Abstract
Background
The World Health Organization Regional Office for Eastern Mediterranean has partnered with the United States Centers for Disease Control and Prevention (CDC) to strengthen pandemic influenza preparedness and response in the Region since 2006. This partnership focuses on pandemic preparedness planning, establishing and enhancing influenza surveillance systems, improving laboratory capacity for detection of influenza viruses, estimating the influenza disease burden, and providing evidence to support policies for the introduction and increased use of seasonal influenza vaccines.
Methods
Various published and unpublished data from public and WHO sources, programme indicators of the CDC cooperative agreement and Pandemic Influenza Preparedness Framework were reviewed and analysed. Analyses and review of the programme indicators and published articles enabled us to generate information that was unavailable from only WHO sources.
Results
Most (19/22) countries of the Region have established influenza surveillance system; 16 countries in the Region have designated National Influenza Centres. The Region has seen considerable improvement in geographic coverage of influenza surveillance and influenza detection. Virus sharing has improved and almost all of the participating laboratories have achieved a 100% efficiency score in the WHO external quality assessment programme. At least seven countries have estimated their influenza disease burden using surveillance data and at least 17 are now using seasonal influenza vaccines as a control strategy for influenza illness.
Conclusion
The Region has achieved substantial progress in surveillance and response to seasonal influenza, despite the adverse effects to the health systems of many countries due to acute and protracted emergencies and other significant challenges.
Keywords: Influenza, Eastern Mediterranean Region, SARI
Introduction
Owing to its ability to change due to antigenic drift of viruses and the associated unpredictable nature of influenza pandemics, influenza remains a major public health concern. Recent estimates by the World Health Organization (WHO) indicate that between 291,000 and 646,000 respiratory deaths from seasonal influenza epidemics occur every year [1]. In the WHO Eastern Mediterranean Region, data are still lacking on regional estimates of the influenza disease burden, especially in groups at high risk of complications due to influenza.
The WHO Eastern Mediterranean Region comprises 21 member states and the occupied Palestinian territory (West Bank and Gaza Strip) and stretches from Morocco in the west to Pakistan in the east. It has a population of nearly 583 million people or approximately 8% of the 2017 world population [2]. The Region is frequently affected by conflicts, humanitarian crises and other protracted emergencies. This has led to the displacement of large numbers of people, including skilled healthcare workers, who have had to flee their countries because of war. As a result, health systems are functioning below optimal levels, with an inadequate health workforce and limited disease control activities pushing back the health gains that were achieved in countries across the Region prior to outbreaks of conflict.
While these protracted emergencies have pushed back health gains substantially, influenza surveillance and response in almost all countries in the Region has shown considerable progress. Egypt was the first country in the Region to establish a sentinel-based surveillance system for influenza in 2006 [3], immediately after the country reported its first human case of infection with highly pathogenic avian influenza virus A(H5N1). The scaling up of the influenza surveillance system across the Region was spurred by this event in Egypt due to the pandemic threat associated with this new highly pathogenic zoonotic influenza.
In 2006, the WHO Regional Office for the Eastern Mediterranean entered a 5-year cooperative agreement with the United States Centers for Disease Control and Prevention (CDC) to strengthen the capacity of countries of the WHO Eastern Mediterranean Region for surveillance and laboratory detection of influenza viruses. This funding supported the establishment and enhancement of influenza surveillance and laboratory detection across the Region, and technical support, assistance and guidance to member states were provided by the WHO Regional Office. This programme was extended for another five-year period in 2011, with a primary focus on strengthening capacity in three areas-(1) to enhance epidemiological and virological surveillance of influenza, (2) to estimate the disease burden associated with influenza, and (3) to support policies to introduce and increase the use of seasonal influenza vaccines. The programme’s objectives stemmed from the rationale that those countries with strong influenza surveillance and vaccine programmes would be better prepared to respond to an influenza pandemic. Building on the success of the first five-year period of improving influenza surveillance in WHO Eastern Mediterranean Region during 2006–2011, the second five-year period between 2011 and 2016 saw further improvements in all three areas of enhancing influenza surveillance, estimating disease burden and use of influenza surveillance data to develop informed policies and strategies for control of influenza. The funding support to seven priorities countries via the Pandemic Influenza Preparedness Framework (PIP) in 2014 greatly supplemented the ongoing works of the Regional Office and spurred the momentum that already generated through the support of US CDC to strengthen influenza surveillance programme in the Region.
This paper highlights the capacity achieved in the countries of WHO Eastern Mediterranean Region in the areas of influenza surveillance system between 2011 to 2018 in each of these three areas- influenza surveillance and laboratory detection; influenza disease burden estimation; and use of seasonal influenza vaccines for influenza control. Owing to availability of comparable, consistent and representative data, the progress and improvement of influenza surveillance system, in the Region, was measured between the situations prevailing in 2011 and 2018.
Materials and methods
We collected data from WHO global and regional databases such as the EMFLU, FluID and FluNet. Information on influenza disease burden was collected from the articles published in peer-reviewed medical journals from the Region. We used the following four outcome indicators to measure improvements in three focus areas of influenza surveillance and esponse: (1) evidence that a country collects and reports epidemiological surveillance data; (2) evidence that a country is testing and reporting influenza virus types and subtypes with EMFLU and FluNET; (3) evidence that a country has published influenza disease burden estimation using surveillance data and/or other data or published information; and (4) progress in introducing seasonal influenza vaccines as part of influenza control programme. We further reviewed the progress in each of these four outcome indicators through analysing the influenza surveillance programme performance indicators that were used in the cooperative agreement of CDC and WHO Eastern Mediterranean Regional Office for measuring progress over time in improving influenza surveillance system. Information collected from WHO global and regional databases (EMFLU, FluID and FluNet) were further triangulated through direct oral or written communication with Ministry of Health officials whenever required. The improvement achieved against each of these four outcome indicators by the end of 2018 and further reviewed by available evidence, were compared with the situation prevailing in 2011 (when the first five-year period of CDC Cooperative agreement on influenza surveillance programme ended and the second five-year period began) in order to assess improvement and measure progress.
WHO data sources
To measure progress in enhancing epidemiological and virological surveillance for influenza, in the Region, we accessed sentinel surveillance data (both epidemiological and virological data) for 19 out of 22 countries in the Region that reported data to global and regional influenza surveillance databases (e.g., FluNet, FluID, EMFLU). Using these data, we calculated the increase in the total number of sentinel sites across the region between 2011 and 2018. We also calculated the change in the number of severe acute respiratory infections (SARI) detected and reported to FluID and EMFLU in each year by the countries between 2011 and 2018. The increase in number, wherever reported and evidenced, was used as an indication of improvement in influenza surveillance system in the Region as a whole. We also accessed regional data from FluNet for 16 national influenza centres (NICs) for analysis and calculated the change in the total number of specimens processed and tested annually between 2011 and 2018. Lastly, in order to measure the capacity in the Region to detect, test and ship influenza specimens, we calculated the change in the number of specimens shared with the WHO Collaborating Centres for the bi-annual vaccine strain selection.
We also analysed the change in the number of NICs in the Region that participated in a voluntary WHO external quality assessment project (EQAP) to test the quality of reverse transcription polymerase chain reaction (RT-PCR) diagnostics for influenza. We also compared the previous EQAP score of the NICs with the recent ones. We interpreted that any participating NIC that scored 90% on all efficiency panels indicated progress made in the quality of influenza testing. All data were analysed by using Epi Info version 7.1.2 (CDC, Atlanta, GA, USA). We used non-parametric tests (Kruskal–Wallis and Mann–Whitney) to compare data from 2011 to 2018 and determine the statistical significance at p < 0.05. Statistics were expressed as numbers and percentages for categorical variables for descriptive analyses and as mean, standard deviation (SD), median and 25th percentile (Q1), 75th percentile (Q3) and inter-quartile ranges (IQR) for numerical variables.
Programme indicators
We used thirteen of the sixteen influenza surveillance programme performance indicators defined in the cooperative agreement to quantify achievements and overall improvement in influenza surveillance system in the Region (Box-1). We analysed the progress in each of these indicators, whenever needed, by cross-referencing the information with both WHO data sources and published and unpublished articles. The difference between the situation of 2011 and 2018 in each these programme performance indicators was identified as improvement if the difference was significant.
Box-1: Programme indicators used in CDC Cooperative Agreement to measure progress in influenza surveillance system in the WHO Eastern Mediterranean Region:
-
aImprovement of surveillance and response for seasonal and pandemic influenza in the Eastern Mediterranean Region
-
•Number of countries with functioning surveillance system for influenza within its routine disease surveillance system;
-
•Number of countries using EMFLU platform (or FluID) for timely reporting of influenza surveillance data;
-
•Number of coordination and scientific meetings of the regional network of influenza surveillance and laboratories organized;
-
•Number of functioning National Influenza Centers;
-
•Number of designated NICs with capacities to detect and isolates seasonal influenza virus;
-
•Number of NICs regularly sharing seasonal influenza virus specimens or viral isolates to WHO CC
-
•Number of NICs reporting virological surveillance data timely to EMFLU (or FluNet);
-
•Number of NICs in the region able to characterize the influenza virus using sequencing and viral neutralization assay;
-
•Number of functioning NICs in the region regularly participating in the WHO External Quality Assurance Program (EQAP) for the detection of influenza virus by PCR;
-
•
-
bEstimation of disease burden for influenza in the Eastern Mediterranean Region
-
•Number of countries completed and published influenza disease burden estimates in peer reviewed journals;
-
•Number of regional and national training workshops organized on estimating burden of disease;
-
•Number of surveillance personnel trained on disease burden estimation;
-
•
-
cEnhancement of rapid response capacity for timely investigation and effective response to outbreaks
-
•Number of countries in the region have trained multi-disciplinary rapid response team at the national level with the ability to facilitate in-country investigation within 48 h of notification
-
•Number of simulation exercises conducted during the project period.
-
•
-
dDevelopment of supportive policies for introduction and increased use of seasonal influenza vaccines
-
•Availability of a comprehensive regional assessment report for seasonal influenza vaccination policies, and availability, accessibility and uptake of seasonal influenza vaccines;
-
•Number of the countries in the region introduced seasonal influenza vaccine targeting high-risk groups and increased the update and coverage of the vaccine.
-
•
Other data sources
We reviewed published and unpublished literature that reported estimates of the influenza burden conducted by countries using influenza surveillance data. We used a systemic review of published articles on influenza vaccination policies and coverage in the Eastern Mediterranean Region from 2006 to 2018 to assess progress made in the use of the seasonal influenza vaccines in the region.
Results
Progress in capacity strengthening
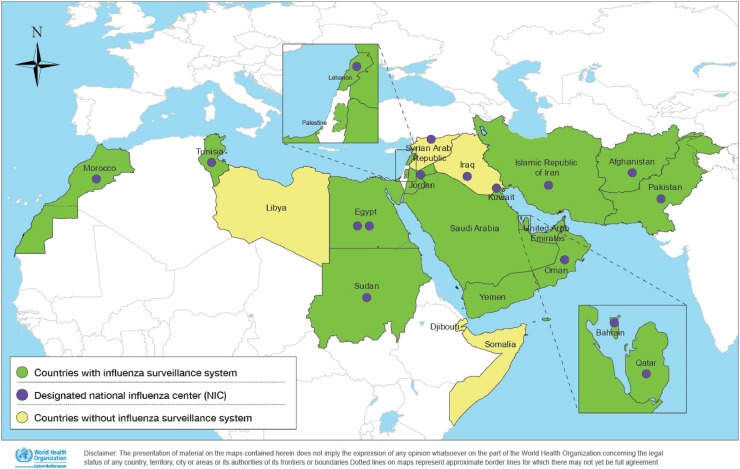
The progress in capacity strengthening was assessed and measured against in each of the four output indicators as well as against the programme performance indicators (Table 1 ). Of the 22 countries in the region, 19 (86%) have established a surveillance system for influenza (Fig. 1 ) in 2018 compared with only 10 in 2011. Fifteen of the twenty-two countries have 16 designated national influenza centers (NICs) compared to 13 in 2011. These influenza laboratories have demonstrated and maintained different levels of capacity for influenza virus detection and characterization. Seven of these sixteen NICs have the capacity to characterize influenza viruses via sequencing and undertake virus neutralization assays. All WHO-designated NICs participate in the EQAP (Table 1).
Table 1.
Progress in capacity strengthening for influenza surveillance in the 22 countries of the WHO Eastern Mediterranean Region between 2011 and 2018.
| Indicator | 2011 | 2018 | Difference | P value |
|---|---|---|---|---|
|
||||
| No. of countries with a functioning influenza surveillance systema | 10/22(50%) | 19/22 (86.3%) | 36.3% | 0.0008 |
| No. of countries with more than 1 sentinel site for ILI/SARI | 8 | 19 | 57.9% | 0.0001 |
| No of countries reporting influenza epidemiological data to FluID for <90% of the weeks a year | 5/10 (50%) | 9/19 (47.38%) | 2.62% | 0.89 |
|
||||
| No. of NICs | 13 | 16 | 15.79% | 0.25 |
| No. of countries reporting influenza virological surveillance data to FluNET for 90% of the weeks a year | 9 | 16 | 36.84% | 0.01 |
| No. of NICs and influenza laboratories with RT-PCR capacity | 9/13 (69.2%) | 19/19 (100%)b | 30.8% | 0.01 |
| No. of NICs and influenza laboratories with capacity for influenza virus isolation | 9/13 (69.2%) | 12/16 (75%) | 5.8% | 0.73 |
| No. of NICs and other influenza laboratories with sequencing capacity (including testing for antiviral susceptibility) | 3/13 (23%) | 7/16 (43.7) | 20.7% | 0.25 |
| No. of NICs and other influenza laboratories participating in WHO EQAP | 9/13 (69.2%) | 19/19 (100%)b | 30.8% | 0.01 |
| No. of NICs and other influenza laboratories achieving a 90% efficiency score on WHO EQAP | 11/14 (78.5%)c | 19/19 (100%)b | 21.5% | 0.03 |
|
||||
| No. of countries with influenza surveillance system estimating influenza disease burden using surveillance datad | 0/10 (0%) | 6/19 (31.57%) | 31.57 | 0.04 |
|
||||
| No. of countries using seasonal influenza vaccines | 12/22 (55%) | 17/22 (77%) | 22% | 0.11 |
SARI: severe acute respiratory illness, NIC: national influenza centre, RT-PCR: real-time polymerase chain reaction,EQAP: external quality assessment programme).
measured against all 21 countries and occupied Palestinian territory of the WHO Eastern Mediterranean Region.
includes three influenza laboratories which are not designated as NIC as of 2018.
includes one influenza laboratory which was not designated as NIC in 2011.
out of the countries with influenza surveillance system in 2011 (10) and 2018 (19).
Fig. 1.
Countries with epidemiological and virological surveillance capacity for influenza in the Eastern Mediterranean Region and designated national influenza centers.
Enhanced capacity for influenza surveillance
Increase in influenza sentinel surveillance sites
The number of sentinel sites for influenza surveillance, increased substantially during the period from 2011 to 2018. In 2011, there were 36 sentinel sites for influenza surveillance in 10 countries of the Region compared to 132 sentinel sites in 19 countries at the end of 2018. The mean number of sentinel sites per country increased from 1.8 among the 10 countries in 2011 to 6.9 among 19 countries by the end of 2018 (P = <0.01). Since the initiation of activities under the PIP framework in 2014, 26 additional sentinel surveillance sites were added across the Region.
Improvement in weekly reporting of epidemiological surveillance data
The total number of SARI patients enrolled at the influenza sentinel surveillance sites for influenza surveillance increased from 2011 to 2018. The number of patients enrolled in all SARI sentinel sites during 2011 was 31,179 (median 357, IQR 260–786) which increased to 111,228 (median 2039, IQR 1140–3001) in 2018. The increase might have possibly been spurred by the start of the Pandemic Influenza Preparedness (PIP) activities in 2014 (Table 2 ).
Table 2.
Comparison of selected indicators of influenza surveillance system, WHO Eastern Mediterranean Region, between 2011 and 2018.
| 2011 |
2018 |
P value | |||
|---|---|---|---|---|---|
| Number | Median value (IQR) | Number | Median value (IQR) | ||
| Number of SARI patients enrolled at influenza sentinel sites | 31,179 | 357 (260–786) | 111,228 | 2039 (1140–3001) | <0.001 |
| Number of influenza specimens tested and reported to FluNet/EMFLU | 30,670 | 351 (253–776 | 109,607 | 2016 (1115–2956) | <0.001 |
| The proportion of influenza positive cases detected | 20.64% | 10.60% (5.8–19.8) | 20.07% | 16.37% (8.3–22) | <0.001 |
| The number of seasonal influenza virus samples sent to WHO CCs for vaccine strain selection | 142 | 11 (4–16) | 1473 | 89 (82–174) | <0.001 |
Improvement in virological surveillance, influenza detection and reporting
The capacity of NICs to detect influenza virus and characterize influenza virus subtypes has markedly increased from 2011 to 2018 and information is currently available on the circulating virus type and subtype of seasonal influenza virus (Figs. 2 and Table 3 ).
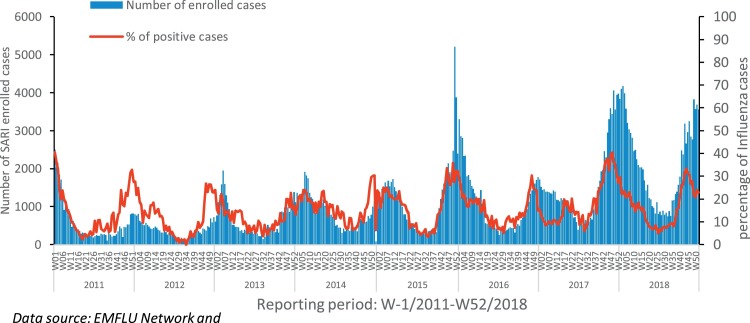
Fig. 2.
Number of seasonal influenza cases detected and percentage of influenza positive cases in the Eastern Mediterranean Region, week no 1/2011-52/2018.
Table 3.
Capacities of countries to conduct influenza virological surveillance in the WHO Eastern Mediterranean Region, 2018.
| Country | Designated NICs | SARI Surveillance system | NICs and Influenza laboratories sharing influenza virus with WHOCC | NICs and Influenza laboratories participating in EQAP | NICs and Influenza laboratories sharing data with Flunet/EMFLU | NICs and Influenza laboratories with RT PCR capacities | NICs and Influenza laboratories performing influenza virus isolation | NICs and Influenza laboratories with sequencing capacities | NICs conducting antiviral susceptibility test |
|---|---|---|---|---|---|---|---|---|---|
| Afghanistan | Y | Y | Y | Y | Y | Y | Y | N | N |
| Bahrain | Y | Y | Y | Y | Y | Y | Y | N | N |
| Djibouti | N | N | N | N | N | N | N | N | |
| Egypt | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Iran | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Iraq | Y | N | Y | Y | Y | Y | N | N | |
| Jordan | Y | Y | Y | Y | Y | Y | N | N | N |
| Kuwait | Y | Y | In progress | Y | In progress | Y | Y | N | N |
| Lebanon | Y | Y | Y | N | Y | Y | N | N | |
| Libya | N | N | N | N | N | N | N | N | N |
| Morocco | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Palestine | Y | Y | Y | Y | Y | N | |||
| Oman | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Pakistan | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Qatar | Y | Y | Y | Y | Y | Y | N | N | N |
| Saudi Arabia | In progress | Y | Y | N | Y | Y | N | N | N |
| Somalia | N | N | N | N | N | N | N | N | N |
| Sudan | Y | Y | Y | Y | N | N | N | ||
| Syrian Arab Republic | Y | N | N | Y | N | Y | N | N | N |
| Tunisia | Y | Y | Y | Y | Y | Y | N | Y | N |
| United Arab Emirates | In progress | Y | Y | N | Y | Y | Y | N | N |
| Yemen | N | Y | N | N | N | N | N | N | N |
Y = Yes; N = No.
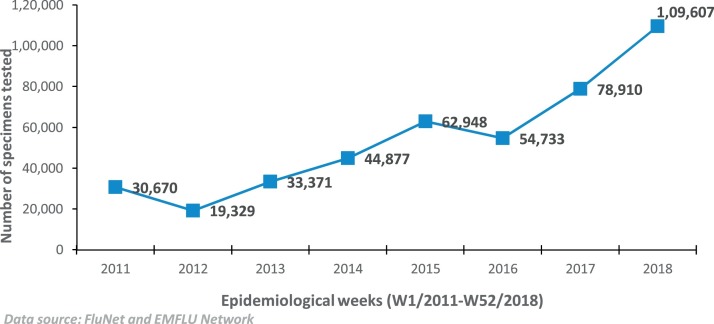
The total number of specimens tested annually and reported to FluNET/EMFLU increased substantially from 30,670 (median 351, IQR 253–776) in 2011 to 109,607 specimens (median 2016, IQR 1115–2956) in 2018 (Fig.3 and Table 2). There has been some variation regarding number of specimens tested and reported by the NICs from one year to another owing to seasonal effect or possibly due to other technical or operational issues, however, the increase in number of influenza specimens tested and reported in 2018 compared to 2011 has been statistically significant (p < 0.001). Currently, 16 of the 22 countries are reporting virological data to FluNet or EMFLU on a weekly basis. The number of countries reporting data to FluNet for all the 52 weeks also increased considerably between 2011 to 2018. The average number of countries reporting to FluNet every week during 2011 was 9.3 (SD ± 1.10) while in 2018, this number increased to 14.2 (SD ± 0.79) with the increase in number being statistically significant (P < 0.0001).
Fig. 3.
Number of influenza specimens tested and reported to FluNet/EMFLU from national influenza centres and influenza laboratories in the Eastern Mediterranean Region, 2011–2018.
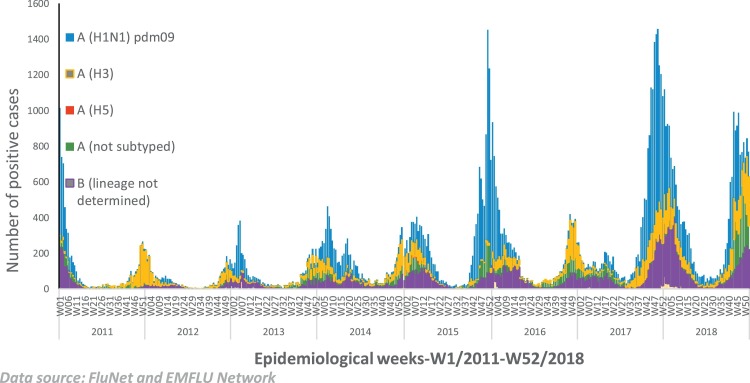
During the same period of 2011 to 2018, an increasing number of influenza positive cases were detected reflecting enhanced ability of the national influenza centers to test and detect influenza positive cases. The proportion of influenza positive cases increased slightly from 20.64% in 2011 (median 10.6%, IQR 5.8 to 19.8) to 20.742% in 2018 (median 16.37%, IQR 8.3 to 22), although the increase in proportion of influenza positive cases from 2011 to 2018 was statistically significant (P < 0.001). Overall, the mean influenza positivity rate of WHO Eastern Mediterranean Region during the period of 2011–2018 was 19% (median 19.3; IQR 16.4–21.5) which was higher than the WHO Africa Region during the same period with mean influenza positivity rate of 15.1% (median 13.9%; IQR 13.9–15.8), Region of America with mean value of 14.5% (median 14.9%, IQR 13.3–15.7), Region of Western Pacific with mean value of 17.6% (median 16.3%, IQR 14.6–20.2). During the same period, the mean influenza positivity rate for WHO European Region was 20.2% (median 20.4%, IQR 16.5–24.5) and the mean influenza positivity rate for WHO South East Asia Region during the same period was 20.9% (median 20.1%, IQR 18.6–24.3). Amongst the influenza virus subtypes, influenza A(H1N1)pdm09 continued to be the predominant virus circulating in the WHO Eastern Mediterranean Region since 2011 (Fig. 4 ).
Fig. 4.
Number of influenza-positive specimens tested and reported to FluNet by subtypes by the national influenza centres and influenza laboratories in the Region, 2011–2018.
Improved performance of influenza laboratories
Of the 16 functioning NICs in the Region, molecular diagnostic capacity exists in almost all (Table 3) and RT-PCR is the primary method to detect circulating seasonal influenza virus. Other important capacities such as influenza virus isolation, sequencing and antiviral susceptibility testing exist in 10, 6 and 2 NICs respectively in the Region (Table 3).
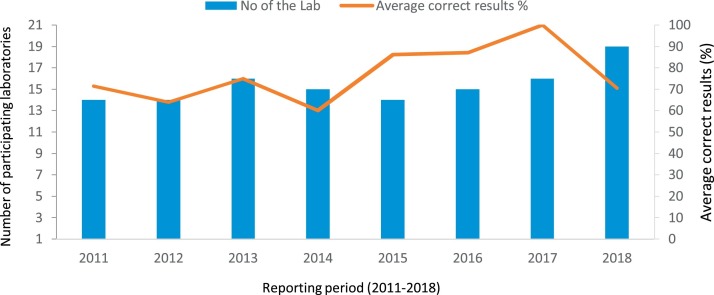
Since 2011, the number of influenza laboratories participating in the WHO EQAP quality assurance test for RT-PCR diagnosis of seasonal influenza has increased. In 2011, 14 laboratories participated in WHO EQAP while in 2018, this number increased to 19. The percentage of countries getting correct results (zero errors) on the EQAP panels increased (Fig. 5 ) from 71.5% in 2011 (with mean correct score of 90%; SD ± 16.64) to 100% (with mean correct score-100%; SD ± 0) in 2017, however this dropped to 70.6% in 2018 although the number of countries participating in the EQAP increased in 2018. The increase in percentage of countries with correct result (with no error) was statistically significant (P < 0.005).
Fig. 5.
Performance of influenza laboratories from the Eastern Mediterranean Region participating in the WHO External Quality Assessment Programme (EQAP) for seasonal influenza, 2011–2018.
Improved participation in WHO influenza vaccine strain selection
The number of NICs and influenza laboratories contributing influenza virus isolates or specimens from the Region to WHO Collaborating Centers for inclusion in the global vaccine strain selection increased from 8 in 2011 to 15 in 2018. A total of 4172 seasonal influenza virus specimens were sent to the WHO Collaborating Centres by 15 NICs and influenza laboratories of the Region during the period from 2011 to 2018. The number of seasonal influenza virus samples sent to WHO CCs for vaccine strain selection increased from 142 specimens in 2011 (median 11, IQR 4–16) to 1473 specimens at the end of 2018 (median 89, IQR 82–174). During the same period, the number of shipments also increased from 11 shipments per year from 8 national influenza centers during 2011 to 33 shipments per year from 15 national influenza centers and influenza laboratories at the end of 2018. Both the increase in number of shipments (P = <0.001) and the increase in number of specimens sent to WHO CCs for vaccine strain selection (P = <0.001) was statistically significant. Most (78%) of the specimens were sent to the WHO collaborating centre in London, 21% to Atlanta and 0.5% to Tokyo. Just over half (52%) of the virus samples were sent from influenza centres in Egypt and Jordan; Bahrain, Iran, Morocco, Oman, Pakistan and Palestine provided 47–100 samples each; and Iraq, Qatar, Syria and Tunisia together contributed 6% of all the specimens received (6–30 samples).
Improved knowledge of the burden of disease associated with influenza
In 2011, when the second phase of the enhanced influenza surveillance programme began, no country in the Region had published any information on influenza disease burden. However, by 2018 at least eight countries in the Region had published data on the influenza disease burden and seasonality in peer-reviewed medical journals [[3], [4], [5], [6], [7], [8], [9], [10], [11]] leading to improved knowledge of the epidemiology of seasonal influenza in the Region (Table 4 ). Although, data on influenza disease burden across countries are not consistent and comparable owing to different study methods and designs used by the countries- from descriptive studies to modelling.
Table 4.
Summary of influenza disease burden estimation studies conducted in the WHO Eastern Mediterranean Region.
| Country | Study title | Indicator | Study method | Study population | Study period | Findings | Seasonality | Status |
|---|---|---|---|---|---|---|---|---|
| Egypt (8) | Incidence of influenza virus-associated severe acute respiratory infection in Damanhour district, Egypt, 2013 | IR | Healthcare use survey to identify the catchment population | 685,641 (Damanhur district) | January-December 2013 |
|
|
Published |
| Iran (9) | Estimation of influenza and SARI incidence (burden) in three provinces of the Iran, 2012 and 2013 | IR | Population census | 3,279,823 (Western Azerbaijan, Alborz and Hamedan) | 2012 to 2013 |
|
|
Published |
| Jordan (6) | Descriptive epidemiology of SARI cases and estimation the proportion of SARI cases attributable to influenza | Proportion of positive influenza cases | Total number of SARI cases admitted | 2891 (admitted patients in the four sentinel sites) | January 2008 to February 2014 |
|
|
Published |
| Lebanon (7) | Seasonal influenza-associated SARI in a southern governmental sentinel site in Lebanon | Proportion of SARI/influenza cases | Total number of all-cause admissions | 468,464 (patients admitted in two sentinel sites) | 1 September 2015 to 31 August 2016 |
|
|
Published |
| Morocco | Estimating influenza disease burden in a sentinel site in Morocco | IR | Health admission survey | 15,985 (Meknes-Tafilalet) | September 2008-August 2012 |
|
The cyclic influenza epidemic generally begins in November and starts to go down in March | In process |
| Oman (4) | Estimating the burden of influenza-associated hospitalization and deaths in Oman (2012-2015) | Proportion of influenza-associated hospitalizations and in-hospital deaths | Discharge records based on ICD-10 codes (J 09- 18) | 4,588,683 (total Oman population) | 2012-2015 |
|
|
Published |
| Tunisia (10) | Estimating the seasonal influenza burden in Tunisia [8] | IR | Population census | 11,151,874 (population covered by ILI sites) | 3 seasons (2012-2013, 2013-2014, 2014-2015) |
|
|
Published |
| Saudi Arabia (11) | Influenza is more common than Middle East Respiratory Syndrome Coronavirus (MERS-CoV) among hospitalized adult Saudi patients | Proportion of influenza positive cases amongst community acquired pneumonia cases | Analysis of surveillance data on community acquired pneumonia in a general hospital over a four-year period | 2657 patients records | 2012-2016 |
|
|
Published |
IR: incidence rate, SARI: severe acute respiratory illness, ILI: influenza-like illness, CI: confidence interval.
Improvement in developing policies for the use of seasonal influenza vaccine
A literature review on the use of seasonal influenza vaccines in the Eastern Mediterranean Region showed that 17 of the 22 countries are now using some form of seasonal influenza vaccines compared with 2011, when only 12 countries were using vaccines against seasonal influenza (Table 1). We found at least 10 studies that discussed policies and recommendations about seasonal influenza vaccination. The studies were from Egypt, Iran, Kuwait, Oman, Saudi Arabia and United Arab Emirates [[12], [13], [14], [15]]. Seven studies were specific to the use of seasonal influenza vaccines amongst the pilgrims for hajj and Umrah and originated from Saudi Arabia and Egypt. Kuwait, Oman and United Arab Emirates. However, it was not clear from these published studies whether or not the influenza disease burden data and influenza seasonality as determined by the disease burden data contributed to policy formulation or recommendation for vaccination or in determining the timing of vaccination.
Other programme improvements
One of the other notable achievements during this period was that all 22 countries established and trained multidisciplinary rapid response teams at the national level. Of the 22 countries, 15 also reported having subnational rapid response teams.
The Eastern Mediterranean Acute Respiratory Illness Surveillance Network (EMARIS) that was established in 2007 [16] and involved only three countries, has expanded to involve all the countries of the Region. The EMARIS network has met every other year since 2012. EMFLU, developed as a regional platform for sharing of epidemiological and virological data on influenza, is now fully functional with 15 of the countries regularly sharing their influenza surveillance data. EMFLU connects with the global influenza surveillance databases housed at WHO Headquarters in Geneva, FluID and FluNet. A weekly epidemiological report on seasonal influenza, avian influenza and other acute respiratory infections is published by the WHO Eastern Mediterranean Regional Office.
Discussion
The importance of having improved capacity for surveillance of and response to influenza and other respiratory diseases in the region cannot be overemphasized. The Eastern Mediterranean Region of WHO has reported the highest number of human infections with highly pathogenic avian influenza A(H5N1) virus to date [17], a virus with pandemic potential. The region has also experienced the emergence of Middle East Respiratory Syndrome (MERS-CoV), another new respiratory pathogen capable of causing a global health emergency. The creation of stronger respiratory disease surveillance systems ensures that the outbreak potential capacity in the region is strong.
Despite the problems in the Region from acute and protracted emergencies which are adversely affecting the performance of the health systems, considerable progress has been made in surveillance of and response to seasonal influenza in the Region as a whole. All indicators examined using WHO data sources have shown improvement and evidence from published information supports this.
Despite protracted conflicts and emergencies throughout the region, countries have prioritized the capacity-strengthening programme for influenza surveillance and testing. They have established and enhanced influenza surveillance through increasing the number of sentinel sites and have improved their testing capacity for influenza and other non-influenza respiratory viruses. More cases of SARI are being detected, surveillance is being conducted all year round, and a systematic sampling and testing strategy for detection of influenza virus is being used. As a result, there is a better understanding of the seasonality of influenza in the Region, the circulating influenza virus types, and the epidemiology of illnesses caused by the influenza virus in any age group that result in hospitalization. The surveillance data currently produced in the Region are more representative and comparable due to standardized, integrated influenza surveillance.
Reporting of SARI cases gradually increased during 2011–2018 and a substantial and sustained increase in reporting of influenza virological data to FluNet. Sustained reporting to global and regional database networks demonstrates the effectiveness of the capacity strengthening programme in the Region.
The improved capacity of the countries for better surveillance and laboratory testing of seasonal influenza has had a snowball effect on their efforts to detect and identify other emerging respiratory pathogens using the same surveillance systems. This flexibility has resulted in detection of other pathogens, including respiratory syncytial virus (RSV) and MERS-CoV and better prepares countries in the Region to respond to both influenza and other non-influenza respiratory health threats that have both epidemic and pandemic potential. Similarly, the rapid response teams, whose establishment was supported by the influenza programme, were also used for timely response to other health events like MERS-CoV and the threat of the introduction of Ebola virus. The recent joint external evaluation (JEE) reports on various countries of the Region suggest that the resources invested to improve the influenza surveillance and response capacity have also contributed to countries’ attaining the core capacities within the International Health Regulations to prevent, detect and respond to other health security threats, especially outbreaks and other emergencies with health consequences. However, more evidence is needed on how best such sentinel surveillance platforms can be used to sustain the improved performance of the health systems for outbreak detection.
A perplexing characteristic of the influenza virus is that it keeps changing through antigenic drift. This means regular testing is needed and the influenza laboratories must have the functional capacity to detect and characterize any subtype of influenza virus including any unsubtypeable virus. The number of influenza laboratories in the Region with the capacity for sequencing and characterization of influenza virus is increasing which is a welcome move. This capacity of the influenza laboratories helps countries make evidence-based decisions for influenza preparedness, prevention and control. The fact that all laboratories participating in the WHO EQAP in 2017 scored above the global standard of 90% efficiency demonstrates that the influenza laboratories in the Region have adequate capacity for detection of any new influenza virus that may emerge in the Region. In addition, an increasing number of countries of the Region are now sharing more influenza virus isolates and specimens for seasonal vaccine strain selection [18]. This improvement in sharing is critical to having a global seasonal influenza vaccine that is representative of the predominant influenza virus strains circulating in the Region.
The countries have also made remarkable progress in using surveillance data to generate evidence on the burden of seasonal influenza in the general population and high-risk groups. Such efforts have yielded estimates of the influenza burden in a number of countries of the Region. This information on disease burden is now an important decision-making factor in developing programmes for influenza prevention and control.
The Region has made significant improvements in influenza surveillance; however, capacity building and strengthening of the influenza surveillance should be continued though the following actions: (1) adopting and adapting recent advances in influenza prevention, detection and response, (2) contributing to operational and scientific research on influenza, not only for the Region but also for the world, and (3) sharing these improvements, best practices and lessons learnt through publications, conferences and meetings.
Conclusion
Despite the challenging circumstances in the WHO Eastern Mediterranean Region, steady progress has been made in the countries in influenza surveillance, laboratory-testing capacity and ability to use the influenza surveillance data to generate evidence and use it for decisions-making. This enhances the readiness of the Region to detect and rapidly respond to any new or pandemic influenza virus strain. At the same time, better quality data are now coming out of the Region on the seasonality and epidemiology of influenza in the Eastern Mediterranean Region. This progress needs to be sustained in the countries, not only for influenza but for the detection of and response to other emerging health threats. Despite the considerable progress in most countries of the Region, challenges remain to further improve the capacity in some of the countries affected by conflict and emergencies and to sustain their capacity for influenza detection and response. Strengthening capacity for influenza surveillance and response contributes to meeting the core capacity requirements of the International Health Regulations, which aim at enhanced capacity of the countries for the prevention and detection of and response to infectious disease threats, especially new influenza viruses with pandemic potential.
Funding
No funding Sources.
Competing interests
None declared.
Ethical approval
Ethical approval not required.
Author contributions
All authors contributed equally in writing this manuscript while A Elkholy analysed the data.
Acknowledgements
We would like to thank all the Ministries of Health of the countries of the Eastern Mediterranean Region for their support in establishing and strengthening capacity for influenza surveillance. We thank the Centers for Disease Control and Prevention (CDC) of USA for funding support through the cooperative agreement. We would like to acknowledge the Pandemic Influenza Preparedness Framework for the complementary funding support to the Region on strengthening influenza surveillance. We would also like to thank all those from the Member States, WHO country offices, Regional Office and Headquarters who have contributed to establishing and strengthening influenza surveillance, and the WHO collaborating centres for their active support.
References
- 1.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO EMRO. About us: http://www.emro.who.int/entity/about-us/index.html.
- 3.Kandeel A., Dawson P., Labib M., Said M., El-Refai S., El-Gohari A. Morbidity, mortality, and seasonality of influenza hospitalizations in Egypt, November 2007–November 2014. PLoS One. 2016;(11) doi: 10.1371/journal.pone.0161301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Awaidy S., Hamid S., Al Obaidani I., Al Baqlani S., Al Busaidi S., Bawikar S. The burden of influenza-associated hospitalizations in Oman, January 2008–June 2013. PLoS One. 2015;(10) doi: 10.1371/journal.pone.0144186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel‐Hady D.M., Al Balushi R.M., Al Abri B.A., Al Abri S.S., Al Kindi H.S., AK Al‐Jardani. Estimating the burden of influenza‐associated hospitalization and deaths in Oman (2012–2015) Influenza Other Respir Viruses. 2018;12(1):146–152. doi: 10.1111/irv.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammad A.A., Patrick D., Jeries H.A., Waleed E.S., Erica D., Tarek A.S. Influenza hospitalization epidemiology from a severe acute respiratory infection surveillance system in Jordan, January 2008–February 2014. Influenza Other Respir Viruses. 2016;(10):91–97. doi: 10.1111/irv.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleh M., Bazzi L., Ismail E., Mroueh L., Jammal N., Elkholy A. Influenza‐associated severe acute respiratory infections in 2 sentinel sites in Lebanon — September 2015 to August 2016. Influenza Other Respir Viruses. 2018;(12):331–335. doi: 10.1111/irv.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Refaey S., Hassan M., Mansour A., Kandeel A. Incidence of influenza virus-associated severe acute respiratory infection in Damanhour district, Egypt, 2013. East Mediterr Health J. 2016;(22):503–512. [PubMed] [Google Scholar]
- 9.Gouya M., Rezaei F., Haghdoost A., Nabavi M., Farahi K.S., Mostafavi E. Estimation of influenza and severe acute respiratory illness incidence (burden) in three provinces of the Islamic Republic of Iran, 2012 and 2013. East Mediterr Health J. 2016;(22):432–439. doi: 10.26719/2016.22.7.432. [DOI] [PubMed] [Google Scholar]
- 10.Chlif S., Aissi W., Bettaieb J., Kharroubi G., Nouira M., Yazidi R. Modelling of seasonal influenza and estimation of the burden in Tunisia. East Mediterr Health J. 2016;22:460–467. [PubMed] [Google Scholar]
- 11.Al-Tawfiq J.A., Rabaan A.A., Hinedi K. Influenza is more common than Middle East Respiratory Syndrome Coronavirus (MERS-CoV) among hospitalized adult Saudi patients. Travel Med Infect Dis. 2017;20:56–60. doi: 10.1016/J.TMAID.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandeel A., Deming M., Abdel Kereem E., El-Refay S., Afifi S., Abukela M. Pandemic (H1N1) 2009 and Hajj pilgrims who received predeparture vaccination, Egypt. Emerg Infect Dis. 2011;(17):1266–1268. doi: 10.3201/eid1707.101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honarvar B., Odoomi N., Mahmoodi M., Kashkoli G.S., Khavandegaran F., Bagheri Lankarani K. Acceptance and rejection of influenza vaccination by pregnant women in southern Iran: physicians’ role and barriers. Hum Vaccin Immunother. 2012;8:1860–1866. doi: 10.4161/hv.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Gharbieh E., Fahmy S., Rasool B.A., Khan S. Influenza vaccination: healthcare workers attitude in three Middle East countries. Int J Med Sci. 2010;7:319–325. doi: 10.7150/ijms.7.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayet A.Y., Al-Shaikh G.K., Al-Mandeel H.M., Alsaleh N.A., Hamad A.F. Knowledge, attitudes, beliefs, and barriers associated with the uptake of influenza vaccine among pregnant women. Saudi Pharm J. 2017;25:76–82. doi: 10.1016/j.jsps.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abubakar A., Barakat A., Ahmed A., El Kholy A., Alsawalh L., Al Ariqi L. Fourth meeting of the Eastern Mediterranean Acute Respiratory Infection Surveillance (EMARIS) network and first scientific conference on acute respiratory infections in the Eastern Mediterranean Region, 11–14 December, 2017, Amman, Jordan. J Infect Public Health. 2019;12(4):534–539. doi: 10.1016/j.jiph.2019.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Refaey S., Azziz-Baumgartner E., Amin M.M., Fahim M., Roguski K., HAEA Elaziz. Increased number of human cases of influenza virus A(H5N1) infection, Egypt, 2014–15. Emerg Infect Dis. 2015;21:2171–2173. doi: 10.3201/eid2112.150885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asghar H., Browne H.M., McCauley J., Malik M., Khan W. Contribution of laboratories in the WHO Eastern Mediterranean Region to the selection of candidate seasonal influenza vaccine, 2010–2015. East Mediterr Health J. 2016;22:445–452. doi: 10.26719/2016.22.7.445. [DOI] [PubMed] [Google Scholar]