Highlights
-
•
Platelet count can discriminate between severe and non-severe COVID-19 infections.
-
•
Patients who did not survive have a significantly lower platelet count than survivors.
-
•
Thrombocytopenia is associated with increased risk of severe disease.
-
•
A substantial decrease in platelet count may be an indicator of worsening illness.
Keywords: Platelets; Thrombocytopenia; Coronavirus, COVID-19
Abstract
Background
Coronavirus disease 2019 (COVID-19) is a novel infectious disease with lack of established laboratory markers available to evaluate illness severity. In this study, we investigate whether platelet count could differentiate between COVID-19 patients with or without severe disease. Additionally, we evaluate if thrombocytopenia is associated with severe COVID-19.
Methods
An electronic search in Medline, Scopus and Web of Science was performed to identify studies reporting data on platelet count in COVID-19 patients. A meta-analysis was performed, with calculation of weighted mean difference (WMD) of platelet number in COVID-19 patients with or without severe disease and odds ratio (OR) of thrombocytopenia for severe form of COVID-19.
Results
Nine studies with 1779 COVID-19 patients, 399 (22.4%) with severe disease, were included in the meta-analysis. The pooled analysis revealed that platelet count was significantly lower in patients with more severe COVID-19 (WMD −31 × 109/L; 95% CI, from −35 to −29 × 109/L). A subgroup analysis comparing patients by survival, found an even lower platelet count was observed with mortality (WMD, −48 × 109/L; 95% CI, −57 to −39 × 109/L. In the four studies (n = 1427) which reported data on rate of thrombocytopenia, a low platelet count was associated with over fivefold enhanced risk of severe COVID-19 (OR, 5.1; 95% CI, 1.8–14.6).
Conclusions
Low platelet count is associated with increased risk of severe disease and mortality in patients with COVID-19, and thus should serve as clinical indicator of worsening illness during hospitalization.
1. Introduction
Since its emergence in December 2019, the outbreak of novel Coronavirus Disease 2019 (COVID-19) outbreak has infected over 113,000 people globally with nearly 4000 deaths [1]. COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), produces a respiratory and systemic illness which progresses to a severe form of pneumonia in 10–15% of patients [2]. Severe COVID-19 can lead to critical illness, with acute respiratory distress (ARDS) and multi-organ failure (MOF) as its primary complications, eventually followed by intravascular coagulopathy [3]. In order to optimize patient care and resource allocation during this pandemic, biomarkers are urgently needed for stratifying patients’ risk and for actively monitoring illness severity.
Platelet count is a simple and readily available biomarker, which is independently associated with disease severity and risk of mortality in the intensive care unit (ICU) [4], [5], [6]. Moreover, a low platelet count correlates with higher disease severity scores such as Multiple Organ Dysfunction Score (MODS), Simplified Acute Physiology Score (SAPS) II, and Acute Physiology and Chronic Health Evaluation (APACHE) II [5]. In the severe acute respiratory syndrome (SARS) outbreak, thrombocytopenia was reported to occur in up to 55% of patients and was identified as a significant risk factor for mortality [7], [8]. Platelet count, with hypoxemia, were the only two variables used by Zou et al. for developing a SARS prognostic model which displayed 96.2% accuracy [9]. In the present study, we aim to investigate whether platelet count could differentiate between COVID-19 patients with or without severe disease, and assess if thrombocytopenia may be associated with severe COVID-19.
2. Materials and Methods
We carried out an electronic search in Medline (PubMed interface), Scopus and Web of Science, using the keywords “laboratory” OR “platelets” AND “coronavirus 2019” OR “COVID-19” OR “2019-nCoV” OR “SARS-CoV-2”, between 2019 and present time (i.e., March 6, 2020), without language restriction. The title, abstract and full text of all documents identified using these search criteria were screened independently by two investigators (GL and BMH), and all documents reporting information on platelet count (either the value or the rate of thrombocytopenia) in COVID-19 patients with a clinically validated definition of severe disease were finally included in our meta-analysis. The reference list of all identified documents was scrutinized, with the aim of identifying additional potentially eligible studies.
A meta-analysis was performed, with calculation of weighted mean difference (WMD) and 95% confidence interval (95% CI) of platelet number in COVID-19 patients with or without severe disease, as well as the odds ratio (OR) of thrombocytopenia for severe COVID-19. Subgroup analysis was performed based on study definition of severity. The statistical analysis was performed with MetaXL, software Version 5.3 (EpiGear International Pty Ltd., Sunrise Beach, Australia). When unavailable, mean and standard deviation of platelet count were extrapolated from sample size, median and interquartile range (IQR), according to Hozo et al. [10]. The study was carried out in accordance with the declaration of Helsinki and with the term of local legislation.
3. Results
3.1. Outcome of the electronic search
Overall, 98 documents could be initially identified based on our search criteria and from the reference lists, 89 of which were excluded after title, abstract or full text reading, since they were review articles (n = 8), commentaries or other editorial materials (n = 2), they did not deal with COVID-19 disease (n = 71), or did not expressly reported the number of platelets and/or the rate of thrombocytopenia in COVID-19 patients with or without severe disease (n = 8). Therefore, 9 studies could finally be included in our meta-analysis, totaling 1779 COVID-19 patients, 399 of whom (22.4%) with severe disease [11], [12], [13], [14], [15], [16], [17], [18], [19].
3.2. Characteristics of the included studies
The characteristics of our included studies is presented in table 1 . The sample size varied between 12 and 1099 COVID-19 patients, whilst the rate of those with severe disease ranged between 14 and 62%. Eight studies included Chinese patients, whilst one was based in Singapore [18]. The clinical severity was defined as the composite of ICU admission, use of mechanical ventilation or death in two studies [11], [13], ICU admission in two studies [12], [16], progression towards ARDS in one study [14], death in three studies [15], [17], [19], and need of mechanical ventilation in the remaining study [18]. All eight studies provided data on the platelet count, whilst only four studies provided information on the rate of thrombocytopenia [11], [12], [14], [19], where thrombocytopenia was defined as platelet count <150 × 109/L in two studies [11], [14], whilst was set at <100 × 109/L in the other two investigations [12], [19].
Table 1.
Characteristics of the Included Studies.
| Study ID | Country | N. Cases | Age | Female (%) | Platelet Count: all × 109/L | Platelet Count: Non-Severe × 109/L | Platelet Count: Severe × 109/L | Thrombocytopenia (%): All | Thrombocytopenia (%): Non-Severe | Thrombocytopenia (%): Severe |
|---|---|---|---|---|---|---|---|---|---|---|
| Guan W et al. [11] | China | 1099 (173 severe) | 47 (median) | 41.9% | 168 (132–207) | 172 (139–212) | 137.5 (99.0–179.5) | 36.2% | 31.6% | 57.7% |
| Huang et al. [12] | China | 41 (13 severe) | 49 year (median) | 28.0% | 164.5 (131.5–263.0) | 196.0 (165.0–263.0) | 140.0 (131.0–263.0) | 5.0% | 8.0% | 4.0% |
| Liu et al. [13] | China | 78 (11 severe) | 38 (median) | 50.0% | 169.1 (57.3) | 173.2 (55.4) | 143.9 (64.8) | NR | NR | NR |
| Liu et al. [14] | China | 12 (6 severe) | 54 years (mean) | 33.0% | 160.3 (53.3) | 186.2 (50.7) | 139.5 (31.1) | 41.7% | 66.7% | 16.7% |
| Ruan et al. [15] | China | 150 (68 died) | 67 (median) died, 50 (median) survived | 32.0% | NR | 173.6 (67.7) | 222.1 (78.0) | NR | NR | NR |
| Wang et al. [16] | China | 138 (36 severe) | 56 (median) | 45.7% | 163 (123–191) | 165 (125–188) | 142 (119–202) | NR | NR | NR |
| Yang et al. [17] | China | 52 (20 died) | 59.7 (mean) | 33.0% | NR | 164 (74) | 191 (63) | NR | NR | NR |
| Young et al. [18] | Singapore | 18 (6 severe) | 47 (median) | 50.0% | 159 (116–217) | 159 (128–213) | 156 (116–217) | NR | NR | NR |
| Zhou et al. [19] | China | 137 (54 died) | 56 (median) | 38.0% | 206.0 (155.0–262.0) | 220 (168.0–271.0) | 165.5 (107.0–229.0) | 7% | 1% | 20% |
*Platelet Count presented as median (IQR) or mean (SD). NR = not reported.
3.3. Meta-analysis
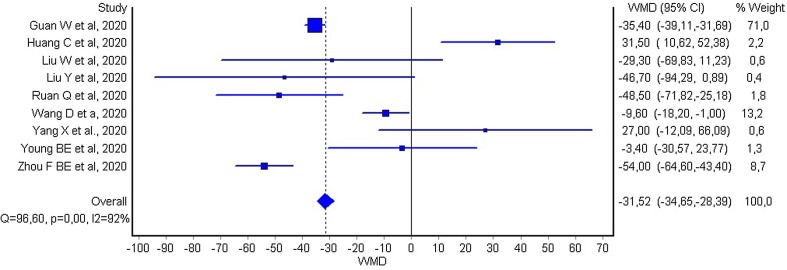
The mean difference in platelet count between COVID-19 patients with or without severe disease in the nine individual studies is shown in Fig. 1 . In seven of the studies, patients with severe COVID-19 displayed a lower platelet count compared to those with milder forms (mean difference ranging between −3 and −54 × 109/L) [11], [13], [14], [15], [16], [18], [19], whilst in the remaining two studies the platelet count was found to be lower in patients with non-severe forms of COVID-19 (mean difference ranging between 27 and 31 × 109/L) [12], [17]. The pooled results of these nine studies revealed that the platelet count was significantly lower in patients with more severe COVID-19 (WMD −31 × 109/L; 95% CI, −35 to −29 × 109/L). The heterogeneity was high (I2, 92%; p < 0.001). The platelet count remained significantly lower (WMD, –22 × 109/L; 95% CI, −26 to −16 × 109/L; I2, 91%; p < 0.001) in patients with more severe COVID-19 after excluding the large study of Guan et al. [11], which accounted for nearly 71% of the overall sample size. In subgroup analysis of three studies [15], [17], [19] whose primary outcome was mortality, a more substantial drop in platelets was observed in non-survivors (WMD, −48 × 109/L; 95% CI, −57 to −39 × 109/L; I2, 91%; p < 0.001). In subgroup analysis of the remaining six studies [11], [12], [13], [14], [16], [18] which used a variable clinical definition of COVID-19 severity of which mortality was not the primary outcome, platelets remained significantly low in the severe form (WMD, −29 × 109/L; 95% CI, –32 to −26 × 109/L; I2, 92%; p < 0.001). In the four studies reporting the rate of thrombocytopenia, a platelet count below the lower limit of the locally defined reference range was associated with a over fivefold enhanced risk of severe COVID-19 (OR, 5.13; 95% CI, 1.81–14.58).
Fig. 1.
Forest plot of mean difference in platelet count between COVID-19 patients with or without severe disease.
4. Discussion
In the presence of this rapidly emerging, novel infection uncharacteristic of the era of modern medicine, identification of biomarkers that could predict disease severity and prognosis are essential to guiding clinical care. Uniquely to COVID-19, a wide range of variability in disease severity is observed ranging from asymptomatic to critical [2]. As such, biomarkers are needed to identify severe disease among hospitalized patients. In this study, we found that platelet count may be a simple, economic, rapid and commonly available laboratory parameter, that could straightforwardly discriminate between COVID patients with and without severe disease. Moreover, we observed that thrombocytopenia is also associated with threefold enhanced risk of severe COVID-19.
Thrombocytopenia is commonplace in critically ill patients, and usually suggests serious organ malfunction or physiologic decompensation as opposed to primary hematologic etiology, as well as the development of intravascular coagulopathy, often evolving towards disseminated intravascular coagulation (DIC) [20]. In COVID-19 patients, the mechanism for thrombocytopenia patients is likely multifactorial. In SARS, it was suggested that the combination of viral infection and mechanical ventilation leads to endothelial damage triggering platelet activation, aggregation and thrombosis in the lung, causing vast platelet consumption [7]. Moreover, as lung may be a site of platelet release from fully mature megakaryocytes, a decrease or morphologic alternation in the pulmonary capillary bed may lead to deranged platelet defragmentation [7]. Coronaviruses may also directly infect bone marrow elements resulting in abnormal hematopoiesis, or trigger an auto-immune response against blood cells [7], [21]. It also has been suggested that a consistently present low grade DIC may propagate a low platelet count in SARS [7]. However, as noted by the World Health Organization (WHO), significant differences are observed between SARS and COVID-19 [2]. As such, the pathophysiologic mechanisms behind each infection are likely to differ [21].
Our study was limited by variable definition of disease severity among the studies, bias of which we in part mitigated through subgroup analysis. Moreover, different cut-offs for thrombocytopenia limits interpretations of that analysis. High heterogeneity suggests inherent variability in platelet levels among patients. At the current time, there is a lack of individual patient data available for more in-depth analysis which may in the future enable more detailed analyses.
Outside findings from the early small, retrospective studies on this emerging pathogen, limited data is available on clinically useful biomarkers for severe COVID-19. In a meta-analysis of early COVID-19 studies, procalcitonin was found to proffer a nearly 5-fold higher risk of severe infection (OR, 4.76; 95% CI, 2.74–8.29) [22]. Future studies should aim to confirm our findings and pool data to identify other biomarkers of severe disease or poor outcomes in COVID-19 infections. Additional research shall also be planned to clarify the precise mechanisms underlying the reduction of platelet count in patients with severe COVID-19, as well as their possible hyper- or hypo-activation.
Author credit statement
All authors directly participated in the design of this study, data collection and analysis, interpretation of the results, and manuscript drafting and revision.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- 1.World Health Organization, Coronavirus disease 2019 (COVID-19) Situation Report – 47, (2020). https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200307-sitrep-47-covid-19.pdf?sfvrsn=27c364a4_2 (accessed March 7, 2020).
- 2.WHO-China Joint Mission, Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19), (2020). https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed March 1, 2020).
- 3.Mattiuzzi C., Lippi G. Which lessons shall we learn from the 2019 novel coronavirus outbreak? Ann. Transl. Med. 2020;8 doi: 10.21037/atm.2020.02.06. http://atm.amegroups.com/post/view/which-lessons-shall-we-learn-from-the-2019-novel-coronavirus-outbreak (accessed March 8, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khurana D., Deoke S.A. Thrombocytopenia in critically Ill patients: clinical and laboratorial behavior and its correlation with short-term outcome during hospitalization. Indian J. Crit. Care Med. 2017;21:861–864. doi: 10.4103/ijccm.IJCCM_279_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderschueren S., De Weerdt A., Malbrain M., Vankersschaever D., Frans E., Wilmer A., Bobbaers H. Thrombocytopenia and prognosis in intensive care. Crit. Care Med. 2000;28:1871–1876. doi: 10.1097/00003246-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Hui P., Cook D.J., Lim W., Fraser G.A., Arnold D.M. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139:271–278. doi: 10.1378/chest.10-2243. [DOI] [PubMed] [Google Scholar]
- 7.Yang M., Ng M.H.L., Li C.K. Thrombocytopenia in patients with severe acute respiratory syndrome (review) Hematology. 2005;10:101–105. doi: 10.1080/10245330400026170. [DOI] [PubMed] [Google Scholar]
- 8.He W., Chen S., Liu X., Li Y., Xiao Z., Zhong N. Death risk factors of severe acute respiratory syndrome with acute respiratory distress syndrome. Chin. Crit. Care Med. 2003;15:336–337. [PubMed] [Google Scholar]
- 9.Zou Z., Yang Y., Chen J., Xin S., Zhang W., Zhou X., Mao Y., Hu L., Liu D., Chang B., Chang W., Liu Y., Ma X., Wang Y., Liu X. Prognostic factors for severe acute respiratory syndrome: a clinical analysis of 165 cases. Clin. Infect. Dis. 2004;38:483–489. doi: 10.1086/380973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Method. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. China medical treatment expert group for covid-19, clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.C. Huang, Y. Wang, X. Li, L. Ren, J. Zhao, Y. Hu, L. Zhang, G. Fan, J. Xu, X. Gu, Z. Cheng, T. Yu, J. Xia, Y. Wei, W. Wu, X. Xie, W. Yin, H. Li, M. Liu, Y. Xiao, H. Gao, L. Guo, J. Xie, G. Wang, R. Jiang, Z. Gao, Q. Jin, J. Wang, B. Cao, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, The Lancet. 395 (2020) 497–506. https://doi.org/10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed]
- 13.Liu W., Tao Z.-W., Lei W., Ming-Li Y., Kui L., Ling Z., Shuang W., Yan D., Jing L., Liu H.-G., Ming Y., Yi H. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respirat. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.-T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K., Anderson D.E., Chan K.S., Tan T.Y., Ng T.Y., Cui L., Said Z., Kurupatham L., Chen M.I.-C., Chan M., Vasoo S., Wang L.-F., Tan B.H., Lin R.T.P., Lee V.J.M., Leo Y.-S., Lye D.C. Epidemiologic Features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang B., Song B., Gu X., Guan L., Wei T., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarychanski R., Houston D.S. Assessing thrombocytopenia in the intensive care unit: the past, present, and future. Hematol. Am. Soc. Hematol. Educ. Program. 2017;2017:660–666. doi: 10.1182/asheducation-2017.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolicoeur P., Lamontagne L. Impairment of bone marrow pre-B and B cells in MHV3 chronically-infected mice. Adv. Exp. Med. Biol. 1995;380:193–195. doi: 10.1007/978-1-4615-1899-0_33. [DOI] [PubMed] [Google Scholar]
- 22.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chim. Acta. 2020 doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]