Abstract
BACKGROUND
Respiratory viral infections can increase the risk of chronic lung allograft dysfunction after lung transplantation, but the mechanisms are unknown. In this study, we determined whether symptomatic respiratory viral infections after lung transplantation induce circulating exosomes that contain lung-associated self-antigens and assessed whether these exosomes activate immune responses to self-antigens.
METHODS
Serum samples were collected from lung transplant recipients with symptomatic lower- and upper-tract respiratory viral infections and from non-symptomatic stable recipients. Exosomes were isolated via ultracentrifugation; purity was determined using sucrose cushion; and presence of lung self-antigens, 20S proteasome, and viral antigens for rhinovirus, coronavirus, and respiratory syncytial virus were determined using immunoblot. Mice were immunized with circulating exosomes from each group and resulting differential immune responses and lung histology were analyzed.
RESULTS
Exosomes containing self-antigens, 20S proteasome, and viral antigens were detected at significantly higher levels (p < 0.05) in serum of recipients with symptomatic respiratory viral infections (n = 35) as compared with stable controls (n = 32). Mice immunized with exosomes from recipients with respiratory viral infections developed immune responses to self-antigens, fibrosis, small airway occlusion, and significant cellular infiltration; mice immunized with exosomes from controls did not (p < 0.05).
CONCLUSIONS
Circulating exosomes isolated from lung transplant recipients diagnosed with respiratory viral infections contained lung self-antigens, viral antigens, and 20S proteasome and elicited immune responses to lung self-antigens that resulted in development of chronic lung allograft dysfunction in immunized mice.
KEYWORDS: exosomes, graft rejection, respiratory viral infection, lung transplantation, chronic rejection, antigens, antibodies
Lung allograft failure from chronic lung allograft dysfunction (CLAD) is the leading cause of death beyond the first year after lung transplant (LTx). Roughly 70% of LTx recipients (LTxRs) with CLAD have bronchiolitis obliterans syndrome (BOS)1 and include both obstructive and restrictive phenotypes.2 The term restrictive allograft syndrome was introduced by Sato et al3 and was diagnosed in 30% of bilateral LTx patients with CLAD. The diagnosis was based on finding a restrictive ventilatory defect and had radiographic findings of interstitial opacities with 41% having upper zone involvement. Previously reported risk factors for CLAD include acute rejection,4, 5, 6 cytomegalovirus (CMV) pneumonitis,7 antibodies (Abs) to donor human leukocyte antigen (HLA),8 , 9 Abs to non-HLA lung-associated self-antigens (SAgs),10, 11, 12 primary graft dysfunction,13 and respiratory viral infections (RVIs).14, 15, 16, 17, 18, 19
The immunologic mechanisms that underlie the development of CLAD remain unknown, and therapy for established CLAD is generally ineffective. RVI after LTx has been associated with increased risk of CLAD.15 , 17 , 18 Fisher et al17 conducted a large retrospective study that used systematic definitions, adjudicated assignment of CLAD by blinded reviewers, and highly sensitive and specific molecular diagnosis of RVI and found a strong and independent association between symptomatic RVI and CLAD17; other studies have also found an association between RVI and CLAD.15 , 18, 19, 20 Potential mechanisms for RVI-induced CLAD pathogenesis were not assessed. We recently demonstrated that LTxRs with acute and chronic rejection have circulating exosomes that contain donor-mismatched HLA, lung SAgs, and immunoregulatory microRNA; exosomes from stable LTxRs do not have these same features.21 A study by Dieudé et al22 demonstrated that the presence of 20S proteasome in exosomes increases their immunogenicity. In this study, we tested the hypothesis that RVI-induced allograft injury may induce circulating exosomes that contain donor HLA, SAgs, and viral antigens, which may activate donor-specific immune responses and increase the risk of CLAD.
Methods
Study population
We performed a retrospective case-control study of 35 adult LTxRs diagnosed with symptomatic upper- and/or lower-tract RVI (cases) and 32 adult LTxRs who had no RVI diagnosis (controls). Patients were eligible for the study if they had undergone LTx at Barnes-Jewish Hospital, Washington University, St. Louis, Missouri, between 2011 and 2015, or at Norton Thoracic Institute, St. Joseph's Hospital, Phoenix, Arizona between 2016 and 2018, and had stored serum available. Baseline patient demographics, transplant details, and laboratory data were collected from patient charts. All patients were followed up for at least 6 years, with clinical and laboratory information collected. The end-point of BOS was diagnosed according to the guidelines from the International Society for Heart and Lung Transplantation.23
RVI testing was performed when indicated for compatible signs and symptoms. Only patients with symptomatic RVI were included. RVI was diagnosed using the BioFire FilmArray PCR (BioMérieux, Marcy-l’Étoile, France), which detects 17 types of respiratory viruses, including adenovirus, coronavirus (types HKU1, NL63, 229E, OC43), human Metapneumovirus, human rhinovirus/enterovirus, influenza (A, A/H1, A/H3, A/H1-2009, B), parainfluenza 1-4, and respiratory syncytial virus (RSV). Both upper (nasopharyngeal swabs) and lower (bronchoalveolar wash or lavage) specimens were included. Patients were considered to have a lower-tract infection if they had a positive lower-tract specimen or upper respiratory specimen along with either lower respiratory symptoms (cough, wheezing) or decline in forced expiratory volume. LTxRs in the control group had no evidence of symptomatic RVI during the period of serum collection. Post-transplant immunosuppression comprised a triple immunosuppressive regimen of tacrolimus or cyclosporine, mycophenolate mofetil or azathioprine, and prednisone. This study was approved by the Institutional Review Boards at Washington University and St. Joseph's Hospital. All laboratory analyses were performed by personnel blinded to clinical outcomes, and all clinical end-points were adjudicated by personnel who were blinded to laboratory results.
Determination of Abs to lung SAgs by enzyme-linked immunosorbent assay (ELISA)
ELISA was used to analyze serum samples from LTxRs diagnosed with RVI and from stable controls for measuring Abs to two SAgs, collagen-V (Col-V) and K-alpha-1 tubulin (Kα1T) detailed in our previous publication.9 In addition to lung SAgs, we used a kidney-associated SAg, collagen-IV (Col-IV) (Meridian, A33125H), as a control. Samples were considered positive if the values were greater than the mean + 2 standard deviations of the healthy controls’ values. Ab concentration was calculated using a standard curve from known concentrations of Col-V and Kα1T Abs (BD Pharmingen 550513, SanJose, CA).
Exosome isolation and validation
Exosomes were isolated from serum samples of LTxRs with RVI and from stable controls by ultracentrifugation as previously described.21 , 24 , 25 Exosome purity was validated using the sucrose cushion method.21 , 26 The presence of the exosome-specific markers CD9 (312102, BioLegend, San Diego, CA) and Alix (634502, BioLegend) was assessed using immunoblot.
Determination of lung SAgs, 20S proteasome, and viral antigens using immunoblot
Immunoblot was used to detect SAgs, 20S proteasome, and viral antigens in exosomes from LTxRs diagnosed with RVI and from stable controls. Total exosome protein (3 µg) was resolved in polyacrylamide gel electrophoresis, and the proteins were transferred into a polyvinylidene difluoride membrane. The membrane was blocked with 5% non-fat milk in 1x phosphate buffered saline and was probed with exosome-specific marker CD9 (312102, BioLegend), Col-V (ab7046, Abcam, Cambridge, United Kingdom), and Kα1T (sc-12462-R, Santa Cruz Biotechnology, Dallas, TX). 20S proteasome subunit α3 (sc-58414, Santa Cruz Biotechnology), rhinovirus VP3 (MA5-18249, Thermo Fisher Scientific, Waltham, MA), coronavirus (NB100-64754, Novus Biologicals, Littleton, CO), and RSV glycoprotein G (7950-0980, Bio-Rad Laboratories, Hercules, CA) were used as primary Abs; secondary Abs conjugated with horseradish peroxidase (HRP) were used specific to primary Ab. The blots were washed with PBS Tween (Thermo Fisher Scientific), developed using chemiluminescent HRP substrate (WBKLS0500, MilliporeSigma, Burlington, MA), and exposed using Odyssey CLx Imaging System (LI-COR Biosciences, Lincoln, NE). The band intensity of target protein was quantified using ImageJ software and normalized with CD9.
Immunization of C57BL/6 mice with exosomes from LTxRs diagnosed with RVI and stable controls
Exosomes (10 µg/100 µl) isolated from LTxRs with RVI or from stable controls were used for immunization of C57BL/6 mice (Days 1, 7, 18, and 25). Prior experiments have demonstrated that injury to the native lungs is required for Abs to lung SAgs to cause lesions.27 Therefore, 0.1 M hydrochloric acid was administered intrabronchially on both groups on Day 0 before immunization with exosomes. Serum samples collected on Days 10 and 30 were used to detect Abs to Col-V and Kα1T by ELISA. On Day 30, the mice were killed and splenocytes isolated to enumerate SAg-specific cytokines producing cells by enzyme-linked ImmunoSpot assay (ELISPOT).
Detection of Abs to lung SAgs in serum samples from mice using ELISA
Serum samples from mice immunized with exosomes of LTxRs diagnosed with RVI and from stable controls were used to measure Abs against Col-V and Kα1T using ELISA as described previously.21 , 27 To detect murine Abs, we used goat–anti-mouse conjugated with HRP (1:10,000) as secondary Ab. The plates were developed with chemiluminescent reagent and the reactions were stopped with 0.1 N hydrochloric acid. The optical density of each well was measured at a wavelength of 420 nm. Serum concentration of Abs to lung SAg was calculated using the standard curve obtained with known concentration of Abs to SAgs. Samples were considered positive if the values were greater than the mean + 2 standard deviations of the healthy controls’ values.
ELISPOT
Splenocytes were isolated from mice immunized with exosomes of LTxRs diagnosed with RVI and of stable controls. ELISPOT was performed as described previously.28 Cytokine-producing cells were analyzed, and the spots were enumerated and subtracted from experimental control wells and reported as spots per million.
Histopathological and morphometric analysis of lungs from mice immunized with exosomes
Lungs from mice immunized with exosomes from LTxRs with RVI and from stable LTxRs were histologically analyzed to detect lesions and cellular infiltration by hematoxylin and eosin and trichrome staining, as described previously.28 Lungs were fixed in 10% formaldehyde and embedded in paraffin blocks. Sections 4 to 5 µm thick were cut and mounted on slides (Leica, Wetzlar, Germany) for hematoxylin and eosin and trichrome staining. Images were obtained on a Leica microscope at × 40 and morphometric analysis was performed using Aperio ImageScope software (Leica). Five different areas were examined for fibroproliferation, epithelial abnormalities, and cellular infiltration.
Morphometric analysis
Slides were scanned and whole slide images were analyzed using Aperio Image Scope (https://www.leicabiosystems.com/digital-pathology/manage/aperio-imagescope/) and ImageJ software (https://imagej.nih.gov/ij/). For analysis of infiltrates, manual annotation of areas with prominent as well as mild or no infiltrate was performed on whole slide images, and the fraction of the total tissue area with prominent infiltrate was determined using Image Scope. For evaluation of fibrosis, whole slide images of lung sections stained with trichrome stain were exported as TIFF files. Color deconvolution of the TIFF files was performed in ImageJ using a color deconvolution plugin (https://imagej.net/Colour_Deconvolution). The extent of blue-staining collagenous fibrosis was then determined using standard tools available in the ImageJ suite.
Statistical analysis
Data analysis was performed using Prism 6 software from GraphPad, Inc. The Ab levels for lung SAgs, optical density of exosomes containing lung SAgs, and viral antigens between RVI LTxRs and controls were compared using Mann–Whitney or two-tailed Student's t-test, as indicated. Statistical data in each cohort was expressed as mean ± standard error. P-values < 0.05 were considered statistically significant in each comparative analysis. The mean optical density of exosomes containing lung SAgs and viral antigens was calculated after normalization with exosome-specific marker CD9 and comparative analysis was performed using Mann–Whitney U test.
Results
Patient demographics
Patient demographics, age, sex, ethnicity, and HLA-mismatch status were not significantly different between groups (Table 1 ). Acute cellular rejection (ACR) occurred after RVI in 2 (A1, ACR) patients and in 1 (A1, ACR) stable LTxR control. Acute antibody-mediated rejection occurred in 5 patients diagnosed with RVI and in none of the stable LTxRs. Donor-specific antibodies developed during follow-up in 8 of 35 (23%) LTxRs with symptomatic RVI and in 4 of 32 (12.5%) stable LTxRs.
Table 1.
Patient Demographics
| Variable | LTxRs with RVI (n = 35) | Stable LTxRs (n = 32) |
|---|---|---|
| Mean recipient age at transplant, years | 58.6 ± 12.8 | 63.5 ± 6.4 |
| Recipient sex (male/female) | 19/16 | 25/7 |
| Race, n (%) | ||
| White | 27 (77) | 30 (94) |
| African American | 1 (3) | 0 (0) |
| American Indian | 1 (3) | 2 (6) |
| Hispanic | 6 (17) | 0 (0) |
| Cause of ESLD, n (%) | ||
| COPD | 14 (40) | 14 (44) |
| IPF | 5 (14) | 10 (31) |
| CF | 3 (9) | 1 (3) |
| Pulmonary fibrosis | 5 (14) | 6 (19) |
| Others | 8 (23) | 1 (3) |
| HLA mismatch | ||
| A | 1.8 ± 0.4 | 1.7 ± 0.5 |
| B | 1.8 ± 0.4 | 1.8 ± 0.4 |
| C | 1.7 ± 0.5 | 1.6 ± 0.6 |
| Prior acute rejection, n (%) | ||
| ACR | 2 (8) | 1 (3) |
| AMR | 5 (21) | 0 |
| Prior chronic rejection, n (%) | ||
| BOS | 2 (8) | NA |
| Infection, n (%) | ||
| RSV | 10 (29) | 0 |
| Rhinovirus | 12 (34) | 0 |
| Coronavirus | 12 (34) | 0 |
| Parainfluenza | 1 (3) | 0 |
| Mean sample collection, years (p < 0.05) | 1.7 ± 1.6 | 3.4 ± 1.4 |
ACR, acute cellular rejection; AMR, antibody-mediated rejection; BOS, bronchiolitis obliterans syndrome; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; ESLD, end-stage lung disease; HLA, human leukocyte antigen; IPF, idiopathic pulmonary fibrosis; LTxRs, lung transplant recipients; NA, not applicable; RSV, respiratory syncytial virus.
Values presented as n (%), unless otherwise noted.
Serum samples from LTxRs diagnosed with symptomatic RVI demonstrated significantly elevated Ab responses to Col-V and Kα1T
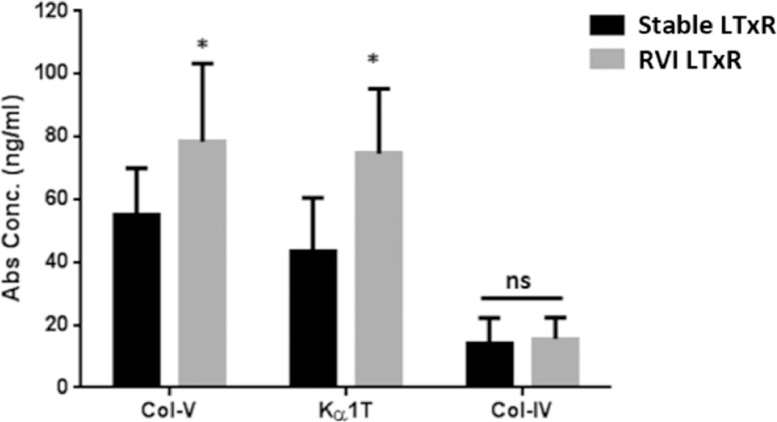
Serum samples collected from patients diagnosed with RVI demonstrated significantly increased Ab titers to SAgs compared with stable LTxRs (Col-V: mean 54.9 ± 15.1 vs 78.3 ± 25.1, p = 0.0169; Kα1T: 43.3 ± 17.2 vs 74.7 ± 20.6, p = 0.0145; Figure 1 ). Both LTxRs with RVI and controls had similar but low levels of Abs to the control SAg, Col-IV.
Figure 1.
Abs to lung-associated SAgs in LTxRs diagnosed with RVI and stable LTxRs. Serum samples collected from LTxRs diagnosed with RVI (n = 35) and from stable LTxRs (n = 32) were used to measure Abs to lung-associated SAgs (Col-V and Kα1T). Abs to Col-V (78.3 ± 25.1 vs 54.9 ± 15.1, p = 0.0169) and Kα1T (74.7 ± 20.6 vs 43.3 ± 17.2, p = 0.0145) were significantly higher in LTxRs with RVI compared with stable LTxRs. Further, Abs to kidney-associated antigen Col-IV were not significantly different between stable LTxRs and LTxRs with RVI (14.2 ± 8 vs 15.6 ± 6.7, p = 0.248). The antibody development to lung SAgs was compared between the stable and RVI LTxR using Mann–Whitney test. Asterisk indicates statistically significant. Ab, antibody; Col-IV, collagen-IV; Col-V, collagen-V; Kα1T, K-alpha-1 tubulin; LTxR, lung transplant recipient; RVI, respiratory viral infection; SAg, self-antigen.
Exosomes isolated from LTxRs diagnosed with RVI contain lung SAgs
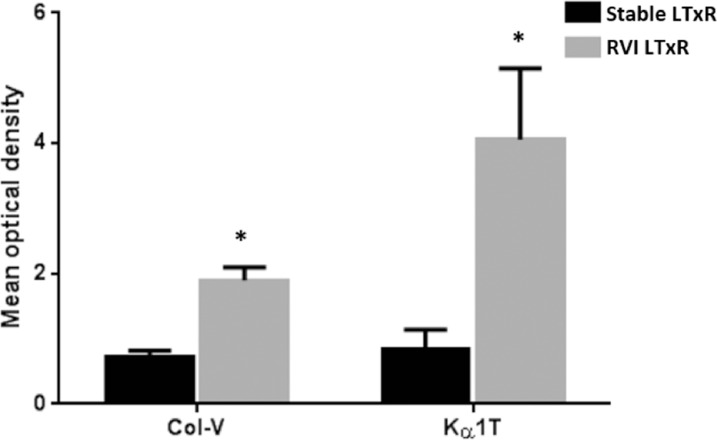
Circulating exosomes in serum samples of both groups were found to contain exosome markers CD9 and Alix. Western blot using Abs to SAgs demonstrated that the exosomes isolated from serum samples from LTxRs with RVI contained significantly higher concentrations of Col-V (mean optical density: LTxR with RVI 1.9 ± 0.2 vs stable LTxR 0.73 ± 0.09, p = 0.0003) and Kα1T (LTxR with RVI 4.06 ± 1.09 vs stable LTxR 0.83 ± 0.31, p = 0.009). Neither cohort had exosomes that contained the control kidney SAg, Col-IV (Figure 2 ).
Figure 2.
Exosomes containing lung-associated SAgs in LTxRs diagnosed with RVI and stable LTxRs. Exosomes isolated from serum samples of LTxRs with RVI (n = 34) and stable LTxRs (n = 30) were analyzed for the presence of lung-associated SAgs (Col-V and Kα1T) by immunoblot. The mean relative optical densities of Col-V (1.9 ± 0.2 vs 0.73 ± 0.09, p = 0.0003) and Kα1T (4.06 ± 1.09 vs 0.83 ± 0.31, p = 0.009) were significantly higher in LTxRs with RVI than stable LTxRs. Optical density was measured using ImageJ software and the OD value of SAgs were calculated in LTxRs with RVI and stable LTxRs after normalization with CD9 OD value. CD9 also served as loading control and exosome-specific markers. The presence of lung SAgs in the exosomes was compared between the cohorts using Mann–Whitney test. Asterisk indicates statistically significant. Col-V, collagen-V; Kα1T, K-alpha-1 tubulin; LTxR, lung transplant recipient; OD, optical density; RVI, respiratory viral infection; SAg, self-antigen.
Viral antigens are detectable in exosomes isolated from LTxRs diagnosed with RVI
RSV
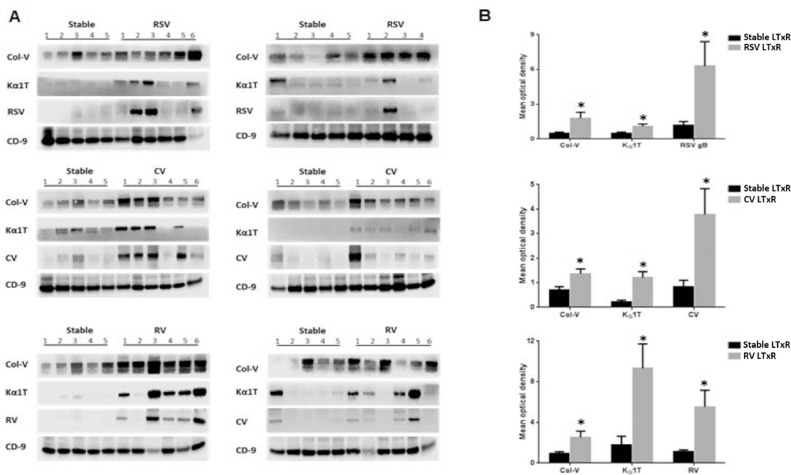
Exosomes from patients diagnosed with RSV were analyzed for the presence of SAgs and RSV glycoprotein G by immunoblot. Viral antigens were seen in 4 of 10 patients with RSV infection; no stable LTxRs had viral antigens (Figure 3 a). Furthermore, significantly increased levels of SAgs and RSV antigens (mean optical intensity: Col-V, 1.8 ± 0.5 vs 0.5 ± 0.1, p = 0.037; Kα1T, 1.1 ± 0.2 vs 0.5 ± 0.1, p = 0.047; RSV, 6.3 ± 2.1 vs 1.2 ± 0.3, p = 0.033) were demonstrated in LTxRs diagnosed with RSV compared with stable LTxRs (Figure 3b).
Figure 3.
Lung-associated SAgs and viral antigens were demonstrable in exosomes isolated from patients with RVI. Exosomes isolated from serum samples of patients with RVI and from stable LTxRs were used to detect the presence of lung-associated SAgs and viral antigens using immunoblot. The results showed a significant increase in lung-associated antigens and viral antigens. (A) RSV (n = 10), coronavirus (n = 12), and rhinovirus (n = 12) in respective patients with viral infection compared with stable LTxRs (n = 30). (B) Graphical representation shows the optical density of lung-associated SAgs and viral antigens measured in RVI and stable LTxRs using ImageJ software. Optical density of lung SAgs and viral antigens were normalized with exosomes specific marker CD9. The presence of lung SAgs and viral antigens in the exosomes was compared between the cohorts using Mann–Whitney test. Asterisk indicates statistically significant. Col-V, collagen-V; CV, coronavirus; Kα1T, K-alpha-1 tubulin; LTxR, lung transplant recipient; RSV, respiratory syncytial virus; RV, rhinovirus; RVI, respiratory viral infection; SAg, self-antigen.
Coronavirus
Immunoblot results showed that coronavirus antigens were detected in exosomes of 5 of 12 patients diagnosed with coronavirus compared with no stable LTxRs (Figure 3a). Levels of SAgs (mean optical intensity: Col-V, 1.37 ± 0.19 vs 0.7 ± 0.14, p = 0.015; Kα1T, 1.2 ± 0.25 vs 0.21 ± 0.08, p = 0.003; coronavirus, 3.78 ± 1.05 vs 0.83 ± 0.27, p = 0.0217) were significantly higher in exosomes from LTxRs with coronavirus than in stable LTxRs (Figure 3b).
Rhinovirus
Twelve patients with rhinovirus infection and 10 stable LTxRs were selected to detect exosomes containing SAgs and rhinovirus antigens. Patients diagnosed with rhinovirus (6/10) showed rhinovirus antigens, but stable LTxRs did not (Figure 3a). The mean optical density of exosomes containing SAgs (mean optical intensity: Col-V, 2.54 ± 0.6 vs 0.92 ± 0.2, p = 0.028; Kα1T, 9.32 ± 2.4 vs 1.78 ± 0.86, p = 0.015; rhinovirus, 5.35 ± 1.63 vs 1.14 ± 0.16, p = 0.030) was significantly higher in exosomes isolated from LTxRs diagnosed with rhinovirus compared with stable LTxRs (Figure 3b).
20S. proteasome subunit α3 is detectable in exosomes from LTxRs with RVI
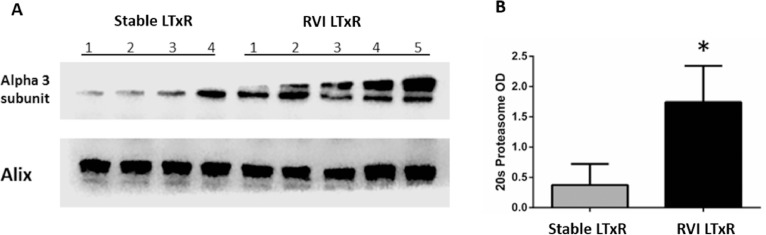
To determine 20S proteasome in exosomes isolated from stable LTxRs (n = 4) and LTxRs diagnosed with RVI (n = 5), we performed immunoblot using Abs to the α3 subunit of 20S proteasome. We found significantly higher levels of 20S proteasome α3 subunit in exosomes isolated from LTxRs diagnosed with RVI compared with stable LTxRs (mean optical density, LTxRs with RVI vs stable LTxRs: 1.74 ± 0.6 vs 0.37 ± 0.35, p = 0.0317). Alix served as an exosome-specific marker and loading control (Figure 4 ).
Figure 4.
Exosomes containing 20S proteasome core in LTxRs with RVI and stable LTxRs. Circulatory exosomes isolated from LTxRs with RVI (n = 5) and stable LTxRs (n = 4) were used to detect the presence of 20S proteasome subunit α3 using immunoblot. (A) The exosomes isolated from patients with RVI showed a significant increase in 20S proteasome compared with exosomes from stable LTxRs (Mean optical density: 1.74 ± 0.6 vs 0.37 ± 0.35, p = 0.0317). Alix served as loading control and exosome-specific marker. (B) Graphical representation shows optical intensity of 20S proteasome α3 subunit abundance in LTxRs with viral infection and stable LTxRs. The presence of 20S proteasome was compared between stable LTxRs and LTxRs with RVI using Student's t-test. LTxR, lung transplant recipient; RVI, respiratory viral infection.
Exosomes containing lung SAgs induce Abs to lung SAgs in mouse model
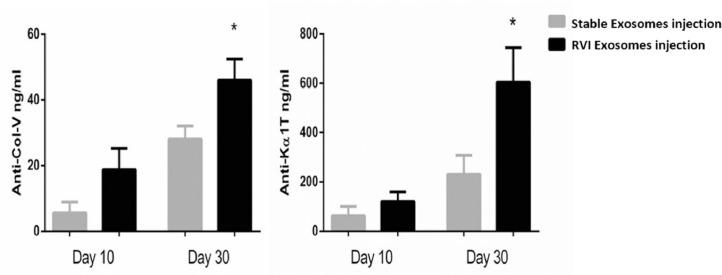
Serum samples collected on Days 10 and 30 were used to measure Abs to SAgs in mice immunized with pooled exosomes isolated from LTxRs diagnosed with RVI and from stable LTxRs. Abs to SAgs in serum samples collected on Day 10 following immunization with the exosomes derived from LTxRs with RVI and stable LTxRs were not significantly different (Col-V, 18.83 ± 6.4 vs 5.50 ± 3.35, p = 0.102; Kα1T, 120.2 ± 39.1 vs 62.8 ± 37.9, p = 0.323). Serum collected on Day 30 showed increased Abs to Col-V and Kα1T in mice immunized with exosomes from LTxRs diagnosed with RVI compared with stable LTxRs (Col-V, 45.9 ± 6.5 vs 28.1 ± 4.0, p = 0.04; Kα1T, 604.6 ± 140 vs 230.4 ± 77.1, p = 0.04; Figure 5 ).
Figure 5.
Exosomes from LTxRs with RVI induce a humoral immune response to lung SAgs. Serum samples collected on Days 10 and 30 from C57BL/6 mice immunized with exosomes isolated from LTxRs with RVI (n = 5) and from stable LTxRs (n = 5) were utilized to measure Abs to lung SAgs by ELISA. Serum samples collected on Day 30 from mice immunized with exosomes from LTxRs with RVI showed significantly increased Abs to SAgs (Col-V, 28.1 ± 4.0 vs 45.9 ± 6.5, p = 0.04; Kα1T, 230.4 ± 77.1 vs 604.6 ± 140, p = 0.04) when compared with mice injected with exosomes from stable LTxRs. The antibody development was compared between the cohorts using Student's t-test. Asterisk indicates statistically significant. Ab, antibody; Col-V, collagen-V; ELISA, enzyme-linked immunosorbent assay; Kα1T, K-alpha-1 tubulin; LTxR, lung transplant recipient; RVI, respiratory viral infection; SAg, self-antigen.
Cellular immune response to exosomes of LTxRs diagnosed with RVI and stable LTxRs in a mouse model
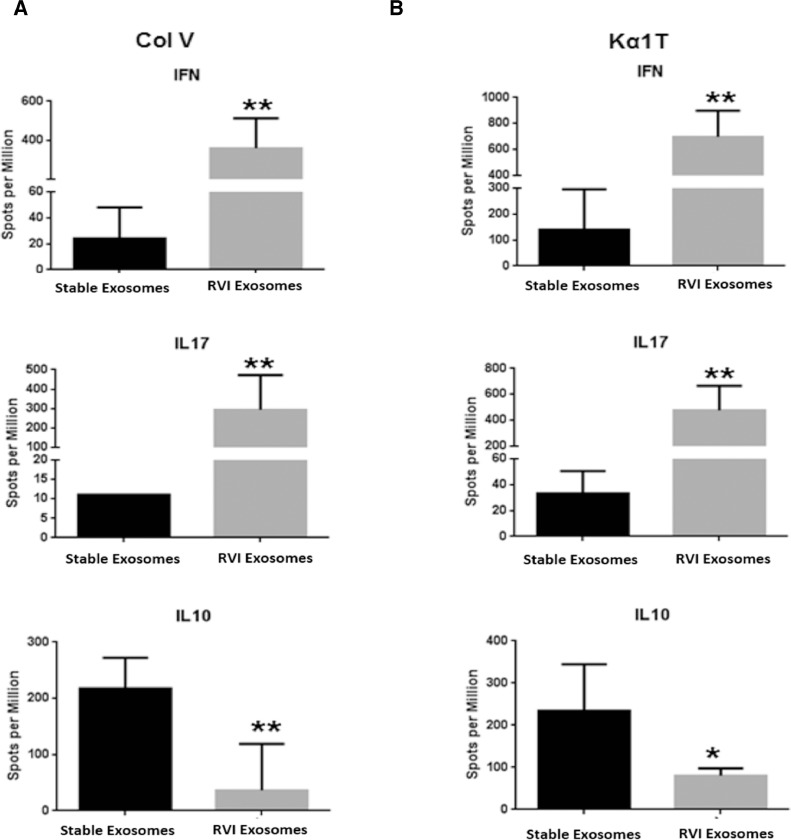
Splenocytes isolated from mice immunized with exosomes from LTxRs with RVI and from stable LTxRs were used to enumerate the cytokine-producing cells against lung SAgs. Mice immunized with exosomes from LTxRs diagnosed with RVI had significantly increased SAg-specific interferon gamma–producing cells (Col-V: 359.3 ± 154 vs 24.2 ± 24, p = 0.002; Kα1T: 696.7 ± 202 vs 140 ± 155, p = 0.004) and interleukin (IL)-17–producing cells (Col-V: 293.3 ± 179 vs 11 ± 0, p = 0.010; Kα1T: 403.3 ± 310 vs 22 ± 17, p = 0.002) than mice immunized with exosomes from stable LTxRs. In contrast, IL-10–producing cells (Col-V: 36.7 ± 83 vs 217.8 ± 55, p = 0.010; Kα1T: 80.7 ± 17 vs 233.2 ± 111, p = 0.036) were significantly reduced in mice immunized with exosomes from LTxRs diagnosed with RVI than in those immunized with exosomes from stable LTxRs (Figure 6 ).
Figure 6.
Exosomes from LTxRs with RVI induce cytokine-producing T cells to lung SAgs. Spleens were collected on Day 30 from C57BL/6 mice immunized with exosomes of LTxRs with RVI (n = 5) and from stable LTxRs (n = 5) were used to measure cytokine-producing T cells against lung SAgs by ELISPOT. Mice immunized with exosomes of RVI showed significant increase in T cells producing IL-17 and interferon gamma to SAgs. Mice injected with exosomes isolated from stable LTxRs showed increased frequency of IL-10–producing T cells compared with mice immunized with RVI exosomes. The cytokine levels were compared between the cohorts using Mann–Whitney test. Asterisks indicate statistically significant. ELISPOT, enzyme-linked ImmunoSpot assay; IL, interleukin; LTxR, lung transplant recipient; RVI, respiratory viral infection; SAgs, self-antigens; ** statistically significant.
Exosomes from LTxRs diagnosed with RVI induce fibrosis in mouse model
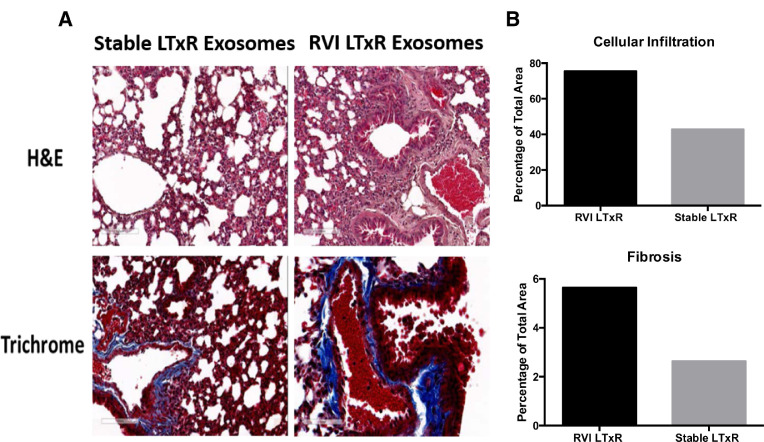
Lungs harvested from mice immunized with exosomes from LTxRs with RVI and stable LTxRs were subjected to histopathological analysis. Mice immunized with exosomes from LTxRs diagnosed with RVI showed inflammatory cells in bronchioles and vessels. Notably, lesions involving bronchioles, cellular infiltration, and increased fibrosis were also observed (Figure 7 ). In contrast, no significant differences in cellular infiltration and lesions were evident in the mice immunized with exosomes from stable LTxRs (Figure 7a). These results demonstrate that circulating exosomes from LTxRs diagnosed with RVI-induced cellular infiltration and alveolar lesions in the lungs of mice. Furthermore, histopathological analysis demonstrates interstitial fibrosis, which after human lung transplant is similar to the pathology seen in restrictive allograft syndrome. The morphometric data (Figure 7b) are given for the representative images.
Figure 7.
Fibrosis and cellular infiltration were demonstrable in mice injected with exosomes isolated from LTxRs with RVI. Mice were killed on Day 30 and their lungs were collected and analyzed using hematoxylin and eosin and trichrome staining. (A) Interstitial and inflammatory infiltrates and fibrosis was more prominent in mice injected with exosomes from LTxRs with RVI compared with mice injected with exosomes from stable LTxRs. Images were obtained on a Leica microscope at × 40 and morphometric analysis was performed using Aperio ImageScope software (Leica). (B) The morphometric data are given for the representative images. LTxR, lung transplant recipient; RVI, respiratory viral infection.
Discussion
Studies have demonstrated an association between RVI and CLAD.15, 16, 17, 18 , 20 Fisher et al16 , 17 applied molecular diagnostic methods to test for RVI in a large cohort of LTxRs. They not only found high rates of RVI but also demonstrated an independent association between RVI and CLAD17 and suggested further study to characterize the viral determinants and to define the mechanisms by which RVI increases the risk for CLAD. RVI after LTx has been shown to dysregulate the regulatory T cells, indicating that RVI can lead to dysregulation of tolerance to SAgs, leading to induction of immune responses to SAgs and increasing the risk of CLAD.14 Studies by our group and others showed that Abs to lung SAgs have been shown to develop and correlate with development of CLAD in LTxRs.11 , 12 , 29 Pre-existing Abs to SAgs have also been reported to increase the incidence of primary graft dysfunction, to induce proinflammatory cytokines, and to increase development of donor-specific antibodies and CLAD after LTx.29
We recently demonstrated that LTxRs diagnosed with acute and chronic rejection have circulating exosomes that express mismatched donor HLA and SAgs. We proposed that the exosomes originating from transplanted lungs may contribute to the immune pathogenesis of CLAD after LTx.21 Based on these findings, we postulated that symptomatic RVI may induce exosomes containing SAgs from the transplanted organ, and that persistence of circulating exosomes with SAgs can lead to immune responses resulting in increased risk of CLAD.
In this study, we determined the development of Abs to SAgs in LTxRs diagnosed with RVI. Our results, presented in Figure 1, demonstrated that Ab titers to SAgs were significantly higher in patients diagnosed with RVI than in stable LTxRs. This demonstrates that RVI can induce a humoral immune response to SAgs. Circulating exosomes isolated from LTxRs diagnosed with BOS express mismatched donor HLA and SAgs (Col-V and Kα1T), confirming their source as the lung allograft and suggesting that exosomes can induce immune responses to alloantigens and SAgs, increasing the risk for CLAD.21 Walker et al30 demonstrated that exosomes released from CMV-infected lung endothelial cells of LTxRs induces CD4 T-cell responses to CMV antigens. Furthermore, a human nasopharyngeal cell line transfected with Epstein-Barr virus (EBV) has been shown to release exosomes containing viral peptide latent membrane protein 1 and fibroblast growth factor 2.31 The exosomes isolated from EBV-transformed B cells contain EBV viral antigen glycoprotein 350, which can specifically bind to B cells.32 These findings support our hypothesis that RVI has the potential to induce exosomes containing lung SAgs and viral antigens from the transplant recipient with RVI.
Our results demonstrate that circulating exosomes isolated from patients diagnosed with symptomatic RVI had not only SAgs but also viral antigens. In this study, we selected patients diagnosed with RSV, coronavirus, and rhinovirus and demonstrated that exosomes isolated from these patients contained specific viral antigens along with SAgs. Therefore, exosomes are induced following viral infection that contain viral antigens and SAgs. Preliminary analysis of serial circulating exosomes containing viral antigens demonstrated that in 3 of 5 LTxRs, there was a transient presence of circulating exosomes. In contrast, 2 of 5 LTxRs with RVI had persistence of circulating exosomes with lung SAgs and viral antigens. This interesting finding needs to be confirmed to determine the role of circulating exosomes in inducing immune responses leading to CLAD. It is likely that the exosomes with viral antigenic epitopes can activate cross-reactive T cells, which can play a role in the pathogenesis of CLAD after LTx.33 , 34 We demonstrated that exosomes isolated from symptomatic patients with RVI contained increased SAgs and viral antigens; if further studies identify a useful threshold concentration, this could potentially serve as a biomarker for CLAD.
A study by Dieudé et al22 demonstrated that exosomes isolated from endothelial cells contained 20S proteasome and therefore increased the immunogenic potential to the kidney-associated SAg perlecan. Intravenous injection of exosomes in C57BL/6 mice led to humoral immune responses to perlecan, suggesting that presence of kidney SAgs, along with 20S proteasome, increases the immunogenicity of the exosomes.22 To demonstrate that exosomes containing SAgs along with viral antigens can be immunogenic, we isolated exosomes from LTxRs with RVI and stable LTxRs and immunized into C57BL/6 mice. Mice immunized with exosomes from RVI developed increased levels of Abs to SAgs than mice immunized with exosomes from stable LTxRs. Additionally, mice immunized with exosomes from LTxRs with RVI showed increased interferon gamma– and IL-17–producing cells and reduced IL-10–producing cells compared with mice injected with exosomes from stable LTxRs. These results confirm that exosomes containing SAgs, viral antigens, and 20S proteasome are immunogenic and can induce Abs to SAgs and alter T-cell cytokine responses, which can lead to CLAD.
Our study is limited in that exosomes isolated from patients diagnosed with RVI were not analyzed in mice models of obliterative airway diseases following lung transplantation. Therefore, we cannot definitively conclude that exosomes from patients with RVI can increase the incidence of CLAD development. The sample size used in the mouse model was small and we used pooled exosomes for immunization. Therefore, the role of individual viruses in inducing exosomes that are immunogenic cannot be concluded from the studies presented. We have shown that serum samples collected from LTxRs with RVI had increased Abs to lung SAgs and exosomes containing lung SAgs and viral antigens compared with stable LTxRs. However, viral RNA in the exosomes and its role in immune activation needs to be determined in future studies. Another limitation is that the role of individual RVI viruses to induce exosomes that can increase the risk for CLAD were not determined because of the limited availability of retrospectively collected samples. Our study, however, demonstrated that exosomes derived from LTxRs with RVI-induced interstitial fibrosis and inflammatory cell infiltration by adoptive transfer of exosomes (gain of function) in a mice model, which suggests that RVI exosomes are sufficient to induce lesions in mice with similarities to the pathology seen in restrictive allograft syndrome in human LTxRs.
We further demonstrated increased humoral and cellular immune responses to lung SAgs in mice immunized with exosomes from LTxRs with RVI compared with mice immunized with exosomes from stable LTxRs. Based on these, we proposed that RVI-induced exosomes containing lung SAgs and viral antigens can augment humoral and cellular immune responses to lung SAgs and alloantigens, increasing the risk of CLAD. These results strongly suggest a biologically plausible mechanistic link between RVI induction and release of circulating exosomes with SAgs and the development of CLAD, which should be assessed in a large prospective cohort.
Disclosure statement
The authors have no conflicts of interest to disclose.
The authors acknowledge Billie Glasscock and Clare Sonntag for their assistance in preparing and submitting this manuscript.
This study was supported by National Institutes of Health grants AI123034, HL056643, and HL092514 (TM).
References
- 1.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 2.Glanville AR. Bronchoscopic monitoring after lung transplantation. Semin Respir Crit Care Med. 2010;31:208–221. doi: 10.1055/s-0030-1249117. [DOI] [PubMed] [Google Scholar]
- 3.Sato M, Waddell TK, Wagnetz U. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30:735–742. doi: 10.1016/j.healun.2011.01.712. [DOI] [PubMed] [Google Scholar]
- 4.Witt CA, Gaut JP, Yusen RD. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2013;32:1034–1040. doi: 10.1016/j.healun.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart S, Fishbein MC, Snell GI. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Arcasoy SM, Berry G, Marboe CC. Pathologic interpretation of transbronchial biopsy for acute rejection of lung allograft is highly variable. Am J Transplant. 2011;11:320–328. doi: 10.1111/j.1600-6143.2010.03382.x. [DOI] [PubMed] [Google Scholar]
- 7.Almaghrabi RS, Omrani AS, Memish ZA. Cytomegalovirus infection in lung transplant recipients. Expert Rev Respir Med. 2017;11:377–383. doi: 10.1080/17476348.2017.1317596. [DOI] [PubMed] [Google Scholar]
- 8.Chalermskulrat W, Neuringer IP, Schmitz JL. Human leukocyte antigen mismatches predispose to the severity of bronchiolitis obliterans syndrome after lung transplantation. Chest. 2003;123:1825–1831. doi: 10.1378/chest.123.6.1825. [DOI] [PubMed] [Google Scholar]
- 9.Saini D, Weber J, Ramachandran S. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624–631. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharat A, Saini D, Steward N. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T. Antibodies to K-alpha 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12:2164–2171. doi: 10.1111/j.1600-6143.2012.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daud SA, Yusen RD, Meyers BF. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 14.Bharat A, Kuo E, Saini D. Respiratory virus-induced dysregulation of T-regulatory cells leads to chronic rejection. Ann Thorac Surg. 2010;90:1637–1644. doi: 10.1016/j.athoracsur.2010.06.048. discussion 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. J Heart Lung Transplant. 2002;21:559–566. doi: 10.1016/s1053-2498(01)00405-3. [DOI] [PubMed] [Google Scholar]
- 16.Fisher CE, Mohanakumar T, Limaye AP. Respiratory virus infections and chronic lung allograft dysfunction: assessment of virology determinants. J Heart Lung Transplant. 2016;35:946–947. doi: 10.1016/j.healun.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Fisher CE, Preiksaitis CM, Lease ED. Symptomatic respiratory virus infection and chronic lung allograft dysfunction. Clin Infect Dis. 2016;62:313–319. doi: 10.1093/cid/civ871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalifah AP, Hachem RR, Chakinala MM. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170:181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 19.Magnusson J, Westin J, Andersson LM, Brittain-Long R, Riise GC. The impact of viral respiratory tract infections on long-term morbidity and mortality following lung transplantation: a retrospective cohort study using a multiplex PCR panel. Transplantation. 2013;95:383–388. doi: 10.1097/tp.0b013e318271d7f0. [DOI] [PubMed] [Google Scholar]
- 20.Bridges ND, Spray TL, Collins MH, Bowles NE, Towbin JA. Adenovirus infection in the lung results in graft failure after lung transplantation. J Thorac Cardiovasc Surg. 1998;116:617–623. doi: 10.1016/S0022-5223(98)70168-0. [DOI] [PubMed] [Google Scholar]
- 21.Gunasekaran M, Xu Z, Nayak DK. Donor-derived exosomes With lung self-antigens in human lung allograft rejection. Am J Transplant. 2017;17:474–484. doi: 10.1111/ajt.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieudé M, Bell C, Turgeon J. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med. 2015;7:318ra200. doi: 10.1126/scitranslmed.aac9816. [DOI] [PubMed] [Google Scholar]
- 23.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127–133. doi: 10.1016/j.healun.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Gunasekaran M, Sharma M, Hachem R, Bremner R, Smith MA, Mohanakumar T. Circulating exosomes with distinct properties during chronic lung allograft rejection. J Immunol. 2018;200:2535–2541. doi: 10.4049/jimmunol.1701587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma M, Liu W, Perincheri S, Gunasekaran M, Mohanakumar T. Exosomes expressing the self-antigens myosin and vimentin play an important role in syngeneic cardiac transplant rejection induced by antibodies to cardiac myosin. Am J Transplant. 2018;18:1626–1635. doi: 10.1111/ajt.14650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30:3.22.1–3.22.29. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian V, Ramachandran S, Banan B. Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am J Transplant. 2014;14:2359–2366. doi: 10.1111/ajt.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukami N, Ramachandran S, Saini D. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiriveedhi V, Gautam B, Sarma NJ. Pre-transplant antibodies to Kalpha1 tubulin and collagen-V in lung transplantation: clinical correlations. J Heart Lung Transplant. 2013;32:807–814. doi: 10.1016/j.healun.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker JD, Maier CL, Pober JS. Cytomegalovirus-infected human endothelial cells can stimulate allogeneic CD4+ memory T cells by releasing antigenic exosomes. J Immunol. 2009;182:1548–1559. doi: 10.4049/jimmunol.182.3.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceccarelli S, Visco V, Raffa S, Wakisaka N, Pagano JS, Torrisi MR. Epstein-Barr virus latent membrane protein 1 promotes concentration in multivesicular bodies of fibroblast growth factor 2 and its release through exosomes. Int J Cancer. 2007;121:1494–1506. doi: 10.1002/ijc.22844. [DOI] [PubMed] [Google Scholar]
- 32.Vallhov H, Gutzeit C, Johansson SM. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J Immunol. 2011;186:73–82. doi: 10.4049/jimmunol.1001145. [DOI] [PubMed] [Google Scholar]
- 33.Fleming A, Sampey G, Chung MC. The carrying pigeons of the cell: exosomes and their role in infectious diseases caused by human pathogens. Pathog Dis. 2014;71:109–120. doi: 10.1111/2049-632X.12135. [DOI] [PubMed] [Google Scholar]
- 34.Yao Z, Qiao Y, Li X. Exosomes exploit the virus entry machinery and pathway to transmit IFN-alpha-induced antiviral activity. J Virol. 2018;92 doi: 10.1128/JVI.01578-18. e01578-18. [DOI] [PMC free article] [PubMed] [Google Scholar]