Abstract
Objective
Language barriers may influence the management of pediatric emergency department (PED) patients who may not align with evidence-based guidelines from the American Academy of Pediatrics. Our objective was to determine if a family's preferred language of Spanish versus English was associated with differences in management of bronchiolitis in the PED.
Methods
We conducted a retrospective study of children ≤2 years old diagnosed with bronchiolitis in a PED over a 7-year period. Rates of PED testing, interventions, and disposition among children whose families’ preferred language was Spanish were compared to children whose families’ preferred language was English. Primary outcomes were frequencies of chest x-ray and bronchodilator orders. Secondary outcomes were diagnostic testing, medication orders, and disposition. Logistic regression was used to calculate adjusted odds ratios after controlling for age, emergency severity index, prior visit, and nesting within attending physicians.
Results
A total of 13,612 encounters were included. Spanish-speaking families were more likely to have chest x-rays (35.8% vs 26.7%, P < .0001; adjusted odds ratio [aOR] 1.5; 95% confidence interval [CI] 1.2–1.9), complete blood counts (8.2% vs 4.9%, P < .005; aOR 1.7; 95% CI 1.2–2.5), and blood cultures ordered (8.1% vs 5.0%, P < .05; aOR 1.7; 95% CI 1.2–2.4). No other differences in bronchodilators, medication orders, or disposition were found between the 2 groups.
Conclusions
Among children diagnosed with bronchiolitis, Spanish-speaking families were more likely to have chest x-rays, complete blood counts, and blood cultures ordered compared to English-speaking families. Further research on how clinical practice guidelines and equity-focused guidelines can impact disparities in diagnostic testing within the PED is warranted.
Keywords: guidelines, health disparities, Latino, limited English proficiency
What's New.
In this retrospective cohort study evaluating children who presented to a pediatric emergency department for bronchiolitis, patients from families with a preferred language of Spanish were more likely to receive diagnostic testing that did not align with the American Academy of Pediatrics bronchiolitis guidelines.
Alt-text: Unlabelled box
Previous research indicates that there are racial and ethnic differences in rates of diagnostic testing and clinical interventions of pediatric patients treated in the pediatric emergency department (PED).1 , 2 In 1 study, non-Hispanic white patients were more likely to receive analgesics for abdominal pain compared to non-Hispanic black or Hispanic patients.2 In another study, non-Hispanic white patients were more likely to receive antibiotics for viral illnesses compared to non-Hispanic black or Hispanic patients, regardless of provider type, insurance status, or acuity.1 Although racial and ethnic disparities among pediatric patients are widely known, disparities in PED management among patients and families with language barriers and limited English proficiency have not been as thoroughly investigated.
Several studies in adult populations have examined how language barriers may contribute to differences in care, such as increased diagnostic testing and admission rates.3 , 4 Among children, several studies have evaluated the impacts of language barriers on overall charges and health care outcomes, but very few studies have examined the association of language and specific PED management. In 1 study, Spanish-speaking families were found to have significant differences in laboratory and radiology charges compared to English-speaking families in the PED.5 , 6 In another study, patients with limited English proficiency were found to have different health care outcomes compared to English-speaking patients. In that study, Hispanic ethnicity with limited English proficiency was shown to be a risk factor for appendiceal perforation in pediatric patients presenting with symptoms consistent with appendicitis.7 However, it is unclear if these differences were due to language barriers or other racial/ethnic factors.
It is known that language barriers may lead to miscommunication and lack of trust between physicians and their patients,8 which can influence PED management. These differences may be more pronounced in disease processes in which there is inconsistent implementation of published guidelines. Bronchiolitis is a commonly diagnosed respiratory illness in the PED with wide variation in practice patterns, which are not always consistent with the American Academy of Pediatrics (AAP) guidelines. Given this practice variation and known racial and ethnic disparities among other common pediatric illnesses, determining if language barriers contribute to variations in the diagnosis and management of bronchiolitis in the PED could potentially help to understand if disparities exist and guide improvement efforts. Therefore, the objective of this study was to determine if a family's preferred language of Spanish versus English was associated with differences in diagnostic testing and management of bronchiolitis in the PED. We hypothesized that there would be a higher frequency of diagnostic tests, medications, treatment orders, and admission rates for Spanish-speaking families compared to English-speaking families who presented to the PED with a child with bronchiolitis.
Methods
Study Design
This was a retrospective cohort study; the PED electronic medical record (EMR) was queried to generate a database that included demographic and clinical information from January 1, 2011 to December 31, 2017. This study was approved by the hospital's institutional review board before commencement.
Setting
The study was conducted at a large tertiary care mid-western children's hospital with 2 PEDs. The PEDs have approximately 92,000 total visits per year. During the PED triage process, families are asked to state their preferred language. If their preferred language is not English, families are asked if they would like to request an interpreter. In order to request an interpreter, the Emergency Services Representative or triage nurse places a request in the patient's chart for the specific language needed in order to use the interpreter services within the hospital. The hospital provides an in-person Spanish interpreter 20 hours every day. If an in-person interpreter is unavailable, providers are able to request a video or phone interpreter. If the family does not request an interpreter, this field remains blank in the chart. The data fields regarding the family's preferred language and whether an interpreter was requested are documented and thus accessible in the patient's EMR.
Study Population
Patients were included if they: were 0 to 24 months old, were seen in the PED with a primary International Classification of Diseases, Tenth Revision (ICD-10) diagnosis code of bronchiolitis, had a preferred language of English, or if they had a preferred language of Spanish and requested an interpreter. Patients were excluded if they were transferred from an outside facility, met Feudtner's complex chronic conditions criteria,9 were not seen by an attending physician, or had discordant language data (ie, if the family's preferred language was English but an interpreter was requested or if the family's preferred language was Spanish but an interpreter was not requested).
Data Collection
We extracted data from EPIC, the hospital's EMR. Extracted variables included: demographics (ie, age, race, ethnicity, insurance status, and if the patient had a primary care physician), date and time of arrival, triage acuity, vital signs, family's preferred language, and if an interpreter was requested. The highest temperature, heart rate and respiratory rates, and the lowest oxygen saturation for each visit were extracted. We also extracted data on the following diagnostic testing orders: chest x-ray, complete blood count (CBC), renal panel (which includes sodium, potassium, chloride, creatinine, calcium, blood urea nitrogen, bicarbonate, and glucose concentrations), viral assays (including rapid influenza A/B antigen, Influenza A/B Polymerase Chain Reaction (PCR), Respiratory Syncytial Virus antigen, Respiratory PCR), and blood culture. Respiratory PCRs are able to detect for viruses including Adenovirus, Coronavirus, Human metapneumovirus, Parainfluenza, Parapertussis, Pertussis, Chlamydia pneumoniae, and Mycoplasma pneumoniae. We extracted data on the following medication orders: bronchodilators (Albuterol, Ipratropium, Racemic Epinephrine), steroids (Methylprednisolone, Prednisolone, Prednisone, and Dexamethasone), and antibiotics (Amoxicillin, Ampicillin, Amoxicillin-Clavulanate, Azithromycin, Cefdinir, Cefotaxime, Ceftriaxone, Cefuroxime, Cephalexin, Clindamycin, Gentamicin, Piperacillin-Tazobactam, Sulfamethoxazole-Trimethoprim, and Vancomycin). It was noted if the medication was administered in the PED and/or prescribed for home. Finally, we extracted data on final disposition: discharge to home, admission to general floor, and admission to intensive care unit.
Independent Variables
The independent variables were defined by the family's preferred language and whether an interpreter was requested. The first independent variable, designated as English-speaking, was defined by patients among families with a preferred language of English and did not request an interpreter. The second independent variable, designated as Spanish-speaking, was defined by patients among families with a preferred language of Spanish who did request an interpreter. Hereafter, the primary independent variables will be referred to as English-speaking and Spanish-speaking for simplicity.
Outcomes
The primary outcome measures were chest x-rays and bronchodilators ordered in the PED. The secondary outcome measures were diagnostic laboratory tests, PED and prescription medication orders for steroids and antibiotics, and disposition.
Statistical Analysis
Descriptive statistics were calculated for demographic characteristics, vital signs, triage acuity, and all outcome data. Differences in bronchiolitis management between English-speaking and Spanish-speaking families were first assessed at the bivariate level, using Chi-square or Fisher exact tests. We used generalized linear mixed models for each outcome and nested for attending physician correlation to account for similar practice patterns among the same provider. We obtained unadjusted and adjusted odds ratios after controlling for the following covariates: patient age (analyzed continuously), triage acuity (categorized using the 5-level Emergency Severity Index10), and whether the patient had been seen in the PED within the prior 48 hours. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC) and STATA (StataCorp LLC, College Station, Tex).
Results
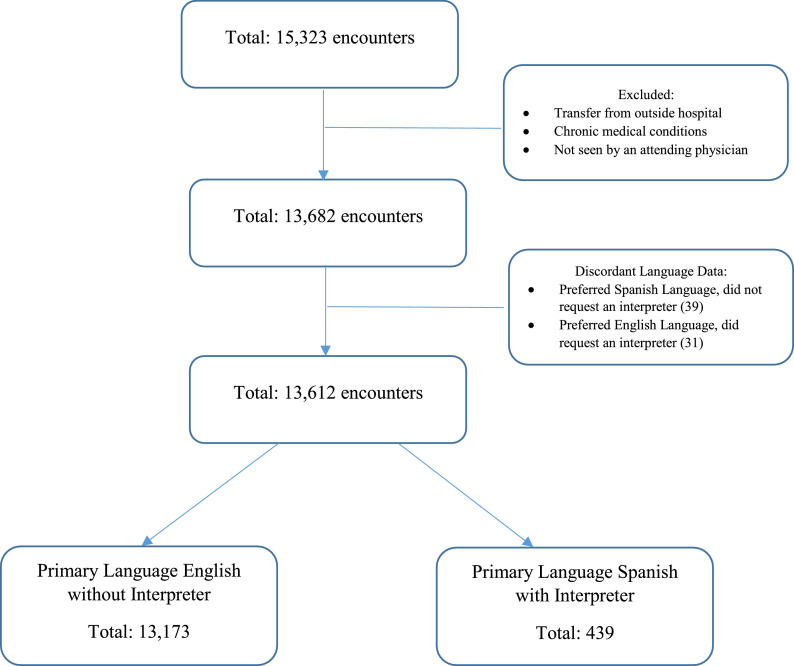
There were a total of 15,323 visits with a primary ICD-10 diagnosis code of bronchiolitis during the study period. A total of 13,612 visits (88.9%) were included in the analysis after application of the exclusion criteria (Figure ); 13,173 (96.8%) visits were by English-speaking families; and 439 (3.2%) were by Spanish-speaking families (Table 1 ). A majority of patients were male and less than 12 months among both groups. Both groups also had similar vital signs during the ED visit (Table 2 ). Patients from Spanish-speaking families had a slightly higher median age, a higher proportion of Medicaid insurance, and a higher percentage of lower triage levels (4 and 5) compared to English-speaking families (Tables 1 and 2).
Figure.
Flow diagram of encounters included in final analysis.
Table 1.
Characteristics of Pediatric Patients With Bronchiolitis, Stratified by Language
| Demographics | English Speakers N = 13,173 |
Spanish Speakers N = 439 |
|---|---|---|
| Median age, months (IQR) | 6 (4, 11) | 7 (4, 11) |
| Age group, N (%) | ||
| 0–12 months | 10,742 (81.5%) | 352 (80.2%) |
| 13–24 months | 2431 (18.5%) | 87 (19.8%) |
| Male, N (%) | 7901 (60.0%) | 285 (64.9%) |
| Ethnicity/race,* N (%) | ||
| Non-Hispanic | 12,518 (95.0%) | 12 (2.7%) |
| White | 4105 | 0 |
| Black | 7053 | 4 |
| Multiracial/other | 1021 | 7 |
| Unknown/missing | 339 | 1 |
| Hispanic | 505 (3.8%) | 426 (97.0%) |
| White | 37 | 0 |
| Black | 258 | 84 |
| Multiracial/other | 176 | 270 |
| Unknown/missing | 34 | 72 |
| Insurance status, N (%) | ||
| Private | 3730 (28.3%) | 3 (0.7%) |
| Medicaid | 9230 (70.1%) | 424 (96.6%) |
| Self-pay | 212 (1.6%) | 12 (2.7%) |
| Other | 1 | 0 |
| Primary care provider, N (%) | 12,916 (98.1%) | 425 (96.8%) |
IQR indicates interquartile range.
Because of missing ethnicity data, percentages do not equal 100%.
Table 2.
Clinical Data of Pediatric Patients With Bronchiolitis, Stratified by Language
| Vital Signs | English Speakers N = 13,173 |
Spanish Speakers N = 439 |
|---|---|---|
| Maximum temperature (°F), mean (SD) | 99.9 (1.52) | 100.2 (1.62) |
| Maximum heart rate, mean (SD) | 161.5 (20.9) | 163.6 (22.8) |
| Maximum respiratory rate, mean (SD) | 53.9 (14.2) | 52.1 (13.6) |
| Lowest oxygen saturation %, mean (SD) | 97.1 (3.7) | 97.2 (3.1) |
| Triage acuity*N (%) | ||
| 1 | 14 (0.1%) | – |
| 2 | 3479 (26.4%) | 79 (18.0%) |
| 3 | 6123 (46.5%) | 184 (41.9%) |
| 4 | 3037 (23.1%) | 148 (33.7%) |
| 5 | 493 (3.7%) | 28 (6.4%) |
SD indicates standard deviation.
Among families with preferred English language, there were 27 charts with missing triage data, thus percentages do not equal 100%.
Chest x-rays were ordered in 3758 visits, which accounted for 27.6% of the study population. Bronchodilators were given in 4219 visits, accounting for 31.0% of the study population. After accounting for attending physician correlation, 26.7% of English speakers received chest x-rays compared to 35.8% of Spanish speakers. Bronchodilators were given to 25% of children from English-speaking families, compared to 23.4% of children from Spanish-speaking families. Spanish-speaking families were more likely to have chest x-rays ordered (odds ratio [OR] 1.29; 95% confidence interval [CI] 1.05–1.59) and were less likely to be admitted (OR 0.80; 95% CI 0.65–0.99). After adjusting for age, triage acuity, and prior visit, Spanish-speaking families had a higher odds of having chest x-rays ordered (adjusted OR [aOR] 1.5; 95% CI 1.2–1.9) and they were also more likely to have CBCs (aOR 1.7; 95% CI 1.2–2.5) and blood cultures ordered (aOR 1.7; 95% CI 1.2–2.4; Table 3 ).
Table 3.
The Association of Spanish Speakers and Pediatric Emergency Department Testing and Interventions for Bronchiolitis*
| OR* (95% CI) | aOR*,† (95% CI) | |
|---|---|---|
| Primary outcomes | ||
| CXR | 1.29 (1.05–1.59)‡ | 1.54 (1.24–1.91)‡ |
| Nebulized treatments (All) | 0.89 (0.72–1.11) | 0.92 (0.73–1.16) |
| Albuterol | 0.91 (0.73–1.13) | 0.92 (0.73–1.16) |
| Ipratropium | 0.97 (0.63–1.49) | 1.09 (0.68–1.73) |
| Racemic epinephrine | 0.77 (0.40–1.46) | 0.96 (0.50–1.86) |
| Secondary outcomes | ||
| Laboratory tests | ||
| CBC | 1.24 (0.90–1.73) | 1.75 (1.23–2.48)‡ |
| Renal panel | 0.77 (0.40–1.51) | 1.05 (0.53–2.08) |
| Viral assays | 1.01 (0.70–1.46) | 1.26 (0.86–1.84) |
| Blood culture | 1.18 (0.85–1.65) | 1.65 (1.16–2.36)‡ |
| Steroids | 0.71 (0.44–1.13) | 0.70 (0.43–1.17) |
| Antibiotics | ||
| ED | 1.13 (0.75–1.70) | 1.15 (0.76–1.72) |
| Discharge | 1.16 (0.89–1.51) | 1.12 (0.86–1.46) |
| Disposition | ||
| Admitted | 0.80 (0.65–0.99)‡ | 1.03 (0.82–1.31) |
| ICU admission | 0.98 (0.56–1.71) | 1.25 (0.70–2.24) |
aOR indicates adjusted odds ratio; CBC, complete blood count; CI, confidence interval; ICU, intensive care unit; OR, odds ratio; CXR, chest x-ray; and ED, emergency department.
English speakers were used at the referent group.
Odds ratio after adjusting for age, triage level, prior visit, and accounting for attending physician correlation.
P value ≤ .05.
To account for the possibility that the presence of pneumonia may contribute to the increased odds of ordering chest x-rays, CBCs, and blood cultures, we conducted 2 sensitivity analyses to account for the diagnosis of pneumonia. A total of 1% of the visits included in our study had a separate diagnosis of pneumonia (including bacterial and viral) for the same index visit. ICD-10 diagnoses that included the word pneumonia were identified. The corresponding ICD-10 codes were added to the regression model as a fourth covariate in the first sensitivity analysis, then the same diagnosis codes were used as an exclusion criteria in a subsequent sensitivity analysis. The results from the repeat analyses were similar to the initial results (Table 4 ).
Table 4.
Sensitivity Analyses of the Association of Spanish Speakers and Pediatric Emergency Department Testing and Interventions for Bronchiolitis*
| Sensitivity Analysis Controlling for Pneumonia | Sensitivity Analysis Excluding Pneumonia | |
|---|---|---|
| aOR*,† (95% CI) | aOR*,‡ (95% CI) | |
| Primary outcomes | ||
| CXR | 1.49 (1.20–1.86)§ | 1.50 (1.19–1.87)§ |
| Nebulized treatments (All) | 0.93 (0.74–1.17) | 0.91 (0.72–1.15) |
| Albuterol | 0.93 (0.74–1.17) | 0.92 (0.73–1.17) |
| Ipratropium | 1.09 (0.69–1.75) | 1.09 (0.67–1.76) |
| Racemic epinephrine | 0.95 (0.49–1.83) | 0.82 (0.39–1.70) |
| Secondary outcomes | ||
| Laboratory tests | ||
| CBC | 1.64 (1.15–2.35)§ | 1.57 (1.07–2.30)§ |
| Renal panel | 0.99 (0.49–1.97) | 1.06 (0.51–2.19) |
| Viral assays | 1.21 (0.82–1.77) | 1.05 (0.69–1.60) |
| Blood culture | 1.54 (1.07–2.22)§ | 1.50 (1.02–2.21)§ |
| Steroids | 0.71 (0.43–1.18) | 0.75 (0.45–1.24) |
| Antibiotics | ||
| ED | 1.11 (0.73–1.67) | 1.15 (0.75–1.76) |
| Discharge | 1.03 (0.78–1.36) | 1.07 (0.80–1.43) |
| Disposition | ||
| Admitted | 0.99 (0.78–1.26) | 0.97 (0.76–1.24) |
| ICU admission | 1.17 (0.652.11) | 1.27 (0.68–2.38) |
aOR indicates adjusted odds ratio; CBC, complete blood count; CI, confidence interval; ICU, intensive care unit; CXR, chest x-ray; and ED, emergency department.
English speakers were used at the referent group.
Odds ratio after controlling for age, triage level, prior visit, diagnosis of pneumonia, and accounting for attending physician correlation.
Odds ratio after excluding those with pneumonia, and controlling for age, triage level, prior visit, and accounting for attending physician correlation.
P value ≤ .05.
Discussion
This is the first study to evaluate differences in management of bronchiolitis based on a preferred language of English or Spanish in the PED. We found that children from Spanish-speaking families were more likely to have chest x-rays, CBCs, and blood cultures ordered compared to children from English-speaking families. Although the mean maximum temperature and heart rates recorded were slightly higher among children from Spanish-speaking families, this small difference neither appears to be clinically significant nor should these vital sign differences prompt increased diagnostic testing. Furthermore, these results were found after controlling for patient age, triage acuity, and prior visit to account for possible differences in disease severity. We did not find significant differences in other diagnostic laboratory testing, such as viral assays. A possible reason for this finding could be that certain tests would not lead to significant changes in the acute management of a nonmedically complex patient. However, if providers were unsure of their diagnosis of bronchiolitis, obtaining a chest x-ray, CBC, or blood culture may prompt change in management such as the consideration of an inpatient admission or the prescription of (or omission of) antibiotics. Given there were no statistically significant differences in the results from the sensitivity analyses, or in admission rates and antibiotic administration, the results suggest there is still diagnostic uncertainty among patients with bronchiolitis when language barriers are present, leading to increased testing without differences in interventions.
These findings contrast with several prior studies that have evaluated management differences by race and ethnicity and have shown that racial/ethnic minority patients are less likely to receive medications, such as pain medications, steroids, or antibiotics1 , 2 , 11 , 12 when compared to nonminority patients. However, in a recent study involving pediatric patients with asthma, there was no difference in pulmonary function testing or rates of asthma exacerbation in patients with limited English proficiency compared to patients who only spoke English.13 The investigators concluded that the lack of differences found in patients with limited English proficiency may be due to the widespread availability of interpreters.13 In our study, we still found differences among families who requested an interpreter. Although there has been a robust body of research that indicates that medical interpreters improve patient satisfaction and understanding,14, 15, 16, 17 exactly how the use of medical interpreters impacts the disparities in patient management in the emergency department is unknown.
The majority of prior studies evaluating differences among patients with language barriers have used hospital charges as a surrogate for testing and interventions done within the emergency department. However, it is difficult to assess how differences in emergency department charges reflect clinical management since charges are affected by many factors beyond the number and types of tests and medications ordered. Very few studies have looked at specific differences in management. In a study by Fields et al, they found that Spanish-speaking patients were less likely to receive interventions, such as nebulized treatments, intravenous medications, laboratory tests, and x-rays.8 They also found significant differences in trust scores among English- and Spanish-speaking patients within the PED, which they proposed could have led to differences in management. However, their study had a small sample size of patients and their results were not statistically significant. Furthermore, in that study, patients’ diagnoses and clinical acuity were not taken into account.
A similar study conducted by Valet et al evaluated health care utilization among Latino infants with acute respiratory illnesses. They found that Latino infants were more likely to receive medications and diagnostic testing compared to infants from African-American families.18 These authors concluded that those differences may have been attributed to language barriers because African-American and Latino families encounter similar socioeconomic and health care disparities.19 Although the authors observed an increasing trend of diagnostic testing among Latino infants from Spanish-speaking families, the study was underpowered to conduct a subgroup analysis evaluating differences due to language alone. In contrast, our study was adequately powered to evaluate differences in outcomes based only on language barriers, irrespective of the family's ethnic background.
When analyzing potential language disparities in clinical management among patients, it is essential that patients with similar diagnoses are evaluated. One prior study by Santiago et al looked at racial and ethnic differences in infants admitted for bronchiolitis.20 Although Hispanic patients did receive more chest x-rays, steroids, and nebulized treatments compared to non-Hispanic patients, their findings were not statistically significant and they did not conduct an analysis on potential differences in management based on language. In contrast, our study only included patients who had the same primary diagnosis of bronchiolitis and we evaluated management differences based on language.
Reasons why language disparities may still exist despite of the use of medical interpreters remain complex. Some providers argue that increased diagnostic testing may persist despite the use of interpreters due to cultural expectations from families. However, cultural expectations are difficult to analyze quantitatively due to multifactorial influences. Although our study was not powered to evaluate differences based on preferred language only among families who identified as Hispanic, there were similar numbers of families who identified as Hispanic in both groups (Table 1). Thus, providers may still face some diagnostic uncertainty irrespective of ethnicity, even when an interpreter is present.
As mentioned previously, the development of evidence-based guidelines alone does not always reflect implementation and clinical practice.21 Despite AAP guidelines and research that indicates that diagnostic testing and interventions are not therapeutically indicated or useful in children with bronchiolitis, physicians still demonstrate varied levels of compliance with these guidelines.22, 23, 24, 25, 26 After the updated AAP bronchiolitis guidelines were published in 2014, approximately 30% of infants hospitalized with bronchiolitis still did not receive the recommended evidence-based supportive therapies,27 despite multiple efforts to reduce diagnostic testing and resource utilization among patients with bronchiolitis.28 , 29 However, the impact of the adherence to clinical practice guidelines on health care disparities remains questionable. In a study conducted by Payne and Puumala, no racial or ethnic disparities were found among pediatric patients who presented with head injuries, for which a head injury algorithm was used.5 Even though differences were not observed, there is insufficient evidence to show that evidence-based guidelines actually reduce disparities among children. Adherence to guidelines may not produce any change (if all patients benefit irrespective of racial, ethnic, or language background) or may even worsen disparities, as shown in some adult studies.30
It is important to note that the differences found in our study were only among diagnostic testing, which would only be mediated by a diagnosis-based guideline. However, there is some rationale that focusing on the development and implementation of comprehensive equity-focused guidelines may be a way to facilitate the reduction of disparities among diagnostic testing, interventions, and health outcomes. Welch et al discussed the necessity of explicitly considering health equity in the Grading of Recommendations, Assessment, Development and Evaluation methodology, and developed an approach for how guideline panels can assess the influence of equity factors on the direction and strength of recommendations.31, 32, 33 For instance, openly asking questions about biases and researching the evidence regarding which populations are potentially disadvantaged in relation to a clinical problem and why (because of race, gender, culture, language, etc.) can help directly inform clinical practice guidelines. Although other frameworks (such as the National Institute for Health and Care Excellence guidelines used in the United Kingdom) explicitly identify certain patient characteristics that must be considered in guideline development, only recently has health equity been included in the Grading of Recommendations, Assessment, Development and Evaluation framework as a consideration for clinical recommendations from an individual perspective. Thus, clinical practice guidelines may contribute to promoting health equity especially when the etiologies of the clinically relevant disparities are thoroughly explored.31
Limitations
There are several limitations that should be noted. First, the retrospective study design used is subject to information and selection bias. Although we used a standardized method to identify patients with the diagnosis code of bronchiolitis, the diagnosis is determined by physicians’ documentation and expertise in making their diagnosis. Furthermore, diagnostic codes may be changed after the chart is reviewed for billing, which may not always be in accord with the physician diagnosis codes. Second, we determined the independent variables (by using preferred language and interpreter requested) from the documented fields in the EMR. Although we attempted to remove potential sources of bias by excluding the encounters with discordant language data, we still could not verify that even if an interpreter was requested, that they were used for the entire visit, when they were used, and to what extent. Third, we developed covariates a priori, and other covariates, such as the diagnosis of reactive airway disease, were difficult to control for given inconsistencies in documentation. These diagnoses may have prompted physicians to use bronchodilators more often. However, we did not see differences in bronchodilator use among the 2 groups of patients, and the history of reactive airway disease should not affect the need for CBCs, blood cultures, and chest x-rays, which were the only significant differences in this study. Last, this was a single-center study and may not be generalizable to other institutions. However, because our study did include 2 emergency departments within a large catchment area, results may be similar at other large children's hospitals.
Conclusions
Among children diagnosed with bronchiolitis, children from Spanish-speaking families were more likely to have chest x-rays, CBCs, and blood cultures ordered compared to children from English-speaking families. Language barriers may be associated with increased diagnostic testing that do not align with AAP bronchiolitis guidelines. Further research on how clinical practice guidelines as well as equity-focused guidelines can impact disparities in diagnostic testing within the PED is warranted.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Goyal MK, Johnson TJ, Chamberlain JM. Racial and ethnic differences in antibiotic use for viral illness in emergency departments. Pediatrics. 2017;140:e20170203. doi: 10.1542/peds.2017-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson TJ, Weaver MD, Borrero S. Association of race and ethnicity with management of abdominal pain in the emergency department. Pediatrics. 2013;132:e851–e858. doi: 10.1542/peds.2012-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waxman MA, Levitt MA. Are diagnostic testing and admission rates higher in non-English-speaking versus English-speaking patients in the emergency department? Ann Emerg Med. 2000;36:456–461. doi: 10.1067/mem.2000.108315. [DOI] [PubMed] [Google Scholar]
- 4.Schulson L, Novack V, Smulowitz PB. Emergency department care for patients with limited English proficiency: a retrospective cohort study. J Gen Intern Med. 2018;33:2113–2119. doi: 10.1007/s11606-018-4493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne NR, Puumala SE. Racial disparities in ordering laboratory and radiology tests for pediatric patients in the emergency department. Pediatr Emerg Care. 2013;29:598–606. doi: 10.1097/PEC.0b013e31828e6489. [DOI] [PubMed] [Google Scholar]
- 6.Hampers LC, Cha S, Gutglass DJ. Language barriers and resource utilization in a pediatric emergency department. Pediatrics. 1999;103:1253–1256. doi: 10.1542/peds.103.6.1253. [DOI] [PubMed] [Google Scholar]
- 7.Levas MN, Dayan PS, Mittal MK. Effect of Hispanic ethnicity and language barriers on appendiceal perforation rates and imaging in children. J Pediatr. 2014;164 doi: 10.1016/j.jpeds.2014.01.006. 1286-91.e2. [DOI] [PubMed] [Google Scholar]
- 8.Fields A, Abraham M, Gaughan J. Language matters: race, trust, and outcomes in the pediatric emergency department. Pediatr Emerg Care. 2016;32:222–226. doi: 10.1097/PEC.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 9.Feudtner C, Feinstein JA, Zhong W. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shelton R. The emergency severity index 5-level triage system. Dimens Crit Care Nurs. 2009;28:9–12. doi: 10.1097/01.DCC.0000325106.28851.89. [DOI] [PubMed] [Google Scholar]
- 11.Zook HG, Payne NR, Puumala SE. Racial/ethnic variation in emergency department care for children with asthma. Pediatr Emerg Care. 2019;35:209–215. doi: 10.1097/PEC.0000000000001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyal MK, Kuppermann N, Cleary SD. Racial disparities in pain management of children with appendicitis in emergency departments. JAMA Pediatr. 2015;169:996–1002. doi: 10.1001/jamapediatrics.2015.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery MP, Allen ED, Thomas O. Association between pediatric asthma care quality and morbidity and English language proficiency in Ohio. J Asthma. 2019;56:603–610. doi: 10.1080/02770903.2018.1474364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karliner L, Jacobs E, Chen A. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2006;42:727–754. doi: 10.1111/j.1475-6773.2006.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lion KC, Brown JC, Ebel BE. Effect of telephone vs video interpretation on parent comprehension, communication, and utilization in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2015;169:1117–1125. doi: 10.1001/jamapediatrics.2015.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagchi AD, Dale S, Verbitsky-Savitz N. Examining effectiveness of medical interpreters in emergency departments for Spanish-speaking patients with limited English proficiency: results of a randomized controlled trial. Ann Emerg Med. 2011;57 doi: 10.1016/j.annemergmed.2010.05.032. 248–256.e1-4. [DOI] [PubMed] [Google Scholar]
- 17.Hampers LC, McNulty JE. Professional interpreters and bilingual physicians in a pediatric emergency department: effect on resource utilization. Arch Pediatr Adolesc Med. 2002;156:1108–1113. doi: 10.1001/archpedi.156.11.1108. [DOI] [PubMed] [Google Scholar]
- 18.Valet RS, Gebretsadik T, Carroll KN. Increased healthcare resource utilization for acute respiratory illness among Latino infants. J Pediatr. 2013;163:1186–1191. doi: 10.1016/j.jpeds.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shone LP, Dick AW, Brach C. The role of race and ethnicity in the State Children's Health Insurance Program (SCHIP) in four states: are there baseline disparities, and what do they mean for SCHIP? Pediatrics. 2003;112(6 Pt 2):e521. [PubMed] [Google Scholar]
- 20.Santiago J, Mansbach JM, Chou SC. Racial/ethnic differences in the presentation and management of severe bronchiolitis. J Hosp Med. 2014;9:565–572. doi: 10.1002/jhm.2223. [DOI] [PubMed] [Google Scholar]
- 21.Eslava-Schmalbach J, Mosquera P, Alzate JP. Considering health equity when moving from evidence-based guideline recommendations to implementation: a case study from an uppermiddle income country on the GRADE approach. Health Policy Plan. 2017;32:1492. doi: 10.1093/heapol/czx158. [DOI] [PubMed] [Google Scholar]
- 22.Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133:e1–e7. doi: 10.1542/peds.2013-2005. [DOI] [PubMed] [Google Scholar]
- 23.Gadomski AM, Bhasale AL. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD001266.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Davison C, Ventre KM, Luchetti M. Efficacy of interventions for bronchiolitis in critically ill infants: a systematic review and meta-analysis. Pediatr Crit Care Med. 2004;5:482–489. doi: 10.1097/01.pcc.0000128891.54799.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knapp JF, Simon SD, Sharma V. Variation and trends in ED use of radiographs for asthma, bronchiolitis, and croup in children. Pediatrics. 2013;132:245–252. doi: 10.1542/peds.2012-2830. [DOI] [PubMed] [Google Scholar]
- 26.Van Cleve WC, Christakis DA. Unnecessary care for bronchiolitis decreases with increasing inpatient prevalence of bronchiolitis. Pediatrics. 2011;128:e1106–e1112. doi: 10.1542/peds.2011-0655. [DOI] [PubMed] [Google Scholar]
- 27.Schuh S, Babl FE, Dalziel SR. Practice variation in acute bronchiolitis: a pediatric emergency research networks study. Pediatrics. 2017;140:e20170842. doi: 10.1542/peds.2017-0842. [DOI] [PubMed] [Google Scholar]
- 28.Ralston SL, Garber MD, Rice-Conboy E. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137:e20150851. doi: 10.1542/peds.2015-0851. [DOI] [PubMed] [Google Scholar]
- 29.Perlstein PH, Kotagal UR, Schoettker PJ. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154:1001–1007. doi: 10.1001/archpedi.154.10.1001. [DOI] [PubMed] [Google Scholar]
- 30.Lion KC, Raphael JL. Partnering health disparities research with quality improvement science in pediatrics. Pediatrics. 2015;135:354–361. doi: 10.1542/peds.2014-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pottie K, Welch V, Morton R. GRADE equity guidelines 4: considering health equity in GRADE guideline development: evidence to decision process. J Clin Epidemiol. 2017;90:84–91. doi: 10.1016/j.jclinepi.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welch VA, Akl EA, Guyatt G. GRADE equity guidelines 1: considering health equity in GRADE guideline development: introduction and rationale. J Clin Epidemiol. 2017;90:59–67. doi: 10.1016/j.jclinepi.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welch VA, Akl EA, Pottie K. GRADE equity guidelines 3: considering health equity in GRADE guideline development: rating the certainty of synthesized evidence. J Clin Epidemiol. 2017;90:76–83. doi: 10.1016/j.jclinepi.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]