Abstract
WHO interim guidelines recommend offering extracorporeal membrane oxygenation (ECMO) to eligible patients with acute respiratory distress syndrome (ARDS) related to coronavirus disease 2019 (COVID-19). The number of patients with COVID-19 infection who might develop severe ARDS that is refractory to maximal medical management and require this level of support is currently unknown. Available evidence from similar patient populations suggests that carefully selected patients with severe ARDS who do not benefit from conventional treatment might be successfully supported with venovenous ECMO. The need for ECMO is relatively low and its use is mostly restricted to specialised centres globally. Providing complex therapies such as ECMO during outbreaks of emerging infectious diseases has unique challenges. Careful planning, judicious resource allocation, and training of personnel to provide complex therapeutic interventions while adhering to strict infection control measures are all crucial components of an ECMO action plan. ECMO can be initiated in specialist centres, or patients can receive ECMO during transportation from a centre that is not specialised for this procedure to an expert ECMO centre. Ensuring that systems enable safe and coordinated movement of critically ill patients, staff, and equipment is important to improve ECMO access. ECMO preparedness for the COVID-19 pandemic is important in view of the high transmission rate of the virus and respiratory-related mortality.
Introduction
The WHO-declared pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is causing clusters of fatal pneumonia from coronavirus disease 2019 (COVID-19), with reports of some patients receiving extracorporeal membrane oxygenation (ECMO) support.1, 2, 3, 4 WHO interim guidelines5 for the management of suspected COVID-19 recommend administering venovenous ECMO to eligible patients with COVID-19-related acute respiratory distress syndrome (ARDS) in expert centres with sufficient case volumes to ensure clinical expertise. However, its effectiveness will be influenced by the initial experience and preparedness of the health-care system.6 Although there are observational data on the use of rescue ECMO during previous outbreaks of emerging infectious diseases, mainly in the context of influenza A(H1N1) ARDS, the real need for ECMO during such outbreaks is unclear. For reference, the incidence of ECMO use in patients with 2009 influenza A(H1N1) ARDS in Australia and New Zealand was estimated to be 2·6 cases per million, whereas 5·8% of the critically ill patients in Saudi Arabia were supported with ECMO for Middle East respiratory syndrome coronavirus-related ARDS.7, 8 Therefore, the overall impact of the COVID-19 outbreak on potential ECMO use is currently unclear, but there could be an increased need for ECMO worldwide.
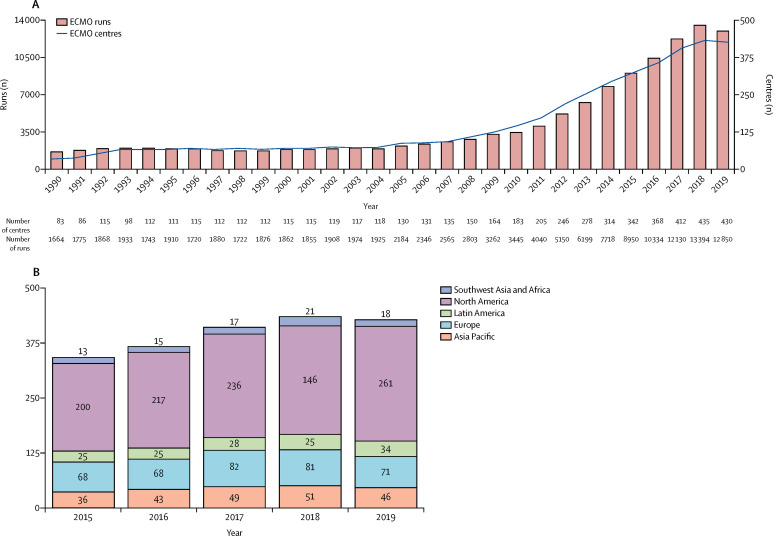
This outbreak is occurring at a time when worldwide ECMO infrastructure and resources for education9 and research10 are considerably more evolved and organised than they were a decade ago. Most importantly, there is now substantially more evidence to support the efficacy and safety of ECMO in patients with ARDS than during previous outbreaks.11, 12, 13, 14 Although during the 2009 influenza A(H1N1) pandemic few ECMO centres were included in the Extracorporeal Life Support Organization (ELSO) registry of active centres (figure 1 ),15 many were urgently established during the outbreak. Notably, despite a steady increase in ELSO-registered centres, there are many centres that are still not registered, which makes it challenging to assess the actual global ECMO capacity and capability.
Figure 1.
Global ECMO capacity according to ELSO
(A) Number of ECMO centres registered with ELSO and number of ECMO runs per year. (B) Geographical distribution of ELSO-registered ECMO centres as of January, 2020. Adapted from the ECMO registry of ELSO,15 by permission of ELSO. ECMO=extracorporeal membrane oxygenation. ELSO=Extracorporeal Life Support Organization.
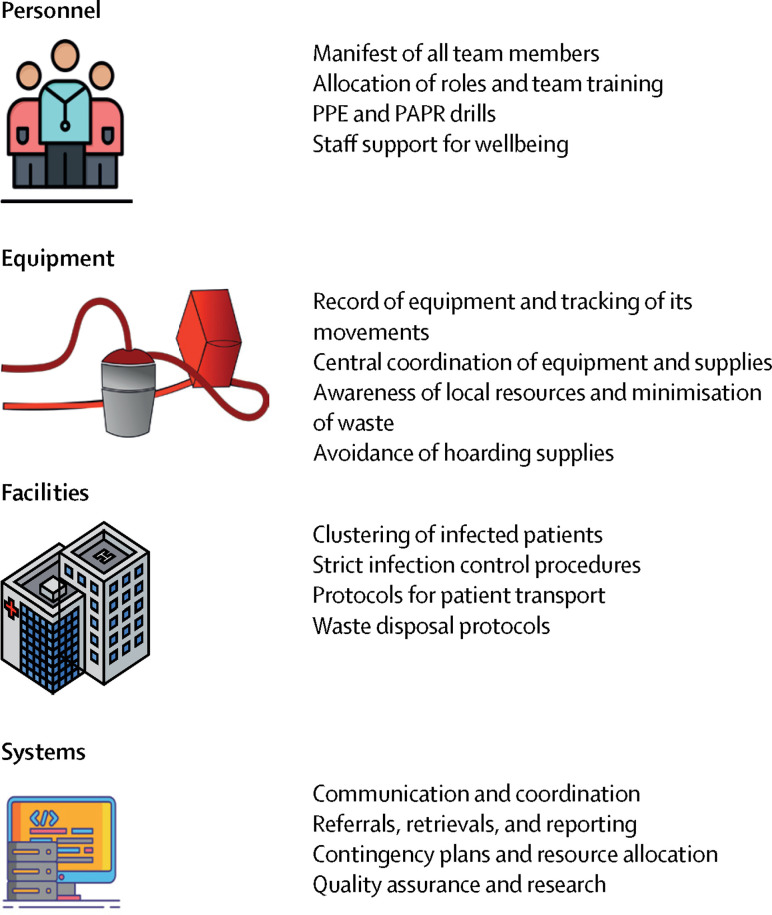
The planning of ECMO services during an outbreak of an emerging infectious disease such as COVID-19 can be broadly categorised into ensuring appropriate organisation of personnel, equipment, facilities, and systems (figure 2 ).16 As with any scarce resource in times of high demand, a mismatch in these factors can develop. During the 2009 influenza A(H1N1) pandemic, inadequate surge capacity highlighted the need for intensive-care reserves and improved health-care resource planning at all levels.17, 18 In an effort to improve ECMO preparedness19 for an outbreak of an emerging infectious disease, we highlight the ten key components of an ECMO action plan (panel ) based on these four categories, with practical recommendations for health centres to ensure appropriate training, capacity, and planning, depending on local priorities and resources. The focus is on building sustainable systems that ensure quality care for patients and their families and staff safety and wellbeing. Details on the organisation of ECMO programmes have been reported elsewhere. 20, 21
Figure 2.
Key elements for planning of ECMO services
Developing organisations that support systems, equipment, facilities, and personnel is crucial to ensure optimal patient care, as well as family and staff safety during outbreaks of emerging infectious diseases. PAPR=power air-purifying respirator. PPE=personal protective equipment.
Panel. Key components in the planning and provision of ECMO during an outbreak of an emerging infectious disease.
ECMO resource planning and allocation
Embed ECMO response into the broad epidemic response strategy; identify and engage all key stakeholders; maintain a log of multidisciplinary team members specifically trained in the care of patients receiving ECMO; maintain a contemporaneous log of ECMO machines serviced and ready to deploy; ensure supply of disposables and develop a regional tracking mechanism; prepare for surge of patients
Personnel assignment
Establish chain of command at ECMO centre level and regional level; formulate minimum and ideal staffing requirements; allocate roles in the ECMO process and allow for staff attrition; develop urgent mechanisms for ECMO credentials across institutions
Personnel training and ECMO use
Provide site-specific training and involve multidisciplinary team members; ensure correct use of PPE and the handling of infected bodily secretions; reinforce need for optimal conventional management of patients and review ECMO indications; simulate ECMO initiation and troubleshooting scenarios
Infection control measures before and during ECMO initiation
Group patients into cohorts for ECMO therapy and clearly mark all areas of increased precaution; ensure PPE stations are strategically placed to enhance compliance; strictly enforce all infection control protocols and PPE use for ECMO processes
Transport of patients receiving ECMO
Devise eligibility criteria for inter-hospital transfer and share them with referral centres; ensure effective communication and coordination; identify and address rate-limiting steps; strictly adhere to infection control protocols throughout patient transport
ECMO weaning, decannulation, and rehabilitation
Carry out ECMO weaning, decannulation and rehabilitation under strict infection control and personal protection measures; use caution while predicting native lung recovery on ECMO; arrange follow-up of patients who have been weaned from ECMO to ensure long-term outcomes
Post-mortem care
Map capacity of mortuary facilities in ECMO centres and review guidelines for post-mortem care; carry out post-mortem decannulation and transport of deceased under strict infection control and personal protection measures; follow national and international guidelines on disposal of infectious biological hazardous materials
Staff support
Routinely monitor staff for their health and wellbeing; put in place protocols for contact tracing; ensure provision of a team of psychiatrists, psychologists, and social workers to monitor and treat the psychological effects on staff; ensure that sick personnel are quarantined for 2 weeks with a clear follow-up and escalation plan in place; provide protocols for contact tracing of staff members
Ethical considerations
Use predetermined consensus criteria for rationing of ECMO if indicated; reassess all aspects of a patient's treatment plan regularly, including the need to continue or terminate ECMO; invoke distributive justice only in circumstances where rationing precludes the ability to care for each individual optimally; seek opinions from hospital ethics and medicolegal committees in ethically challenging scenarios
Quality assurance and collaborative research
Maintain quality assurance and clinical governance frameworks with frequent ECMO quality reviews; ensure collection and sharing of data to inform preparedness and patient care; aim to have ethics approval in place or develop mechanisms for expedient ethics review and approval processes
ECMO=extracorporeal membrane oxygenation. PPE=personal protective equipment.
ECMO resource planning and allocation
Essential elements
ECMO centres should maintain a dedicated manifest of all members of their multidisciplinary team who are specifically trained in the care of patients receiving ECMO. A contemporaneous log of machines serviced and ready to deploy should be maintained. Although stockpiling of ECMO-related equipment in specific centres might be appropriate, care must be taken not to hoard supplies. Hospital operations teams and regional tracking of disposables can help with the distribution of these supplies on the basis of need. All ECMO centres should have designated areas for putting on and removing of personal protective equipment (PPE) and power air-purifying respirators (PAPR). Regional planning should also predict and prepare for a surge of patients to designated expert ECMO centres.
Desirable elements
Clustering patients with COVID-19 requiring ECMO within an expert centre should be considered for ease of resource management.22 It would be desirable to have a regional outbreak response system that stratifies the extent of the disease spread, the risk of extended transmission, and the effect on the wider community23 to allow hospital authorities to allocate appropriate resources and reduce elective services and admissions within a suitable timeframe. As supply chains might be compromised by excessive demand, the components essential to operating an ECMO service should be centrally coordinated. However, individual centres must focus on conservation of scarce supplies to ensure readiness in case of surges in demand and to support neighbouring hospitals. This strategy will require frequent reporting by centres to the local or regional organising body.
In the event that ECMO centres exceed their capacity, a list of regional expert centres with contact details and directions that clearly delineate arrival areas with adequate infection control precautions should be readily available. All ECMO centres should identify key rate-limiting factors to target in the event of a surge in demand with respect to the four key categories of resource allocation (figure 2). It is probable that when all resources are stretched during an outbreak, the use of ECMO might be reconsidered and available resources targeted at caring for more patients with interventions that are less resource-intensive.6 Expert centres might consider offering ECMO services to less experienced centres by sending in a satellite team or using telemedicine24 until resources are re-established in the expert centre. A regional plan for monitoring available beds and supplies could assist in coordinating the distribution of transported patients to avoid overloading a referral site. For decisions involving resource allocation and complex inter-hospital transfers, a local and regional ECMO coordinator could help to strengthen this model of care.
Personnel assignment
Essential elements
The chain of command for dealing with high demand in every ECMO centre should be established and widely publicised with clear instructions, allocated roles, and appropriate simulation drills.25 The ECMO director would lead the team in managing patients receiving ECMO at an institutional level. The director should also identify teams of doctors, perfusionists, nurses, respiratory therapists, and physiotherapists who can be rostered in groups after ECMO has been initiated. Multidisciplinary team involvement with an infectious disease specialist should be seen as a minimum requirement to further patient management, given the need for subspecialty knowledge regarding testing, monitoring, therapeutics, and containment.26 We recommend that ECMO centres formulate a minimum and an ideal staffing requirement and communicate them to the local, regional, and national networks to facilitate staffing allocation in case ECMO team members become ill or otherwise unavailable.
Desirable elements
A dedicated ECMO team should care for all patients, but ongoing training to increase the number of staff members with less experience should be encouraged whenever safe and feasible. This approach has two advantages: preventing burnout in the short term and improving preparation for future pandemics. Globally, technicians and biomedical engineers could also participate in such teams. As part of the clinical interdisciplinary team, a surgeon or clinician with an extended skillset (eg, experienced intensivist with cannulation skills) could be involved to enhance the safety and success of cannulation while reducing the incidence of vascular complications on decannulation.
Personnel training and ECMO use
Essential elements
Personnel training is essential to ensure preparedness and success in dealing with a pandemic. Ensuring staff safety must be a high institutional priority. Training in use of ECMO should be site-specific and should involve team members with interdisciplinary experience. Apart from ECMO-specific training, team members should become familiar with general intensive-care management practices (largely supportive)26, 27, 28, 29 of infected patients during an outbreak. Training programmes should be led by expert members of the ECMO team, with support from other specialised staff (eg, infection control), and formulated into guidelines for clinical management; such guidelines should be made available to all members of the ECMO team. All ECMO team members should be educated on the eligibility criteria of patients with emerging infectious diseases to receive ECMO support. From the reported clinical characteristics of patients with COVID-19 thus far,2, 30, 31 patient selection and the decision to initiate ECMO can be based on established criteria, such as those for severe ARDS from other causes.20, 32
The correct use of personal protection devices and handling of infected bodily secretions and laboratory specimens are essential skills to empower health-care workers and all other institutional staff to manage any pandemic. After adequate PPE training, use of all ECMO-related equipment must be practiced as a team in a high-fidelity simulation environment while wearing PPE or PAPR. Practice scenarios should simulate critical events that might be stressful even without added burdens such as accidental decannulation, pump failure, air in the circuit, and cardiac arrest. Operators should be familiar with the ECMO cannula sizes and dilators and be able to deal with cannulation complications while wearing PPE or PAPR. Since verbal communication, peripheral vision, and vision clarity through a respirator lens or face shield are impaired, simulation training should also ensure that these nuances are not experienced for the first time at the bedside.
Although ECMO can be a viable rescue strategy for some patients,32 it is not a substitute for optimal conventional ARDS management. Typically, this management includes ventilation with low tidal volumes and at least moderate positive end-expiratory pressure, while restricting plateau airway and driving pressures,33 and accepting some hypercapnia. This strategy can include a neuromuscular blockade and should always include prone positioning unless contraindicated or technically unfeasible. Some patients can be considered too unstable for prone positioning and might have to be administered rescue ECMO expeditiously if possible. Equally, in unstable patients and in patients who are likely to deteriorate during transfer, ECMO can be used to facilitate safe transport from a non-expert centre to an expert centre.34 All infection control and personal protection measures should be followed when ECMO is being initiated for a patient in extremis, as such uncontrolled situations can put team members at high risk of exposure to pathogens.
Once the patient is safely administered ECMO, it is important to ensure that an ultra-protective mechanical ventilation strategy is used.11, 35, 36 There is no consensus on the most appropriate ventilatory strategy for ECMO. Two randomised trials of ventilation during ECMO for patients with ARDS (EOLIA;11 plateau pressure of ≤24 cm H2O while applying a positive end-expiratory pressure of at least 10 cm H2O; respiratory rate of 10–30 breaths per min) and conventional ventilatory support versus ECMO for severe adult respiratory failure (CESAR;37 peak inspiratory pressure 20–25 cm H2O; positive end-expiratory pressure 10 cm H2O; respiratory rate of 10 breaths per min) could serve as useful guides pending further evidence on ventilation strategies for ECMO in patients with ARDS. Prone positioning could be considered during ECMO, although there are few data to support this practice.38
The benefits of ECMO for ARDS largely stem from its ability to confer lung protection, which is associated with decreased adverse outcomes. It should be noted that although extracorporeal CO2 removal for lung-protective ventilation is feasible, it is neither evidence-based nor is it universally available.39 Occasionally, patients develop concomitant or discrete cardiac failure—mainly from myocarditis, myocardial infarction, or sepsis-related cardiomyopathy—and might need venoarterial ECMO. Provision of ECMO in the setting of both pulmonary and cardiac dysfunction is particularly complex and, where possible, requires close liaison with an expert extracorporeal life support centre before ECMO initiation. ECMO-assisted cardiopulmonary resuscitation (ECPR) can be considered in carefully selected patients who develop cardiac arrest. However, given the likelihood of poor outcomes and the considerable risk of infection to staff responding to these patients, ECPR should be undertaken with great caution.
Routine use of steroids is not recommended on the basis of data from influenza studies that suggest potential harm.40, 41 Although the usefulness of antiviral medications in the context of the COVID-19 outbreak is unclear, an understanding of altered pharmacokinetics and pharmacodynamics during ECMO42, 43 will enable optimal dosing of antiviral drugs.
Desirable elements
Frequent ECMO simulation exercises should also be considered outside a state of emergency to develop and reinforce cannulation skills, deal with circuit or pump emergencies, and practise other troubleshooting exercises all with PPE or PAPR donned.
Infection control measures before and during ECMO initiation
Essential elements
Infection control precautions are essential to prevent further spread of emerging infectious diseases and to keep the treating team, other patients, and family members safe.8 Areas of increased precaution should be clearly marked and visual and audio guidance (where possible) should be provided to staff trying to enter these areas. Pre-existing security measures must be maintained (eg, swipe card and code access), and mask users must always check the seals before entry into any patient area. Additional security personnel to ensure staff and patient safety in what can be a difficult and emotionally charged environment might be necessary. The stations for personal protection devices must be strategically placed to enhance compliance.
WHO recommends that health-care workers performing aerosol-generating procedures such as endotracheal intubation and bronchoscopy should do them in an adequately ventilated room—ie, with natural ventilation (airflow of at least 160 L/s per patient) or in negative-pressure rooms (at least 12 air changes per h). When using mechanical ventilation devices, a controlled direction of airflow is required. Use of N95 or Fluid/Firm Particle-2 masks is essential and previous fit-testing is desirable.44
ECMO centres should revisit their existing infection control guidelines and protocols and ensure that all members of the ECMO team receive adequate training.44, 45 If ECMO needs to be initiated at another health centre, the team should either carry the PPE or PAPR resources with them or be familiar with using equipment at another hospital. Personnel performing ECMO cannulation must wear sterile attire in addition to personal protection devices.
Desirable elements
To reduce the infection control resources required to transport highly contagious patients receiving ECMO, ECMO centres should consider developing facility-level plans to group such patients.46 It would be desirable to have harmonised infection-control practices in regional hospitals so that ECMO teams are used to a standardised approach to personal protection devices.
Transport of patients receiving ECMO
Expert centres should devise eligibility criteria (specific to the current outbreak) for inter-hospital transfer and share them with referral centres. An experienced ECMO clinician should evaluate referrals and enact retrieval plans as required. If the rapidity of disease progression from dyspnoea to ARDS is short or unknown, we recommend early transfer (eg, after tracheal intubation) to an ECMO centre, if feasible. If ECMO needs to be initiated in a peripheral hospital, the ECMO team should be aware of the local resources and make a concerted effort not to deplete their supplies. Guidance for preparing the patient and the area for cannulation on the team's arrival should be forwarded to the local hospital to shorten turnaround time. Any rate-limiting steps should be identified early by a dedicated ECMO retrieval coordinator. Effective communication and coordination will be crucial for a successful and efficient interhospital transfer.47 Declined referrals should be clearly communicated to the treating team.
Patients receiving ECMO might also require transportation within the same institution to the radiology suite, cardiac catheterisation laboratory, or operating room. The risk-benefit profile of all transports must include the possibility of disease dissemination to health-care workers and the public. This risk should be meticulously assessed before ECMO is initiated. Strict continuous adherence to infection control policies is required, with immediate disinfection of the route and transport vehicles, to prevent and reduce the risk of cross-contamination.
ECMO weaning, decannulation, and rehabilitation
Essential elements
The natural history of COVID-19 pneumonia and the median time to resolution are currently unknown. All ECMO centres should have standardised ECMO weaning protocols,48, 49 and team members should be trained accordingly. A successful weaning trial should be accompanied by decannulation, and further rehabilitation should follow strict infection control measures with use of PPE or PAPR. Ambulation of the patient outside the negative-pressure room might not be possible as long as the patient is infectious. Follow-up of such patients is important to ensure that their long-term outcomes are acceptable.50
Desirable elements
Weaning and decannulation simulation drills using PPE and PAPR should be considered. Possible exit strategies, including maintaining ECMO until recovery, transplantation, or palliation, should be actively deliberated in the event of a requirement for prolonged ECMO, and options should be based on a realistic understanding of available resources.
Post-mortem care
Essential elements
Until further evidence is available, all deceased patients should be treated as infectious and appropriate precautions adopted. Storage capacities of mortuary facilities in ECMO centres should be mapped, and guidelines for post-mortem care reviewed.51 Decannulation of the deceased if they were treated with ECMO might carry a strong infection risk and should be done while wearing PPE and PAPR. Waste should be disposed of following national and international guidelines on disposal of infectious biological hazardous materials.
Desirable elements
Flowcharts for post-mortem management of patients with COVID-19 following ECMO decannulation should be considered. Such flowcharts should include certification of death, notification of health authorities, and appropriate wrapping of the body. The body handlers and the immediate family members should take infection control precautions.
Staff support
Essential elements
Members of the ECMO team should be frequently monitored for their health and wellbeing, given the risk of infection transmission to health-care providers. Moderate-to-high staff attrition rates as a result of increased psychological stress should be anticipated and ameliorated with provision of stress management and psychosocial support for team members.51 A multidisciplinary team of trained psychiatrists, psychologists, social workers, or psychiatric nurses would be ideal to monitor and treat affected staff. A psychiatric hotline could be made available to staff members in need of urgent attention.52 Centres need to review policies to manage and retain staff trained in ECMO, including insurance, incentives, sick leave, and occupational health and safety.
Personnel who show symptoms of infection should be quarantined for a duration based on the maximal incubation period of the infection (eg, 2 weeks), with a clear follow-up and escalation plan in place if their condition deteriorates. Additionally, protocols for contact-tracing of all non-infected patients who have been cared for by an infected staff member must be available.
Desirable elements
Workplace policies for management of staff wellbeing, including ensuring the presence of staff support services to provide basic amenities, such as meals and drinks, showering facilities, rest areas, and a laundry service for used scrubs or uniforms, could be introduced. Training of staff not specialised in ECMO should be considered to further support crucial roles in the ECMO teams (eg, credentialling of nurses).16, 51 Institutions might consider segregating teams treating highly infectious patients receiving ECMO from other patients in the intensive care unit so that they can function independently of each other to prevent cross-infection. Enhanced use of tele-platforms for meetings and cancellation of educational and recreational activities that bring large groups of frontline staff together should also be considered. Protection of junior and inexperienced staff from involvement in the management of such highly infectious patients in the initial stages of the outbreak is usually possible. However, if an outbreak escalates rapidly, their participation might be necessary, and fast-track training programmes should be devised to ensure readiness in this instance.
Ethical considerations
Essential elements
Ethical challenges53, 54, 55 will affect decision making when offering ECMO therapy in an outbreak. There might be uncertainties regarding the potential benefit and duration of ECMO in this context, which might be dynamic as the outbreak evolves and would require regular review. All aspects of a patient's treatment plan should be regularly reviewed, including the need to continue or terminate ECMO. The decision to stop treatment should not be made purely on the basis of duration of ECMO therapy but only after achieving consensus within the treating team that the patient has minimal potential to recover. Unprecedented situations, such as too few ECMO machines, might necessitate rationing of ECMO services and should involve predetermined consensus criteria formulated to empower clinicians during times of crisis while upholding distributive justice. Distributive justice is only invoked in circumstances in which rationing precludes the ability to care for each individual optimally.56 Appropriate opinions from hospital ethics, social work, and medicolegal committees might be required in ethically challenging scenarios.57, 58
Desirable elements
Shared decision making with family members on aspects of ECMO initiation, continuation, and termination would be desirable. Surrogate decision makers consenting to ECMO do so at a time of high stress related to the disease severity. The involvement of social workers and ethics committee members might help to alleviate some of the ethical hurdles encountered during the management of a patient receiving ECMO in such high-pressure settings.57
Quality assurance and collaborative research
Quality assurance and clinical governance frameworks must be maintained, and ECMO quality reviewed frequently, to measure overall outcomes of ECMO administration, identify problems, and formulate plans for corrective actions. Validated quality metrics specific to ECMO use during outbreaks of emerging infectious diseases are yet to be developed. Lessons learned from management of previous such outbreaks might help to close any gaps in preparedness once they have been identified or to define a consistent approach for improving quality of ECMO delivery. Collection and sharing of data are important to ensure preparedness and patient care, especially in parts of the world that have not yet been affected by the outbreak. The ELSO registry that currently collects de-identified patient data on indication, demography, and haemodynamic and biochemical variables before and after initiating ECMO, as well as details of patient outcomes and complications from more than 400 ECMO centres worldwide, can be a useful resource during an outbreak of an infectious disease, and modifications to existing data collection (eg, information specific to COVID-19 such as swabbing details or cycle threshold values for PCR) might provide valuable data for quality assurance and research. Research during outbreaks of emerging infectious diseases can be further facilitated by global research collaborations, such as the International ECMO Network, International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC), and the Extracorporeal Membrane Oxygenation for 2019 novel Coronavirus Acute Respiratory Disease (ECMOCARD) collaborative. Having research proposals with ethics approval in place or developing pathways for an expedient ethics review and approval are highly desirable to generate and disseminate knowledge that can benefit patients globally.
Conclusions and future perspectives
Periodic outbreaks of emerging infectious diseases, such as COVID-19, are now becoming inevitable in our well connected world. Such global outbreaks demand a strategic global response. Given the propensity of these emerging infections to rapidly cause severe respiratory failure in many patients and, in the case of COVID-19, the high transmission rate of the virus and unclear overall case-fatality rate, the critical care response should be part of a larger pandemic plan to reduce transmission and prevent deaths. These preparations for the COVID-19 outbreak should involve the provision of ECMO in sufficiently resourced settings. Careful patient selection for ECMO is needed because patient age and comorbidities appear to influence outcomes in critically ill patients with COVID-19. Moreover, several variables, such as reversibility of the pulmonary disease process, risk of secondary pulmonary infections, and the role of antiviral drugs and other disease-modifying factors, might affect outcomes with ECMO. In addition to careful patient selection, strict adherence to standard intensive care management practices and infection control protocols should be among the primary goals of care.
Outside infectious disease outbreaks, ECMO for ARDS is an evidence-based, yet resource-intensive, infrequently used service that is typically available only at specialised centres. Although many new centres have been created and ECMO retrieval services are now increasingly available, access to ECMO is restricted, especially in resource-poor settings. Establishing hub-and-spoke models20, 21 of care, in which severely ill patients who are eligible for high-end intensive care support including ECMO are managed at the hub might enable improved stratification of critically ill patients. Well established ECMO retrieval programmes will be an important resource if patients deteriorate in regional centres. Building sustainable systems that can be used to rapidly transfer and group high numbers of critically ill patients would be important to provide high-quality ECMO care during outbreaks of emerging infectious diseases such as COVID-19.
Furthermore, much needs to be done through global collaboration to contain the disease and to prioritise vaccine production to alter the natural history of the pathogen. Real-time data collection and sharing, establishing global biobanks, and fostering an international collaborative research culture that removes geographic boundaries is crucial to rapidly identify populations at risk, the patients that stand to benefit from therapies such as ECMO, and potential therapeutic targets. Moreover, an improved understanding of ARDS, including rapid identification of ARDS phenotypes, could enable more precise use of therapeutics such as ECMO during outbreaks.
Search strategy and selection criteria
We systematically searched PubMed for articles published between Jan 1, 1960, and Feb 25, 2020. We used combinations of search terms that included “extracorporeal membrane oxygenation” OR “ECMO”, “extracorporeal life support” OR “ECLS”, “outbreak”, “emerging infections”, “pandemic”, “severe acute respiratory syndrome”, “influenza A(H1N1)”, and “Middle East respiratory syndrome”. Non-English articles, animal studies, and articles on the use of extracorporeal membrane oxygenation in the neonatal or paediatric populations were excluded. Relevant guidelines for management of suspected COVID-19 published by WHO and the US Centers for Disease Control and Prevention were also reviewed. The final reference list was generated on the basis of relevance to the topics covered here, with the aim of developing an ECMO action plan to guide clinicians in the setting of a pandemic.
Acknowledgments
KS thanks Metro North Hospital and Health Service (Brisbane, QLD, Australia) for research fellowship support. We thank Marta Velia Antonini (1st Intensive Care Unit, University Hospital of Parma, Parma, Italy) for assistance with the artwork.
Contributors
KS, GM, and DB conceived the idea for this paper. KR wrote the original draft. KR, DA, and KS further developed the early drafts. KS coordinated the writing process and designed the figures and panel. DB and GM critically evaluated the drafts regularly and made substantial editorial contributions. AC, MO, MP, and BZ critically evaluated and made substantial edits to the manuscript. All authors approved the final version for submission.
Declaration of interests
KR is a member of the Extracorporeal Life Support Organization (ELSO) Extracorporeal Membrane Oxygenation (ECMO) Educational Task Force (ECMOed) and ELSO's data review committee and scientific overview committee. AC reports grants and personal fees from Getinge and Baxter; he was president of EuroELSO and is a member of the executive and scientific committees of the International ECMO Network (ECMONet). MP is president elect of ELSO. BZ is chair of logistics and education at ELSO. MO is the current president of ELSO. GM is member of the executive committee of ELSO. DB is on the medical advisory boards for Breethe, Xenios, and Hemovent, and is a past medical advisory board member for Baxter and ALung Technologies; he is currently on the trial steering committee for the VENT-AVOID trial sponsored by ALung Technologies; he is a member of the board of directors of ELSO and is chairman of the executive committee of ECMONet. KS is a member of the ECMONet scientific committee, the Asia-Pacific ELSO educational committee, and Australia and New Zealand's Intensive Care Society COVID-19 working group; he is also the lead of an ECMOed research working group. DA declares no competing interests.
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 Hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. published online Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. published online Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. published online Feb 24. [DOI] [PubMed] [Google Scholar]
- 5.WHO Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. 2020. https://apps.who.int/iris/bitstream/handle/10665/330854/WHO-nCoV-Clinical-2020.2-eng.pdf?sequence=1&isAllowed=yWHO/nCoV/Clinical/2020.2
- 6.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020 doi: 10.1001/jama.2020.2342. published online Feb 19. [DOI] [PubMed] [Google Scholar]
- 7.Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 Influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 8.Arabi YM, Al-Omari A, Mandourah Y, et al. Critically ill patients with the Middle East respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med. 2017;45:1683–1695. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 9.Zakhary B, Shekar K, Diaz R, et al. Position paper on global extracorporeal membrane oxygenation education and educational agenda for the future: a statement from the Extracorporeal Life Support Organization ECMOed Taskforce. Crit Care Med. 2019;48:406–414. doi: 10.1097/CCM.0000000000004158. [DOI] [PubMed] [Google Scholar]
- 10.Brodie D, Vincent JL, Brochard LJ, et al. Research in extracorporeal life support: a call to action. Chest. 2018;153:788–791. doi: 10.1016/j.chest.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 12.Goligher EC, Tomlinson G, Hajage D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 13.Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7:163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 14.Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;322:557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 15.Extracorporeal Life Support Organization International summary–January 2020. 2020. https://www.elso.org/Registry/Statistics/InternationalSummary.aspx
- 16.Hamele M, Neumayer K, Sweney J, Poss WB. Always ready, always prepared-preparing for the next pandemic. Transl Pediatr. 2018;7:344–355. doi: 10.21037/tp.2018.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolay N, Callaghan MA, Domegan LM, et al. Epidemiology, clinical characteristics and resource implications of pandemic (H1N1) 2009 in intensive care units in Ireland. Crit Care Resusc. 2010;12:255–261. [PubMed] [Google Scholar]
- 18.Webb SAR, Pettilä V, Seppelt I, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 19.Ronco C, Navalesi P, Vincent JL. Coronavirus epidemic: preparing for extracorporeal organ support in intensive care. Lancet Respir Med. 2020;8:240–241. doi: 10.1016/S2213-2600(20)30060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combes A, Brodie D, Bartlett R, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190:488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 21.Abrams D, Garan AR, Abdelbary A, et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. 2018;44:717–729. doi: 10.1007/s00134-018-5064-5. [DOI] [PubMed] [Google Scholar]
- 22.Patroniti N, Zangrillo A, Pappalardo F, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–1457. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Health Singapore Being prepared for a pandemic. 2019. https://www.moh.gov.sg/diseases-updates/being-prepared-for-a-pandemic
- 24.Ostermann M, Vincent JL. How much centralization of critical care services in the era of telemedicine? Crit Care. 2019;23:423. doi: 10.1186/s13054-019-2705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westergaard JM. Contingency planning: preparation of contingency plans. Zoonoses Public Health. 2008;55:42–49. doi: 10.1111/j.1863-2378.2007.01088.x. [DOI] [PubMed] [Google Scholar]
- 26.Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46:315–328. doi: 10.1007/s00134-020-05943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu H, Tong Z, Ma P, et al. Intensive care during the coronavirus epidemic. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05966-y. published online Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung JC-H, Ho LT, Cheng JV, Cham EYK, Lam KN. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30084-9. published online Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kain T, Fowler R. Preparing intensive care for the next pandemic influenza. Crit Care. 2019;23:337. doi: 10.1186/s13054-019-2616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang D, Lin M, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1623. published online Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao X, Wang B, Kang Y. Novel coronavirus infection during the 2019–2020 epidemic: preparing intensive care units-the experience in Sichuan province, China. Intensive Care Med. 2020;46:357–360. doi: 10.1007/s00134-020-05954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abrams D, Ferguson ND, Brochard L, et al. ECMO for ARDS: from salvage to standard of care? Lancet Respir Med. 2019;7:108–110. doi: 10.1016/S2213-2600(18)30506-X. [DOI] [PubMed] [Google Scholar]
- 33.Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 34.Abrams D, Fan E, Ferguson ND, Brodie D. Unproven and expensive may still be justifiable. Am J Respir Crit Care Med. 2018;198:140. doi: 10.1164/rccm.201801-0112LE. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt M, Pham T, Arcadipane A, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome. an international multicenter prospective cohort. Am J Respir Crit Care Med. 2019;200:1002–1012. doi: 10.1164/rccm.201806-1094OC. [DOI] [PubMed] [Google Scholar]
- 36.Abrams D, Schmidt M, Pham T, et al. Mechanical ventilation for ARDS during extracorporeal life support: research and practice. Am J Respir Crit Care Med. 2019;201:514–525. doi: 10.1164/rccm.201907-1283CI. [DOI] [PubMed] [Google Scholar]
- 37.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 38.Guervilly C, Prud'homme E, Pauly V, et al. Prone positioning and extracorporeal membrane oxygenation for severe acute respiratory distress syndrome: time for a randomized trial? Intensive Care Med. 2019;45:1040–1042. doi: 10.1007/s00134-019-05570-9. [DOI] [PubMed] [Google Scholar]
- 39.Boyle AJ, Sklar MC, McNamee JJ, et al. Extracorporeal carbon dioxide removal for lowering the risk of mechanical ventilation: research questions and clinical potential for the future. Lancet Respir Med. 2018;6:874–884. doi: 10.1016/S2213-2600(18)30326-6. [DOI] [PubMed] [Google Scholar]
- 40.Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23:99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shekar K, Fraser JF, Smith MT, Roberts JA. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care. 2012;27:741.e9–741.e18. doi: 10.1016/j.jcrc.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Shekar K, Roberts JA, Mcdonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012;16:R194. doi: 10.1186/cc11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. https://apps.who.int/iris/rest/bitstreams/1266296/retrieve
- 45.WHO How to put on and take off personal protective equipment (PPE) 2008. https://www.who.int/csr/resources/publications/PPE_EN_A1sl.pdf?ua=1
- 46.Sukhal S, Sethi J, Ganesh M, Villablanca PA, Malhotra AK, Ramakrishna H. Extracorporeal membrane oxygenation in severe influenza infection with respiratory failure: a systematic review and meta-analysis. Ann Card Anaesth. 2017;20:14–21. doi: 10.4103/0971-9784.197820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis R. Caring for critically ill patients with novel coronavirus. 2020. https://www.sccm.org/getattachment/Blog/January-2020/Caring-for-Critically-Ill-Patients-with-Novel-Coro/SCCM-Caring-for-Critically-Ill-Patients-with-2019-nCoV.pdf?lang=en-US
- 48.Vasques F, Romitti F, Gattinoni L, Camporota L. How I wean patients from veno-venous extra-corporeal membrane oxygenation. Crit Care. 2019;23:316. doi: 10.1186/s13054-019-2592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broman LM, Malfertheiner MV, Montisci A, Pappalardo F. Weaning from veno-venous extracorporeal membrane oxygenation: how I do it. J Thorac Dis. 2018;10(suppl 5):S692–S697. doi: 10.21037/jtd.2017.09.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodgson CL, Burrell AJC, Engeler DM, Pellegrino VA, Brodie D, Fan E. Core outcome measures for research in critically ill patients receiving extracorporeal membrane oxygenation for acute respiratory or cardiac failure: an international, multidisciplinary, modified Delphi consensus Study. Crit Care Med. 2019;47:1557–1563. doi: 10.1097/CCM.0000000000003954. [DOI] [PubMed] [Google Scholar]
- 51.WHO A checklist for pandemic influenza risk and impact management 2018 update: building capacity for pandemic response. 2018. https://apps.who.int/iris/bitstream/handle/10665/259884/9789241513623-eng.pdf?sequence=1
- 52.Loutfy MR, Wallington T, Rutledge T, et al. Hospital preparedness and SARS. Emerg Infect Dis. 2004;10:771–776. doi: 10.3201/eid1005.030717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abrams DC, Prager K, Blinderman CD, Burkart KM, Brodie D. Ethical dilemmas encountered with the use of extracorporeal membrane oxygenation in adults. Chest. 2014;145:876–882. doi: 10.1378/chest.13-1138. [DOI] [PubMed] [Google Scholar]
- 54.Abrams D, Pham T, Burns KEA, et al. Practice patterns and ethical considerations in the management of venovenous extracorporeal membrane oxygenation patients: an international survey. Crit Care Med. 2019;47:1346–1355. doi: 10.1097/CCM.0000000000003910. [DOI] [PubMed] [Google Scholar]
- 55.Abrams D, Curtis JR, Prager KM, Garan AR, Hastie J, Brodie D. Ethical considerations for mechanical support. Anesthesiol Clin. 2019;37:661–673. doi: 10.1016/j.anclin.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Kissoon N, Bohn D. Use of extracorporeal technology during pandemics: ethical and staffing considerations. Pediatr Crit Care Med. 2010;11:757–758. doi: 10.1097/PCC.0b013e3181e288a4. [DOI] [PubMed] [Google Scholar]
- 57.Ramanathan K, Cove ME, Caleb MG, Teoh KL, Maclaren G. Ethical dilemmas of adult ECMO: emerging conceptual challenges. J Cardiothorac Vasc Anesth. 2015;29:229–233. doi: 10.1053/j.jvca.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 58.Bein T, Brodie D. Understanding ethical decisions for patients on extracorporeal life support. Intensive Care Med. 2017;43:1510–1511. doi: 10.1007/s00134-017-4781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]