Highlights
-
•
Coronaviruses (CoV) represent current and future risk for pandemic zoonotic infections.
-
•
Nucleoside analogues are highly active across multiple virus families.
-
•
CoVs encode a proofreading exoribonuclease that opposes inhibition by many nucleoside analogues.
-
•
Remdesivir is an adenosine analogue that acts against a broad spectrum of CoVs.
-
•
Other nucleoside analogues show promise against CoVs.
Abstract
Recent outbreaks of SARS-Coronavirus and MERS-Coronavirus (CoV) have heightened awareness about the lack of vaccines or antiviral compounds approved for prevention or treatment of human or potential zoonotic CoVs. Anti-CoV drug development has long been challenged by the activity of a 3′ to 5′ proofreading exoribonuclease unique to CoVs. Recently, a promising nucleoside analogue with broad-spectrum activity against CoVs has been identified. This review will discuss progress made in the development of antiviral nucleoside and nucleotide analogues targeting viral RNA synthesis as effective therapeutics against CoV infections and propose promising strategies for combination therapy.
Current Opinion in Virology 2019, 35:57–62
This review comes from a themed issue on Antiviral strategies
Edited by Margo A Brinton and Richard K Plemper
For a complete overview see the Issue and the Editorial
Available online 21st May 2019
https://doi.org/10.1016/j.coviro.2019.04.002
1879-6257/© 2019 Published by Elsevier B.V.
Introduction
Coronaviruses (CoVs) are a family of enveloped viruses containing a positive-sense RNA genome. CoVs belong to the family Coronaviridae within the order Nidovirales. The Coronaviridae, henceforth referred to as CoVs, infect a broad range of vertebrates including mammals and birds. Of the six CoV strains isolated from humans, four strains (HCoV-229E, HCoV-NL63, HCoV-HKU1, and HCoV-OC43) cause a usually mild self-limiting upper respiratory infection accounting for an estimated 15–29% of common colds [1]. Two other human CoV strains have produced more serious respiratory disease epidemics in recent years. Severe acute respiratory syndrome (SARS)-CoV originated in China in 2002 and 2003 and spread to a total of 37 countries, causing more than 8000 cases of SARS with an estimated fatality rate of 9% [2]. The cause of an ongoing epidemic of Middle East respiratory syndrome, MERS-CoV was first identified in Saudi Arabia in 2012 and to date has caused more than 2374 cases in 27 countries with a fatality rate >34% [3,4]. The use of human host cell receptors by both human and bat CoVs supports predictions of significant risk for emergence of new potential pandemic zoonotic CoVs [5,6]. Notably, no vaccines or antiviral compounds are approved for prevention or treatment of human or potential zoonotic CoVs. Emerging CoVs have been accorded priority status by WHO and government agencies for development of prevention and treatment strategies due to severity of these infections and credible pandemic potential [7, 8, 9]. This review will focus on opportunities for and challenges to development of antiviral nucleoside and nucleotide analogues targeting viral RNA synthesis as effective therapeutics against CoV infections.
CoV antiviral strategies
Drugs currently under investigation for use against CoV disease include monoclonal antibodies; direct-acting antivirals (DAAs) including as protease, helicase, and polymerase inhibitors; and immunomodulators such as interferons and corticosteroids. Challenges to CoV antivirals development are both general to RNA viruses and specific to CoVs. The replication of positive-sense RNA virus genomes is generally characterized by high error rates, high viral yields, short replication times, and abundant homologous and nonhomologous recombination [10]. In consequence, ‘viral swarms’ are generated which consist of a diverse population of genome mutants with varying degrees of fitness. This genetic plasticity challenges development of broadly useful antivirals by enabling rapid development of drug resistance while preserving overall viral fitness.
Development of nucleoside analogue inhibitors of CoVs is further hampered by the novel RNA-dependent RNA proofreading activity of CoV nonstructural protein 14, a 3′ to 5′ exoribonuclease (nsp14-ExoN), which confers up to 20-fold increase in replication fidelity compared with other RNA viruses. The nsp14-ExoN activity is responsible for native CoV high resistance to many nucleoside analogues including ribavirin and 5-fluorouracil [11, 12, 13]. Nevertheless, the recent development of nucleotide and nucleoside analogue inhibitors with a high barrier for resistance, broad-spectrum activity against multiple CoVs, and the ability to inhibit WT CoVs in the presence of nsp14-ExoN holds promise for the treatment of CoV disease.
Nucleotide and nucleoside analogue inhibitors for the treatment of CoV infections
Nucleotide and nucleoside analogue inhibitors, hereafter abbreviated NI, are chemically synthesized analogues of purines and pyrimidines in which the heterocyclic ring or sugar moiety has been altered. Currently used to treat both chronic and acute viral infections, NIs are administered as nucleotide or nucleoside precursors or prodrugs, which are metabolized by host or viral kinases to their active triphosphate once inside the cell.
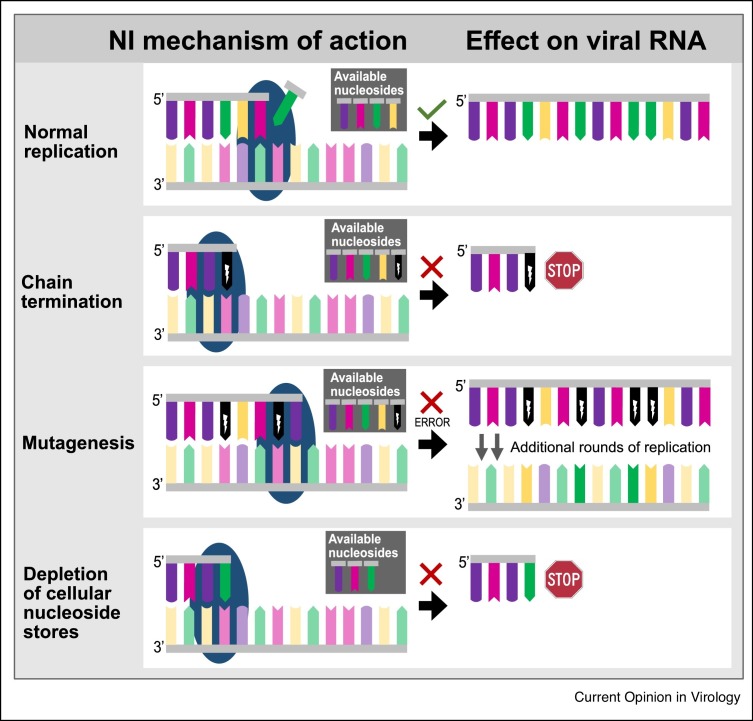
NIs exert inhibitory effects on viral replication by one or more non-mutually exclusive mechanisms (Figure 1 ). First, mis-incorporation of foreign nucleotides in replicating viral genomes may cause chain termination and disrupt subsequent replication or transcription. Chain termination may be immediate (obligate) or may occur following a limited extent of continued RNA or DNA synthesis (non-obligate). Second, NIs may incorporate into elongating nucleotide chains, mispairing with and/or substituting natural nucleotides, thereby introducing mutations that potentially impair RNA synthesis, structure, or RNA–protein interactions or protein functions. Accumulation of mutations and loss of virus viability are referred to as lethal mutagenesis and error catastrophe [14]. Through these mechanisms, NIs alter the genetic makeup of the virus, leading to a decrease in viral fitness with every consecutive replication cycle. Finally, NIs may cause depletion of pools of naturally occurring nucleotides by mimicking [15].
Figure 1.
Mechanisms of inhibition by nucleoside and nucleotide analogues. Schematic representation illustrates normal replication by the RdRp (blue sphere), premature chain termination caused by an obligate chain terminator, reduced replication fidelity due to mutagen incorporation, and depletion of pools of naturally occurring nucleotides.
NIs demonstrate a relatively high barrier to resistance emergence because the structural conservation of the binding site of their polymerase targets is high among virus families, and resistance mutations generally incur a fitness cost for the enzyme and the virus [16]. For CoVs, amino acid conservation of the viral RdRp ranges from 70 to near 100% and is maintained across genera, suggesting NIs could potentially serve as broad-spectrum inhibitors of CoV infection [17••]. However, proofreading activity of nsp14-ExoN activity protects CoVs from many NIs effective against other RNA viruses [12,18]. To effectively inhibit CoVs, an NI needs to either evade recognition by ExoN or undergo uptake into the elongating strand at a rate exceeding ExoN excision kinetics. We will next discuss several antiviral NIs described in the literature and evidence supporting their efficacy against CoVs.
Ribavirin (1-β-d-ribofuranosyl-1, 2,4-triazole-3-carboxyamide) is a guanosine analogue with broad-spectrum antiviral activity against RNA viruses. It is used to treat hepatitis C and E virus, respiratory syncytial virus, Lassa virus, and hantavirus infections. In its monophosphate form, ribavirin interactions with inosine monophosphate dehydrogenase (IMPDH), a host enzyme vital to nucleotide biosynthesis, result in decreased guanosine production leading to inhibition of viral RNA synthesis though depletion of cellular GTP pools [19]. Furthermore, incorporation of its triphosphate form by the viral polymerase leads to lethal mutagenesis [20]. The proofreading activity of ExoN leaves CoVs impervious to doses of ribavirin that inhibit other viruses effectively [12]. Although high dose ribavirin showed partial inhibition of SARS-CoV and MERS-CoV replication in vitro (Table 1 ) [18,21], drug treatment increased viral load and exacerbated disease in a mouse model of SARS-CoV disease [22]. Ribavirin treatment did not improve the clinical outcome of SARS-CoV disease in human subjects and caused significant toxicity [23,24]. Synergistic activity against MERS-CoV of ribavirin combined with IFNα2b was observed in vitro and in rhesus macaques, suggesting that IFN increases the potency of ribavirin at lower, more tolerable concentrations [25,26]. However, five critically ill MERS-CoV-positive patients who were treated with a combination of ribavirin and IFNα2b showed no clinical improvement [27]. Treatment of 20 MERS patients with a combination of ribavirin and IFNα2a showed significantly improved survival at 14 days but not at 28 days [28], whereas treatment of MERS patients with a combination of IFNα2a or IFNβ1a and ribavirin yielded no survival benefit in another study [29]. Thus, although ribavirin shows some efficacy in vitro, it does not provide clinical benefit to humans with SARS-CoV or MERS-CoV infections.
Table 1.
Nucleoside analogues with demonstrated activity against human CoVs
| Nucleoside analogue | EC50 or IC50 | Citation |
|---|---|---|
| Ribavirin | 50–819 μM (SARS-CoV) | [21,51] |
| 20 μM (MERS-CoV) | ||
| Remdesivir | 0.07 μM (SARS-CoV) | [17••,32••] |
| 0.03–0.07 μM (MERS-CoV) | ||
| 0.03 μM (MHV) | ||
| β-d-N4-hydroxycytidine | 0.4 μM (HCoV-NL63) | [36,38] |
| 5 μM (SARS-CoV) | ||
| BCX4430 | 68.4 μM (MERS-CoV) | [31] |
| 57.7 μM (SARS-CoV) | ||
| Gemcitabine hydrochloride | 1.22 μM (MERS-CoV) | [40] |
| 4.96 μM (SARS-CoV) | ||
| 6-Azauridine | 0.03 μM (HCoV-NL63) | [36] |
| Mizoribine | 13.5 μM (MERS-CoV) | [18] |
| 62 μM (SARS-CoV) | ||
| Acyclovir fleximer | 23 μM (MERS-CoV) | [41] |
| 8.8 μM (HCoV-NL63) | ||
Remdesivir (Gilead Science (GS)-5734) is a phosphoramidate prodrug of the adenosine NI GS-441524 which is effective against filoviruses, pneumoviruses, and paramyxoviruses and is currently in a Phase I dose-escalation trial for Ebola virus infection [30]. Biochemical studies indicate that remdesivir acts as a non-obligate chain terminator [31]. Remdesivir is effective against a broad spectrum of human and pre-epidemic zoonotic CoVs and potently inhibits replication of SARS-CoV and MERS-CoV in primary human airway epithelial cultures (Table 1) [17••,32••]. Mice infected with a lethal dose of SARS-CoV displayed less weight loss, lower lung viral titer, and reduced lung pathology following prophylactic or therapeutic administration of remdesivir [17••]. A partial resistance phenotype was attributed to two mutations in the viral RdRp, suggesting remdesivir acts on this target [32••]. Importantly, the fitness and virulence of remdesivir-resistant SARS-CoV was reduced compared to WT SARS-CoV, indicating the barrier to remdesivir resistance is high [17••]. Increased potency of remdesivir against CoV lacking ExoN catalytic activity suggests that the drug is sensitive to proofreading by ExoN, albeit to a markedly lower extent than other NIs [32••]. This raises important questions about the mechanism of CoV inhibition by remdesivir, which may be recognized by ExoN but removed less efficiently or may be less visible to ExoN compared with other NIs. Studies probing the interactions between remdesivir and the CoV replication machinery will likely yield crucial insights into how this NI circumvents or overcomes CoV proofreading activity, which can in turn be applied to modeling the development of new NIs and enhancing potency of existing NIs.
Beta- d -N4-hydroxycytidine (NHC): NHC is a cytidine analogue that has demonstrated potent, broad-spectrum antiviral activity against Venezuelan equine encephalitis virus (VEEV), respiratory syncytial virus (RSV), influenza A virus (IAV), influenza B virus (IBV), chikungunya virus (CHIKV), and CoVs (Table 1). NHC exerts its antiviral effect primarily through mutagenesis of viral RNA [22,33,34,35•,36]. Serial passaging in the presence of NHC led to low level resistance for VEEV but not RSV, IAV, and bovine viral diarrhea virus, thus indicating a high resistance barrier [33,35•,37]. Potent anti-CoV activity of NHC was demonstrated for SARS-CoV and HCoV-NL63 [36,38]. Although the mechanism of CoV inhibition has not been determined, micromolar-range EC50s suggests that like remdesivir, NHC may also have a novel way of interacting with the CoV replicase. The potent, broad-spectrum antiviral activity exhibited by NHC warrants further investigation into use of NHC for the treatment of CoV infections, either alone or in combination with other DAAs and immunomodulators.
Other NIs with in vitro activity against CoVs and low cytotoxicity include the adenosine analogue BCX4430, which has broad spectrum activity against positive and negative sense RNA viruses and has shown antiviral activity against MERS-CoV and SARS-CoV [31,39]; the deoxycytidine analogue gemcitabine hydrochloride, a chemotherapy drug that inhibits SARS-CoV and MERS-CoV [40]; the uridine analogue 6-azauridine with activity against HCoV-NL63 [36]; and the immunosuppressant imidazole nucleoside mizoribine which inhibits SARS-CoV (Table 1) [18]. Flex-base modification of the guanosine analog acyclovir (acyclovir fleximer) yielded activity against HCoV-NL63 and MERS-CoV (Table 1) [41]. More research into the efficacy, potency, and mechanism of CoV inhibition is necessary to determine whether further development of these compounds as CoV antivirals is warranted.
Conclusions and outlook
As with SARS-CoV and MERS-CoV, new zoonotic CoVs likely will emerge from divergent virus pools in animal reservoirs. It is therefore critical to develop broad-spectrum anti-CoV strategies aimed at multiple conserved targets and functions. Despite high conservation of the viral RdRp and low tolerance for mutations at key residues, resistance against NIs due to mutations in the viral RdRp has been observed in CoVs and other RNA viruses [32••,42,43•,44,45]. Treatment with combinations of potent anti-CoV NIs could increase the barrier to resistance and enhance efficacy, especially if additive or synergistic interactions occur. Moreover, a therapeutic regimen that combines drugs with distinct modes of action or which interfere at different steps in the viral replication cycle could simultaneously increase antiviral potency, broaden the activity spectrum, and reduce the emergence of drug resistance against CoVs. Classes of candidate companion compounds include NIs, helicase inhibitors, protease inhibitors, monoclonal antibodies, viral entry inhibitors, and DEDDh family exoribonuclease inhibitors with potential activity against ExoN [46,47•,48], the latter deserving special interest. DEDDh inhibitors aurintricarboxylic acid and pontacyl violet 6R effectively inhibited Lassa virus NP exonuclease in a proof-of-concept biochemical assay [48]. The structural and functional conservation across CoV family members and the absence of redundant functions elsewhere in the genome makes ExoN a potential Achilles’ heel. Combining compounds that inhibit ExoN activity with one or more NIs would simultaneously reduce CoV replication fidelity, boost the potency of the NI, mitigate selective pressures leading to drug resistance, and ultimately attenuate viral disease.
Finally, both SARS-CoV and MERS-CoV infections spur exuberant host inflammatory responses that rapidly progress toward severe immunopathology, the most probable driver of morbidity and death rather than direct viral damage to pulmonary tissues [49]. This potentially limits the therapeutic window for DAAs. Thus, combinations of DAAs and targeted immunomodulators may be necessary to halt lethal progression of immunopathology and extend the therapeutic window for intervention. A multicenter, placebo-controlled, double-blind randomized trial (MIRACLE: NCT02845843) is currently in progress to determine the efficacy of combining the immunomodulator IFNβ1b with lopinavir-ritonavir, a protease inhibitor cocktail used to treat HIV that also inhibits MERS-CoV in vitro [50]. Adequately controlled prospective studies like MIRACLE are urgently needed to assess efficacy of candidate CoV drugs. These studies should include compounds that have demonstrated broad-spectrum in vivo efficacy against CoVs, such as remdesivir, knowing that additional novel zoonotic CoVs are an inevitable future occurrence.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank Jim Chappell and Maria Agostini for careful review of this manuscript. This work was supported by the National Institutes of Health [U19 AI109680, R01 AI132178, R01 AI108197].
References
- 1.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revised U.S. Surveillance Case Definition for Severe Acute Respiratory Syndrome (SARS) and Update on SARS Cases — United States and Worldwide, December 2003. [date unknown]. [PubMed]
- 3.WHO | Middle East respiratory syndrome coronavirus (MERS-CoV).WHO [date unknown.
- 4.Arabi Y.M., Balkhy H.H., Hayden F.G., Bouchama A., Luke T., Baillie J.K., Al-Omari A., Hajeer A.H., Senga M., Denison M.R. Middle east respiratory syndrome. N Engl J Med. 2017;376:584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo C.-M., Wang N., Yang X.-L., Liu H.-Z., Zhang W., Li B., Hu B., Peng C., Geng Q.-B., Zhu G.-J. Discovery of novel bat coronaviruses in South China that use the same receptor as middle east respiratory syndrome coronavirus. J Virol. 2018;92 doi: 10.1128/JVI.00116-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu H., Chan C.-M., Zhang X., Wang Y., Yuan S., Zhou J., Au-Yeung R.K.-H., Sze K.-H., Yang D., Shuai H. Middle east respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells. J Biol Chem. 2018;293:11709–11726. doi: 10.1074/jbc.RA118.001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modjarrad K., Moorthy V.S., Ben Embarek P., Van Kerkhove M., Kim J., Kieny M.-P. A roadmap for MERS-CoV research and product development: report from a world health organization consultation. Nat Med. 2016;22:701–705. doi: 10.1038/nm.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brende B., Farrar J., Gashumba D., Moedas C., Mundel T., Shiozaki Y., Vardhan H., Wanka J., Røttingen J.-A. CEPI-a new global R&D organisation for epidemic preparedness and response. Lancet Lond Engl. 2017;389:233–235. doi: 10.1016/S0140-6736(17)30131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehand M.S., Al-Shorbaji F., Millett P., Murgue B. The WHO R&D blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Res. 2018:63–67. doi: 10.1016/j.antiviral.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perales C., Domingo E. Antiviral strategies based on lethal mutagenesis and error threshold. Curr Top Microbiol Immunol. 2016;392:323–339. doi: 10.1007/82_2015_459. [DOI] [PubMed] [Google Scholar]
- 11.Eckerle L.D., Lu X., Sperry S.M., Choi L., Denison M.R. High fidelity of murine hepatitis virus replication is decreased in nsp14 exoribonuclease mutants. J Virol. 2007;81:12135–12144. doi: 10.1128/JVI.01296-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith E.C., Blanc H., Vignuzzi M., Denison M.R. Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferron F., Subissi L., Silveira De Morais A.T., Le N.T.T., Sevajol M., Gluais L., Decroly E., Vonrhein C., Bricogne G., Canard B. Structural and molecular basis of mismatch correction and ribavirin excision from coronavirus RNA. Proc Natl Acad Sci U S A. 2018;115:E162–E171. doi: 10.1073/pnas.1718806115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tejero H., Montero F., Nuño J.C. Theories of lethal mutagenesis: from error catastrophe to lethal defection. In: Domingo E., Schuster P., editors. Quasispecies: From Theory to Experimental Systems. Springer International Publishing; 2016. pp. 161–179. [Google Scholar]
- 15.Te H.S., Randall G., Jensen D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol Hepatol. 2007;3:218–225. [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan P.C., Stevens S.K., Deval J. Nucleosides for the treatment of respiratory RNA virus infections. Antivir Chem Chemother. 2018;26 doi: 10.1177/2040206618764483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]; Although nucleoside analogs have been shown effective against many RNA viruses, many do not potently inhibit CoVs. This study demonstrates potent activity of nucleoside analogue GS-5734 against a diverse spectrum of human and zoonotic CoV in human airway epithelial cultures and in mouse models of SARS-CoV and MERS-CoV disease. This is the first study to demonstrate activity of GS-5734 against CoVs.
- 18.Saijo M., Morikawa S., Fukushi S., Mizutani T., Hasegawa H., Nagata N., Iwata N., Kurane I. Inhibitory effect of mizoribine and ribavirin on the replication of severe acute respiratory syndrome (SARS)-associated coronavirus. Antiviral Res. 2005;66:159–163. doi: 10.1016/j.antiviral.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feld J.J., Hoofnagle J.H. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 20.Cameron C.E., Castro C. The mechanism of action of ribavirin: lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr Opin Infect Dis. 2001;14:757–764. doi: 10.1097/00001432-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Chan J.F.W., Chan K.-H., Kao R.Y.T., To K.K.W., Zheng B.-J., Li C.P.Y., Li P.T.W., Dai J., Mok F.K.Y., Chen H. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnard D.L., Day C.W., Bailey K., Heiner M., Montgomery R., Lauridsen L., Winslow S., Hoopes J., JK-K Li, Lee J. Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antiviral Res. 2006;71:53–63. doi: 10.1016/j.antiviral.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 24.Gross A.E., Bryson M.L. Oral Ribavirin for the treatment of noninfluenza respiratory viral infections: a systematic review. Ann Pharmacother. 2015;49:1125–1135. doi: 10.1177/1060028015597449. [DOI] [PubMed] [Google Scholar]
- 25.Falzarano D., de Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P., Brining D., Bushmaker T., Martellaro C., Baseler L. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep. 2013;3 doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Tawfiq J.A., Momattin H., Dib J., Memish Z.A. Ribavirin and interferon therapy in patients infected with the middle east respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., Almakhlafi G.A., Albarrak M.M., Memish Z.A., Albarrak A.M. Ribavirin and interferon alfa-2a for severe middle east respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalhoub S., Farahat F., Al-Jiffri A., Simhairi R., Shamma O., Siddiqi N., Mushtaq A. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L., Flint M., McMullan L.K., Siegel D., Clarke M.O. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep. 2017;7 doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren T.K., Wells J., Panchal R.G., Stuthman K.S., Garza N.L., Van Tongeren S.A., Dong L., Retterer C.J., Eaton B.P., Pegoraro G. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]; Low level resistance against the potent, broad-spectrum anti-CoV nucleoside analog GS-5734 (remdesivir) was selected by serial passage and is associated with two mutations in the viral RdRP. Remdesivir resistance decreased fitness of MHV and attenuated pathogenesis of SARS-CoV in a mouse model of SARS-CoV disease, supporting further development of remdesivir as a pan-CoV antiviral and raising important questions about the mechanism by which remdesivir overcomes CoV nsp14-ExoN activity.
- 33.Urakova N., Kuznetsova V., Crossman D.K., Sokratian A., Guthrie D.B., Kolykhalov A.A., Lockwood M.A., Natchus M.G., Crowley M.R., Painter G.R. β-d-N4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J Virol. 2018;92 doi: 10.1128/JVI.01965-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehteshami M., Tao S., Zandi K., Hsiao H.-M., Jiang Y., Hammond E., Amblard F., Russell O.O., Merits A., Schinazi R.F. Characterization of β-d-N4-hydroxycytidine as a novel inhibitor of chikungunya virus. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.02395-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Yoon J.-J., Toots M., Lee S., Lee M.-E., Ludeke B., Luczo J.M., Ganti K., Cox R.M., Sticher Z.M., Edpuganti V. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00766-18. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies the modified cytidine ribonucleoside analogue β-d-N4-hydroxycytidine as a potent broad-spectrum inhibitor of respiratory viruses including respiratory syncytial virus, influenza B virus, and influenza A viruses of human, avian, and swine origins and has a high resistance barrier. This compound also is a candidate for the treatment of CoV infection.
- 36.Pyrc K., Bosch B.J., Berkhout B., Jebbink M.F., Dijkman R., Rottier P., van der Hoek L. Inhibition of human coronavirus NL63 infection at early stages of the replication cycle. Antimicrob Agents Chemother. 2006;50:2000–2008. doi: 10.1128/AAC.01598-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuyver L.J., Whitaker T., McBrayer T.R., Hernandez-Santiago B.I., Lostia S., Tharnish P.M., Ramesh M., Chu C.K., Jordan R., Shi J. Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture. Antimicrob Agents Chemother. 2003;47:244–254. doi: 10.1128/AAC.47.1.244-254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnard D.L., Hubbard V.D., Burton J., Smee D.F., Morrey J.D., Otto M.J., Sidwell R.W. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARSCoV) by calpain inhibitors and beta-d-N4-hydroxycytidine. Antivir Chem Chemother. 2004;15:15–22. doi: 10.1177/095632020401500102. [DOI] [PubMed] [Google Scholar]
- 39.Taylor R., Kotian P., Warren T., Panchal R., Bavari S., Julander J., Dobo S., Rose A., El-Kattan Y., Taubenheim B. BCX4430 – a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J Infect Public Health. 2016;9:220–226. doi: 10.1016/j.jiph.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jahrling P.B., Laidlaw M. Repurposing of clinically developed drugs for treatment of middle east respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters H.L., Jochmans D., de Wilde A.H., Posthuma C.C., Snijder E.J., Neyts J., Seley-Radtke K.L. Design, synthesis and evaluation of a series of acyclic fleximer nucleoside analogues with anti-coronavirus activity. Bioorg Med Chem Lett. 2015;25:2923–2926. doi: 10.1016/j.bmcl.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deval J., Fung A., Stevens S.K., Jordan P.C., Gromova T., Taylor J.S., Hong J., Meng J., Wang G., Dyatkina N. Biochemical effect of resistance mutations against synergistic inhibitors of RSV RNA polymerase. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Eyer L., Kondo H., Zouharova D., Hirano M., Valdés J.J., Muto M., Kastl T., Kobayashi S., Haviernik J., Igarashi M. Escape of tick-borne flavivirus from 2′-C-methylated nucleoside antivirals is mediated by a single conservative mutation in NS5 that has a dramatic effect on viral fitness. J Virol 91. 2017:e01028-17. doi: 10.1128/JVI.01028-17. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that the nucleoside analog 7-deaza-2′-C-methyladenosine (7-deaza-2′-CMA) demonstrates potent antiviral activity against tick-borne encephalitis virus. Resistance generated by serial passaging in the presence of 7-deaza-2′-CMA was associated with a single mutation in the polymerase and resulted in resistance against a broad spectrum of derivative compounds. This finding highlights the importance combination therapy to reduce the emergence of resistant virus.
- 44.Diphoko T., Gaseitsiwe S., Kasvosve I., Moyo S., Okatch H., Musonda R., Wainberg M., Makhema J., Marlink R., Novitsky V. Prevalence of rilpivirine and etravirine resistance mutations in HIV-1 subtype C-infected patients failing nevirapine or efavirenz-based combination antiretroviral therapy in botswana. AIDS Res Hum Retroviruses. 2018;34:667–671. doi: 10.1089/aid.2017.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valdés J.J., Butterill P.T., Růžek D. Flaviviridae viruses use a common molecular mechanism to escape nucleoside analogue inhibitors. Biochem Biophys Res Commun. 2017;492:652–658. doi: 10.1016/j.bbrc.2017.03.068. [DOI] [PubMed] [Google Scholar]
- 46.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Liang R., Wang L., Zhang N., Deng X., Su M., Su Y., Hu L., He C., Ying T., Jiang S. Development of small-molecule MERS-CoV inhibitors. Viruses. 2018;10:721. doi: 10.3390/v10120721. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive review of small-molecule inhibitors targeting viral proteins at different stages of the MERS-CoV viral replication cycle. Illustrates chemical structure formulas of inhibitors and summarizes potency, cytotoxicity, and testing model of each inhibitor in a table.
- 48.Huang K.-W., Hsu K.-C., Chu L.-Y., Yang J.-M., Yuan H.S., Hsiao Y.-Y. Identification of inhibitors for the DEDDh family of exonucleases and a unique inhibition mechanism by Crystal structure analysis of CRN-4 bound with 2-Morpholin-4-ylethanesulfonate (MES) J Med Chem. 2016;59:8019–8029. doi: 10.1021/acs.jmedchem.6b00794. [DOI] [PubMed] [Google Scholar]
- 49.Gralinski L.E., Baric R.S. Molecular pathology of emerging coronavirus infections. J Pathol. 2015;235:185–195. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., AlJohani S., Al Harbi S., Kojan S., Al Jeraisy M., Deeb A.M., Assiri A.M. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen F., Chan K.H., Jiang Y., Kao R.Y.T., Lu H.T., Fan K.W., Cheng V.C.C., Tsui W.H.W., Hung I.F.N., Lee T.S.W. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]