased the IL-6 levels at 24 and 72 h and IL-8 levels at 72 h after infection (data at 24 h not shown). Pretreatment with glycopyrronium, formoterol, budesonide, or GFB reduced the infection-induced increase in IL-6 and IL-8 secretion compared with that of the vehicle (Fig. 7E and F). The IL-6 and IL-8 concentrations in GFB-treated cells were lower than those in cells treated with glycopyrronium, formoterol, or budesonide alone (Fig. 7E and F). CAPE (25 μg/mL) reduced the infection-induced increase in IL-6 and IL-8 secretion (Fig. 7E and F).
ICI (1 μM) reversed the inhibitory effects of formoterol on viral titers, mRNA, and protein levels of CD13, acidic endosomes, and the production of IL-6 and IL-8 (Fig. 7A–7F).
The mRNA levels of IFN-β, IFN-λ1, and IFN-γ in HTE cells increased after infection. Pretreatment with glycopyrronium, formoterol, budesonide, and GFB decreased these levels (Fig. 7G-I). In contrast, the protein concentrations of IFN-β, IFN-λ1, and IFN-γ in ASL and the basolateral supernatant were below the detection limits.
4. Discussion
We demonstrated that pretreatment of primary HNE and HTE cell cultures with glycopyrronium, formoterol, or a combination of the three drugs (glycopyrronium, formoterol, and budesonide; GFB) reduced HCoV-229E replication and cytokine (IL-6 and IL-8) concentrations in ASL. The selective β2-adrenergic receptor antagonist ICI 118,551 reversed the inhibitory effects of formoterol. Formoterol and GFB both reduced the expression of CD13, the HCoV-229E receptor [5]. An inhibitor of CD13, 2, 2′-dipyridyl, reduced the viral titers. These findings suggest that these drugs inhibit HCoV-229E replication partly via the regulation of receptor expression.
There was a reduction in the HCoV-229E titer and CD13 expression in HNE cells pretreated with cAMP at similar levels to those secreted into the supernatant when cells were treated with formoterol. The inhibitory effects of formoterol on CD13 expression were consistent with the results of a previous report by Danielsen et al., which found that CD13 expression in the intestinal mucosa was decreased by forskolin, an activator of adenylate cyclase [25], which is activated by formoterol [26]. Therefore, formoterol may decrease HCoV-229E replication partly by modulating receptor expression via cAMP production.
In addition, pretreatment with glycopyrronium, formoterol, and GFB reduced the number and fluorescence intensity of acidic endosomes where HCoV-229E RNA enters the cytoplasm [7]. Therefore, these drugs may also inhibit HCoV-229E replication by inhibiting the functioning of acidic endosomes.
Endosomal pH is regulated by the vacuolar H+-ATPase [27] and ion transport across Na+/H+ exchangers [28]. cAMP increases endosomal pH by inhibiting an Na+/H+ exchanger [29]. We demonstrated that formoterol increased the cAMP levels in HNE cells, which decreased the number of acidic endosomes, as previously reported for HTE cells [30]. Therefore, formoterol may inhibit the functioning of Na+/H+ exchangers via the production of cAMP in cells.
Furthermore, acetylcholine activates H+/K+-ATPase in gastric cells [31], while bafilomycin A1, an inhibitor of H+-ATPase [32], inhibits the acetylcholine-induced increase in cytoplasmic pH [33]. Therefore, glycopyrronium may inhibit the activity of H+/K+-ATPase and/or H+-ATPases in airway epithelial cells.
The glycopyrronium concentration in the lungs is estimated to be 19.5 ng/mL (49 nM), according to the delivery rate in the lungs [34,35]. Furthermore, we found that formoterol and GFB decreased the viral titers at a concentration of 10 nM. Similar levels of plasma formoterol have been reported previously in the literature [36]. Therefore, glycopyrronium and formoterol may reduce HCoV-229E replication at safe clinical doses.
IL-6 and IL-8 are related to airway inflammation in COPD and bronchial asthma exacerbation induced by viral infection [4,13]. The decreased production of IL-6 and IL-8 in cells pretreated with glycopyrronium, formoterol, and budesonide, as well as the increased inhibitory effects of GFB observed in the present study, may be associated with the inhibitory effects of these drugs on COPD and bronchial asthma exacerbation [9,10].
In the present study, significant mRNA levels of IFN-β, IFN-λ1 and IFN-γ were detected in cells, the production of which was reduced by treatment with drugs, although the concentrations of IFN-β, IFN-λ1, and IFN-γ in the ASL and basolateral supernatant were undetectable. These findings are consistent with those of a previous study demonstrating that rhinoviral infection induced innate immunity by stimulating the interferon system in human airway epithelial cells and that treatment with fluticasone reduced IFN production [37]. The reduced production of IFN-β, IFN-λ1, and IFN-γ could be associated with decreased HCoV-229E replication or the suppression of toll-like receptor 3 [37,38].
Acetylcholine induces NF-κB activation [39], which is associated with cytokine production [39,40]. Formoterol and budesonide have been shown to inhibit NF-κB activity [11]. In addition, the NF-κB inhibitor CAPE decreased the production of IL-6 and IL-8 after HCoV-229E infection. Therefore, reduced NF-κB activation could be associated with the inhibition of cytokine production by the drugs used in the present study.
Budesonide did not decrease the HCoV-229E titers and CD13 expression; however, budesonide inhibits rhinoviral replication by reducing the expression of its receptor, ICAM-1 [11,41]. Although the subjects from whom nasal tissues were obtained were treated with various drugs, including corticosteroids, pretreatment with budesonide did not reduce HCoV-229E replication in HTE cells from patients who were not treated with corticosteroids. Sorrell et al. demonstrated that corticosteroids do not reduce CD13 expression in fibroblasts [42]. Therefore, the different effects of budesonide on receptor expression may lead to differences in the effects on the replication of HCoV-229E and rhinoviruses.
HNE and HTE cells have been used in several studies of respiratory viral infection [[43], [44], [45]]. Here, we used HNE and HTE cells because we previously used these cells in infection studies [11,12,15,46]. However, because bronchi and alveoli are the main sites of the pathogenesis of COPD and bronchial asthma exacerbations, bronchial and alveolar epithelial cells were used to reveal the effects of viral infection on exacerbations and the effects of drugs [37,47]. Therefore, further studies using bronchial and alveolar epithelial cells will be needed to confirm HCoV-229E replication and the effects of drugs.
5. Conclusions
This study is the first to demonstrate that glycopyrronium, formoterol, and a combination of glycopyrronium, formoterol, and budesonide may reduce HCoV-229E replication and the secretion of cytokines from human nasal and tracheal epithelial cells. Our findings suggest that these drugs are able to modulate airway inflammation after HCoV-229E infection.
Declaration of Competing Interest
This work was supported by a research support grant from AstraZeneca KK (NCR-17-12892). Glycopyrronium, formoterol, and budesonide were obtained from AstraZeneca PLC.
Acknowledgements
We thank the staff at the Biomedical Research Unit and the Department of Pathology at Tohoku University Hospital for providing technical support.
References
- 1.El-Sahly H.M., Atmar R.L., Glezen W.P., Greenberg S.B. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shirato K., Kawase M., Watanabe O., Hirokawa C., Matsuyama S., Nishimura H. Differences in neutralizing antigenicity between laboratory and clinical isolates of HCoV-229E isolated in Japan in 2004- 2008 depend on the S1 region sequence of the spike protein. J Gen Virol. 2012;93:1908–1917. doi: 10.1099/vir.0.043117-0. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson K.G., Kent J., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seemungal T., Harper-Owen R., Bhowmik A., Moric I., Sanderson G., Message S. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 5.Yeager C.L., Ashmum R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram S., Dijkman R., Habjan M., Heurich A., Gierer S., Glowacka I. TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium. J Virol. 2013;87:6150–6160. doi: 10.1128/JVI.03372-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawase M., Shirato K., Matsuyama S., Taguchi F. Protease-mediated entry via the endosome of human coronavirus 229E. J Virol. 2009;83:712–721. doi: 10.1128/JVI.01933-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirato K., Kanou K., Kawase M., Matsuyama S. Clinical isolates of human coronavirus 229E bypass the endosome for cell entry. J Virol. 2016;91:e01387. doi: 10.1128/JVI.01387-16. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global Initiative for Obstructive Lung Disease . 2019. GOLD 2019 report global strategy for the diagnosis, management and prevention of COPD.https://goldcopd/org [Google Scholar]
- 10.U.S. Department . Global initiative for asthma: global strategy for asthma management and prevention. 2017. Of health and human services, national institutes of health, national heart, lung, and blood institute.www.ginasthma.org [Google Scholar]
- 11.Yamaya M., Nishimura H., Nadine L., Kubo H., Nagatomi R. Formoterol and budesonide inhibit rhinovirus infection and cytokine production in primary cultures of human tracheal epithelial cells. Respir Investig. 2014;52:251–260. doi: 10.1016/j.resinv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Yamaya M., Nishimura H., Hatachi Y., Yasuda H., Deng X., Sasaki T. Inhibitory effects of tiotropium on rhinovirus infection in human airway epithelial cells. Eur Respir J. 2012;40:122–132. doi: 10.1183/09031936.00065111. [DOI] [PubMed] [Google Scholar]
- 13.Barnes P.J. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allegy Clin Immunol. 2015;136:531–545. doi: 10.1016/j.jaci.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 14.Yamaya M., Finkbeiner W.E., Chun S.Y., Widdicombe J.H. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 15.Lusamba Kalonji N., Nomura K., Kawase T., Ota C., Kubo H., Sato T. The non-antibiotic macrolide EM900 inhibits rhinovirus infection and cytokine production in human airway epithelial cells. Phys Rep. 2015;3 doi: 10.14814/phy2.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condit R.C. Principles of virology. In: Knipe D.M., Howley P.M., editors. Fields virology. sixth ed. Lippincott Williams and Wilkins; Philadelphia, PA: 2013. pp. 21–51. [Google Scholar]
- 17.Nakayama K., Jia Y.X., Hirai H., Shinkawa M., Yamaya M., Sekizawa K. Acid stimulation reduces bactericidal activity of surface liquid in cultured human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:105–113. doi: 10.1165/ajrcmb.26.1.4425. [DOI] [PubMed] [Google Scholar]
- 18.van Elden L.J., van Loon A.M., van Alphen F., Hendriksen K.A., Hoepelman A.I., van Kraaij M.G. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. J Infect Dis. 2004;189:652–657. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wentworth D.E., Holmes K.V. Molecular determinants of species specificity in the coronavirus receptor aminopeptidase N (CD13): influence of N-linked glycosylation. J Virol. 2001;75:9741–9752. doi: 10.1128/JVI.75.20.9741-9752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathinam C., Poueymirou W.T., Rojas J., Murphy A.J., Valenzuela D.M., Yancopoulos G.D. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood. 2011;118:3119–3128. doi: 10.1182/blood-2010-12-326926. [DOI] [PubMed] [Google Scholar]
- 21.Comar C.E., Goldstein S.A., Li Y., Yount B., Baric R.S., Weiss S.R. Antagonism of dsRNA-induced innate immune pathways by NS4a and NS4b accessory proteins during MERS coronavirus infection. mBio. 2019;10 doi: 10.1128/mBio.00319-19. : pii: e00319-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munk R.B., Sugiyama K., Ghosh O., Sasaki C.Y., Rezanka L., Banerjee K. Antigen-independent IFN-γ production by human naïve CD4 T cells activated by IL-12 plus IL-18. PloS One. 2011;6 doi: 10.1371/journal.pone.0018553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilski A.J., Halliday S.E., Fitzgerald J.D., Wale J.L. The pharmacology of a β2-selective adrenoceptor antagonist (ICI 118,551) J Cardiovasc Pharmacol. 1983;5:430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Natarajan K., Singh S., Burke T.R., Jr., Grunberger D., Aggarwal B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Nat Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danielsen E.M., Hansen G.H., Cowell G.M. Biosynthesis of intestinal microvillar proteins. Forskolin reduces surface expression of aminopeptidase N. Eur J Cell Biol. 1987;44:273–277. [PubMed] [Google Scholar]
- 26.Tescemacher A., Lemoine H. Kinetic analysis of drug-receptor interactions of long-acting β2 sympathomimetics in isolated receptor membranes: evidence against prolonged effects of salmeterol and formoterol on receptor-coupled adenylyl cyclase. J Pharmacol Exp Therapeut. 1999;288:1084–1092. [PubMed] [Google Scholar]
- 27.Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 28.Nass R., Rao R. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J Biol Chem. 1998;273:21054–21060. doi: 10.1074/jbc.273.33.21054. [DOI] [PubMed] [Google Scholar]
- 29.Gekle M., Serrano O.K., Drumm K., Mildenberger S., Freudinger R., Gassner B. NHE3 serves as a molecular tool for cAMP-mediated regulation of receptor-mediated endocytosis. Am J Physiol. 2002;283:F549–F558. doi: 10.1152/ajprenal.00206.2001. [DOI] [PubMed] [Google Scholar]
- 30.Yamaya M., Nishimura H., Nadine L., Kubo H., Nagatomi R. Tulobuterol inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. Phys Rep. 2013;1 doi: 10.1002/phy2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao X., Forte J.G. Cell biology of acid secretion by the parietal cell. Annu Rev Physiol. 2003;65:103–131. doi: 10.1146/annurev.physiol.65.072302.114200. [DOI] [PubMed] [Google Scholar]
- 32.Bowman E.J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou Y., Wu Q., Delamere N.A. H+-ATPase-mediated cytoplasmic pH-responses associated with elevation of cytoplasmic calcium in cultured rabbit nonpigmented ciliary epithelium. J Membr Biol. 2001;182:81–90. doi: 10.1007/s00232-001-0028-y. [DOI] [PubMed] [Google Scholar]
- 34.Colthorpe P., Voshaar T., Kieckbusch T., Cuoghi E., Jauemig J. Delivery characteristics of a low-resistance dry-powder inhaler used to deliver the long-acting muscarinic antagonist glycopyrronium. J Drug Assess. 2013;2:11–16. doi: 10.3109/21556660.2013.766197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis B., Morris T. Physiological parameters in laboratory animals and humans. Pharm Res (N Y) 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 36.Campestrini J., Lecaillon J.B., Godbillon J. Automated and sensitive method for the determination of formoterol in human plasma by high-performance liquid chromatography and electrochemical detection. J Chromatogr B. 1997;704:221–229. doi: 10.1016/s0378-4347(97)00425-8. [DOI] [PubMed] [Google Scholar]
- 37.Singanayagam A., Glanville N., Girkin J.L., Ching Y.M., Marcellini A., Porter J.D. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat Commun. 2018;9:2229. doi: 10.1038/s41467-018-04574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slater L., Bartlett N.W., Haas J.J., Zhu J., Message S.D., Walton R.P. Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Profita M., Bonanno A., Siena L., Ferraro M., Montalbano A.M., Pompeo F. Acetylcholine mediates the release of IL-8 in human bronchial epithelial cells by a NFkB/ERK-dependent mechanism. Eur J Pharmacol. 2008;582:145–153. doi: 10.1016/j.ejphar.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Z., Tang W., Ray A., Wu Y., Einarsson O., Landry M.L. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor κB-dependent transcriptional activation. J Clin Invest. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greve J.M., Davis G., Meyer A.M., Forte C.P., Yost S.C., Marlor C.W. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 42.Sorrell J.M., Brinon N., Baber M.A., Caplan A.I. Cytokines and glucocorticoids differentially regulate APN/CD13 and DPPIV/CD26 enzyme activities in cultured human dermal fibroblasts. Arch Dermatol Res. 2003;295:160–168. doi: 10.1007/s00403-003-0417-4. [DOI] [PubMed] [Google Scholar]
- 43.Roberts N., Al Mubarak R., Francisco D., Kraft M., Chu H.W. Comparison of paired human nasal and bronchial airway epithelial cell responses to rhinovirus infection and IL-13 treatment. Clin Transl Med. 2018;7:13. doi: 10.1186/s40169-018-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pech M., Weckmann M., König I.R., Franke A., Heinsen F.A., Oliver B. Rhinovirus infections change DNA methylation and mRNA expression in children with asthma. PloS One. 2018;13 doi: 10.1371/journal.pone.0205275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lachowicz-Scroggins M.E., Boushey H.A., Finkbeiner W.E., Widdicombe J.H. Interleukin-13-induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am J Respir Cell Mol Biol. 2010;43:652–661. doi: 10.1165/rcmb.2009-0244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaya M., Nomura K., Arakawa K., Nishimura H., Lusamba Kalonji N., Kubo H. Increased rhinovirus replication in nasal mucosa cells in allergic subjects is associated with increased ICAM-1 levels and endosomal acidification and is inhibited by L-carbocisteine. Immun Inflamm Dis. 2016;4:166–181. doi: 10.1002/iid3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rider C.F., Miller-Larsson A., Proud D., Giembycz M.A., Newton R. Modulation of transcriptional responses by poly (I:C) and human rhinovirus: effect of long-acting β2-adrenoceptor agonists. Eur J Pharmacol. 2013;708:60–67. doi: 10.1016/j.ejphar.2013.02.056. [DOI] [PubMed] [Google Scholar]