Summary
Since its discovery in 1947 in Uganda and control and eradication efforts have aimed at its vectors (Aedes mosquitoes) in Latin America in the 1950s, an absolute neglect of Zika programs and interventions has been documented in Aedes endemic and epidemic-prone countries. The current unprecedented Zika viral epidemics and rapid spread in the Western hemisphere pose a substantial global threat, with associated anxiety and consequences. The lack of safe and effective drugs and vaccines against Zika or dengue epidemics further buttresses the realization from the West Africa Ebola outbreak that most emerging disease-prone countries are still poorly prepared for an emergency response. This paper examines knowledge gaps in both emerging and neglected arthropod-borne flavivirus infectious diseases associated with poverty and their implications for fostering local, national and regional emerging disease preparedness, effective and robust surveillance–response systems, sustained control and eventual elimination. Strengthening the regional and Global Health Flavivirus Surveillance-Response Network (GHFV-SRN) with other models of socio-economic, climatic, environmental and ecological mitigation and adaptation strategies will be necessary to improve evidence-based national and global maternal–child health agenda and action plans.
Keywords: Zika virus, Epidemics, Health, Preparedness, Surveillance, Maternal–child
Introduction
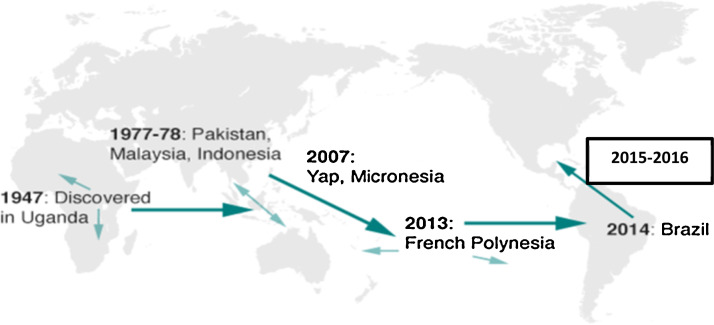
The unprecedented and emerging Zika epidemic is a public health threat of international concern in the Western hemisphere that is spreading quickly and causing havoc. More than two million people have been affected to date, with multiple health and socio-economic consequences. This infectious disease of poverty was first discovered in Uganda in 1947; only 15 cases were documented prior to 2007, and the disease remained in a state of dormancy until now [1]. It has been identified in more than 20 countries in South and Central America, the Caribbean, Asia-Pacific and Africa and some Pacific islands (Fiji, Vanuatu, Micronesia) have also reported sporadic outbreaks [2], [3], [4]. Arbovirus disease epidemics have the potential to worsen the global maternal–child health burden in most vector-borne disease-prone countries [5] (Fig. 1 ).
Figure 1.
Reported and active transmission of Zika viral disease countries.
Zika is caused by an arthropod-borne virus “arbovirus” transmitted primarily by Aedes mosquito (Aedes aegypti and Aedes albopictus) bite. Zika is also part of the flavivirus family that also includes dengue, West Nile and yellow fever. Zika virus (ZIKV) can be transmitted through blood and other bodily fluids such as semen during sexual intercourse or mother-to-fetus transmission throughout pregnancy [2]. ZIKV disease outbreaks have been linked with pregnancy syndrome, newborn and child defects and premature deaths [1], [2]. Colombia has recoded 13,500 suspected cases and predicts 600,000 cases by the end of 2016 with significant social, health and economic impacts. In comparison, Brazil reported 4000 cases of fetal microcephaly during pregnancy yet had only 150 cases total in 2015 and 2014, respectively. In addition, of the 25,165 infected cases, more than 3177 pregnant women have been documented with the ZIKV in Colombia [6], [7]. There is an urgent need for innovative approaches and tools for early detection and rapid confirmation of ZIKV-linked microcephaly and early warning systems to provide timely evidence-based information to guide decision making policies and strategies including pre- and ante-natal counseling and strict abortion measures. We also need new methods for managing climate change, environmental and ecological systems in hospital and maternity settings and hygiene and sanitation efforts to interrupt any potential sources of transmission.
This paper examines the knowledge gaps of these emerging and neglected infectious diseases of poverty epidemics (Zika and dengue) implications in fostering local, national and regional emerging threats and epidemics preparedness, surveillance and smart response systems for sustained maternal–child health.
Methodology
Search strategy
Systematic review results from searches for Zika and dengue outbreak-affected countries using key MeSH terms including “Zika or dengue outbreak, ZIKV/DENV, Zika or dengue arthropod-borne flaviviruses vector threat and epidemics surveillance, Zika viral outbreak response, Aedes mosquito control, dengue control programs and maternal–child health interventions” along with terms referring to different types of outbreak responses and interventions, those indexed in PubMed, data published in acknowledged websites and national and regional reports were reviewed. This was done to assess the emerging Zika and dengue public health threat and burden, surveillance and control programs and effectiveness of interventions in affected countries.
Inclusion and selection criteria
Systematic review information and data from the affected countries and maternal–child populations were scrutinized and documented. Data sources included the results from all acknowledged web-based searches useful in providing detailed analyses of national and regional trends, health strategies and measures and WHO guidelines for outbreak surveillance and response in humanitarian emergencies, global infectious disease surveillance, epidemic preparedness and response, Global Alert and Response and the Global Outbreak Alert and Response Network documents specifically focused on arbovirus/flavivirus (ZIKV and DENV). Inclusion criteria for studies were those conducted in the Western hemisphere, Africa and Pacific islands; epidemics analyzed over time and space; studies on the incidence, prevalence and fatality rates in affected countries; and studies focusing on programs and emergency response. The Centers for Disease Control and Prevention's National Notifiable Dengue Surveillance databases and World Health Global Outbreak Emergency Response data were also used. The data obtained were summarized and used to describe the public health burdens of ZIKV and DENV diseases.
Results
A total of 174 papers were analyzed; 31 were selected based on ZIKV and DENV surveillance programs and maternal–child health interventions in Aedes arthropod-borne flavivirus-affected countries. Only 31 publications that met the inclusion criteria were fully assessed and analyzed, with respect to the prevention of disease co-infection burden and maternity and pediatric care delivery services.
Chronological trend and general characteristics of Zika and dengue viral diseases epidemics
From its discovery in Uganda in 1947 and its isolation in Nigeria in 2005 and 2007 and Senegal in 2007, the Zika virus migrated to the Middle East and Southeast Asia, to the Pacific islands and to the Western hemisphere and the Americas. In 2015 and 2016, Brazil recorded the highest number of cases of microcephaly (more than 3800), which was over 30 times more than in any year since 2010 (Fig. 1).
Zika and dengue viral disease impact implications on global maternal–child health burden
Both Zika and dengue are emerging or re-emerging viral diseases that are both caused by the arbovirus “Aedes species”. Both Zika and dengue infections appear to have similar early signs and symptoms including maculo-papular rash, fever, back/body pain; they differ in their late stage or complication symptoms, such as hemorrhagic bleeding in dengue. Previous dengue epidemics have been documented but less virulent and localized than the ongoing widespread ZIKV epidemics. Nevertheless, there is still much to be learned regarding the full human impact of ZIKV compared to the pathophysiological and immunological consequences of dengue virus. ZIKV can be transmitted to humans by infected Aedes mosquito bites, infected mother-to-child trans-placenta bodily fluid diffusion, blood transfusion, unprotected sexual intercourse with an infected individual and perhaps breastfeeding. More short and long-term operational research is needed to further establish the risk factors and determinants and to confirm the association between Zika virus infections, microcephaly and related neurological aberrations suspected in fetal and childhood development (Table 1 ).
Table 1.
Key comparative characteristics between Zika and dengue viral disease threats and epidemics.
| Characteristics | Zika | Dengue | References |
|---|---|---|---|
| Discovery year | 1947 Uganda Zika virus isolation in monkey and human in 1952 and 1954 in Nigeria, respectively |
265–420 AD Aedes confirmation in 1906 |
[1], [4] |
| Disease type | Viral arthropod-borne flavivirus | Viral arthropod-borne flavivirus | |
| Vector mosquito | Aedes aegypti, Aedes albopictus | Aedes aegypti, Aedes albopictus | |
| Genius, family and member | Genus Aedes, Arbovirus/flavivirus, Flaviviridae | Genus Aedes, Arbovirus/flavivirus, Flaviviridae | [3], [10] |
| Virus types | Zika virus (ZIKV1, 2, 3) | DENV1, 2, 3, 4 virus | |
| Transmission | Infected vector/Aedes bite Infected fluids: blood, semen, rectal fluids, vaginal fluids, mother-to-child transmission via breast milk. Blood transfusion or needle exchange |
Mosquito Aedes bite Unprotected sexual intercourse Infected fluids: blood, semen, rectal fluids, vaginal fluids, mother-to-child transmission via breast milk or blood transfusion suspected |
[11], [13], [23] |
| Route of transmission | Human-to-human transmission Wildlife-to-human by infected Aedes bite Direct contact with infected body fluids, blood of infected human and animal including blood, urine, sweat, semen and breast milk (sweat, semen, saliva) |
Human-to-human transmission Wildlife-to-human by infected Aedes bite Direct contact with infected body fluids, blood of infected human and animal including blood, urine, sweat, semen and breast milk (sweat, semen, saliva) |
[1], [12], [13], [15], [16], [22] |
| Incubation | 2–23 days | Usually 2–21 days | |
| Host reservoir | Human, gorillas, monkeys, apes and chimpanzees | Human and animals | |
| Susceptible countries | >50 Africa (Cape Verde, Central African Republic, Egypt, Gabon, Sierra Leone, Tanzania, Nigeria and Uganda), Southeast Asia and the Pacific Islands (including India, Indonesia, Malaysia, the Philippines, Thailand and Vietnam, Samoa), South and Central America (Brazil, Colombia, Mexico, Venezuela, Ecuador, Jamaica, El Salvador, Haiti, Honduras, Puerto Rico), Asia-Pacific Islands (Fiji, Vanuatu, Micronesia) | >100 Countries in Africa, Southeast Asia, the Pacific Islands and middle East including (Uganda, South and Central America, Brazil, Colombia, Mexico, Venezuela, El Salvador, Haiti, Honduras, Puerto Rico), Asia-Pacific (China, Malaysia, India, Pakistan, Fiji, Vanuatu, Micronesia) and Saudi Arabia | [1], [2], [3], [14] |
| Risk factors/determinants | Global trade and travel, Tourism in Enzootic or Aedes-prone settings, Unsafe sexual behavior, probably from patients’ body fluids, breastfeeding or transplacental or virus infected materials | Poverty and insecurity Unsafe sexual behavior, probably from patients’ body fluids, breast milk, unsterilized virus infected needles and materials |
[3], [6], [22], [23] |
| Signs and symptoms | Fever and arthralgia Joints pain and conjunctivitis, Rash, headaches, Guillain–Barre syndrome, increasing congenital microcephaly or birth defects or neurological disorders in babies/newborns, stillbirths and miscarriage, and poor pregnancy outcomes |
High fever (40 °C/104 °F) Severe headache, Pain behind the eyes Muscle and severe abdominal joint pain Swollen glands or rash nausea, vomiting Complications include fluid accumulation, respiratory distress, severe bleeding, Restlessness and blood in vomit or organ impairment |
[4], [7], [14], [25] |
| Global incidence | – NA | <284–528 million infections annually | [1], [2] |
| Global cumulative cases | >2 million | >652,212 cases of dengue hemorrhagic fever 67–136 million cases dengue fever |
|
| Global cumulative deaths | – NA | 22,000 deaths mainly among children | |
| Diagnostic methods | Syndromic screening in most cases. Travel and work history exposure to wildlife Prognosis Differential diagnosis by detecting of viral RNA by PCR/RT-PCR and proteins antibodies against the virus in infected patient's blood by ELISA or Serum neutralization test. |
Syndromic screening in most cases. Travel and work history exposure to wildlife prognosis Differential diagnosis by detecting of viral RNA by PCR/RT-PCR and proteins antibodies against the virus in infected patient's blood by ELISA or Serum neutralization test |
[14], [24] |
| Preventive and control options | – No vaccine or drug available – Health education and community social mobilization, awareness, empowerment and resilience culture – Scaling up the usage of repellents containing DEET, picaridin and IR3535, condom promotion and ABC HIV/STIs approach – Family planning and abortion reforms as well as personal protection, improved hygiene and sanitation – Intensive care with the very few antiviral treatment options including anti-inflammatory drugs and oral rehydration therapy – Integration of active and comprehensive threat and disease surveillance and actions systems (1) Integration of a comprehensive threat and disease surveillance and actions systems (2) Increasing community awareness education and risk communication (3) Scaling up Zika and dengue screening for effective treatment and care (4) Partnership and cooperation among stakeholders in emerging infectious prevention and control programs and measures |
[15], [23], [26], [33] | |
| Research and development needs | – Effective contextual vector control programs and measures – Strengthening integrated and effective ZIKV cross-border preparedness and emergency response – Establishment of contextual local surveillance–response systems and capacity development (laboratories, clinical and field) – Community/environmental health workers, case management Safe and effective drugs and vaccines development with sustained political commitment and financial investment in ZIKV research – Sexual, reproductive and mental health education as well as strict legal measures on abortion – Climate change mitigation, environmental management and adaptation evidence-based strategies and policies – Understanding maternal vulnerability and impact on reproductive health and family planning policy – Addressing co-infection with ZIKV and HIV in vulnerable populations on maternal–child health stigmatization and fear – New global alliance for arbovirus/flavivirus or vector-borne diseases agenda |
[1], [6], [15], [26], [33] | |
NA, not available data.
Treatment or vaccination against ZIKV and DENV disease prevention and control
In the absence of safe and effective Zika or dengue drugs and vaccines, addressing the local and national Zika public health emergency and global concern requires a full understanding of the social, ecological and contextual risk factors and determinants of emerging disease outbreaks. Currently, only palliative treatments including anti-inflammatory and antiviral treatment options are readily available for patient management.
Enhancing community-based social mobilization and engagement, awareness campaigns and outreach, vigilance and monitoring of early warning signals fosters health system capacity development (e.g., infrastructure and human resources) and community partnerships and leadership. In addition, effective community resilience and empowerment, prevention and care management and containment emergency responses across endemic or epidemic prone countries are vital. Adequate training of healthcare workers is needed to effectively carry out effective and efficient communication operations as well as community literacy, prevention, care and containment (e.g., patient isolation) and management approaches.
The differential diagnosis of ZIKV and DENV mosquito-borne illnesses by health workers or local physicians can be very challenging because of the similarities in signs and symptoms. Molecular diagnostic assay results from sophisticated and differential medical interpretations (e.g., ultrasonography, MRI, RT PCR assay) are not often available and accessible and are also expensive for vulnerable populations in remote rural and semi-urban settings. Other alternative solutions include providing advocacy and mitigation platforms against climate changes, urbanization and globalization approaches to reducing mother-to-child transmission, comprehensive antenatal and postnatal programs for women, sexual education and use of protective tools and measures, adherence and quality outcomes. Containing the current public health scourge of ZIKV and DENV diseases will require the integration of population-based mass drug administration and immunization interventions supported by robust evidence-based and integrated epidemiological, socio-cultural, climatic and environmental surveillance and information management to guide decision-making policy and rapid responses. Nevertheless, implementation of real time, contextual and practical laws and regulations focusing on positive social behavioral attitudes and perceptions is needed to strengthen health systems, nurture safe motherhood and childhood practices and foster evidence-driven community project ownership to accelerate recovery programs and activities in affected communities.
Strengthening vector-borne disease preparedness and surveillance–response in maternal and neonatal child health
Weak or lack of health system preparedness and emergency response, lack or inadequacies in early detection and diagnostics, lack of surveillance indicators and benchmarks, lack of tracing and tracking and lack of effective risk communication and reporting tools and resources in Zika and other emerging infectious diseases are major challenges in most affected countries. The poor accessibility and availability of care services to most vulnerable populations during Zika epidemics increase maternal–child vulnerability in remote rural settings. Improving the health system capacity to respond to maternal and neonatal health challenges can be accomplished by timely, credible and actionable data inputs, coverage and the quality of services provided. Implementers require “early-warning systems” to provide reliable, user-friendly and cost-effective programs to monitor progress, to foster evidence-based decision making and optimal resilient culture and integrated quality packages, including mosquito-borne disease awareness and health education campaigns, mitigation and recovery measures. ZIKV rates might be underestimated and could even be higher because confusion with other diseases with similar symptoms could be misleading due to acquired immunity to other tropical infections in ZIKV human reservoirs, which might also complicate the actual Zika cases. ZIKV transmission in human reservoirs can be interrupted with DEET/permethrin clothing repellents as Zika prevention and control methods.
Evidence-based interventions and support programming can be provided by strengthening local and national preparedness as well as surveillance–response system implementation in maternal, neonatal and child health. Accelerating the process of local/national ZIKV and DENV immunization is critical and should be included in family and reproductive health planning, nutrition and poverty alleviation programs, programs and services linking communities and primary health facilities and referral hospitals. Moreover, effective and sustainable programs and measures are crucial to increase access and coverage rates to reduce health inequities, poverty and the maternal–child health burden in most emerging infectious disease epidemic-prone countries.
Discussion
Zika and dengue viral diseases have posed great challenges to the most affected countries, mainly in terms of maternal–child health impact. Increasing our knowledge regarding co-infections and health complications requires rapid global response for effective treatment or vaccines; revamping innovative socio-cultural, health education and communication approaches require more research in the future. Tackling persistent disease resurgence by intensifying information dissemination and innovative actions requires educational reforms at all levels, faith-based and community-based interventions for emerging disease outbreak preparedness, prevention and emergency response are all vital.
ZIKV and DENV infections have important health consequences; geographical, structural and biological risk factors, including poor community engagement and lack of viral disease surveillance–response systems, have several related implications [6], [8]. Table 1 shows that Zika and dengue have similar structural characteristics including fear, discrimination and even stigmatization [2], [4], [10], [21]. Biologically, the viruses have similar host reservoirs, likely modes of transmission and spread in human populations, mainly through human-to-human behavioral spread [22], [25]. This includes exposure to bodily fluids such as blood and semen. Zika is mainly transmitted via unprotected sex and via semen during poor sanitary practices [15]; however, contact with infected bodily fluids is one route of person-to-person transmission [12], [21]. The incubation period of both viral diseases is short and varies between 2 and 23 days; progression to infectiousness is between 4 and 7 days [21]. Blood-fed Zika mosquito screening and monitoring of emerging viral diseases, especially in maternal and child populations, are needed. Unraveling Aedes vector competence and genetic evolution determinants could not only provide significant insights into sudden ZIKV outbreaks, but can also provide a better understanding of virus epidemiology and ecology, including water breeding containers, buckets, flower pots, tires or construction sites [14], [15], [22].
It is important to understand the risk factors and transmission dynamics of ZIKV and DENV to plan for future infection resurgence, especially in vulnerable regions. Because most African countries have limited surveillance indicators to assess the threat of re-emergence, early warning disease alerts, community awareness outreach and education are essential. Both Zika and dengue are spread in a similar way, via body fluids, and they currently do not have an effective antiretroviral cure [8], [15], [21]. Infections caused by Ebola hemorrhagic fever have killed 50–70% of their victims; 70% of people living with Zika or dengue are in sub-Saharan Africa [3]. Although Zika can be highly lethal in the fetus during pregnancy, more so than dengue [23], [24]. The number of dengue-infected individuals globally is far greater than the number of Zika-infected individuals [3], [15]. Additionally, Zika and dengue are largely restricted to low-income countries affected by poverty, civil war, high maternal and infant mortality rates, low education levels and internal strife [16], [18]. The areas currently most affected by Zika include forest fringes and unstable African countries [20], [28], [29]. These areas have very weak health systems, which accounts for the re-emergence of these infectious diseases [15], [20]. Therefore, strengthening community health systems and fostering effective prevention and care delivery capacity are paramount. Tourists or mobile cross-border migrants in affected areas are at higher risk of a disproportionate burden, especially vulnerable newborns, children and mothers, who easily travel from one area to another [30], [31]. This can stretch the already fragile health systems of these affected settings and increase the vector or arbovirus transmission rates, which can spread unrecognized and from underdiagnosed reservoirs [16], [32].
Preventing a Zika-style epidemic crisis requires more evidence-based innovative prevention and control approaches and interventions. Implementation of effective and sustainable interventions such as community social engagement and participation outreach, prompt identification and case management, surveillance and preparedness systems, contact tracing, laboratory diagnosis, community mobilization, recovery systems for survivors, safe burial practices and behavioral changes (e.g., promotion of condom usage. abstinence, antenatal counseling) among ZIKV victims offer new opportunities to eliminate ZIKV, other sexually transmitted diseases (STIs) and dengue in the Western hemisphere, Pacific islands and some parts of sub-Saharan Africa [15], [21]. Maternal perinatal education and increasing household income in affected communities can have a significant impact in reducing child deaths and maternal vulnerability. Moreover, there is an emerging resurgence of dengue patterns and trend in poor countries and the Americas, likely related to climate changes, global warming and intense environmental perturbations. Strengthening the development of more effective and affordable early warning signals and surveillance indicators and tools, preventive and curative treatments to improve community care and wellness are needed [1], [5], [19], [21].
As indicated in Fig. 1, the geographical distribution of ZIKV and dengue from Africa to the Asian Pacific and Latin America requires further detailed data to model the dynamics of vectors and potential ecological including climatic impacts over time in old and new affected or vulnerable countries. For example, the Pan-American Health Organization (PAHO) is making robust strides in identifying and supporting research areas as part of its response. National governments and stakeholders should improve efforts to reduce inequality and implement fundamental reforms such as delaying pregnancy and limiting unnecessary travel [11]. Previous studies have shown that male circumcision, which is highly practiced in West Africa, is associated with a lower risk of ZIKV and other sexually transmitted diseases compared to other countries with low/no male circumcision rates [27], [28]. Local and international partnerships with sustainable commitment and support are required to halt these re-emerging epidemics by leveraging documented progress and addressing knowledge gaps in achieving significant reduction, effective control and containment efforts.
Similar to Ebola outbreaks in West Africa, re-emerging vector-borne infections could have severe impacts on maternal–child healthcare and worsening socio-economic challenges in sub-Saharan Africa. Tackling emerging or re-emerging vector-borne viral disease requires more proactive and concrete efforts and commitment in terms of climate changes and environmental protection, mitigation and appropriate adaptation measure development and deployment to address the growing trends. Innovative approaches in vector or virus sustained surveillance, monitoring and containment are needed and require significant attention similar to what has been done with MERS-CoV and dengue in Saudi Arabia, H1N1 and H7N9 avian influenza in China [2], [5], [9], [17], [26], [28]. Additionally, further research is needed to identify and map the growing patterns of resurgence. Timely exploration of the impacts of climate changes, changing host interactions and environmental impacts on vector-virus genetic evolution and flow as well as population immunity, viral competence and virulence advantages will require further research.
Currently, there is no vaccine or curative drug available, although highly active antiretroviral therapy can prolong the lives of infected individuals [11]. Early in the course of ZIKV and DENV research, the mysterious diseases were perceived as illnesses of forest regions [15]. Therefore, infected individuals were able to silently and unknowingly spread the infection for years [32]. In contrast, Ebola and HIV infections and related deaths are rapid and therefore more terrifying; however, dengue cases can be identified and isolated and patient contacts can be traced and monitored [15], [21]. As indicated in early arbovirus research, most healthcare workers and blood-transfused patients might be infected with Zika or dengue because of a lack of proper diagnostic capacities, poor elucidation of the routes of transmission, pathogenesis and spread. A similar effect was observed among dengue or ZIKV-infected health workers during the early history of the disease. In addition, lessons learned from the global dengue response should be extended to ZIKV in a timely manner [25]. Apart from that, Ebola virus requires more sophisticated isolation measures to contain the disease [21], [29] than does Zika or dengue.
There is an urgent need to support Zika and dengue research aimed at the development and implementation of safe and effective vaccine and drugs for millions of vulnerable mothers, newborns and children. It is also important to expand the coverage of innovative high-impact interventions including existing immunization campaigns to limit threatening co-infections and ensure the survival of women and children [28], [29], [31], [32]. Addressing mosquito vector and viral surveillance challenges in the context of limited resources or urbanized settings is necessary for effective treatment and vaccine accessibility and availability to the most hard-to-reach remote areas and vulnerable populations. Also critical is addressing the shortage of trained field and community healthcare workers, weak health systems, weak community engagement, lack of participation in early identification, challenging diagnostics and self-reporting, stigmatization and weak prompt isolation/quarantine facilities [28], [31], [32], [33]. The burden faced by children and mothers highlights the importance of increasing access to community social mobilization and participation, health education and awareness programs, facilities and effective emergency responses, including delivery of palliative antiretroviral drugs to affected communities, tracking and tracing of suspected cases and sexual partners, monitoring of risk factors and determinants of ZIKV and DENV threats and epidemic resurgence [33], [34], [35]. Improving health systems in LIMCs against the transmission dynamics and spread of ZIKV and DENV and their overlapping characteristic signs and symptoms is challenging due to clinical and diagnostic limitations.
Conclusion
There is an urgent need to improve investment in Zika, dengue and other infectious disease research and development on evidence-informed and sustainable policies making and strategies implementation to avert potential impacts in low and middle income countries (LIMCs). Moreover, strengthening the Global Health Flavivirus Network (GHFVN) for timely, reliable and detailed data in revamping pragmatic, evidence-based and action-oriented maternal–child health and global women's wellbeing is imperative. Implementing aggressive and strategic innovative interventions to prevent or control these viral diseases will be tantamount to directly or indirectly in promoting capacity building and empowerment in understanding ZIKV impacts on the maternal–child health burden. Ultimately, there is also a need to promote proactive and evidence-informed programs and intervention delivery, preparedness structural changes, health planning and emergency response programs and quality outcomes.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
Authors’ contributions
ET conceived the idea and built the conceptual framework. ET screened the synthetic primary information, analyzed and wrote the primary draft of the manuscript. ET, HAK and EIMK provided additional evidence and insights. All authors read and approved the final version of the manuscript.
Acknowledgments
This paper received no funding support.
References
- 1.Fauci A.S., Morens D.M. Zika virus in the Americas – yet another arbovirus threat. N Engl J Med. 2016;(January) doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 2.Attar N. ZIKA virus circulates in new regions. Nat Rev Microbiol. 2016;14(February (2)):62. doi: 10.1038/nrmicro.2015.28. [DOI] [Google Scholar]
- 3.McCarthy M. Zika virus outbreak prompts US to issue travel alert to pregnant women. BMJ. 2016;352(January):i306. doi: 10.1136/bmj.i306. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira Melo A.S., Malinger G., Ximenes R., Szejnfeld P.O., Alves Sampaio S., Bispo de Filippis A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(January (1)):6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 5.Firoz T., Chou D., von Dadelszen P., Agrawal P., Vanderkruik R., Tunçalp O. Measuring maternal health: focus on maternal morbidity. Bull World Health Organ. 2013;91:794–796. doi: 10.2471/BLT.13.117564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tambo E., Ugwu E.Ch., Ngogang J.Y. Need of surveillance response systems to combat Ebola outbreaks and other emerging infectious diseases in African countries. Inf Dis Poverty. 2014;3(August):29. doi: 10.1186/2049-9957-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ventura C.V., Maia M., Bravo-Filho V., Góis A.L., Belfort R., Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;(January) doi: 10.1016/S0140-6736(16)00006-4. pii: S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- 8.Villamil-Gómez W.E., González-Camargo O., Rodriguez-Ayubi J., Zapata-Serpa D., Rodriguez-Morales A.J. Dengue, chikungunya and Zika co-infection in a patient from Colombia. J Infect Public Health. 2016;January doi: 10.1016/j.jiph.2015.12.002. pii: S1876-0341(15)00221-X. [DOI] [PubMed] [Google Scholar]
- 9.Bogoch I.I., Brady O.J., Kraemer M.U., German M., Creatore M.I., Kulkarni M.A. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;(January) doi: 10.1016/S0140-6736(16)00080-5. pii: S0140-6736(16)00080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tambo E. Non-conventional humanitarian interventions in Ebola outbreak crisis in West Africa: health, ethics and legal implications. Infect Dis Poverty. 2014;3(November):42. doi: 10.1186/2049-9957-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cristiane W.C., Igor A.D.P., Mariana K., Moreno S.R., Monaise M.O.S., Gubio S.C. Outbreak of exanthematous illness associated with Zika, chikungunya, and dengue viruses, Salvador, Brazil. Emerg Infect Dis J. 2015;21(12) doi: 10.3201/eid2112.151167. 2274.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musso D., Nilles E.J., Cao-Lormeau V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014;20(October (10)) doi: 10.1111/1469-0691.12707. O595-6.6. [DOI] [PubMed] [Google Scholar]
- 13.Diallo D., Sall A.A., Diagne C.T., Faye O., Faye O., Ba Y. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0109442. e109442.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M.I., Wong P.S., Ng L.C., Tan C.H. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis. 2012;6(8) doi: 10.1371/journal.pntd.0001792. e1792.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen E.E., Staples J.E., Meaney-Delman D., Fischer M., Ellington S.R., Callaghan W.M. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. Morb Mortal Wkly Rep. 2016;65(January (2)):30–33. doi: 10.15585/mmwr.mm6502e1. [DOI] [PubMed] [Google Scholar]
- 16.Korhonen E.M., Huhtamo E., Smura T., Kallio-Kokko H., Raassina M., Vapalahti O. Zika virus infection in a traveller returning from the Maldives, June 2015. Euro Surveill. 2016;21(January (2)) doi: 10.2807/1560-7917.ES.2016.21.2.30107. [DOI] [PubMed] [Google Scholar]
- 17.Dyer O. Jamaica advises women to avoid pregnancy as Zika virus approaches. BMJ. 2016;352(January):i383. doi: 10.1136/bmj.i383. [DOI] [PubMed] [Google Scholar]
- 18.Diagne C.T., Diallo D., Faye O., Ba Y., Faye O., Gaye A. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect Dis. 2015;15(November):492. doi: 10.1186/s12879-015-1231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morens D.M., Fauci A.S. Emerging infectious diseases: threats to human health and global stability. PLoS Pathog. 2013;9(7):e1003467. doi: 10.1371/journal.ppat.1003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fauci A.S., Morens D.M. The perpetual challenge of infectious diseases. N Engl J Med. 2012;366:454–461. doi: 10.1056/nejmra1108296. [DOI] [PubMed] [Google Scholar]
- 21.Tambo E., Chidiebere E.U., Olowasogo A.O., Isatta W., Jeannetta K.J. Values and hopes of Ebola vaccines mass immunization programs and treatments adoption and implementation benefits in Africa. Int J Vaccines Vaccine. 2015;1(2):00011. doi: 10.15406/ijvv.2015.01.00011. [DOI] [Google Scholar]
- 22.Patiño-Barbosa A.M., Medina I., Gil-Restrepo A.F., Rodriguez-Morales A.J. Zika: another sexually transmitted infection? Sex Transm Infect. 2015;91(August (5)):359. doi: 10.1136/sextrans-2015-052189. [DOI] [PubMed] [Google Scholar]
- 23.Althouse B.M., Hanley K.A., Diallo M., Sall A.A., Ba Y., Faye O. Impact of climate and mosquito vector abundance on sylvatic arbovirus circulation dynamics in Senegal. Am J Trop Med Hyg. 2015;92(January (1)):88–97. doi: 10.4269/ajtmh.13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinohara K., Kutsuna S., Takasaki T., Moi M.L., Ikeda M., Kotaki A. Zika fever imported from Thailand to Japan, and diagnosed by PCR in the urines. J Travel Med. 2016;23(January (1)) doi: 10.1093/jtm/tav011. pii: tav011. [DOI] [PubMed] [Google Scholar]
- 25.Tetro J.A. Zika and microcephaly: causation, correlation, or coincidence? Microbes Infect. 2016;(January) doi: 10.1016/j.micinf.2015.12.010. pii: S1286-4579(16)00008-3. [DOI] [PubMed] [Google Scholar]
- 26.Tambo E., Oljira T., Oluwasogo O.A., Khater E.I.M., Xiao-Nong Z. Averting MERS-Cov emerging threat and epidemics: the importance of community alertness and preparedness policies and programs. J Prev Infect Control. 2015;1(1):2. [Google Scholar]
- 27.Roth A., Mercier A., Lepers C., Hoy D., Duituturaga S., Benyon E. Concurrent outbreaks of dengue, chikungunya and Zika virus infections – an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Eurosurveillance. 2014;19(41) doi: 10.2807/1560-7917.es2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 28.Faye O., Freire C.C., Iamarino A., Faye O., de Oliveira J.V., Diallo M. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis. 2014;8(1) doi: 10.1371/journal.pntd.0002636. e2636.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duffy M.R., Chen T.H., Hancock W.T., Powers A.M., Kool J.L., Lanciotti R.S. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(June (24)) doi: 10.1056/NEJMoa0805715. 2536-43.4. [DOI] [PubMed] [Google Scholar]
- 30.Wong P.S., Li M.Z., Chong C.S., Ng L.C., Tan C.H. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis. 2013;7(August (8)):e2348. doi: 10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tappe D., Pérez-Girón J.V., Zammarchi L., Rissland J., Ferreira D.F., Jaenisch T. Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol. 2015;(December) doi: 10.1007/s00430-015-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grard G., Caron M., Mombo I.M., Nkoghe D., Mboui Ondo S., Jiolle D. Zika virus in Gabon (Central Africa) – 2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8(February (2)):e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginier M., Neumayr A., Günther S., Schmidt-Chanasit J., Blum J. Zika without symptoms in returning travellers: what are the implications? Travel Med Infect Dis. 2016;14(January–February (1)):16–20. doi: 10.1016/j.tmaid.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Goorhuis A., von Eije K.J., Douma R.A., Rijnberg N., van Vugt M., Stijnis C. Zika virus and the risk of imported infection in returned travelers: implications for clinical care. Travel Med Infect Dis. 2016;14(January–February (1)):13–15. doi: 10.1016/j.tmaid.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Oster A.M., Brooks J.T., Stryker J.E., Kachur R.E., Mead P., Pesik N.T. Interim guidelines for prevention of sexual transmission of Zika virus – United States, 2016. Morb Mortal Wkly Rep. 2016;65(February (5)):120–121. doi: 10.15585/mmwr.mm6505e1. [DOI] [PubMed] [Google Scholar]