Abstract
Most of traditional Chinese medicine substances come from herbal plants. The medicinal quality of herbal plants varies with the locations of cultivation, the parts of the herb collected, the season of the herb collected, and the herb processing method. Polysaccharides are major components of the herb plants and their biosynthesis is partly controlled by the genes but mostly influenced by the availability of the nutrition and determined by the various environmental factors. In recent decades, polysaccharides isolated from different kinds of Chinese herbs have received much attention due to their important biological activities, such as anti-tumor, anti-oxidant, anti-diabetic, radiation protecting, antiviral, hypolipidemic, and immunomodulatory activities. Interestingly, different batches of the same herb can obtain different polysaccharide fractions with subtle differences in molecular weight, monosaccharide compositions, glycosidic linkages, and biological functions. Even with these variations, a large number of bioactive polysaccharides from different kinds of traditional Chinese herbs have been purified, characterized, and reported. This review provides a comprehensive summary of the latest polysaccharide extraction methods and the strategies used for monosaccharide compositional analysis plus polysaccharide structural characterization. Most importantly, the reported chemical characteristics and biological activities of the polysaccharides from the famous traditional Chinese herbs including Astragalus membranaceus, Ginseng, Lycium barbarum, Angelica sinensis, Cordyceps sinensis, and Ophiopogon japonicus will be reviewed and discussed. The published studies provide evidence that polysaccharides from traditional Chinese herbs play an important role in their medical applications, which forms the basis for future research, development, and application of these polysaccharides as functional foods and therapeutics in modern medicine.
Keywords: Traditional Chinese herbs, Polysaccharides, Extraction method, Chemical structure, Biological activity
1. Introduction
The biological information flows from DNA to RNA to protein with template-based precision.1 However, thorough understanding the information residing in DNAs, RNAs, and proteins cannot explain the makeup of cells, tissues, and organs as well as the pathophysiological and physiological processes because the environment is in charge of both the building materials and waste management to keep the organisms alive. Moreover, polysaccharides/glycans and lipids are constantly synthesized and metabolized by concerted effort of at least hundreds of proteins from inorganic elements and small organic molecules without a template in living organisms. Furthermore, polysaccharides are heterogeneous biomolecules containing far more structural information than that are carried by protein-, nucleic acid-, and lipid-combined.2, 3 Such paradigm is applicable to all living systems including animals, plants, fungi, and microbes.4, 5, 6, 7 Thus, polysaccharides are positioned to serve important energy, structural, and biological functions in all living organisms.
Traditional Chinese medicine is one of the oldest medical systems in the world. Most traditional Chinese medicine substances come from herbal plants. The individual plant cell makes a cell wall enriched in cellulose, non-cellulosic polysaccharides and lignin. The non-cellulosic polysaccharides are heterogeneous.4 Spatially and temporally controlled heterogeneity in the physicochemical properties of cell wall non-cellulosic polysaccharides is observed at the tissue and individual cell levels, which plays important role in defensing and survival of the plants. It has been found that the herb polysaccharides have important biological activities such as anti-tumor, anti-oxidant, anti-diabetic, radiation protecting, antiviral, hypolipidemic, immunomodulatory, and other activities8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 with lower toxicity and side effects. Therefore, the isolation, characterization, and biological activity testing of polysaccharides in herbs have become a hot research field in China.20
This review provides a comprehensive summary of the extraction, isolation, identification, structural analysis and biological activity from many important traditional Chinese Herbs, such as Astragalus membranaceus, Ginseng, Lycium barbarum, Angelica sinensis, Cordyceps sinensis, and Ophiopogon japonicus. The published studies provide solid evidence that polysaccharides from traditional Chinese herbs play an important role in their medical applications, which forms the basis for future research, development, and application of these polysaccharides as functional foods and therapeutics in modern medicine.
2. Extraction, separation, purification and structural analysis method of polysaccharide
The most common method for preparing herbal medicine is to boil the herb in hot water and the liquid is then taken as the medicine. Most of the non-cellulosic polysaccharides are polar macromolecules that are readily soluble in water. Thus, the traditional polysaccharide extraction method is a water leaching extraction method, which is usually extracted by hot water leaching, and the polar macromolecular compound polysaccharide is dissolved in a polar solvent such as water to extract by using the principle of “similar compatibility.” In addition, according to the structure and properties of polysaccharides, some auxiliary means are introduced.20 On the basis of traditional water extraction, acid–base extraction method, enzymatic extraction method, microwave-assisted extraction method, ultrasonic assisted extraction method and ultrahigh-pressure extraction method have been developed.20, 21, 22, 23, 24, 25, 26, 27, 28 Then the separation and purification process of the polysaccharide is generally carried out from the water extracted materials by removing the non-polysaccharide components first. Common methods for polysaccharide purification include precipitation, gel chromatography, anion exchange chromatography, macro porous resin column chromatography, ultrafiltration, and other methods alike.29, 30, 31, 32, 33, 34 Most of the polysaccharides obtained by the extraction, separation and purification techniques at this stage are still crude products because the quality of polysaccharides is difficult to control. First, the medicinal quality of herbal plants varies with the locations of cultivation, the parts of the herb collected, the season of the herb collected, and the herb processing method. Second, the extraction methods used vary from lab to lab. Third, unlike DNA, RNA, and proteins, the non-template synthesized polysaccharides are never pure compounds no matter how many procedures have been used for purification. The obtained polysaccharides are always associated with either narrow or wide molecular weight ranges. The biological activities of the polysaccharides purified by the same starting materials vary as well. Therefore, special attention has to be paid during the extraction, separation and purification of herb polysaccharides.35, 36, 37, 38 The advantages and disadvantages of each method are shown in Table 1 .
Table 1.
Polysaccharide extraction, separation and purification methods from traditional Chinese herbs.
| Contents | Methods | Features | ||
|---|---|---|---|---|
| Extraction | Acid–base or water | Prevent glycosidic bond break | ||
| Enzymatic | Mild conditions, lower damage, higher yield, avoiding changes in physiological activity | |||
| Microwave-assisted | High yield, shorter extraction time and lower cost | |||
| Ultrasound-assisted | Faster, energy-saving, higher yield | |||
| Ultra high pressure | Shorter time and high efficiency | |||
| Isolation and purification | Miscellaneous | Removing proteins | Sevage method | Troublesome, time-consuming, large amount of reagents, structural damage, and large losses |
| Trichloroacetic acid | Effect but destroying structure | |||
| Protease | Mild and efficient | |||
| Decolorization | Activated carbon adsorption | Stronger affinity adsorption, larger loss | ||
| Hydrogen peroxide | Pigment containing unsaturated double bonds, hydroxyl groups and aromatic rings | |||
| Ion exchange | High decolorization and retention rate | |||
| Small molecule impurities | Dialysis | – | ||
| Fractional purification | Precipitation | Polysaccharides with differences in solubility | ||
| Gel chromatography | – | |||
| Anion exchange chromatography | Crude purification of polysaccharide | |||
| Macroporous resin column chromatography | Have no effect on the biological activity | |||
| Ultrafiltration | High separation efficiency, low energy consumption, no pollution and no damage to polysaccharide activity, easy to be contaminated | |||
Modified from Xie MY, Nie SP. A review about the research of structure and function of polysaccharides from natural products. J Chin Inst Food Sci Technol 2010;10(02):1–11.
The structure of polysaccharides is more complex than that of proteins and DNAs. From a chemical point of view, the complexity of the polysaccharides undoubtedly brings great difficulties to its structural analysis. The structural classification of polysaccharides follows the suit of proteins and DNAs, i.e., the structure of polysaccharides can be divided into primary, secondary, tertiary and quaternary structures.39
Like other biomolecules, the higher structure of the polysaccharide chain is based on its primary structure. The difference is that primary structure of the polysaccharide chain having much more “meaning” than the protein or DNAs. To determine the primary structure of a polysaccharide chain, the following problems have to be solved: (1) relative molecular mass; (2) monosaccharide compositions of the polysaccharide chain; (3) presence or absence of uronic acid and specific uronic acid type and ratio; (4) d- or l-configuration of each monosaccharide residue, pyran ring or furan ring form; (5) sequence of linkage between individual monosaccharide residues; the α- or β-anomeric form of each glycosidic bond; (7) the substitution of a hydroxyl group on each monosaccharide residue; (8) the attachment of a polysaccharide chain to a non-polysaccharide moiety; (9) the backbone of the polysaccharide chain and the branch linkage site; and (10) a polysaccharide residue may be modified by a sulfate group, an acetyl group, a phosphate group, a methyl group, or the like. There are many analytical methods for polysaccharide structural characterization,40, 41 not only instrumental analysis methods such as infrared, nuclear magnetic resonance, mass spectrometry, etc., but also chemical methods such as partial acid hydrolysis, complete acid hydrolysis, periodic acid oxidation, Smith degradation, etc., as well as biological methods such as specific glycosidase digestion, immunological methods, etc. Polysaccharide extraction, separation and purification methods from traditional Chinese herbs are shown in Table 1.39
3. Overview of biological activities of polysaccharides purified from traditional Chinese herbs
3.1. Astragalus membranaceus
Astragalus membranaceus (A. membranaceus) (Table 3) belongs to the family Leguminosae and is widely distributed in Asia, Europe and North America.43, 79 According to reports, there are > 3000 different types of A. membranaceus, and the roots are collected and dried for use. It has been known for centuries as a treatment for various diseases in traditional Chinese medicine. Such as in wound healing, diabetes, leukemia, hypertension, eye disease, nephritis, cirrhosis, uterine cancer.80 In recent years, many phytochemistry and pharmacological studies show that the polysaccharide part is one of the major bioactive components of A. membranaceus. It has a variety of health benefits, including immune regulation, anti-inflammatory, anti-oxidation, anti-glomerulonephritis, anti-atherosclerosis, anti-diabetes and anti-tumor ability.42
Table 3.
Summary of structural and biological activities of polysaccharides from six traditional Chinese herbs.
| Species | Glycans | Extraction methods | Major monosaccharides | Glycosidic linkage in backbone | MW (Da) | Bioactivities | References |
|---|---|---|---|---|---|---|---|
 |
Astragalus membranaceus Polysaccharides |
Hot water,ultrasonic and microwave extraction, DEAE-Sephadex A-25, Sephadex G-100 | Rhamnose, arabinose, xylose, ribose, galactose, glucose, mannose, fructose, fucose | α-(1 → 4)-Glc; α(1 → 3)-Gal | 8.7–4800 K | Immunomodulation | 42 |
| Anti-inflammation | 43 | ||||||
| Anti-oxidant | 44 | ||||||
| Anti-glomerulonephritis | 45 | ||||||
| Anti-atherosclerosis | 46 | ||||||
| Anti-diabetes | 47 | ||||||
| Anti-tumor | 49 | ||||||
 |
Ginseng Polysaccharide | Hot water, ethanol fractionation, DEAE-Sepharose-CL-6B, Sepharose-CL-6B, Sephadex-G-75 | l-arabinose, d-galactose, l-rhamnose, d-galacturonic acid, d-glucuronic acid | α-(1 → 3)-Ara; β-(1 → 3) or β-(1 → 4) Gal | 3.2–1900 K | Antibacterial | 48 |
| Anti-oxidant | 49 | ||||||
| Anti-inflammatory | 50 | ||||||
| Anti-depressant | 51 | ||||||
| Anti-tumor | 52 | ||||||
| Immunomodulation | 53 | ||||||
 |
Lycium Barbarum Polysaccharide | Warm water extraction, DEAE cellulose column, Sephadex G-150 | Glucose, arabinose, galactose, mannose, xylose, rhamnose, fucose, galacturonic acid, glucuronic acid | β-(1 → 3) or β-(1 → 4) Gal; α-(1 → 6)-Glc | Average 49.1 K | Anti-oxidant | 54 |
| Anti-tumor | 55 | ||||||
| Anti-radiation | 56 | ||||||
| Anti-fatigue | 57 | ||||||
| Anti-aging | 58 | ||||||
| Anti-inflammation | 59 | ||||||
| Immunomodulation | 60 | ||||||
 |
Angelica Polysaccharide | Water extraction, SephadexG-100, DEAE-52 | Glucose, mannose, galactose, rhamnose, arabinose, xylose | α(1,4)-Glc | 5.1–2300 K | Immunomodulation | 61, 62 |
| Anti-tumor | 63, 64 | ||||||
| Hepatoprotective | 65 | ||||||
| Anti-diabetic | 66 | ||||||
| Gastrointestinal protection | 66, 67 | ||||||
 |
Cordyceps sinensis Polysacchride | Hot water extraction, DEAE-Sepharose Fast Flow, Sephadex G-75 | Mannose, glucose, galactose, galacturonic acid | α(1 → 2) or α(1 → 4)-Man-α(1 → 4)-Glc | 7.7–210 K | Immunomodulation | 30, 68 |
| Anti-tumor | 69 | ||||||
| Anti-oxidant | 70 | ||||||
| Anti-diabetes | 71 | ||||||
| Anti-aging | 72 | ||||||
| Anti-scald | 73 | ||||||
 |
Ophiopogon japonicus Polysaccharide | Hot water, ultrasonic and enzymatic water extraction, DEAE-52,Sephadex G-100 | Fructose, glucose, arabinose, mannose | Fru-β (2 →,→2)-Fru-β(6 →,→6)-Glc-α(1 → and → 1.2)-Fru-β (6 →) | 3.4–48.7 K | Anti-myocardial infarction | 74 |
| Anti-diabetes | 75 | ||||||
| Anti-oxidant | 76 | ||||||
| Immunomodulation | 77 | ||||||
| Anti-thrombotic | 78 |
A number of polysaccharides are isolated from the roots and aerial parts of A. membranaceus. Jin et al. listed 24 polysaccharides isolated from the roots of A. membranaceus, and most of them are heteropolysaccharides.42 These heteropolysaccharides have molecular weights ranging from 8.7 to 4800 kDa, with different proportions of the monosaccharides, including arabinose, fructose, fucose, galactose, glucose, mannose, rhamnose, ribose and xylose. Kiyohara et al. separated 13 different types of polysaccharides from the aerial part of A. membranaceus, 9 of which consist of arabinogalactans and pectic acid arabinogalactan or pectin.44
Structural analysis of the water-soluble heteropolysaccharide (APSID3) isolated from A. membranaceus showed that the minimal repeat unit consists of one terminal arabinose, one 1,5-linked arabinose, one 1,3-linked rhamnose, one 1,3,4-linked rhamnose, six 1,4-linked glcuronic acid and five 1,4-linked galacturonic acid residues, with the backbone of which consists of 1,4-linked galacturonic acid, 1,4-linked glcuronic acid and a small amount of 1,3-linked rhamnose is attached and the side chain consists of a 1,5-linked arabinose on the C-4 of the 1,3-linked rhamnose.45
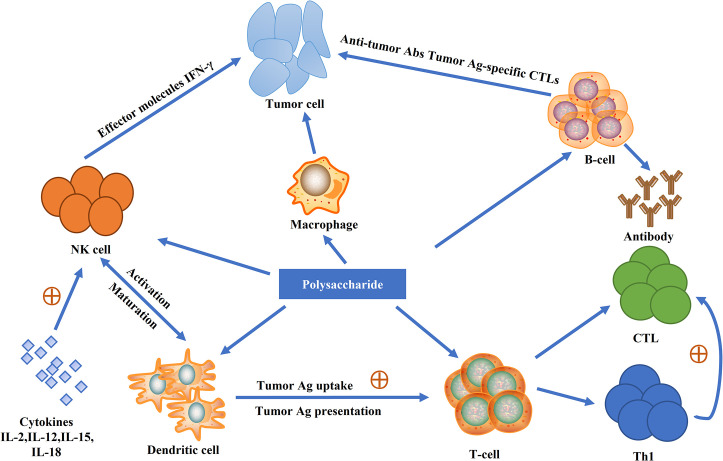
The immunomodulatory activity of the APS polysaccharide has been extensively studied both in vitro and in vivo. APS has been reported to improve the function of T cells, B cells, macrophages, lymphocytes and dendritic cells42 (Fig. 1 ). Abuelsaad46 studied the immunomodulatory effects of APS treatment on mice infected with Aeromonas hydrophila and found that APS treatment reduces ROS production, downregulates neutrophil activity and the proportion of CD4 +/CD8 + T cells is increased. Yang et al.47 reported the immunomodulatory activity of APS in experimental rat model of colitis induced by trinitrobenzene sulfonic acid. It is reported that APS can significantly improve experiment rat TNBS-induced colitis by regulating the expression of TNF-α, IL-1β and NFATc4.47
Fig. 1.
Immune regulatory mechanisms of herb polysaccharides.
Modified from Jin M, Zhao K, Huang Q, Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol 2014;64:257–266.
3.2. Ginseng
Ginseng (Table 3) has a long history of use as a traditional herb in many Eastern countries including Korea, China and Japan. There are two main types of ginseng: Panax ginseng and Panax quinquefolius. The common medicinal “ginseng” is mainly derived from the roots. It has been found to play a role in the improvement of various injuries and diseases from the central nervous system, the cardiovascular system to endocrine secretion and the reproductive and immune systems.52 The main active compound ginseng is ginsenoside, which is a steroidal saponin conjugated to different sugar moieties and polysaccharides (10–20% by weight).
To date, several components of ginseng polysaccharides have been identified and studied, including arabinogalactan, pectin and acidic polysaccharides, which are mainly composed of monosaccharides such as l-arabinose, d-galactose, l-rhamnose, d-galacturonic acid and d-glucuronic acid.53 Molecular weights are ranged from 3.2 to 1900 kDa.48 However, there is a lack of detailed information on the actual structural characteristics and heterogeneity of these polysaccharide components. Many studies have shown that ginseng polysaccharides have antibacterial, anti-oxidant, anti-inflammatory, anti-depressant, anti-tumor and immunomodulatory properties both in vitro and in vivo.48
Anti-tumor and chemical protective effects of ginseng polysaccharide have received a lot of attention in the past decade. In a tumor-bearing mouse model, a sublethal dose of cyclophosphamide (CP) after treatment with 100 mg/kg Ganshan injection significantly reduced mortality and promoted recovery.49 Ginseng polysaccharide is also found to inhibit cell proliferation and to induce apoptosis in HCT116 human colon cancer cells through the cyclin inhibitor protein p21.50 And in both sexes of mice, a 100 mg/kg dose of Ginsan (the polysaccharide fraction of ginseng) significantly enhances liver endogenous anti-oxidant levels with no significant hepatotoxicity.51 In a mouse model of γ-radiation-induced spleen injury, Ginsan (100 mg/kg) can be used to restore endogenous anti-oxidant enzymes heme oxygenase-1 (HO-1), SOD and GPx by the action of cytokines.54, 81
3.3. Lycium barbarum
Lycium barbarum (Table 3) polysaccharide (LBP) is derived from the Lycium barbarum (wolfberry) fruit in the Solanaceae family. LBP is a well-known traditional Chinese herbal formula that has been used in China for > 2300 years.55 The Chinese believe that it can nourish the eyes, liver and kidneys, and balance the “yin” and “yang” in the human body.56 Today, it has become a popular food or food supplement in East and West. Recent studies have confirmed the beneficial effects of wolfberry on human health, including anti-oxidative stress, anti-tumor, anti-radiation, anti-fatigue, anti-aging, anti-inflammatory and immunomodulatory properties.57
Under optimized extraction conditions, the polysaccharide component is 23% of the cognac mass.58 Up to 95% of the LBP consists of glycans including glucose, arabinose, galactose, mannose, xylose, rhamnose, and fucose.59 Recent studies have found that water-soluble polysaccharides derived from hydrazine have an average molecular weight of 49.1 kDa and an average protein content of 3.75%. The molar ratio of arabinose to galactose is 5.6:1. In addition, LBP is a highly branched polysaccharide with a backbone of (1 → 6) Galp linked galactose replaced by galactosyl or arabinose at O-3.82
Studies have confirmed LBP's beneficial effects on various diseases such as acute liver injury,60 alcoholic liver injury,83 and nonalcoholic fatty liver disease,60, 84 performance impairment,85 brain I/R injury,86 retinal degeneration,82 stroke87 and Alzheimer's disease.55, 88
3.4. Angelica sinensis
Angelica sinensis (A. sinensis) (Table 3) is a well-known Chinese herbal medicine that has been used as a nourishing and hematopoietic agent for the treatment of gynecological diseases61 for thousands of years. Recent studies have shown that polysaccharides in A. sinensis (APS) are the main bioactive components with various biological activities.62
Many ASP polysaccharides have been identified from the roots of A. sinensis. Their main structural features such as the monosaccharide compositions and molecular weight ranges have been summarized.62 Several components of ASP are mainly composed of monosaccharides such as glucose, mannose, galactose, rhamnose, arabinose, and xylose. Molecular weights range from 5.1 to 2300 kDa. Most of the polysaccharides isolated from A. sinensis reported in the literature are heteropolysaccharides. Cao et al.63 studied the structural characteristics of an anti-tumor polysaccharide named APS-1d isolated from A. sinensis. It was found that the backbone of APS-1d consists of α (1,4)-d-glucopyranosyl residues. Branches consist of α (1,6)-d-Glcp residues and terminating with β-l-arabinofuranose residues.
In addition, some glucans are isolated and purified from A. sinensis. As-IIIa with a M w of 850 kDa from A. sinensis consists of α-(1 → 3)-glucan.64 It is also reported that a glucan from A. sinensis has an M w of 100 kDa and consists of α-(1 → 6)-linked glucose.65 Cao et al.63 studied the structural characteristics of two glucans (APS-1cI and APS-1cII) from A. sinensis, and found that APS-1cI is a linear α-glucan composed only of α-(1 → 6)-d-Glcp. And APS-1cII has a repeating unit consisting of α(1 → 6)-d-Glcp and α-(1 → 4)-d-Glcp in a molar ratio of 1:4, α-(1 → 4)-d-Glcp-α-(1 → 6)-d-Glcp-α-(1 → 4)-d-Glcp-α-(1 → 4)-d-Glcp α(1 →]n is a repeating unit of APS-1cII.47
The immunomodulatory activity of ASP has been extensively studied both in vitro and in vivo. It has been found that ASP increases the proliferation of total spleen cells, macrophages, and T cells by primary activation of non-specific immunity and secondary activation of helper T cells. ASP also enhances the gene expressions of IL-2 and IFN-γ.89 The anti-tumor activity of ASP is revealed in that ASP can significantly inhibit the proliferation of HeLa cells and lung cancer cells. ASP also inhibits the growth of transplanted sarcoma-180 tumors in mice.63 Other biological activities have also been found in ASP, such as hematopoietic activity, anti-oxidant activity, hepatoprotective activity, anti-osteoarthritis activity,60 gastrointestinal protection66, 90 and anti-diabetic activity.47, 66
3.5. Cordyceps sinensis
Cordyceps sinensis (Table 3), the Chinese caterpillar fungus or DongChongXiaCao (winter worm-summer grass) in Chinese or Tochukaso in Japanese, is a valuable traditional Chinese medicine. C. sinensis has been used in China for > 700 years, mainly as a tonic for nourishing the lungs and nourishing the kidneys.91 Modern pharmacological studies have shown that it has a therapeutic effect on a variety of diseases and conditions such as the respiratory system, kidneys, liver, nervous system and cardiovascular diseases, as well as tumors, aging, hyposexuality, and hyperlipidemia.92, 93, 94, 95, 96, 97, 98, 99 Since 1964, C. sinensis has been listed as the official Pharmacopeia of the Chinese Ministry of Health by the Pharmacopeia and the Chinese Ministry of Education's Committee of Herbs in the Severe Acute Respiratory Syndrome (SARS) outbreak in China in 2003, with a significant increase in the use of C. sinensis.100, 101
Polysaccharides have become the target of the development and quality control of C. sinensis. And they can be classified into two types according to their position in fungal cells, intracellular polysaccharides (IPSs) and extracellular polysaccharides (extracellular polysaccharides, EPS).68, 101, 102, 103, 104, 105 The monosaccharide composition is usually glucose, mannose and galactose in different molar ratios. Different molecular weights have been found under various source materials and experimental conditions of C. sinensis, ranging from 1 to 1000 kDa.68, 100, 106, 107, 108, 109, 110, 111 Recently, some water-soluble IPSs isolated from cultured C. sinensis are identified as glucomannan, whose backbone is mainly composed of (1 → 2) and (1 → 4)-mannose, (1 → 3)-galactose, (1 →) and (1 → 3,6)-the linkage of glucose.100, 112 Wang et al.30 reported that the chemical structure of the isolated water-soluble polysaccharide (CPS-2) is derived from cultured C. sinensis, which is mainly composed of α-(1 → 4)-d-glucose and α-(1 → 3)-d-mannose branched with (1 → 4,6)-d-glucose every 12 residues on averages.101 We found that salinity-induced anti-angiogenesis activities and structural changes of the polysaccharides from cultured Cordyceps militaris. 113, 114
Based on the theory of traditional Chinese medicine, the main role of C. sinensis is to “enrich lung yin and yang.” Its uses include treatment of chronic low back pain, colds, excessive mucus and tears, chronic cough and wheezing, and sputum caused by kidney yang (shenyangqu). According to Western medicine, C. sinensis also has antibacterial activity, which reduces asthma, lowers blood pressure and enhances heartbeat. According to a large number of animal and clinical studies, polysaccharides represent a large class of biologically active components of C. sinensis, contributing to its health and pharmacological activity. The various biological activities and health benefits of IPS and EPS are summarized in Table 3. Both IPS and EPS obtained from wild or cultured C. sinensis show immunomodulation, anti-tumor, anti-oxidant and hypoglycemic effects, as well as other important biological activities, including anti-fibrosis, anti-fatigue, kidney protection, increasing plasma testosterone levels, and radiation protection.30, 69, 70, 71, 72, 73, 111, 113
3.6. Ophiopogon japonicus
Ophiopogon japonicus (Maidong in Chinese) (Table 3), is a widely used traditional Chinese herbal medicine (Chinese Pharmacopeia Commission, 2015). According to traditional Chinese medicine theory, O. japonicas can nourish yin deficiency, promote body fluid production, nourish the lungs, relieve the mind, and eliminate heart fire. O. japonicas is listed as an edible Chinese medicine by the Ministry of Public Health of China because of its high efficiency, high availability and safety.112 To date, China Food and Drug Administration (CFDA) has approved patent drugs namely Shen Mai injection/granule, Xuan Mai Gan Jie capsule/granule, etc., which contain O. japonicas as the main medicinal ingredient.74, 115
The polysaccharides, the main composition of O. japonicas with an extraction rate up to 35%.75 The molecular weights of O. japonicas polysaccharides are inconsistent, ranging from 2.74 to 325 kDa. In general, O. japonicus polysaccharide are mainly composed of β-fructose and a small amount of α-glucose. The backbone of OJP is formed by Fru-β(2 →,→2)-Fru-β(6 →,→6)-Glc-α(1 → and → 1,2)-Fru-β(6 →), and the molar ratios were 6.8:15.8:1.0:5.8.75
O. japonicus is rich in polysaccharides, which are possibly responsible for its biological activities, such as anti-diabetic activity, cardiovascular protection, immunomodulatory activity, anti-oxidant activity, anti-obesity activity, therapeutic effect on Sjogren's syndrome, etc.74, 76, 77, 78, 116, 117, 118, 119, 120
4. Future perspectives
Bioactive polysaccharides from traditional Chinese Herbal medicines are well known. During the past few decades, considerable efforts have been dedicated to the development of bioactive polysaccharides from the traditional Chinese Herbs. The main focus of these studies has been the purification of the polysaccharides from the traditional Chinese herbs, which are followed by monosaccharide compositional analysis, structural characterization and biological activity studies (Fig. 1 and Table 1, Table 2, Table 3 ). The polysaccharides from Astragalus membranaceus, Ginseng, Lycium barbarum, Angelica sinensis, Cordyceps sinensis, Ophiopogon japonicus have been systematically studied by many different research groups. Both structural and biological information obtained are plentiful and accountable.
Table 2.
Analytical methods for identifying monosaccharide compositions, molecular weight distributions, and glycosidic linkages of polysaccharides.
| Items | Methods |
|---|---|
| Determination of purity and relative molecular weight distributions of polysaccharides | HPGPC, osmotic pressure, viscosity method, light scattering method, polyacrylamide gel electrophoresis |
| Monosaccharide compositional analysis | Complete acid hydrolysis, HPLC, GC, GC–MS, ion chromatography |
| Glycoside ring form (pyran, furan) | Infrared spectrum |
| Glycosidic linkages of the polysaccharide | Methylation analysis, GC–MS, LC–MS |
| The anomeric forms substituted by glycosides (α- and β-) | Glycosidase hydrolysis, nuclear magnetic resonance, infrared spectroscopy, laser Raman spectroscopy, etc. |
| Sequence of the oligosaccharides | Elective acid hydrolysis, sequential hydrolysis by glycosidases, nuclear magnetic resonance, etc. |
| The hydroxyl positions in the monosaccharide | Methylation, periodate oxidation, Smith degradation, GC–MS, nuclear magnetic resonance, etc. |
| Polysaccharide-peptide linkage | Dilute alkali hydrolysis method, hydrazine reaction, amino acid composition analysis, etc. |
The unique and distinctive monosaccharide compositions, structural diversity, and remarkable biological activities of polysaccharides from the traditional Chinese herbs represent rich natural sources for drug development. The information reviewed here may be helpful in the definition of structure and function relationships necessary to design biological active polysaccharides with potential for the therapeutical use or to be used as ingredients in functional foods. Both advantages and disadvantages of polysaccharides as drugs rely on its complicated molecular structures, multiple biological functions, and multiple molecular targets. Thus, there is a great need for further clarifying the active ingredients in the polysaccharides and its molecular targets responsible for the observed drug effects. In addition, how to comprehend the pharmacodynamics of these polysaccharides, how to standardize the quality of polysaccharides, and how to perform reliable pharmacokinetic studies of polysaccharides also should be addressed in the near future. The relatively inexpensive polysaccharides from the traditional Chinese herbs should be useable for the development of novel therapeutic agents, functional food, or adjuvants for preventing or treating different pathological conditions as those fungal polysaccharide-based drugs approved and used in China.6, 121, 122, 123, 124, 125, 126
Acknowledgments
Acknowledgments
This research was supported by the Natural Science Foundation of China (Grant 81672585), Key Technology Fund of Shandong Province (Grant 2016ZDJS07A07), the Taishan Scholar Fellowship, and the “Double First Class fund” of Shandong Province in China to L.Z.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Pengjiao Zeng, Email: pjzeng219@163.com.
Lijuan Zhang, Email: zhanglj@qduhospital.cn.
References
- 1.Varki A., Kornfeld S. 3rd ed. Cold Spring Harbor Laboratory Press; NY: 2017. Historical Background and Overview. [Google Scholar]
- 2.Lowe J., Marth J. A genetic approach to Mammalian glycan function. Annu Rev Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L. Glycosaminoglycans in development, health and disease. Preface. Prog Mol Biol Transl Sci. 2010;93:xvii–xviii. doi: 10.1016/S1877-1173(10)93026-3. [DOI] [PubMed] [Google Scholar]
- 4.Burton R.A., Gidley M.J., Fincher G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol. 2010;6(10):724–732. doi: 10.1038/nchembio.439. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L. Glycosaminoglycan (GAG) biosynthesis and GAG-binding proteins. Prog Mol Biol Transl Sci. 2010;93:1–17. doi: 10.1016/S1877-1173(10)93001-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z., Qiu P., Zeng Y. Chinese FDA approved fungal glycan-based drugs: an overview of structures, mechanisms and clinical related studies. Transl Med. 2014;4(141):1–11. [Google Scholar]
- 7.Porter N.T., Martens E.C. The critical roles of polysaccharides in gut microbial ecology and physiology. Annu Rev Microbiol. 2017;71:349–369. doi: 10.1146/annurev-micro-102215-095316. [DOI] [PubMed] [Google Scholar]
- 8.Liang L.J., Tu P.F., Zhao K.J. Advances in pharmacological studies of Astragalus polysaccharides. China Pharm. 2010;21(43):4113–4116. [Google Scholar]
- 9.Shu R.G., Jiang Y.P., Cai Y.H. Exploration of extraction and isolation method of plant polysaccharides. China Pharm. 2011;22(11):1052–1055. [Google Scholar]
- 10.An X.J., Feng L., Song H.P., Li S.W. Advances in structural analysis and pharmacological activities of plant polysaccharides. Chin Pharm J. 2012;47(16):1271–1275. [Google Scholar]
- 11.Wang P.C., Zhao S., Yang B.Y., Wang Q.H., Kuang H.X. Anti-diabetic polysaccharides from natural sources: a review. Carbohydr Polym. 2016;148:86–97. doi: 10.1016/j.carbpol.2016.02.060. [DOI] [PubMed] [Google Scholar]
- 12.Gao Q.H., Fu X., Zhang R., Wang Z., Guo M. Neuroprotective effects of plant polysaccharides: a review of the mechanisms. Int J Biol Macromol. 2018;106:749–754. doi: 10.1016/j.ijbiomac.2017.08.075. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z., Cheng L., He Y., Wei X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: a review. Int J Biol Macromol. 2018;120(pt B):2076–2085. doi: 10.1016/j.ijbiomac.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Song Y.H., Liu Q., Lv Z.P., Chen Y.Y., Zhou Y.C., Sun X.G. Protection of a polysaccharide from Salvia miltiorrhiza, a Chinese medicinal herb, against immunological liver injury in mice. Int J Biol Macromol. 2008;43(2):170–175. doi: 10.1016/j.ijbiomac.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai M.H., Matsumoto T., Kiyohara H., Yamada H. B-cell proliferation activity of pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L. and its structural requirement. Immunology. 1999;97(3):540–547. doi: 10.1046/j.1365-2567.1999.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nosal'ova G., Kardosova A., Franova S. Antitussive activity of a glucuronoxylan from Rudbeckia fulgida compared to the potency of two polysaccharide complexes from the same herb. Pharmazie. 2000;55(1):65–68. [PubMed] [Google Scholar]
- 17.Matsumoto T., Moriya M., Sakurai M.H., Kiyohara H., Tabuchi Y., Yamada H. Stimulatory effect of a pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L., on G-CSF secretion from intestinal epithelial cells. Int Immunopharmacol. 2008;8(4):581–588. doi: 10.1016/j.intimp.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y., Matsumoto T., Kikuchi Y., Ikejima T., Wang B., Yamada H. Effects of a pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L. on interleukin 6 production of murine B cells and B cell lines. Immunopharmacology. 2000;49(3):307–316. doi: 10.1016/s0162-3109(00)00245-9. [DOI] [PubMed] [Google Scholar]
- 19.Chiu L.C., Zhu W., Ooi V.E. A polysaccharide fraction from medicinal herb Prunella vulgaris downregulates the expression of herpes simplex virus antigen in Vero cells. J Ethnopharmacol. 2004;93(1):63–68. doi: 10.1016/j.jep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Li C.L., Wang W., Zhang Y., Li J.A., Lao F.Y. Research progress on extraction, separation and purification of polysaccharides from traditional Chinese medicine. China Pharm. 2016;27(19):2700–2703. [Google Scholar]
- 21.Wu Y.F., Wang X.S., Zhang Y.P., Li N., Shi Q.L. Microwave-assisted extraction of polysaccharide from Fructus corni. Hubei Agric Sci. 2011;50(03):570–572. [Google Scholar]
- 22.Chen Y., Zhao L., Liu B., Zuo S. Application of response surface methodology to optimize microwave-assisted extraction of polysaccharide from Tremella. Phys Procedia. 2012;24:429–433. [Google Scholar]
- 23.Han H.D., Wang X.L. Optimization of acid extraction of polysaccharides in Gonostegia hirta (BL.) Miq. by response surface analysis. J Southwest Univ (Nat Sci Ed) 2013;39(01):54–60. [Google Scholar]
- 24.Ahmad A., Alkharfy K.M., Wani T.A., Raish M. Application of Box–Behnken design for ultrasonic-assisted extraction of polysaccharides from Paeonia emodi. Int J Biol Macromol. 2015;72:990–997. doi: 10.1016/j.ijbiomac.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Zeng H.L., Zhang Y., Xue Y.R., Liu J., Zheng B.D. Optimization of the alkali extraction technology of Fortunella margarita polysaccharides via respons surface methodology. Chin J Trop Crops. 2015;36(01):179–184. [Google Scholar]
- 26.Pan L., Wang J., Ye X., Zha X.Q., Luo J. Enzyme-assisted extraction of polysaccharides from Dendrobium chrysotoxum and its functional properties and immunomodulatory activity. LWT—Food Sci Technol. 2015;60(2):1149–1154. [Google Scholar]
- 27.Sun L., Wu D., Ning X. α-Amylase-assisted extraction of polysaccharides from Panax ginseng. Int J Biol Macromol. 2015;75:152–157. doi: 10.1016/j.ijbiomac.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Zeng H.L., Zhang Y., Lin S., Jian Y., Miao S., Zheng B. Ultrasonic–microwave synergistic extraction (UMSE) and molecular weight distribution of polysaccharides from Fortunella margarita (Lour.) Swingle. Sep Purif Technol. 2015;144:97–106. [Google Scholar]
- 29.Guo Y.D., Shan B., Li M.Y. Comparative study on deproteinization methods of Momordica charantia L. J Anhui Agric Sci. 2009;37(07):3225–3227. [Google Scholar]
- 30.Wang Y.H., Yin H., Lv X., Wang Y.H., Gao H., Wang M. Protection of chronic renal failure by a polysaccharide from Cordyceps sinensis. Fitoterapia. 2010;81(5):397–402. doi: 10.1016/j.fitote.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Zhang R.N., Zhang H.S., Zhao Y., Zhang Z.Y., Zhang Y., Du X.X. Separation and structural analysis of persimmon polysaccharide. Nat Prod Res Dev. 2012;24(12):1761–1765. [Google Scholar]
- 32.Li H.F., Guo S.B., Man S.L. Graded ethanol precipitation method on physicochemical properties and antioxidant activities of polysaccharides extracted from Astragalus radix. China J Chin Mater Med. 2015;40(11):2112–2116. [PubMed] [Google Scholar]
- 33.Liao N., Zhong J., Ye X. Ultrasonic-assisted enzymatic extraction of polysaccharide from Corbicula fluminea: characterization and antioxidant activity. LWT–Food Sci Technol. 2015;60:1113–1121. 2 pt 2. [Google Scholar]
- 34.Zou S., Xu Y., Zhang Q. Review on extraction and purification technology of polysaccharides from natural plants. Nat Prod Res Dev. 2015;27(08):1501–1509. [Google Scholar]
- 35.Fan Y.J., Yu F.F., Zhang P., Bao Q.G. Study on the separation of Lycium polysaccharide by ultrafiltration. J Anhui Agric Sci. 2011;39(22):13400–13403. [Google Scholar]
- 36.He Y.T., Pan X.M., Gong Z.J. Study on decolorization of corn silk polysaccharide by macroporous resin. Sci Technol Food Ind. 2011;32(05):299–301. [Google Scholar]
- 37.Ren H.W., Chen H.X., Tang X.H., Li X., Li S.Z. Purification of Lycium barbarum polysaccharide by macroporous. Sci Technol Food Ind. 2012;33(03) 249–251 + 254. [Google Scholar]
- 38.Qi Y.M., Chen T. Isolation, purification and antioxidant activity of extracellular polysaccharide from Zizhi. Sci Technol Food Ind. 2013;34(04):105. 108 + 113. [Google Scholar]
- 39.Xie M.Y., Nie S.P. A review about the research of structure and function of polysaccharides from natural products. J Chin Inst Food Sci Technol. 2010;10(02):1–11. [Google Scholar]
- 40.Fang J.N. Structural analysis of polysaccharides. J Int Pharm Res. 1981;04:222–228. [Google Scholar]
- 41.Li Y.H., Wang F.S., He Y.L. General situation of research on chemical modification of polysaccharides; Paper presented at: Shandong Pharmaceutical Association 2006 Biochemistry and Biotechnology Drug Symposium; 2006. Liaocheng, Shandong, China. [Google Scholar]
- 42.Jin M., Zhao K., Huang Q., Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol. 2014;64:257–266. doi: 10.1016/j.ijbiomac.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Rios J.L., Waterman P.G. A review of the pharmacology and toxicology of Astragalus. Phytother Res. 1997;11(6):411–418. [Google Scholar]
- 44.Kiyohara H., Uchida T., Takakiwa M. Different contributions of side-chains in beta-D-(1-->3,6)-galactans on intestinal Peyer's patch-immunomodulation by polysaccharides from Astragalus mongholics Bunge. Phytochemistry. 2010;71(2–3):280–293. doi: 10.1016/j.phytochem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Wang S., Shan J.J., Wang Z., Hu Z. Isolation and structural analysis of an acidic polyaccharide from Astragalus membranaceus (Fisch.) Bunge. J Integr Plant Biol. 2006;48(11):1379–1384. [Google Scholar]
- 46.Abuelsaad A.S. Supplementation with Astragalus polysaccharides alters Aeromonas-induced tissue-specific cellular immune response. Microb Pathog. 2014;66:48–56. doi: 10.1016/j.micpath.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Xie J.H., Jin M.L., Morris G.A. Advances on bioactive polysaccharides from medicinal plants. Crit Rev Food Sci Nutr. 2016;56(suppl 1):S60–S84. doi: 10.1080/10408398.2015.1069255. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng C.A. Meyer: an overview. Carbohydr Polym. 2011;85(3):490–499. [Google Scholar]
- 49.Shim J.Y., Han Y., Ahn J.Y., Yun Y.S., Song J.Y. Chemoprotective and adjuvant effects of immunomodulator ginsan in cyclophosphamide-treated normal and tumor bearing mice. Int J Immunopathol Pharmacol. 2007;20(3):487–497. doi: 10.1177/039463200702000307. [DOI] [PubMed] [Google Scholar]
- 50.King M.L., Murphy L.L. Role of cyclin inhibitor protein p21 in the inhibition of HCT116 human colon cancer cell proliferation by American ginseng (Panax quinquefolius) and its constituents. Phytomedicine. 2010;17(3–4):261–268. doi: 10.1016/j.phymed.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song J.Y., Akhalaia M., Platonov A. Effects of polysaccharide ginsan from Panax ginseng on liver function. Arch Pharm Res. 2004;27(5):531–538. doi: 10.1007/BF02980127. [DOI] [PubMed] [Google Scholar]
- 52.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 53.Wang M., Guilbert L.J., Li J. A proprietary extract from north American ginseng (Panax quinquefolium) enhances IL-2 and IFN-gamma productions in murine spleen cells induced by Con-A. Int Immunopharmacol. 2004;4(2):311–315. doi: 10.1016/j.intimp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Jin M., Huang Q., Zhao K., Shang P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int J Biol Macromol. 2013;54:16–23. doi: 10.1016/j.ijbiomac.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 55.Jiao R., Liu Y., Gao H., Xiao J., So K.F. The anti-oxidant and antitumor properties of plant polysaccharides. Am J Chin Med. 2016;44(3):463–488. doi: 10.1142/S0192415X16500269. [DOI] [PubMed] [Google Scholar]
- 56.Chang R.C., So K.F. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol. 2008;28(5):643–652. doi: 10.1007/s10571-007-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang H.L., Chen C., Wang S.K., Sun G.J. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int J Biol Macromol. 2015;77:235–242. doi: 10.1016/j.ijbiomac.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 58.Yin G., Dang Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr Polym. 2008;74(3):603–610. [Google Scholar]
- 59.Wang C.C., SCg C., Chen B.H. Chromatographic determination of polysaccharides in Lycium barbarum Linnaeus. Food Chem. 2009;116(2):595–603. [Google Scholar]
- 60.Xiao J., Liong E.C., Ching Y.P. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress and necroinflammation. J Ethnopharmacol. 2012;139(2):462–470. doi: 10.1016/j.jep.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 61.Cao W., Li X.Q., Wang X. A novel polysaccharide, isolated from Angelica sinensis (Oliv.) Diels induces the apoptosis of cervical cancer HeLa cells through an intrinsic apoptotic pathway. Phytomedicine. 2010;17(8–9):598–605. doi: 10.1016/j.phymed.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 62.Jin M., Zhao K., Huang Q., Xu C., Shang P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: a review. Carbohydr Polym. 2012;89(3):713–722. doi: 10.1016/j.carbpol.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 63.Cao W., Li X., Liu L. Structure of an anti-tumor polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr Polym. 2006;66(2):149–159. [Google Scholar]
- 64.Zhang L.W., Huang R.D. Purification, characterization and structure analysis of polysaccharide As-IIIa and As-IIIb from Angelica sinensis. Acta Laser Biol Sin. 1999;8(2):123–126. [Google Scholar]
- 65.Chen R., Liu Z., Zhao J. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011;127(2):434–440. doi: 10.1016/j.foodchem.2010.12.143. [DOI] [PubMed] [Google Scholar]
- 66.Cho C.H., Mei Q.B., Shang P. Study of the gastrointestinal protective effects of polysaccharides from Angelica sinensis in rats. Planta Med. 2000;66(04):348–351. doi: 10.1055/s-2000-8552. [DOI] [PubMed] [Google Scholar]
- 67.Sun Y., Tang J., Gu X., Li D. Water-soluble polysaccharides from Angelica sinensis (Oliv.) Diels: preparation, characterization and bioactivity. Int J Biol Macromol. 2005;36(5):283–289. doi: 10.1016/j.ijbiomac.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y.H., Wang M., Ling Y., Fan W., Wang Y.H., Yin H. Structural determination and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps sinensis. Am J Chin Med. 2009;37(05):977–989. doi: 10.1142/S0192415X09007387. [DOI] [PubMed] [Google Scholar]
- 69.Wong W.C., Wu J.Y., Benzie I.F. Photoprotective potential of Cordyceps polysaccharides against ultraviolet B radiation-induced DNA damage to human skin cells. Br J Dermatol. 2011;164(5):980–986. doi: 10.1111/j.1365-2133.2010.10201.x. [DOI] [PubMed] [Google Scholar]
- 70.Yan F., Zhang Y., Wang B. Effects of polysaccharides from Cordyceps sinensis mycelium on physical fatigue in mice. Bangladesh J Pharmacol. 2012;7:217–221. [Google Scholar]
- 71.Yao X., Meran S., Fang Y. Cordyceps sinensis: in vitro anti-fibrotic bioactivity of natural and cultured preparations. Food Hydrocoll. 2014;35:444–452. [Google Scholar]
- 72.Zhang X., Liu B., Al-Assaf S., Phillips G.O., Phillips A.O. Cordyceps sinensis decreases TGF-β1 dependent epithelial to mesenchymal transdifferentiation and attenuates renal fibrosis. Food Hydrocoll. 2012;28(1):200–212. [Google Scholar]
- 73.Zhong S., Pan H., Fan L. Advances in research of polysaccharides in Cordyceps species. Food Technol Biotechnol. 2009;47(3) [Google Scholar]
- 74.Fang J., Wang X., Lu M., He X., Yang X. Recent advances in polysaccharides from Ophiopogon japonicus and Liriope spicata var. prolifera. Int J Biol Macromol. 2018;114:1257–1266. doi: 10.1016/j.ijbiomac.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 75.Gong Y., Zhang J., Gao F. Structure features and in vitro hypoglycemic activities of polysaccharides from different species of Maidong. Carbohydr Polym. 2017;173:215–222. doi: 10.1016/j.carbpol.2017.05.076. [DOI] [PubMed] [Google Scholar]
- 76.Ding L., Li P., Lau C.B.S. Mechanistic studies on the antidiabetic activity of a polysaccharide-rich extract of radix ophiopogonis. Phytother Res. 2012;26(1):101–105. doi: 10.1002/ptr.3505. [DOI] [PubMed] [Google Scholar]
- 77.Shuang L., Li A., Huang N., Lu F., Hou D. Antioxidant and immunoregulatory activity of different polysaccharide fractions from tuber of Ophiopogon japonicus. Carbohydr Polym. 2011;86(3):1273–1280. [Google Scholar]
- 78.Chen M., Chen X., Wang M., Lin L., Wang Y. Ophiopogon japonicus—a phytochemical, ethnomedicinal and pharmacological review. J Ethnopharmacol. 2016;181:193–213. doi: 10.1016/j.jep.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 79.Mamedova R.P., Isaev M.I. Triterpenoids from Astragalus plants. Chem Nat Compd. 2004;40(4):303–357. [Google Scholar]
- 80.Djimtombaye B.J., Alankus-Caliskan O., Gulcemal D., Khan I.A., Anil H., Bedir E. Unusual secondary metabolites from Astragalus halicacabus LAM. Chem Biodivers. 2013;10(7):1328–1334. doi: 10.1002/cbdv.201200436. [DOI] [PubMed] [Google Scholar]
- 81.Han Y., Son S.J., Akhalaia M. Modulation of radiation-induced disturbances of antioxidant defense systems by ginsan. Evid Based Complement Alternat Med. 2005;2(4):529–536. doi: 10.1093/ecam/neh123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H., Zhang X., Li Y. Antitumor activity of a polysaccharide from longan seed on lung cancer cell line A549 in vitro and in vivo. Tumour Biol. 2014;35(7):7259–7266. doi: 10.1007/s13277-014-1927-8. [DOI] [PubMed] [Google Scholar]
- 83.Xiao J., Liong E.C., Ching Y.P. Lycium barbarum polysaccharides protect rat liver from non-alcoholic steatohepatitis-induced injury. Nutr Diabetes. 2013;3 doi: 10.1038/nutd.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao J., Xing F., Huo J. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci Rep. 2014;4:5587. doi: 10.1038/srep05587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lau B.W., Lee J.C., Li Y. Polysaccharides from wolfberry prevents corticosterone-induced inhibition of sexual behavior and increases neurogenesis. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0033374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu M., Chen X., Gu Y. Baicalin can scavenge peroxynitrite and ameliorate endogenous peroxynitrite-mediated neurotoxicity in cerebral ischemia-reperfusion injury. J Ethnopharmacol. 2013;150(1):116–124. doi: 10.1016/j.jep.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 87.Yang D., Li S.Y., Yeung C.M. Lycium barbarum extracts protect the brain from blood-brain barrier disruption and cerebral edema in experimental stroke. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ho Y.S., Yu M.S., Yik S.Y., So K.F., Yuen W.H., Chang R.C. Polysaccharides from wolfberry antagonizes glutamate excitotoxicity in rat cortical neurons. Cell Mol Neurobiol. 2009;29(8):1233–1244. doi: 10.1007/s10571-009-9419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang T., Jia M., Meng J., Wu H., Mei Q. Immunomodulatory activity of polysaccharide isolated from Angelica sinensis. Int J Biol Macromol. 2006;39(4–5):179–184. doi: 10.1016/j.ijbiomac.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 90.Wen Y., Li J., Tan Y. Angelica Sinensis polysaccharides stimulated UDP-sugar synthase genes through promoting gene expression of IGF-1 and IGF1R in chondrocytes: promoting anti-osteoarthritic activity. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0107024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dong C.H., Yao Y.J. In vitro evaluation of antioxidant activities of aqueous extracts from natural and cultured mycelia of Cordyceps sinensis. LWT—Food Sci Technol. 2008;41(4):669–677. doi: 10.1016/j.lwt.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu J.S., Halpern G.M., Jones K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: part II. J Altern Complement Med. 1998;4(4):429–457. doi: 10.1089/acm.1998.4.429. [DOI] [PubMed] [Google Scholar]
- 93.Liu Z., Li P., Zhao D., Tang H., Guo J. Protective effect of extract of Cordyceps sinensis in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Behav Brain Funct. 2010;6:61. doi: 10.1186/1744-9081-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song L.-Q., Si-Ming Y., Xiao-Peng M., Li-Xia J. The protective effects of Cordyceps sinensis extract on extracellular matrix accumulation of glomerular sclerosis in rats. Afr J Pharm Pharmacol. 2010;4(7):471–478. [Google Scholar]
- 95.Ding C., Tian P.X., Xue W. Efficacy of Cordyceps sinensis in long term treatment of renal transplant patients. Front Biosci (Elite Ed) 2011;3:301–307. doi: 10.2741/e245. [DOI] [PubMed] [Google Scholar]
- 96.Marchbank T., Ojobo E., Playford C.J., Playford R.J. Reparative properties of the traditional Chinese medicine Cordyceps sinensis (Chinese caterpillar mushroom) using HT29 cell culture and rat gastric damage models of injury. Br J Nutr. 2011;105(9):1303–1310. doi: 10.1017/S0007114510005118. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Z., Wang X., Zhang Y., Ye G. Effect of Cordyceps sinensis on renal function of patients with chronic allograft nephropathy. Urol Int. 2011;86(3):298–301. doi: 10.1159/000323655. [DOI] [PubMed] [Google Scholar]
- 98.Lo H.C., Hsieh C., Lin F.Y., Hsu T.H. A systematic review of the mysterious caterpillar fungus Ophiocordyceps sinensis in Dong-ChongXiaCao (Dong Chong Xia Cao) and related bioactive ingredients. J Tradit Complement Med. 2013;3(1):16–32. doi: 10.4103/2225-4110.106538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yue K., Ye M., Zhou Z., Sun W., Lin X. The genus Cordyceps: a chemical and pharmacological review. J Pharm Pharmacol. 2013;65(4):474–493. doi: 10.1111/j.2042-7158.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 100.Yan J., Wang W., Li L., Wu J. Physiochemical properties and antitumor activities of two α-glucans isolated from hot water and alkaline extracts of Cordyceps (Cs-HK1) fungal mycelia. Carbohydr Polym. 2011;85(4):753–758. [Google Scholar]
- 101.Yan J., Wang W., Wu J. Recent advances in Cordyceps sinensis polysaccharides: mycelial fermentation, isolation, structure, and bioactivities: a review. J Funct Foods. 2014;6:33–47. doi: 10.1016/j.jff.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kiho T., Tabata H., Ukai S., Hara C. A minor, protein-containing galactomannan from a sodium carbonate extract of Cordyceps sinensis. Carbohydr Res. 1986;156:189–197. [Google Scholar]
- 103.Kiho T., Ookubo K., Usui S., Ukai S., Hirano K. Structural features and hypoglycemic activity of a polysaccharide (CS-F10) from the cultured mycelium of Cordyceps sinensis. Biol Pharm Bull. 1999;22(9):966–970. doi: 10.1248/bpb.22.966. [DOI] [PubMed] [Google Scholar]
- 104.Wu Y., Sun C., Pan Y. Studies on isolation and structural features of a polysaccharide from the mycelium of an Chinese edible fungus (Cordyceps sinensis) Carbohydr Polym. 2006;63(2):251–256. [Google Scholar]
- 105.Guan J., Zhao J., Feng K., Hu D.J., Li S.P. Comparison and characterization of polysaccharides from natural and cultured Cordyceps using saccharide mapping. Anal Bioanal Chem. 2011;399(10):3465–3474. doi: 10.1007/s00216-010-4396-y. [DOI] [PubMed] [Google Scholar]
- 106.Miyazaki T., Oikawa N., Yamada H. Studies on fungal polysaccharides. XX. Galactomannan of Cordyceps sinensis. Chem Pharm Bull. 1977;25(12):3324–3328. [Google Scholar]
- 107.Gong M., Zhu Q., Wang T., Wang X.L., Ma J.X., Zhang W.J. Molecular structure and immunoactivity of the polysaccharide from Cordyceps sinensis (Berk.) Sacc. Sheng Wu Hua Hsueh Tsa Chih. 1990;6:486–492. [Google Scholar]
- 108.Li S.P., Zhao K.J., Ji Z.N. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003;73(19):2503–2513. doi: 10.1016/s0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- 109.Cha S.H., Lim J.S., Yoon C.S., Koh J.H., Chang H.I., Kim S.W. Production of mycelia and exo-biopolymer from molasses by Cordyceps sinensis 16 in submerged culture. Bioresour Technol. 2007;98(1):165–168. doi: 10.1016/j.biortech.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 110.Nie S.P., Cui S.W., Phillips A.O. Elucidation of the structure of a bioactive hydrophilic polysaccharide from Cordyceps sinensis by methylation analysis and NMR spectroscopy. Carbohydr Polym. 2011;84(3):894–899. [Google Scholar]
- 111.Nie S.P., Cui S.W., Xie M.Y., Phillips A.O., Phillips G.O. Bioactive polysaccharides from Cordyceps sinensis: isolation, structure features and bioactivities. Bioact Carbohydr Diet Fibre. 2013;1(1):38–52. [Google Scholar]
- 112.Nie S.P., Xie M. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 2011;25(2):144–149. [Google Scholar]
- 113.Zeng Y., Han Z., Qiu P. Salinity-induced anti-angiogenesis activities and structural changes of the polysaccharides from cultured Cordyceps militaris. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0103880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zeng Y., Han Z., Yu G., Hao J., Zhang L. Polysaccharides purified from wild Cordyceps activate FGF2/FGFR1c signaling. J Ocean Univ China. 2015;14(1):171–177. [Google Scholar]
- 115.Liang H., Xing Y., Chen J., Zhang D., Guo S.B., Wang C. Antimicrobial activities of endophytic fungi isolated from Ophiopogon japonicus (Liliaceae) BMC Complement Altern Med. 2012;12(1):238. doi: 10.1186/1472-6882-12-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y.H., Yan T., Shen J., Guo H., Xiang X. Preventive effect of Ophiopogon japonicus polysaccharides on an autoallergic mouse model for Sjogren's syndrome by regulating the Th1/Th2 cytokine imbalance. J Ethnopharmacol. 2007;114(2):246–253. doi: 10.1016/j.jep.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 117.Wang S., Zhang Z., Lin X., Xu D., Feng Y., Ding K. A polysaccharide, MDG-1, induces S1P1 and bFGF expression and augments survival and angiogenesis in the ischemic heart. Glycobiology. 2009;20(4):473–484. doi: 10.1093/glycob/cwp199. [DOI] [PubMed] [Google Scholar]
- 118.Zheng Q., Feng Y., Xu D., Lin X., Chen Y. Influence of sulfation on anti-myocardial ischemic activity of Ophiopogon japonicus polysaccharide. J Asian Nat Prod Res. 2009;11(4):306–321. doi: 10.1080/10286020902727363. [DOI] [PubMed] [Google Scholar]
- 119.Wang X., Sun R., Zhang J., Chen Y., Liu N. Structure and antioxidant activity of polysaccharide POJ-U1a extracted by ultrasound from Ophiopogon japonicus. Fitoterapia. 2012;83(8):1576–1584. doi: 10.1016/j.fitote.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 120.Wang H. Preventive effects of ophiopogon-polysaccharide on apiponectin in gestational diabetes mellitus rat. Asian Pac J Trop Med. 2013;6(4):296–299. doi: 10.1016/S1995-7645(13)60059-0. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Y., Zhang M., Jiang Y. Lentinan as an immunotherapeutic for treating lung cancer: a review of 12 years clinical studies in China. J Cancer Res Clin Oncol. 2018;144(11):2177–2186. doi: 10.1007/s00432-018-2718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zeng P., Guo Z., Zeng X. Chemical, biochemical, preclinical and clinical studies of Ganoderma lucidum polysaccharide as an approved drug for treating myopathy and other diseases in China. J Cell Mol Med. 2018;22(7):3278–3297. doi: 10.1111/jcmm.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li X., He Y., Zeng P. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J Cell Mol Med. 2019;23(1):4–20. doi: 10.1111/jcmm.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang Y., Chang Y., Liu Y. Overview of Ganoderma sinense polysaccharide-an adjunctive drug used during concurrent chemo/radiation therapy for cancer treatment in China. Biomed Pharmacother. 2017;96:865–870. doi: 10.1016/j.biopha.2017.09.060. [DOI] [PubMed] [Google Scholar]
- 125.He Y., Li X., Hao C. Grifola frondosa polysaccharide: a review of antitumor and other biological activity studies in China. Discov Med. 2018;25(138):159–176. [PubMed] [Google Scholar]
- 126.Chang Y., Zhang M., Jiang Y. Preclinical and clinical studies of Coriolus versicolor polysaccharopeptide as an immunotherapeutic in China. Discov Med. 2017;23(127):207–219. [PubMed] [Google Scholar]