Abstract
Resistance to respiratory pathogens, including coronavirus-induced infection and clinical illness in chickens has been correlated with the B (MHC) complex and differential ex vivo macrophage responses. In the current study, in vitro T lymphocyte activation measured by IFNγ release was significantly higher in B2 versus B19 haplotypes. AIV infection of macrophages was required to activate T lymphocytes and prior in vivo exposure of chickens to NP AIV plasmid enhanced responses to infected macrophages. This study suggests that the demonstrated T lymphocyte activation is in part due to antigen presentation by the macrophages as well as cytokine release by the infected macrophages, with B2 haplotypes showing stronger activation. These responses were present both in CD4 and CD8 T lymphocytes. In contrast, T lymphocytes stimulated by ConA showed greater IFNγ release of B19 haplotype cells, further indicating the greater responses in B2 haplotypes to infection is due to macrophages, but not T cells. In summary, resistance of B2 haplotype chickens appears to be directly linked to a more vigorous innate immune response and the role macrophages play in activating adaptive immunity.
Keywords: Macrophage, T lymphocytes, Major histocompatibility complex, B haplotype, Avian influenza virus, Nucleocapsid protein
Abbreviations: Avian influenza virus, AIV; B2 and B19 refer to the homozygous, B2/B2 or B19/B19 haplotypes, respectively; Concanavalin A, ConA; imunofluorescent antibody analysis, IFA; infectious bronchitis virus, IBV; interferon, IFN; major histocompatibility complex, MHC; nucleocapsid protein, NP; peripheral blood mononuclear cells, PBMC; polyriboinosinic acid:polyribocytidylic acidpoly, I:C
Highlights
-
•
B haplotypes in chickens correlate with resistance to disease.
-
•
Influenza virus infected macrophages from resistant lines are highly effective at activating T lymphocytes.
-
•
Cell-cell interactions are not required.
-
•
Both CD4 and CD8 T lymphocytes respond to infected macrophages.
-
•
T lymphocyte mitogen stimulation does not correlate with disease.
1. Introduction
Disease resistance to a variety of pathogens has been associated with MHC (major histocompatibility complex) haplotypes in several species, including mice and chickens (Banat et al., 2013, Briles and Briles, 1982, Dunnington et al., 1992, Heinzelmann et al., 1981, Joiner et al., 2007, Kim et al., 2008, Lamont, 1998, Briles and Briles, 1987; Mays et al., 2005, Mills, 2015, Yoo and Sheldon, 1992). In the chicken, MHC B haplotypes have been shown to display differential resistance to several viruses; including Marek's disease virus, avian leukosis virus, Newcastle disease virus, Rous sarcoma virus, infectious bronchitis virus (IBV) and avian influenza virus (AIV), as well as Salmonella (Banat et al., 2013, Briles and Briles, 1982, Lambrecht et al., 2004, Dunnington et al., 1992, Heinzelmann et al., 1981, Joiner et al., 2007, Kim et al., 2008, Lamont, 1998, Mays et al., 2005, Yoo and Sheldon, 1992).
Following infection with IBV, birds with the homozygous B2 haplotype have been shown to be more resistant to respiratory illness than B19 haplotype birds (Banat et al., 2013). Furthermore, B19 monocytes from peripheral blood mononuclear cells (PBMC) were slower to differentiate into macrophages upon in vitro culture than monocytes from the PBMC of birds with the B2 homozygous haplotype (Dawes et al., 2014). Macrophages, important cells of innate immunity, are directly involved in cellular interactions with pathogens, resulting in release of cytokines that activate other immune cells and in antigen presentation to cells responsible for adaptive immunity (Medzhitov and Janeway, 1997, Medzhitov and Janeway, 2000a, Medzhitov and Janeway, 2000b, Romagnani, 1992). Dawes et al. (2014) demonstrated that macrophages isolated from B2 birds were significantly more responsive than B19 derived macrophages to polyriboinosinic acid:polyribocytidylic acid (poly I:C), which simulates viral RNA replication and to IFNγ, an indicator of lymphocyte activation.
Considering the ease of activating B2 macrophages compared with the B19 macrophages, it was of interest to compare their relative capacity to stimulate T lymphocytes and what conditions, such as prior exposure to antigen, are essential for activation. In our previous studies, using infected homozygous kidney cells as antigen presenting cells, in vivo vaccination of vectors expressing the NP of AIV were strong inducers of MHC restricted CD8 T lymphocytes (Singh et al., 2010a, Singh et al., 2010b).
The current study compares the roles of macrophages in the activation of homologous and heterologous T lymphocytes. Unlike kidney cells, macrophages are considered professional antigen presenting cells, expressing both MHC I and MHC II and thus are capable of activating CD8 and CD4 T lymphocytes, respectively. AIV infection in chicken macrophages has been demonstrated previously (Xing et al., 2010, Barjesteh et al., 2014). Therefore, macrophages infected in vitro with AIV were ideal in evaluating differences in ex vivo activation of T lymphocytes purified from B2 and B19 birds vaccinated with the AIV NP.
2. Materials and methods
2.1. Experimental animals
Animal procedures were approved and conducted according to guidelines established by the Western University of Health Sciences, Pomona, California (Western University) Institutional Animal Care and Use Committee. Fertilized eggs (from either B2/B2 or B19/B19 haplotype birds), acquired from the laboratory of Dr. W. Elwood Briles at Northern Illinois University, were incubated and hatched under standard conditions at Western University (38 °C and 50–65% humidity). Post hatch, chicks were held in an incubator maintained at 38 °C and 50–65% humidity for 24 h, before transferring to a brooder, pre-heated to 35 °C, in the vivarium of the University Research Center at Western University. In addition to daily health monitoring, fresh food and water were provided ad libitum. Room temperature was adjusted to and maintained at 32 °C until 3 weeks of age when chicks were transferred to open cages in the vivarium. To minimize the risk of pecking disorders, chicks were kept under restricted lighting conditions throughout the study. Peripheral blood was collected from the jugular or wing web veins. Experimental animals were euthanized by insufflation of isoflurane.
2.2. Vaccination of chicks
The NP of the AIV plasmid was prepared as described by Singh et al. (2010a) using the Qiagen Plasmid Midi kit according to manufacturer's instructions. Chicks were vaccinated intramuscularly (i.m.) with 500 μg of cDNA expressing the NP cDNA plasmid (Singh et al., 2010a).
2.3. Macrophage preparations
Peripheral blood mononuclear cells (PBMCs) were isolated using differential centrifugation as previously described (Seo and Collisson, 1997, Drechsler et al., 2009, Dawes et al., 2014). Briefly, 3 ml of peripheral blood was mixed with 3 ml of phosphate buffered saline (PBS) (Sigma-Aldrich, St. Louis, MO) after which this mixture was layered over an equal volume of Ficoll-Hypaque (1.083) (Sigma-Aldrich, St. Louis, MO; Dawes et al., 2014). Samples were centrifuged for 35 min (400 x g; 23 °C; brake off) before collecting the interphase cells. Isolated cells were washed twice in 5 ml PBS (400×g; 10 min, 23 °C), counted and viability of ≥90% was confirmed based on the exclusion of 0.1% trypan blue dye. Cells were re-suspended in RPMI to a final concentration of 5 × 107 cells/ml. Five hundred μl of the cell suspensions were added to each well in 24 well plates and incubated at 37 °C for 6 days until the monocytes differentiated into macrophages at 70 to 80 percent confluence. Macrophages were counted in wells after adherence via the NIS Elements program, with cells being counted per mm2 and calculated per well as previously described (Dawes et al., 2014).
2.4. AIV infection of macrophages
Low pathogenic AIV, H5N9 (A/Turkey/Wis/68), was propagated in the allantoic sacs of 10-day-old embryonating chicken eggs (ECE) (Singh et al., 2010a, Singh et al., 2010b). The allantoic fluid was harvested 48 h post inoculation and virus was quantified by titrating in ECE and expressed as embryo infectious dose 50 (EID50) (Beard, 1989). Each well with a macrophage preparation was inoculated with 250 μl of virus preparation from a stock of 102 EID50 of virus. Macrophages infected with virus were incubated at 39 °C for 45 min before washing twice with RPMI medium, followed by overnight culture. Infection was confirmed after 24 h of incubation by indirect immunofluorescence (IFA) (Singh et al., 2010a). Primary antiserum used was AIV (+) serum from the APHIS National Veterinary Services Laboratories, and the secondary antibody used was goat anti-chicken IgG (Alexa 488, Molecular Probes). Viral load in macrophages was determined with the TCID titer 10−2.2/500 μl at 16 h after infection by real time PCR. Primer sequences: cDNA synthesis: TGCTCTCTCGAATGGAAGGT, PCR primers: GAATCCTGGGAATGCTGAAA and GTGCTGGATTTTCGTTTGGT. PCR conditions were as follows: 95 °C for 10 min-hot start, 40 cycles of 95 °C for 15 s, 60 °C for 30 s according to manufacturer instructions for the Biotool 2x Sybr Green qPCR Mix (Biotool, Houston, Tx). Viral infections were also confirmed with TCID50 as previously described, using cytopathic effects (CPE) and the hemagglutination (HA) assay (Moresco et al., 2010, Reed and Muench, 1938). Briefly, macrophages were isolated as described above and 500 μl of PBMC in media were placed in 48 well plates at 5 × 107 cells/ml. Stocks of 102 EID50 of virus were used to infect wells at 1:10 dilutions in duplicates and CPE was evaluated by microscopy. Plates were frozen at −80 °C for 24 h before thawing, when supernatants were used for HA assays (RBC from uninfected chickens incubated with freeze thaw supernatant (Moresco et al., 2010) and TCID50 determined.
2.5. T lymphocyte preparation
The T lymphocytes were prepared from 3 ml of peripheral blood diluted in an equal volume of PBS from the interphase after Ficoll-histopaque density (1.077) gradient centrifugation (Pei et al., 2003, Seo and Collisson, 1997, Singh et al., 2010a). After collecting cells from the interface and washing with PBS, mononuclear cells were suspended in 3 ml of RPMI 1640, supplemented with 10% FBS (Invitrogen, La Jolla, CA) and incubated for at least 45 min in tissue culture plates to allow for macrophage attachment. After removing the unattached cell population, the B lymphocyte population was depleted using an RPMI equilibrated nylon wool column, as previously described (Seo and Collisson, 1997, Singh et al., 2010a). The T lymphocyte enriched preparations were washed twice and suspended in RPMI before use.
2.6. Ex vivo activation of T lymphocytes
T lymphocytes, purified from PBMC, were incubated with or without macrophages as described for stimulation with AIV infected kidney cells (Seo and Collisson, 1997, Singh et al., 2010a). After removing the media from each well when macrophages were 70–80% confluent (approximately 5 × 105 cells per well of a 24 well plate), T lymphocytes were added at a concentration of 5 × 106 in 500 μl per well of RPMI 1640 with 10% FBS. The infected or uninfected control macrophages were incubated with T lymphocytes for 24–48 h before quantifying the amount of IFNγ produced in each well as the indicator of T lymphocyte activation. Studies included the co-culture of 1. Heterologous, NP vaccinated T lymphocytes and AIV infected macrophages, 2. Homologous T lymphocytes from birds that were not vaccinated with NP with infected macrophages and 3. NP vaccinated T lymphocytes with homologous, uninfected macrophages.
2.7. Detection of activated T Lymphocytes
Activation of T lymphocytes was evaluated by determining the concentration of IFNγ in the clarified supernatants from cultured T lymphocytes using a commercial ELISA specific for ch-IFNγ (Invitrogen, La Jolla, CA, Pei et al., 2003, Singh et al., 2010a, Singh et al., 2010b). Nitric oxide secretions induced in the cultured macrophage and T lymphocyte combinations were quantified using a Griess reagent assay according to the manufacturer's protocol (Sigma-Aldrich, St. Louis, MO) and described by Singh et al. (Singh et al., 2010a, Dawes et al., 2014). The concentration of nitrite produced was determined using sodium nitrite solutions with concentrations of 1–20 μm as standards. The concentration of any non-specific production of nitric oxide by soluble factors was adjusted by subtracting the nitrite concentration of supernatants from macrophages cultured without T lymphocytes from the supernatants of the macrophages cultured with T lymphocytes.
2.8. Interactions in the absence of macrophage-T lymphocyte, cell-to-cell interactions
Supernatant fluids collected from infected or uninfected macrophages were placed on homologous T lymphocytes collected as described above. After 24 h of exposure, the supernatant fluids from these T lymphocytes were collected and clarified by centrifuging at 1500 RPM for 10 min before quantifying IFNγ in the supernatant using ELISA as described above.
2.9. Depletion of CD4 and CD8 T lymphocytes
T lymphocytes were purified before incubating with mouse, anti-chicken CD8 antibody as described by Singh et al. (2010b). The CD8 and CD4 T lymphocytes were separated by antibody-mediated depletion using Dynabeads (Invitrogen, La Jolla, CA; Singh et al., 2010a). T lymphocytes were labeled with either mouse anti-chicken CD8 or mouse anti-chicken CD4 monoclonal antibodies (MAb) (Southern Biotech, Birmingham, AL) at a concentration of 1 μg/106 cells in PBS containing 0.1% bovine serum albumin fraction V (Sigma- Aldrich, St. Louis, MO) before incubating at 4 °C for 30 min. The cells were washed twice with PBS to remove unattached antibodies before incubating with rat anti-mouse IgG coated Dynabeads, DynaMag-2 (Invitrogen, La Jolla, CA) according to manufacturer's instructions. Unlabeled cells in the supernatants were collected before confirming the purity of CD4 or CD8 depleted T lymphocyte preparation by FACS analysis using fluorescent labeling with CD4 or CD8 MAbs (Bohls et al., 2006). The purity of CD4 or CD8 T lymphocyte subpopulations after antibody depletion of either CD8 or CD4, respectively, was 95%.
2.10. ConA stimulation of T lymphocytes
T lymphocytes, purified as described above, were incubated in 48 well microtiter plates at a concentration of 5 × 107 cells/ml with 250 μl per well. 2.5–5 μg of ConA per ml (Sigma) were added to each well before incubating for 16–24hr, when the supernatants were removed and the concentration of IFNγ was determined using ELISA. Sample readings were equivalent after either 16hr or 24 h and whether using 2.5 or 5 μg/ml of ConA.
2.11. Statistical analyses
The GraphPad Prism 6 (GraphPad Software Inc, La Jolla, CA) was used for statistical analyses. Statistical tests performed were unpaired t-tests, non-parametric, (Mann Whitney test or Kruskall Wallis test) with statistical significance considered at p < 0.05. The data is represented in figures as averages with standard errors.
3. Results
3.1. In vitro AIV infection of macrophages
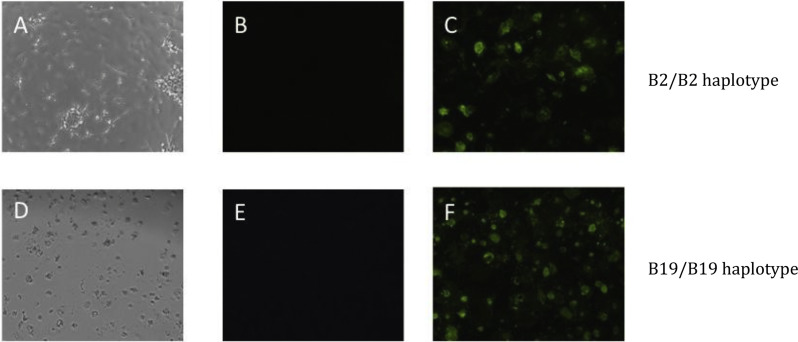
Chicken macrophages are readily infected with AIV (Lyon and Hinshaw, 1991, Xing et al., 2010, Barjesteh et al., 2014). Therefore, in order to compare the potential for macrophages from B2 or B19 defined birds to activate T lymphocytes, chicken macrophages were infected with the low pathogenic H5N9 strain (A/Turkey/Wis/68) of AIV (Singh et al., 2010a). Infection was detected by immunofluorescent staining of preparations of macrophages derived from birds of either haplotype (Fig. 1 ). TCID50 was determined by CPE and HA assays to be 10−2.2/500 μl, with no detectable difference for either haplotype. Viral RNA detection in B2 and B19 macrophages 45 min (after viral uptake) and 16 h incubation after infection indicated that there were no significant differences between B2 and B19 haplotypes (n = 3 uninfected controls/haplotype, n = 5 infected/haplotype). Viral replication calculated with the delta ct method against viral load at the 45 min infection time point was 38 fold more in infected B2 macrophages and 34 fold more in infected B19 macrophages, showing comparable replication. These results demonstrate that there were no significant differences (p > 0.05) in viral replication and infectivity in B2 and B19 haplotype macrophages.
Fig. 1.
Infection of macrophages with low pathogenic H5N9 AIV (A/Turkey/Wis/68). Infection of cell culture differentiated B2 or B19 derived macrophages with AIV. 10× objective was used for all microscopy pictures. (A) Brightfield microscopy of AIV infected B2 macrophages, (B) IFA stained uninfected B2 macrophages and (C) IFA stained AIV infected B2/B2 macrophages. (D) Brightfield microscopy of AIV infected B19 macrophages, (E) IFA stained uninfected B19 macrophages and (F) IFA stained AIV infected B19 macrophages.
3.2. B2 derived AIV infected macrophages are better at activating NP primed homologous T lymphocytes than B19 derived infected macrophages
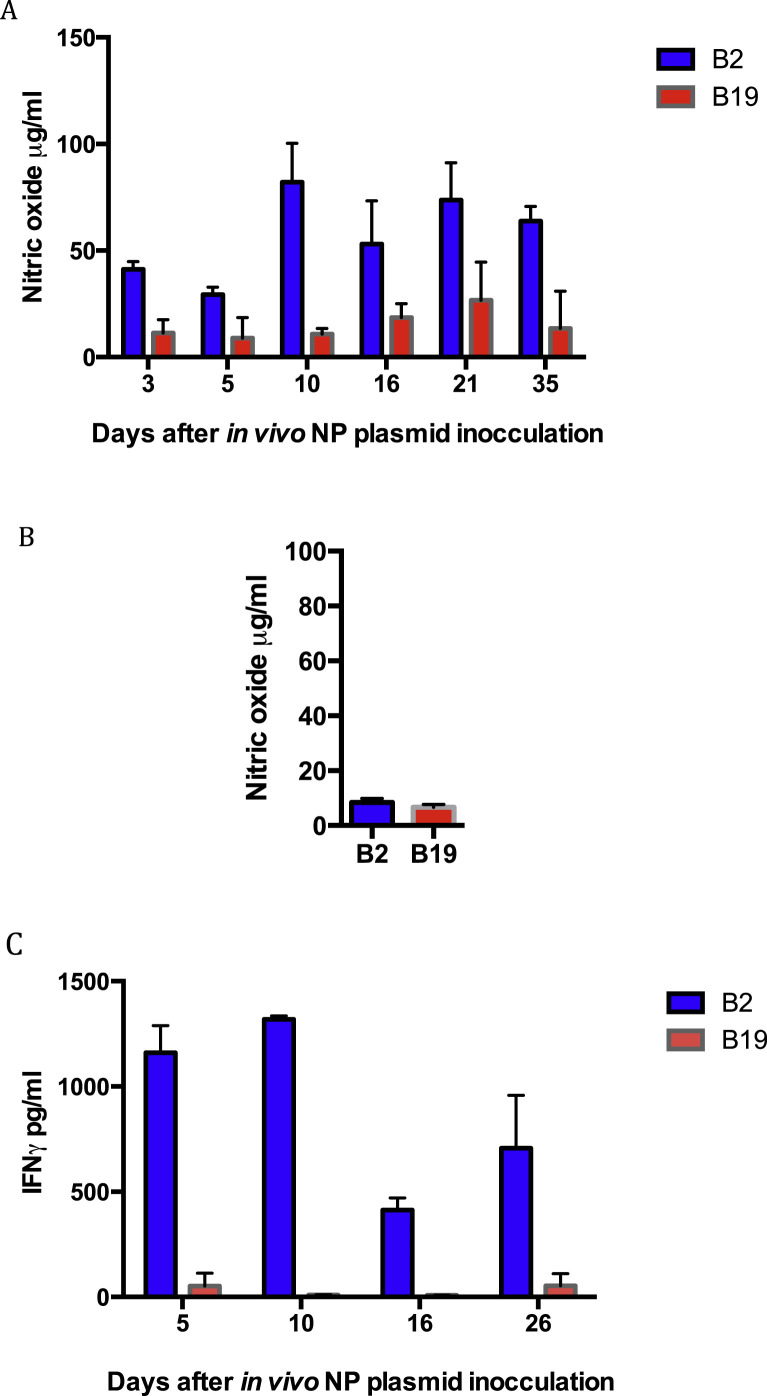
We have previously shown that the NP of AIV expressed in cDNA or an adenovirus vector is an exceptional CD8 T lymphocyte antigen (Singh et al., 2010a, Singh et al., 2010b). Therefore, chicks vaccinated with the NP cDNA were used as a source of influenza primed T lymphocytes. The potential for B2/B2 or B19/B19 macrophages to stimulate NP primed T lymphocytes was examined using AIV infected macrophages cultured with homologous T lymphocytes purified from NP vaccinated birds (Fig. 2 A).
Fig. 2.
Homozygous B2 AIV infected macrophages are significantly better at stimulating B2 NP primed T lymphocytes than B19 AIV infected macrophages at stimulating NP primed B19 T lymphocytes. AIV infected macrophages were cultured with homologous NP vaccinated B2 or B19 T lymphocytes. NO was produced by macrophages co-cultured with T cells from NP vaccinated birds (A), while AIV infected macrophages in the absence of T cells did not release significant levels of NO (B). Stimulation of T lymphocytes from vaccinated birds by AIV infected homologous macrophages was quantified with IFNγ ELISA (C). Significant differences (p < 0.05) between the two haplotypes were determined at all days post-infection.
Activation of macrophages at 3, 5, 10, 16, 21 and 35 days post-vaccination with the NP cDNA, was measured by quantitation of NO, reflective of a response to stimuli including cytokines such as IFNγ secreted by the co-cultured T lymphocytes (Fig. 2A). Macrophages from both haplotypes show activation, however, stimulation of B2 macrophages was considerably greater at any day post-vaccination than that of the B19 macrophage cultures. Infected macrophages alone in the absence of T lymphocytes produced negligible NO (Fig. 2B), indicating the stimulation of macrophages and consequent NO release observed was due to co-culture and activation of Tcells.
Activation of T lymphocytes from 5 to 26 days post-vaccination was evaluated using ch-IFNγ ELISA (Fig. 2C). The differences seen between the haplotypes were even more pronounced than measuring NO release from macrophages. There was essentially no detectable activation of B19 T lymphocytes by homologous infected macrophages on days 10 and 16 after NP vaccination. The B2 derived primed T lymphocytes were consistently more potently activated by infected macrophages than B19 derived infected T cells from the influenza NP vaccinated birds.
3.3. AIV infection of macrophages was required for T lymphocyte activation
T lymphocytes, collected at varying times between 3 and 42 days after vaccination of birds with the NP cDNA, were cultured with uninfected macrophages. Macrophages that were not infected, regardless of the haplotype, were incapable of activating T lymphocytes as evidenced by lack of detectable IFNγ in the ELISA (data not shown).
3.4. Prior exposure to NP is not required for stimulation of T lymphocytes by infected macrophages
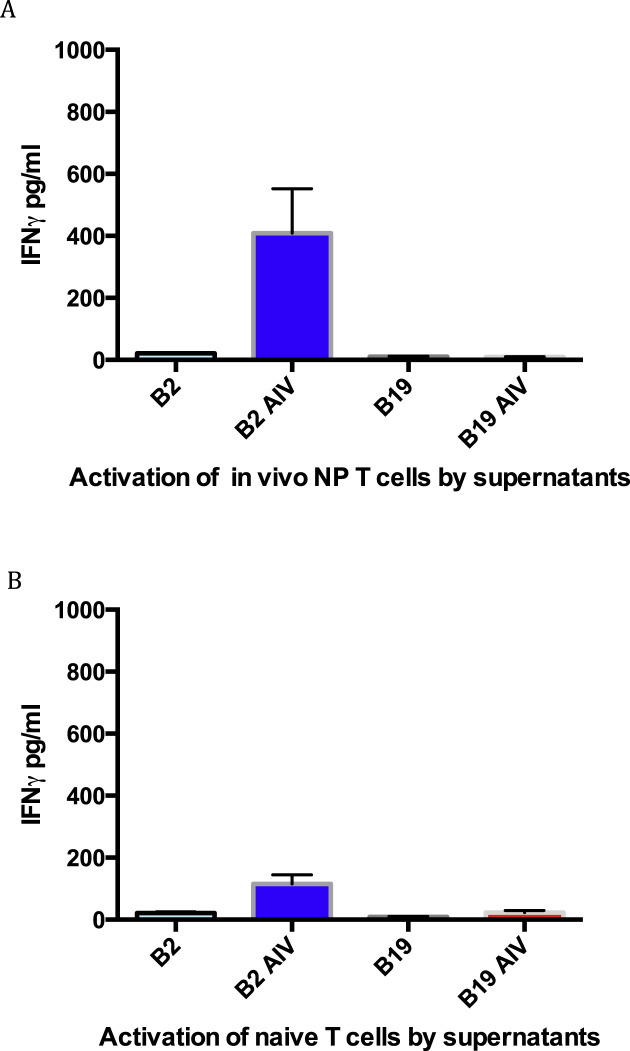
Macrophage stimulation did not require that the source of T lymphocytes be from chickens vaccinated with the NP cDNA. Naïve T cells from either B2 or B19 unvaccinated chicks were stimulated with homologous infected macrophages resulting in IFNγ release from T cells. However, the levels of stimulation were lower when compared with T cells derived from NP vaccinated chicks. In co-culture experiments of infected macrophages and naïve T-cells, B2 macrophages released more nitric oxide than B19 cells (Fig. 3 A). Whereas IFNγ release of naïve B2 T cells could be detected when co-cultured with AIV infected B2 macrophages, B19 infected macrophage mediated stimulation of B19 naïve T cells was again significantly less as determined by the IFNγ ELISA (Fig. 3B).
Fig. 3.
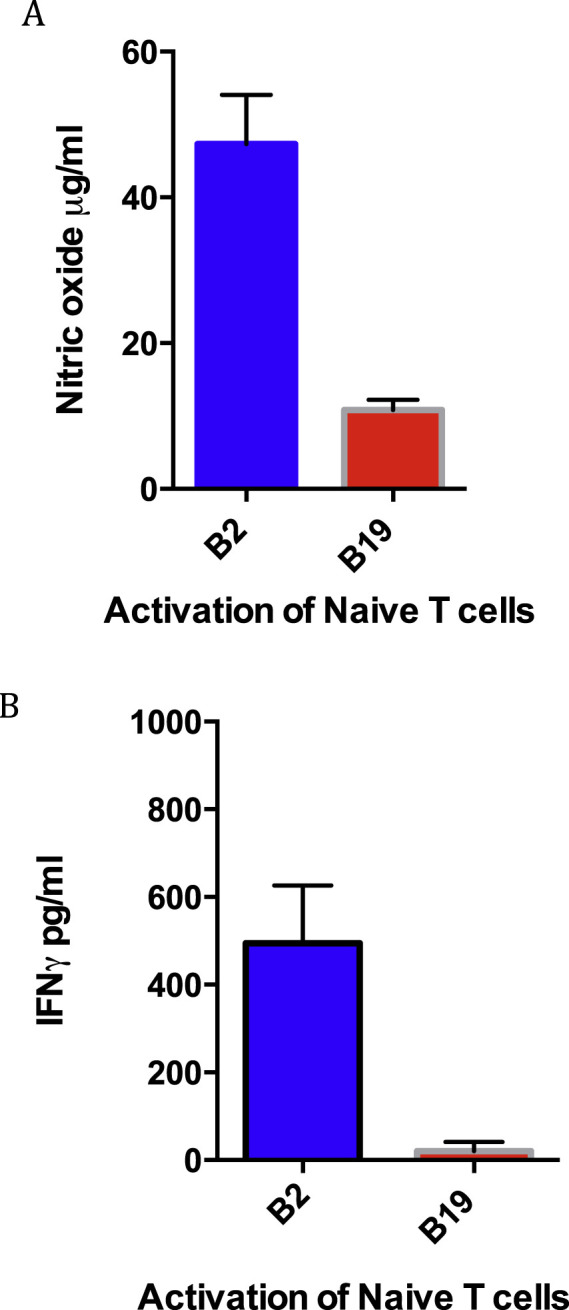
Infected B2 macrophages stimulated homologous T cells from unvaccinated chicks. Production of NO by macrophages co-cultured with T lymphocytes (A). Activation of T lymphocytes was determined by detection of IFNγ (B). The differences between the B2 and B19 macrophage stimulation not exposed to the NP cDNA vaccine were p < 0.001 for both figures A and B.
3.5. T lymphocyte activation by infected macrophages does not require cell-to-cell interaction
Antigen presentation implies direct interaction between the macrophage and T cell. In order to examine the necessity for cell-to-cell interaction, T lymphocytes were cultured with supernatants collected from infected macrophages rather than with the infected macrophages. Just as B19 infected macrophages had little influence on B19 T lymphocytes in the previous experiments, the supernatants from the B19 infected macrophages had no impact on the B19 T lymphocytes. In the absence of cell-to-cell interaction, supernatants from B2 infected macrophages did stimulate homologous T lymphocytes (Fig. 4 A). However, the magnitude of IFNγ released was significantly less compared to cells that were co-cultured and allowed cell-cell interaction between T lymphocytes and infected macrophages (Fig. 4B), indicating that antigen presentation plays an important role in this activation.
Fig. 4.
Supernatants from B2 infected macrophages stimulated B2 T lymphocytes to produce IFNγ. Supernatants from infected macrophages were used to stimulate homologous T lymphocytes from vaccinated (A) or unvaccinated (B) birds. Differences in both figures were p < 0.01.
3.6. Infected B2 macrophages activate IFNγ production from B19 T lymphocytes
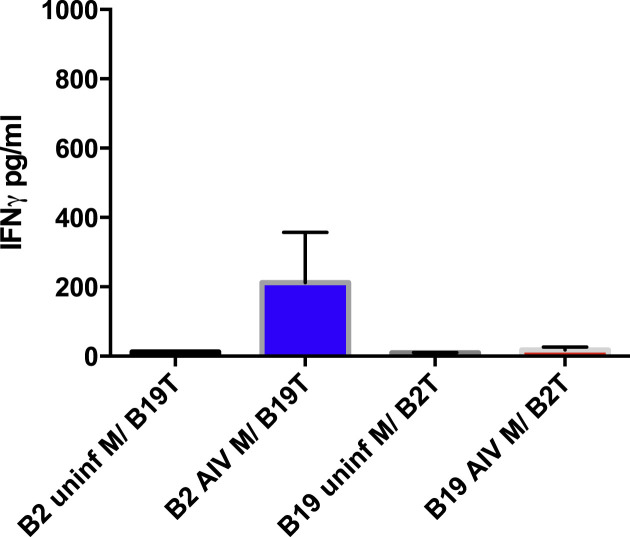
In order to determine requirements for MHC compatibility between the infected macrophage and the T lymphocyte, MHC cross-matched experiments between AIV infected macrophages and T lymphocytes were performed. Infected B2 macrophages were able to stimulate B19 T cells (Fig. 5 ). In contrast, B19 macrophages did not stimulate B2 T lymphocytes, further confirming that the B19 macrophages are poorly stimulated by AIV infection themselves and consequently not activating the Tcells via cytokines.
Fig. 5.
Stimulation of T lymphocytes by infected B2 macrophages was not entirely MHC restricted. The B2 infected macrophages stimulated heterologous B19 T lymphocytes while B19 infected macrophages had essentially no impact on B2 T lymphocytes (p < 0.05). B2 uninf M or B19 uninf M represent uninfected macrophages cultured with heterologous /B19 or /B2 T cells from vaccinated birds. B2 AIV M represent infected B2 derived macrophages cultured with B19 derived T lymphocytes and B19 AIV M represent infected B19 macrophages cultured with B2 derived T lymphocytes.
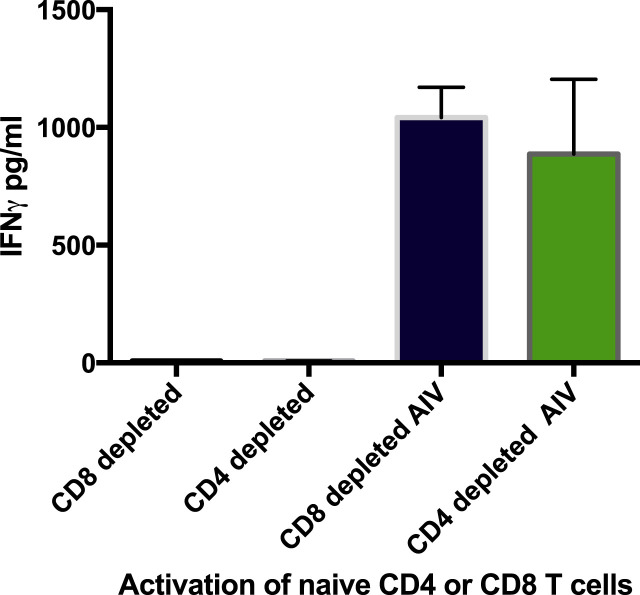
3.7. Both CD4 and CD8 T lymphocytes are activated by infected macrophages
In order to determine the T cell source of activation, either CD8 or CD4 T lymphocytes from vaccinated B2 birds were purified prior to exposure to AIV infected homologous macrophages (Fig. 6 ). Both CD4 and CD8 depleted T lymphocytes, respectively, were activated in the presence of infected macrophages. Therefore, both T cell phenotypes from B2 vaccinated chicks responded similarly to homologous AIV infected macrophages with no significant difference. In the absence of infected macrophages neither CD4 nor CD8 control T lymphocytes were stimulated.
Fig. 6.
Both CD4 and CD8, B 2 T lymphocytes from vaccinated B2 birds were activated by B2 infected macrophages. T cell phenotypes were enriched by depletion of CD8 and CD4 antibody, respectively. Controls represent T lymphocyte responses in the absence of macrophages. AIV indicates infection of macrophages cultured with either phenotype.
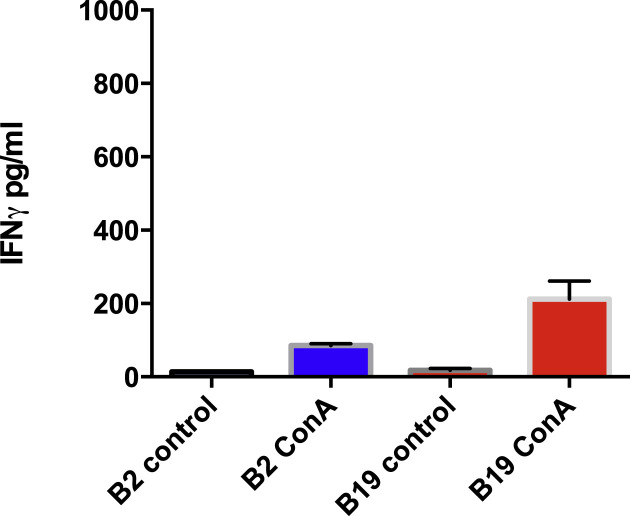
3.8. T cells from B19 haplotype chicks were better responders to nonspecific ConA stimulation
To assess whether the T cells of either B2 or B19 haplotypes have the potential for non-specific activation by a lymphocyte mitogen in the absence of macrophages, both T lymphocytes from either B2 or B19 birds were exposed to ConA. Surprisingly, while both types of lymphocytes responded to ConA, the B19 T lymphocytes responded significantly better than the B2 derived T lymphocytes (Fig. 7 ). Differences in the activation of the T lymphocytes thus could not be attributed generally to a lack of B19 lymphocyte function.
Fig. 7.
ConA stimulation of B19 derived T lymphocytes was significantly better than that of B2 T lymphocytes (p < 0.05). Neither B2 nor B19 derived T lymphocytes were stimulated in the controls in the absence of ConA.
4. Discussion
These studies demonstrate dramatic functional differences in the impact of the MHC B2 and B19 haplotype defined macrophages on adaptive immunity, specifically, activation of T lymphocytes. Quantification of macrophage responses co-cultured with T cells consistently demonstrated higher nitric oxide responses by the B2 haplotypes. In addition, activation of IFNγ responses from either homologous B2 or even B19 derived T lymphocytes co-cultured with AIV infected macrophages were demonstrated, despite viral load and infectivity being comparable in both haplotypes. Thus, the B19 infected macrophages were poor at stimulating T lymphocytes.
The AIV specific responses of T cells have been described after inoculation of birds with an AIV cDNA expressing the NP (Singh et al., 2010a, Singh et al., 2010b). The NP cDNA had been previously shown to encode a more effective T lymphocyte antigen than even the AIV hemagglutinin protein. Although B2 derived T lymphocytes from birds that had never been exposed to AIV NP also responded to homologous infected macrophages, prior exposure of the B2 birds inoculated with NP cDNA consistently resulted in significantly greater responses to AIV infected B2 derived macrophages.
It was of interest to determine if T cell stimulation required cell-to-cell interaction with the infected macrophage or if supernatants from macrophages alone activate lymphocyte production of IFNγ. T cells incubated with supernatants from B2 AIV infected macrophages in the absence of macrophages were able to stimulate T cells to release IFNγ. However, we also found that the amount of IFNγ released was lower than in experiments with T cell-macrophage contact.
B2 infected macrophages could stimulate IFNγ from heterologous B19 T lymphocytes, most likely by release of cytokines, as antigen presentation does not play a role in the MHC mismatched cell culture. However, the homologous cultures consistently resulted in greater induction of B2 T lymphocyte IFNγ compared to the heterologous cultures, indicating that although cytokines released by the infected macrophages play a role in the stimulation of Tcells, antigen presentation with direct cell-cell contact has a significant effect on this activation. Another indication of a feedback activation in this co-culture system is the increase in NO levels released by B2 macrophages co-cultured with T lymphocytes. This NO release is likely caused by release of IFNγ by the Tcells after activation, which has been previously shown to be the major activator of macrophages, or possibly by other cytokines that stimulate the nitric oxide release.
The previous studies by Singh et al., 2010a, Singh et al., 2010b indicated that the CD8 rather than CD4 T cells were induced ex vivo with homologous AIV infected kidney cell, that is, the nonprofessional cell cultures. Infected macrophages in the current study induced both CD4 and CD8 T cells, as indicated by the release of IFNγ by both CD4 and CD8 depleted Tcells. Since macrophages, as professional antigen presenting cells, are active players for both innate and adaptive immunity, they would be expected to be more comprehensive at alerting the T lymphocyte population of infection. Whereas kidney cells express MHC I, macrophages express both MHC class I and MHC class II antigens and thus have the potential to activate CD8 and CD4 T cells, respectively (Burgdorf and Kurts, 2008). In addition the release of cytokines by infected macrophages would equally affect CD4 and CD8 positive T lymphocytes, although the release of IFNγ was too great to be just mediated by cytokines alone.
Detection of macrophage activation using nitric oxide measurements by co-culturing with T lymphocytes is complicated by the fact that the macrophages are infected with AIV. Therefore, it was critical that direct determination of IFNγ confirm the macrophage and T lymphocyte interactions and that in the absence of infection, macrophages did not stimulate T cells to produce IFNγ or other cytokines that might in turn activate the macrophages to release nitric oxide.
Cytokine production, specifically secretion of pro-inflammatory molecules, has also been associated with increased resistance against disease in chickens (Ferro et al., 2004, Swaggerty et al., 2004). Dawes et al. (2014) showed that, in response to poly I:C or IFNγ stimuli, responses of macrophages derived from the more resistant B2 chickens, were dramatically greater than the responses of macrophages from the more IBV susceptible B19 haplotype chickens.
The demonstration of differences early in infection described by Banat et al. (2013) had suggested that innate immunity initially contributed to the enhanced resistance of the B2/B2 birds to IBV associated clinical illness. In the current study, we used AIV rather that IBV because macrophages are readily infected with AIV. Although belonging to different viral families with distinctly different properties, both viruses have single-stranded RNA genomes and both have enveloped virion particles. It should be assumed that IBV, although not readily infecting macrophages, infects epithelial cells and consequential pathology would be expected to activate innate immunity.
The current studies suggest that the B19/B19 derived macrophages are deficient in their response to external stimuli, that is, macrophage infection. However, the deficiency is not universal for all immune cells from the B19 haplotypes, since B19 T lymphocytes surprisingly not only responded to ConA stimulation, but responded significantly better than the B2 derived T lymphocytes. Upon stimulation of macrophages with ch-IFNγ, Irizarry and Drechsler (personal observations) also have now found differences in a number of cellular pathways in macrophages from either B19 or B2 birds.
The current studies, focusing on T lymphocyte responses, confirm and highlight the importance of the interphase in the chicken between macrophages and adaptive, specifically T lymphocyte, immunity. These studies strongly suggest that macrophage function rather than T lymphocyte function, is the driving factor in the differences observed between B2 versus B19 activation of T lymphocytes. Differences in macrophage function, correlating with differential innate immune responses (Dawes et al., 2014), corroborate recent studies that demonstrate a much more important role macrophages play, in general, in directing the adaptive immunity, particularly T cell responses, in a variety of diseases including cancer and autoimmunity (Mills et al., 2000, Mosser, 2003, Mills, 2012, Biswas et al., 2012).
5. Conclusions
B haplotype chicken lines provide an excellent resource for studying the genetic bases of MHC associated disease resistance and susceptibility, and the relationship between macrophage function and disease resistance. Further studies elucidating molecular mechanisms of this activation are needed in order to understand the underlying genetic causes.
Acknowledgements
The authors give special recognition to Dr. W. Elwood Briles and Mrs. Ruth Briles for whom this manuscript is dedicated and who were responsible for the early recognition of the chicken B haplotypes. We also thank Dr. Briles' technician Ms. Linda Yates and his assistant, Dr. Rene Kopulos for their essential contributions to the development and characterization of these chicken haplotypes. Dr. Maisie Dawes' contributions to the initial development of the macrophage culture technologies cited in this paper are greatly appreciated (Dawes et al., 2014). We also received critical personnel support from the Western University of Health Sciences College of Veterinary Medicine. These studies were funded by grants from the United States Department of Agriculture-National Research Institute (USDA-NRI) #2006-35204-18810, USDA-National Institute of Food and Agriculture (NIFA) #2008-00875 and the USDA-Cooperative State Research Service Competitive Grants Program (2006-35204-16560).
References
- Mills C.D., Kincaid K., Alt J.M., Heilman M.J., Hill A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000 Jun 15;164(12):6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Banat G.R., Tkalcic S., Dzielawa J.A., Jackwood M.W., Saggese M.D., Yates L., Kopulos R., Briles W.E., Collisson E.W. Association of the chicken MHC B haplotypes with resistance to avian coronavirus. Dev. Comp. Immunol. 2013;39:430–437. doi: 10.1016/j.dci.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barjesteh N., Behboudi S., Brisbin J.T., Villanueva A.I., Nagy É., Sharif S. TLR ligands induce antiviral responses in chicken macrophages. PLoS ONE. 2014;9(8) doi: 10.1371/journal.pone.0105713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C.W. A laboratory manual for the isolation and identification of avian pathogens. In: Purchase L.H.A.H.G., Domermuth C.H., Pearson J.E., editors. third ed. Kendall/Hunt Publishing Co; Dubuque, IA: 1989. [Google Scholar]
- Biswas S.K., Chittezhath M., Shalva I.N., Lim J.Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012 Sep;53(1–3):11–24. doi: 10.1007/s12026-012-8291-9. Review. [DOI] [PubMed] [Google Scholar]
- Bohls R.L., Smith R., Ferro P.J., Silvy N.J., Li Z., Collisson E.W. The use of flow cytometry to discriminate avian lymphocytes from contaminating thrombocytes. Dev. Comp. Immunol. 2006;30(9):843–850. doi: 10.1016/j.dci.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Briles W.E., Briles R.W. Identification of haplotypes of the chicken major histocompatibility complex (B) Immunogenetics. 1982;15:449–459. doi: 10.1007/BF00345904. [DOI] [PubMed] [Google Scholar]
- Briles W.E., Briles R.W. Genetics and classification of the major histocompatibility complex antigens of the chicken. Poult. Sci. 1987;66:776–781. doi: 10.3382/ps.0660776. [DOI] [PubMed] [Google Scholar]
- Burgdorf S., Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr. Opin. Immunol. 2008;20:89–95. doi: 10.1016/j.coi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Dawes M.E., Griggs L.M., Collisson E.W., Briles W.E., Drechsler Y. Dramatic differences in Interferon gamma and polyinosinic:polycytidylic acid -stimulations of macrophages from chickens with B2 and B19 MHC-defined haplotypes. Poult. Sci. 2014 Apr;93(4):830–838. doi: 10.3382/ps.2013-03511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler Y., Bohls R.L., Smith R., Silvy N., Lillehoj H., Collisson E.W. An avian, oncogenic retrovirus replicates in vivo in more than 50% of CD4+ and CD8+ T lymphocytes from an endangered grouse. Virology. 2009;386:380–386. doi: 10.1016/j.virol.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Dunnington E.A., Larsen C.T., Gross W.B., Siegel P.B. Antibody responses to combinations of antigens in White Leghorn chickens of different background genomes and major histocompatibility complex genotypes. Poult. Sci. 1992;71:1801–1806. doi: 10.3382/ps.0711801. [DOI] [PubMed] [Google Scholar]
- Ferro P.J., Swaggerty C.L., Kaiser P., Pevzner I.Y., Kogut M.H. Heterophils isolated from chickens resistant to extra-intestinal Salmonella enteritidis infection express higher levels of pro-inflammatory cytokine mRNA following infection than heterophils from susceptible chickens. Epidemiol. Infect. 2004 Dec;132(6):1029–1037. doi: 10.1017/s0950268804002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzelmann E.W., Clark K.K., Collins W.M., Briles W.E. Host age and major histocompatibility genotype influence on Rous sarcoma regression in chickens. Poult. Sci. 1981 Oct;60(10):2171–2175. doi: 10.3382/ps.0602171. [DOI] [PubMed] [Google Scholar]
- Kim D.K., Lillehoj H.S., Hong Y.H., Park D.W., Lamont S.J., Han J.Y., Lillehoj E.P. Immune-related gene expression in two B-complex disparate genetically inbred Fayoumi chicken lines following Eimeria maxima infection. Poult. Sci. 2008 Mar;87(3):433–443. doi: 10.3382/ps.2007-00383. [DOI] [PubMed] [Google Scholar]
- Joiner K.S., Hoerr F.J., Ewald S.J., van Santen V.L., Wright J.C., van Ginkel F.W., Toro H. Pathogenesis of infectious bronchitis virus in vaccinated chickens of two different major histocompatibility B complex genotypes. Avian Dis. 2007;51:758–763. doi: 10.1637/0005-2086(2007)51[758:POIBVI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lambrecht B., Gonze M., Meulemans G., van den Berg T.P. Assessment of the cell-mediated immune response in chickens by detection of chicken interferon-gamma in response to mitogen and recall Newcastle disease viral antigen stimulation. Avian Pathol. 2004 Jun;33(3):343–350. doi: 10.1080/0307945042000220318. [DOI] [PubMed] [Google Scholar]
- Lamont S. Impact of genetics on disease resistance. Poult. Sci. 1998 Aug;77(8):1111–1118. doi: 10.1093/ps/77.8.1111. Review. [DOI] [PubMed] [Google Scholar]
- Lyon J.A., Hinshaw V.S. Replication of influenza A viruses in an avian macrophage cell line. J. Gen. Virol. 1991 Aug;72(Pt 8):2011–2013. doi: 10.1099/0022-1317-72-8-2011. [DOI] [PubMed] [Google Scholar]
- Mays J.K., Bacon L.D., Pandiri A.R., Fadly A.M. Response of white leghorn chickens of various B haplotypes to infection at hatch with subgroup J avian leukosis virus. Avian Dis. 2005;49:214–219. doi: 10.1637/7315-120104R. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. Innate immunity; impact on the adaptive immune response. Curr. Opin. Immunol. 1997 Feb;9(1):4–9. doi: 10.1016/s0952-7915(97)80152-5. Review. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C., Jr. Innate immunity. N. Engl. J. Med. 2000 Aug 3;343(5):338–344. doi: 10.1056/NEJM200008033430506. Review. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C., Jr. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 2000 Feb;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. Review. [DOI] [PubMed] [Google Scholar]
- Mills C.D. M1 and M2 macrophages: oracles of health and disease. Crit. Rev. Immunol. 2012;32(6):463–488. doi: 10.1615/critrevimmunol.v32.i6.10. Review. [DOI] [PubMed] [Google Scholar]
- Mills C.D. Anatomy of a discovery: M1 and M2 macrophages. Front. Immunol. 2015 May;5(6):212. doi: 10.3389/fimmu.2015.00212. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moresco K.A., Stallknecht D.E., Swayne D.E. Evaluation and attempted optimization of avian embryos and cell culture methods for efficient isolation and propagation of low pathogenicity avian influenza viruses. Avian Dis. 2010;54(1Suppl. l):622–626. doi: 10.1637/8837-040309-Reg.1. [DOI] [PubMed] [Google Scholar]
- Mosser D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003 Feb;73(2):209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Pei J., Briles W.E., Collisson E.W. Memory T cells protect chicks from acute infectious bronchitis virus infection. Virology. 2003;306:376–384. doi: 10.1016/s0042-6822(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol. Today. 1992 Oct;13(10):379–381. doi: 10.1016/0167-5699(92)90083-J. Review. [DOI] [PubMed] [Google Scholar]
- Seo S.H., Collisson E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 1997;71:5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Briles W.E., Lupiani B., Collisson E.W. Avian influenza viral nucleocapsid and hemagglutinin proteins induce chicken CD8+ memory T lymphocytes. Virology. 2010;399:231–238. doi: 10.1016/j.virol.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Toro H., Tang D.C., Briles W.E., Yates L.M., Kopulos R.T., Collisson E.W. Non-replicating adenovirus vectors expressing avian influenza virus hemagglutinin and nucleocapsid proteins induce chicken specific, effector memory and effector memory CD8+ T lymphocytes. Virology. 2010;405:62–69. doi: 10.1016/j.virol.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaggerty C.L., Kogut M.H., Ferro P.J., Rothwell L., Pevzner I.Y., Kaiser P. Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and -susceptible chickens. Immunology. 2004 Sep;113(1):139–148. doi: 10.1111/j.1365-2567.2004.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z., Cardona C.J., Anunciacion J., Adams S., Dao N. Roles of the ERK MAPK in the regulation of proinflammatory and apoptotic responses in chicken macrophages infected with H9N2 avian influenza virus. J. Gen. Virol. 2010 Feb;91(Pt 2):343–351. doi: 10.1099/vir.0.015578-0. 2010. [DOI] [PubMed] [Google Scholar]
- Yoo B.H., Sheldon B.L. Association of the major histocompatibility complex with avian leukosis virus infection in chickens. Br. Poult. Sci. 1992;33:613–620. doi: 10.1080/00071669208417500. [DOI] [PubMed] [Google Scholar]