Highlights
-
•
Virus-Like Particles (VLPs) present several advantages over conventional vaccines which make them attractive for vaccination.
-
•
Interest in VLP design and production has been increasing in recent years.
-
•
Mammalian cell production approach can produce more immunogenic VLPs due to the post-translation modifications accessed.
-
•
VLP vaccines against several infectious diseases are already on the market, with others in preclinical and clinical trials.
Keywords: Recombinant vaccines, virus-like particles, culture mode, immunogen, production platform
Abbreviations: VLP, virus-like particle; B/IC, baculovirus-insect cell expression system
Abstract
Virus-like particles (VLPs) are nanostructures that resemble the structures of viruses. They are composed of one or more structural proteins that can be arranged in several layers and can also contain a lipid outer envelope. VLPs trigger a high humoral and cellular immune response due to their repetitive structures. A key factor regarding VLP safety is the lack of viral genomic material, which enhances safety during both manufacture and administration. Contemporary VLP production may take advantage of several systems, including bacterial, yeast, insect and mammalian cells. The choice of production platform depends on several factors, including cost and the need for post-translational modifications (PTMs), which can be essential in generating an optimal immune response. Some VLP-based vaccines designed to prevent several infectious diseases are already approved and on the market, with many others at the clinical trial or research stage. Interest in this technology has recently increased due to its advantages over classical vaccines. This paper reviews the state-of-the-art of VLP production systems and the newest generation of VLP-based vaccines now available.
Introduction: viral vaccines
Types of vaccine
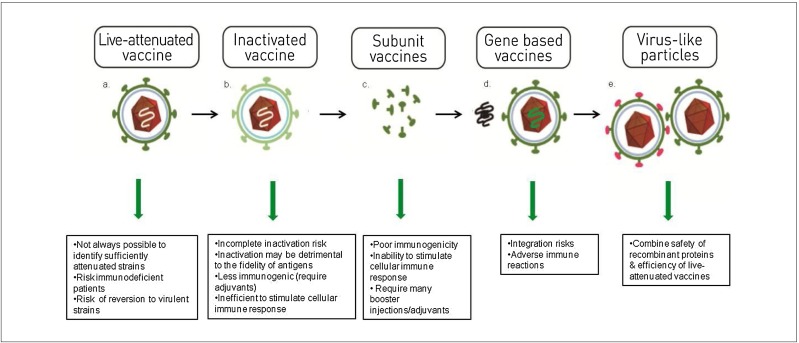
The vast majority of preventative viral vaccines consist of an attenuated or inactivated virus, for administration to the individual to provoke a protective immune response. These types of vaccines are very effective, and generally a second administration or the use of adjuvants is not required. Nevertheless, attenuated vaccines in particular present a risk at the level of manufacture or administration since they can in principle revert to a pathogenic form [1], [2], [3]. Newer generation vaccines improve safety by removing whole viruses from the formulation altogether, instead utilizing protein subunits, DNA, or virus-like particles (VLPs) (Fig. 1 ). Subunit vaccines are composed of recombinant viral proteins or purified proteins from the wild-type virus, which act as antigens [4]. DNA vaccination is a technique that consists of the direct administration to the recipient of plasmid DNA encoding an antigenic protein which is expressed by the recipient’s cells, subsequently generating an immune response [5].
Fig. 1.
Types of vaccines: a. Live-attenuated vaccine; b. Inactivated vaccine; c. Subunit vaccines; d. Gene based vaccines; e. Virus-like particles.
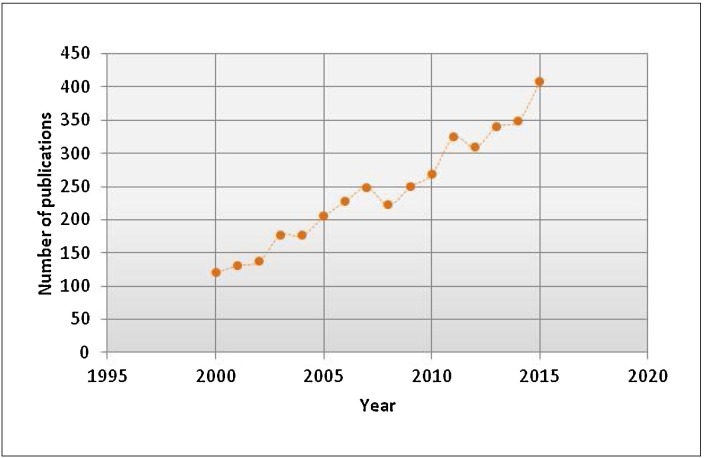
Virus-like particles are artificial nanostructures that resemble a virus. They are composed of all or some of the proteins that form the viral capsid but lack genomic material, precluding any possibility of reversion mutations or pathogenic infection. VLPs are unable to replicate in the recipient but stimulate the immune system through recognition of repetitive subunits, producing a high cellular and humoral immune responses [6]. Due to the advantages compared to other types of vaccines, the interest in VLP technology has increased in recent years (Fig. 2 ).
Fig. 2.
Increase in the interest in Virus-Like Particles. Total number of publications from PubMed about VLPs.
Types of virus-like particles
Virus-like particles are composed of one or several structural proteins that have the ability to self-assemble when recombinantly expressed. The proteins can be arranged in single, double or triple layers [7]. In the case of human papillomavirus (HPV) [8], the VLPs are formed by a single structural protein that forms the basic capsid of the particle. Other more complex VLPs comprise several structural proteins, e.g. VLPs of the Reoviridae family are formed by 2 to 4 different proteins disposed in several layers [7]. VLPs can also have an external lipid envelope. In this case, the structural protein core exits the cell through a budding process, enveloping the capsid within part of the cell membrane. This is the case for HIV-1 VLPs, which are formed by the Gag polyprotein and take part of the host cell membrane as the envelope [9]. Influenza VLPs are also formed by the protein core and the hemagglutinin spikes that are displayed on its surface [10]. Hence, the choice of the producer cell line is very important since enveloped-VLPs will contain the proteins expressed on its membrane.
Recently, a novel type of VLP, termed “chimeric VLPs”, has been developed. Their structure is composed of a viral protein while the envelope proteins are derived from a second virus. Recently, a porcine circovirus type 2 VLP was developed displaying a porcine reproductive and respiratory syndrome virus GP5 epitope B [11]. This opens the possibility of using VLPs as a delivery system. Envelope proteins can act as signals for specific tissue receptors. In this way, VLPs may be targeted to a given tissue, with capsid proteins linked to components for delivery to the targeted tissue. Thus, VLPs have new applications in drug delivery, gene therapy, and cancer treatment [12].
Production methods
The expression system chosen for VLP production must take into consideration the requirements for protein folding and post-translational modifications. Several expression systems are available, and the main advantages and disadvantages of each system are summarised in Table 1 .
Table 1.
Advantages and disadvantages of the different VLP production platforms.
| Production platform | Advantages | Disadvantages |
|---|---|---|
| E. coli | • Ease of expression • Ability to scale-up • Low production cost |
• Does not allow for glycosylation. • Endotoxins |
| Yeast | • Ease of expression • Ability to scale-up • Low production cost |
• Non-appropriate protein glycosylation (i.e. high mannose glycoprotein modification). • Risk of incorrect folding & assembly. |
| Insect cells | • Can produce large amounts of correctly folded VLP in high density cell culture conditions • Ability to scale-up • The risk of culturing opportunistic pathogens is minimised compared to mammalian cell culture • Host-derived insect cell/baculovirus components may act as vaccine adjuvants, help trigger a more effective immune response |
• Limited to high mannose glycoprotein modification. • Baculovirus contaminants may be difficult to remove • Host-derived insect cell/baculovirus components may also mask the immune response against the desired epitope |
| Mammalian cells | • Producer cells more closely related to the natural host • Appropriate PTMs and authentic assembly of VLPs |
• Higher production cost • Lower productivities |
| Plants | • Ease of expression • Ability to scale-up • No human-derived virus contamination |
• Cannot undergo PTMs and VLP assembly • Low expression levels • Stability: antigen degradation |
Bacteria and yeast
Bacteria and yeast represent easily scaled up and cost-effective production systems. Bacteria are a more suitable expression system for VLPs formed with just one or two structural proteins and no envelope. The main advantage is the high yield of the proteins of interest; however, bacteria are unable to perform post-translational modifications, which can be very important for VLP immunogenicity [6]. Production of HPV Type 16 L1 VLPs has been successfully carried out using Lactobacillus casei, where immunofluorescence was used to confirm the presence of conformational epitopes [13]. Conversely, E. coli bacteria were used for the production of recombinant norovirus capsid, which was found to be useful in antigenic and also receptor-binding studies, but not as a vaccine candidate [14].
Due to the ability of yeast to perform post-translational modifications, it represents a step forward in VLP production. Recently, Chikungunya VLPs were produced using P. pastoris and promising results were obtained in terms of murine immunisation [15]. Indeed, several yeast-produced VLPs have already reached approval by regulatory agencies, such as papillomavirus VLP [16]. Nevertheless, their PTM pattern is not exactly the same as in humans.
Baculovirus/insect cell (B/IC) expression system
The B/IC system process is divided into two phases: an infection phase and a production phase. Baculovirus design is a fast and easy procedure, which makes it suitable for the production of vaccines for viruses whose surface protein can vary between each outbreak [17]. The B/IC system can produce protein quantities comparable with those achieved with bacteria or yeast, but its capacity to perform complex PTMs is greater [6]. Two main insect cell lines are used for recombinant protein production using B/IC expression, Sf9 (Spodoptera frugiperda) and High Five cells (Trichoplusia ni). Many VLP types have been produced using the B/IC system, including Chikungunya, HIV or porcine parvovirus-like particles [18], [19], [20]. The main disadvantage is that enveloped baculoviruses are also produced at the same time as VLPs, making purification a difficult and expensive step [10].
There are currently other platforms to produce VLPs that avoid the use of baculovirus, hence simplifying purification. Stable cell lines can be generated in which the protein of interest is continuously expressed. HIV-1 VLPs were produced by stably transfected Drosophila S2 cells [21]. If the protein produced has a cytotoxic effect, it can be regulated by an inducible promoter. Transient transfection can also be carried out in insect cells. Cellfectin has been used for the production of influenza A VLPs consisting of haemagglutinin (HA) and matrix protein (M1) in Sf9 cells [22]. Little research has investigated the use of cheaper transfection reagents, such as polyethyleneimine (PEI), for recombinant protein production in insect cells [23].
Mammalian cells
Several mammalian cell types are suitable for VLP production. Although mammalian cells produce less of the protein of interest compared to other systems, they have the capacity to produce more complex and accurate PTMs [24]. For this reason, mammalian cells are typically used to produce complex enveloped VLPs composed of multiple structural proteins. Several mammalian cell lines are available for recombinant protein production and are adapted to grow in suspension using serum-free chemically defined media [25]. One of the most extensively utilised is the Chinese Hamster Ovary (CHO) cell line. In comparison with other mammalian cell lines, it has the advantage that it is not human-derived and therefore presents a lower risk of contamination by human viruses [26]. CHO cells have already been used for the generation of hantavirus-like particles, which were able to induce a specific immune response in mice [27]. The HEK293 cell line is another widely used mammalian production platform, which has been tested for the production of many different types of VLP, such as rabies, HIV, and influenza [9], [28], [29]. Other human cell lines being evaluated for the production of complex recombinant proteins include CAP-T cells, derived from human amniotic fluid, for HIV-1 VLP production [30].
There are two methods for producing VLPs in mammalian cell cultures. The classical method is the generation of a stable cell line in which the gene encoding the protein of interest is integrated. This process starts with the transfection of a cell culture followed by a single clone selection process in which high producers are selected [28]. Up to six months later, a stable cell line can be obtained. Transient transfection is a much faster process. In this case, VLPs can be harvested approximately 48 to 72 h post transfection [9], generating an appreciable quantity of the product of interest within two weeks. This process is suitable when small quantities of different VLPs are needed, such as in initial research phases. It is also useful when the wild-type antigen of interest undergoes frequent mutations, requiring the vaccine to be frequently modified, or when one of the VLP proteins is toxic for the producer cell line.
Plants
Transgenic plants have also been used for VLP production. Agrobacterium tumefaciens is commonly used for infection and transformation of the cells [31]. These bacteria can infect plant cells and introduce a specific gene of interest into the host genome. Several examples are available of VLP production in plants, such as for HPV type 16 or influenza [32], [33]. The most commonly used plants for recombinant protein production are Nicotiana tabacum and Arabidopsis thaliana [34]; others include potato or tomato [35], [36].
Production yields
Comparison of production yields among different systems is not always straightforward, since production is dependent not only on the system but also on the complexity of the VLP. Nevertheless, a wide range of yields can be estimated. As previously discussed, bacteria and yeast are high-concentration production systems, and yields can vary from 0.75 to 700 μg of protein per ml of culture [37], [38]. Animal-based systems achieve lower production yields: between 0.2 and 18 μg/ml in the case of B/IC system [39], [40] and between 0.018 and 10 μg/ml for mammalian cell technology [41], [42]. Animal cells tend to be lower VLP-producers, but for complex enveloped VLPs, they have become the platform of choice. Transgenic plants are the most difficult to compare with the other systems, since their production is generally calculated per mg of vegetal tissue, with yields ranging from 4 to 2380 pg/mg of leaf [43], [44].
Culture modes
For VLP production, there are three different culture modes: batch, fed-batch, and continuous cultivation. In the batch mode, all the elements needed are added at the beginning of the culture. This has the advantage that the medium is well utilised, and the product is highly concentrated. It is the method most commonly used for VLP production, and has been used for many types of VLP, including HIV [30], Chikungunya virus [18], and Ebola virus [45]. To extend the exponential growth phase, the fed-batch mode can be implemented. Here, small quantities of nutrients or medium are added during the culture to supply the cells with specific components depleted. Fed-batch strategies can be used to reach high cell concentrations. Fed-batch was tested for the production of parvovirus-like particles in the B/IC system [20].
Finally, in continuous cultivation mode, fresh medium is added while the conditioned medium is extracted. With this method, continuous production is obtained and the product must be stored under proper conditions while it is being produced. Product concentrations remain the same as in the batch mode, but higher total quantities are obtained. However, continuous production requires large amounts of medium, which presents difficulties in adapting this method to large-scale production. Recently, a novel production strategy termed Extended Gene Expression (EGE), using the HEK293 mammalian cell line and transient transfection, was used for HIV1 VLP production. In this case, HEK293 cell medium was renewed every 48 h and two retransfection rounds were carried out at Erlenmeyer flask scale [46]. Continuous cultivation has also been used to produce rabies VLPs in HEK293. The HEK293 cell line was made to stably expresses the VLPs in a 5L bioreactor scale [28].
Fed-batch and continuous cultivation modes can also be used when genes encoding VLP proteins are stably expressed in cell line platforms regulated by inducible promoters. After attaining high density cell growth, gene expression is induced obtaining higher protein concentrations.
Classically, bioreactors for recombinant protein production are stainless steel vessels. Nevertheless, single-use technology is gaining importance in the manufacture of biopharmaceuticals. Most useful for small to medium scale production, it has certain advantages for VLP production. It does not require an in situ cleaning and sterilisation process, precluding cross-contamination. It has been reported that operation with single-use bioreactors reduces significantly both the investment as well as the operating costs. However, single-use vessels are less useful for large volume production, with the maximum production volume (200 L) limited by the process dependence on bags. The bags may also release leachables and extractables to the cell culture, and obtaining them can be a limiting factor in production. The probes needed for monitoring the culture are also not well adapted to the single-use technology [47].
VLP-based vaccines
Hepatitis B virus
Hepatitis B virus (HBV), a small DNA virus from the family of Hepadnaviridae approximately 42 nm in size, is composed of a lipid envelope and a capsid that contains the viral circular DNA genome (3-3.3 kb) and a DNA polymerase. The core protein of the virus is HBcAg and is coded by the C gene. The enveloped proteins (HBsAg) are coded by the gene S in the viral genome [48].The first HBV vaccines were derived from inactivated HBsAg particles from sera of HBV-positive patients [49]. The development of recombinant DNA technology and safety concerns regarding human plasma-derived vaccines spurred interest in the development of new-generation vaccines. HBsAg was initially produced in E. coli [50], but was not secreted and the protein was misfolded. Eukaryotic cell lines were then sought to produce the recombinant protein. Yeast and mammalian cells are the two systems used for the production of the HBV vaccine. Yeast lines stably express the enveloped protein HBsAg, which can self-assemble and is secreted by the yeast cell, resulting in 20 nm size particles similar to those produced by infected human cells. The mammalian CHO cell line has also been used for the production of HBsAg VLPs. These tend to be larger than the ones produced in yeast and are composed of a mixture of glycosylated and non-glycosylated HBsAg, in contrast with yeast VLPs which are composed of only non-glycosylated HBsAg. This difference in size and composition leads to the higher immunogenicity of the particles [51]. This vaccine is also produced by various transgenic plants [35]. Hepatitis E VLP vaccine has also been approved in China under the name of Hecolin. Capsid protein from Hepatitis E is expressed in E. coli and purified. The HEK 293 protein self-assembles into homodimers resulting in the formation of VLPs. A VLP-based Hepatitis C vaccine is also currently in the research stage [52].
Malaria
RTS,S/AS01 (Mosquirix) is the first vaccine generated against a parasitic disease [53]. It is a VLP-based vaccine composed of the surface of hepatitis B virus (S) and parts of the secreted circumsporozoite protein (CSP) from the malaria parasite. These are the central tandem repeat (R) and epitopes from the CSP carboxy-terminal (T). The three parts (RTS) are engineered into the hepatitis B surface antigen (HBsAg). Upon expression in yeast cells, the fusion protein forms VLPs, which present the antigens to the immune system, thus provoking a response [54].
Human papillomaviruses
VLP vaccines against HPV are based on the structural capsid protein L1 [55]. There are two available vaccines for HPV prevention: Gardasil and Cervarix. They protect against HPV types 16 and 18, which are both cancer-associated serotypes. Gardasil VLP is produced by S. cerevisiae, and protects against HPV types 6 and 11. The recombinant protein L1 has the ability to self-assemble inside the yeast and form the VLPs. To purify them, a cell disruption process is carried out followed by a series of chemical and physical purification steps. Cervarix is produced by Trichoplusia ni cells infected with a recombinant baculovirus containing the L1 gene. A cell disruption process is followed by several purification steps to obtain the protein. After the purification process, L1 is assembled into VLPs [56]. There are also other HPV VLPs for protection from other HPV types produced in yeast and even in bacteria at a research stage [57].
Influenzavirus A
Influenzavirus A is between 80 and 120 nanometers in diameter. The envelope is composed of two proteins: hemagglutinin (HA) and neuraminidase (NA), which are the commonly targeted protein in antiviral treatments. The B/IC platform has been used for the production of influenza VLPs. Sf9 cells were infected using three different baculoviruses. Each baculovirus encodes for one gene: HA, NA and matrix protein gene (M1). The co-expression of these three proteins leads to the formation of VLPs that can be harvested from the culture supernatant. These VLPs provide a broader immune response than the inactivated virus or the recombinantly produced hemagglutinin [58]. Transgenic plant technology has also been used for the production of influenza VLPs, which have provided good results in the preclinical phases [33], [59].
Human immunodeficiency virus (HIV)
A complete HIV particle measures approximately 80–120 nm. The genome contains 3 major genes: gag, pol and env. Gag encodes the three domains of the capsid of the virion. Specifically, the matrix (MA) subdomain binds to the inner phase of the lipid envelope that surrounds the particle. The capsid (CA) domain forms a conical and more condensed core that contains the viral RNA bound to the nucleocapsid (NC) domain and the viral enzymes. The envelope proteins are encoded by the env gene and are gp120 and gp41, which are placed in the lipid bilayer and displayed on the viral surface. Finally, pol encodes the enzymatic proteins involved in the viral cycle [60]. HIV VLPs have been produced using different expression platforms. Expressed Gag polyprotein has the ability to migrate to the cell membrane, self-assemble, and bud from the cell. HIV VLPs produced by S. cerevisiae have already reached the clinical trial phase. They are composed of the structural proteins p17 and p24 [61], [62]. HEK293, among other mammalian cell lines, have been used for the production of HIV VLPs based on the Gag and/or Env proteins using either transient transfection or the generation of a stable cell line [9], [63]. Insect cell/Baculovirus systems have also been used for the production of Gag-Env VLPs and stable insect cell lines producing the Gag polyprotein have been developed for the expression of these VLPs [64].
Human parvovirus
Human parvovirus is a small, non-enveloped DNA virus of the family Parvoviridae. It has two main structural proteins, VP1 and VP2 [65]. Human parvovirus B19 (HPVB19) VLPs have reached the clinical trial stage, and are composed of the proteins VP1 and VP2 produced in the B/IC system. Sf9 cells are infected by two baculoviruses, which leads to the production and self-assembly of immunogenic VLPs [6].
Norovirus
Norovirus (NV) is a 27-nm-diameter RNA virus responsible for acute viral gastroenteritis and belongs to the Caliciviridae. It is non-enveloped with a 7.5 kb genome encoding a large polyprotein that is cleaved into both structural (VP1 and VP2) and regulatory proteins (NS1/2 to NS7). NV VLPs in clinical trials are composed of the capsid protein. The main structural protein VP1 has been expressed in the B/IC system (Sf-9), and is producing promising results in clinical trials. NV VLPs formed by this capsid protein have also been produced by transgenic plants at the clinical trial level [66].
Severe acute respiratory syndrome-related coronavirus (SARS-CoV)
SARS-CoV belongs to the Coronaviridae. It is enveloped, with an unusually large (29.7 kb) single-stranded RNA genome encoding 14 proteinswhich have either regulatory or structural roles. The virus is composed of four structural proteins: nucleocapsid (NP), spike (SP), membrane (MP) and an envelope (EP). It has also been produced by the B/IC system in Sf21 cells by the expression of SP, EP and MP through infection of the culture with three different baculoviruses, one per protein to be expressed, at a research level [67].
Conclusions
Virus-like particles represent a step forward in vaccine development. They resemble the actual structure of a virus, which provokes a humoral and cellular immune response. Furthermore, they contain no viral genetic material, which makes them safer for vaccine recipients and operators that are in contact with the vaccine. This is an advantage compared with the classical vaccines, such as live-attenuated and inactivated, as there is no danger of accidental infection. Bacteria and yeast are easy and fast platforms for recombinant protein production, but they lack the ability to produce complex structures and PTMs. This makes them suitable for the production of simple and generally non-enveloped VLPs. The B/IC system has the ability to produce much more complex structures due to its ability to glycosylate recombinant proteins. This system can reach high yields that are comparable with those obtained with bacteria and yeast. Baculovirus design, construction, and cell infection are also relatively straightforward processes, further streamlining production. The main drawback of this platform is the purification step, as enveloped baculoviruses are produced at the same time as VLPs and possess very similar physical and chemical characteristics. For this reason, other baculovirus-free systems using insect cells are currently being investigated. Finally, mammalian cells are most suitable for the production of complex structures. This system faithfully replicates human glycosylation patterns, representing a significant advantage. However, yields obtained with mammalian cell lines are generally much lower compared to other systems.
Selection of the producer cell line must consider the needs and characteristics of the VLP being produced. It is an especially important step in enveloped VLP production, since membrane proteins from the producer cell line will be present in the VLP envelope, possibly enhancing immunogenicity and acting as an adjuvant.
A number of VLP-based vaccines are already available on the market with good results. Many others are still in clinical and preclinical trials. The interest in VLP design and production has increased in recent years due to the advantages that they present over classical vaccines. New applications in cell line targeting for drug delivery have been explored, which opens the possibility of new uses of VLP technology.
References
- 1.Ulmer J.B., Valley U., Rappuoli R. Vaccine manufacturing: challenges and solutions. Nat Biotechnol. 2006;24:1377–1383. doi: 10.1038/nbt1261. [DOI] [PubMed] [Google Scholar]
- 2.Mäkelä P.H. Vaccines, coming of age after 200 years. FEMS Microbiol Rev. 2000;24:9–20. doi: 10.1016/S0168-6445(99)00025-X. [DOI] [PubMed] [Google Scholar]
- 3.Plotkin S. History of vaccination. Proc Natl Acad Sci U S A. 2014;2014:1–5. doi: 10.1073/pnas.1400472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiller J.T., Lowy D.R. Raising expectations for subunit vaccine. J Infect Dis. 2015;211:1373–1375. doi: 10.1093/infdis/jiu648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu M. REVIEW DNA vaccines: a review. J Intern Med. 2003;253:402–410. doi: 10.1046/j.1365-2796.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 6.Roldao A., Mellado M.C., Castilho L.R., Carrondo M.J., Alves P.M. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 7.Lua L.H.L., Connors N.K., Sainsbury F., Chuan Y.P., Wibowo N., Middelberg A.P.J. Bioengineering virus-like particles as vaccines. Biotechnol Bioeng. 2014;111:425–440. doi: 10.1002/bit.25159. [DOI] [PubMed] [Google Scholar]
- 8.Abdoli A., Soleimanjahi H., Fotouhi F., Teimoori A., Pour Beiranvand S., Kianmehr Z. Human papillomavirus type16- L1 VLP production in insect cells. Iran J Basic Med Sci. 2013;16:891–895. [PMC free article] [PubMed] [Google Scholar]
- 9.Cervera L., Gutiérrez-Granados S., Martínez M., Blanco J., Gòdia F., Segura M.M. Generation of HIV-1 Gag VLPs by transient transfection of HEK 293 suspension cell cultures using an optimized animal-derived component free medium. J Biotechnol. 2013;166:152–165. doi: 10.1016/j.jbiotec.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Liu F., Wu X., Li L., Liu Z., Wang Z. Use of baculovirus expression system for generation of virus-like particles: successes and challenges. Protein Expr Purif. 2013;90:104–116. doi: 10.1016/j.pep.2013.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu G., Wang N., Yu W., Wang Z., Zou Y., Zhang Y. Generation and immunogenicity of porcine circovirus type 2 chimeric virus-like particles displaying porcine reproductive and respiratory syndrome virus GP5 epitope B. Vaccine. 2016;34:1896–1903. doi: 10.1016/j.vaccine.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 12.Yan D., Wei Y.Q., Guo H.C., Sun S.Q. The application of virus-like particles as vaccines and biological vehicles. Appl Microbiol Biotechnol. 2015;99:10415–10432. doi: 10.1007/s00253-015-7000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aires K.A., Cianciarullo A.M., Carneiro M., Villa L.L., Boccardo E., Pérez-martinez G. Production of human papillomavirus type 16 L1 virus-Like particles by recombinant lactobacillus casei cells production of human papillomavirus type 16 L1 virus-Like particles by recombinant lactobacillus casei cells. Appl Environ Microbiol. 2006;72:745–752. doi: 10.1128/AEM.72.1.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan M., Zhong W., Song D., Thornton S., Jiang X. E. coli-expressed recombinant norovirus capsid proteins maintain authentic antigenicity and receptor binding capability. J Med Virol. 2004;74:641–649. doi: 10.1002/jmv.20228. [DOI] [PubMed] [Google Scholar]
- 15.Saraswat S., Athmaram T.N., Parida M., Agarwal A., Saha A., Dash P.K. Expression and characterization of yeast derived chikungunya virus like particles (CHIK-VLPs) and its evaluation as a potential vaccine candidate. PLoS Negl Trop Dis. 2016;10:1–19. doi: 10.1371/journal.pntd.0004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luna J., Plata M., Gonzalez M., Correa A., Maldonado I., Nossa C. Long-term follow-up observation of the safety, immunogenicity, and effectiveness of Gardasil™ in adult women. PLoS One. 2013:8. doi: 10.1371/journal.pone.0083431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felberbaum R.S. The baculovirus expression vector system: a commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol J. 2015;10:702–714. doi: 10.1002/biot.201400438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metz S.W., Gardner J., Geertsema C., Le TT Goh L, Vlak J.M. Effective chikungunya virus-like particle vaccine produced in insect cells. PLoS Negl Trop Dis. 2013:7. doi: 10.1371/journal.pntd.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buonaguro L., Tornesello M.L., Tagliamonte M., Gallo R.C., Wang L.X., Kamin-Lewis R. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J Virol. 2006;80:9134–9143. doi: 10.1128/JVI.00050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maranga L., Brazao T.F., Carrondo M.J.T. Virus-like particle production at low multiplicities of infection with the baculovirus insect cell system. Biotechnol Bioeng. 2003;84:245–253. doi: 10.1002/bit.10773. [DOI] [PubMed] [Google Scholar]
- 21.Yang L., Song Y., Li X., Huang X., Liu J., Ding H. HIV-1 virus-like particles produced by stably transfected drosophila S2Cells: a desirable vaccine component. J Virol. 2012;86:7662–7676. doi: 10.1128/JVI.07164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pushko P., Tumpey T.M., Van Hoeven N., Belser J.A., Robinson R., Nathan M. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine. 2007;25:4283–4290. doi: 10.1016/j.vaccine.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 23.Shen X., Hacker D.L., Baldi L., Wurm F.M. Virus-free transient protein production in Sf9 cells. J Biotechnol. 2013;171:61–70. doi: 10.1016/j.jbiotec.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol Adv. 2012;30:1158–1170. doi: 10.1016/j.biotechadv.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Wurm F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 26.Jayapal K.P., Wlaschin K.F., Hu W.-S., Yap M.G.S. Recombinant protein therapeutics from CHO cells — 20 years and counting. CEP Mag. 2007:40–47. [Google Scholar]
- 27.Li C., Liu F., Liang M., Zhang Q., Wang X., Wang T. Hantavirus-like particles generated in CHO cells induce specific immune responses in C57BL/6 mice. Vaccine. 2010;28:4294–4300. doi: 10.1016/j.vaccine.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Fontana D., Kratje R., Etcheverrigaray M., Prieto C. Immunogenic virus-like particles continuously expressed in mammalian cells as a veterinary rabies vaccine candidate. Vaccine. 2015;33:4238–4246. doi: 10.1016/j.vaccine.2015.03.088. [DOI] [PubMed] [Google Scholar]
- 29.Thompson C.M., Petiot E., Lennaertz A., Henry O., Kamen A a. Analytical technologies for influenza virus-like particle candidate vaccines: challenges and emerging approaches. Virol J. 2013;10:141. doi: 10.1186/1743-422X-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutiérrez-Granados S., Cervera L., Segura M de las M., Wölfel J., Gòdia F. Optimized production of HIV-1 virus-like particles by transient transfection in CAP-T cells. Appl Microbiol Biotechnol. 2016;100:3935–3947. doi: 10.1007/s00253-015-7213-x. [DOI] [PubMed] [Google Scholar]
- 31.JK-C Ma, Drake P.M.W., Christou P. The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet. 2003;4:794–805. doi: 10.1038/nrg1177. [DOI] [PubMed] [Google Scholar]
- 32.Liu H.-L., Li W.-S., Lei T., Zheng J., Zhang Z., Yan X.-F. Expression of human papillomavirus type 16 L1 protein in transgenic tobacco plants. Acta Biochim Biophys Sin (Shanghai) 2005;37:153–158. doi: 10.1111/j.1745-7270.2005.00027.x. [DOI] [PubMed] [Google Scholar]
- 33.D’Aoust M.A., Couture M.M.J., Charland N., Trépanier S., Landry N., Ors F. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J. 2010;8:607–619. doi: 10.1111/j.1467-7652.2009.00496.x. [DOI] [PubMed] [Google Scholar]
- 34.Greco R., Michel M., Guetard D., Cervantes-Gonzalez M., Pelucchi N., Wain-Hobson S. Production of recombinant HIV-1/HBV virus-like particles in Nicotiana tabacum and Arabidopsis thaliana plants for a bivalent plant-based vaccine. Vaccine. 2007;25:8228–8240. doi: 10.1016/j.vaccine.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 35.Huang Z., Elkin G., Maloney B.J., Beuhner N., Arntzen C.J., Thanavala Y. Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine. 2005;23:1851–1858. doi: 10.1016/j.vaccine.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Fruit L., Jesús T.D.E., Flores O., Arias N., López S., Arias C. Production of rotavirus-like particles in. Tomato. 2006;19:42–53. doi: 10.1089/vim.2006.19.42. [DOI] [PubMed] [Google Scholar]
- 37.Schädlich L., Senger T., Kirschning C.J., Müller M., Gissmann L. Refining HPV 16 L1 purification from E. coli: reducing endotoxin contaminations and their impact on immunogenicity. Vaccine. 2009;27:1511–1522. doi: 10.1016/j.vaccine.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Leavitt A.D., Roberts T.M., Garcea R.L. Polyoma virus major capsid protein, VP1: Purification after high level expression in Escherichia coli. J Biol Chem. 1985;260:12803–12809. [PubMed] [Google Scholar]
- 39.Mortola E., Roy P. Efficient assembly and release of SARS coronavirus-like particles by a heterologous expression system. FEBS Lett. 2004;576:174–178. doi: 10.1016/j.febslet.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aucoin M.G., Jacob D., Chahal P.S., Meghrous J., Bernier A., Kamen A a. Virus-like particle and viral vector production using the baculovirus expression vector system/insect cell system: adeno-associated virus-based products. Methods Mol Biol. 2007;388:281–296. doi: 10.1007/978-1-59745-457-5_14. [DOI] [PubMed] [Google Scholar]
- 41.Taube S., Kurth A., Schreier E. Generation of recombinant Norovirus-like particles (VLP) in the human endothelial kidney cell line 293T. Arch Virol. 2005;150:1425–1431. doi: 10.1007/s00705-005-0517-x. [DOI] [PubMed] [Google Scholar]
- 42.Holzer G.W., Mayrhofer J., Leitner J., Blum M., Webersinke G., Heuritsch S. Overexpression of hepatitis B virus surface antigens including the preS1 region in a serum-free Chinese hamster ovary cell line. Protein Expr Purif. 2003;29:58–69. doi: 10.1016/S1046-5928(03)00011-1. [DOI] [PubMed] [Google Scholar]
- 43.Warzecha H., Mason H.S., Lane C., Tryggvesson A., Rybicki E., Williamson A.-L. Oral immunogenicity of human papillomavirus-like particles expressed in potato. J Virol. 2003;77:8702–8711. doi: 10.1128/JVI.77.16.8702-8711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Z., Santi L., LePore K., Kilbourne J., Arntzen C.J., Mason H.S. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine. 2006;24:2506–2513. doi: 10.1016/j.vaccine.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 45.Ye L., Lin J., Sun Y., Bennouna S., Lo M., Wu Q. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;351:260–270. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Cervera L., Gutiérrez-Granados S., Berrow N.S., Segura M.M., Gòdia F. Extended gene expression by medium exchange and repeated transient transfection for recombinant protein production enhancement. Biotechnol Bioeng. 2015;112:934–946. doi: 10.1002/bit.25503. [DOI] [PubMed] [Google Scholar]
- 47.Eibl R., Kaiser S., Lombriser R., Eibl D. Disposable bioreactors: the current state-of-the-art and recommended applications in biotechnology. Appl Microbiol Biotechnol. 2010;86:41–49. doi: 10.1007/s00253-009-2422-9. [DOI] [PubMed] [Google Scholar]
- 48.Francis V.C., Ferrari C. Hepatitis B virus immunopathogenesis. Annu Revi Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 49.Michel M.-L., Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol. 2010;58:288–295. doi: 10.1016/j.patbio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979;281:646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- 51.Zhou W., Bi J., Janson J.-C., Li Y., Huang Y., Zhang Y. Molecular characterization of recombinant Hepatitis B surface antigen from Chinese hamster ovary and Hansenula polymorpha cells by high-performance size exclusion chromatography and multi-angle laser light scattering. J Chromatogr B. 2006;838:71–77. doi: 10.1016/j.jchromb.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 52.Chua B.Y., Johnson D., Tan A., Earnest-Silveira L., Sekiya T., Chin R. Hepatitis C VLPs delivered to dendritic cells by a TLR2 targeting lipopeptide results in enhanced antibody and cell-mediated responses. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0047492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkes N. European Medicines Agency approves first malaria vaccine. BMJ. 2015;351:h4067. doi: 10.1136/bmj.h4067. [DOI] [PubMed] [Google Scholar]
- 54.Morrison C. Landmark green light for Mosquirix malaria vaccine. Nat Biotechnol. 2015;33:1015–1016. doi: 10.1038/nbt1015-1015. [DOI] [PubMed] [Google Scholar]
- 55.Harper D.M., Franco E.L., Wheeler C., Ferris D.G., Jenkins D., Schuind A. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 56.Einstein M.H., Baron M., Levin M.J., Chatterjee A., Edwards R.P., Zepp F. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5:705–719. doi: 10.4161/hv.5.10.9518. 9518 [pii] [DOI] [PubMed] [Google Scholar]
- 57.Schiller J.T., Nardelli-Haefliger D. Chapter 17: Second generation HPV vaccines to prevent cervical cancer. Vaccine. 2006;24:147–153. doi: 10.1016/j.vaccine.2006.05.123. [DOI] [PubMed] [Google Scholar]
- 58.Bright R.A., Carter D.M., Daniluk S., Toapanta F.R., Ahmad A., Gavrilov V. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 59.Marsian J., Lomonossoff G.P. Molecular pharming-VLPs made in plants. Curr Opin Biotechnol. 2016;37:201–206. doi: 10.1016/j.copbio.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Sundquist W.I. 2015. Kra H. HIV-1 Assembly, Budding, and Maturation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doan L.X., Li M., Chen C., Yao Q. Virus-like particles as HIV-1 vaccines. Rev Med Virol. 2005;15:75–88. doi: 10.1002/rmv.449. [DOI] [PubMed] [Google Scholar]
- 62.Weber J., Cheinsong-Popov R., Callow D., Adams S., Patou G., Hodgkin K. Immunogenicity of the yeast recombinant p17 p24:Ty virus-like particles (p24-VLP) in healthy volunteers. Vaccine. 1995;13:831–834. doi: 10.1016/0264-410X(94)00061-Q. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X., Wang X., Zhao D., Meng X., Zhao X., Yu X. Design and immunogenicity assessment of HIV-1 virus-like particles as a candidate vaccine. Sci China Life Sci. 2011;54:1042–1047. doi: 10.1007/s11427-011-4244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tagliamonte M., Visciano M.L., Tornesello M.L., De Stradis A., Buonaguro F.M., Buonaguro L. HIV-Gag VLPs presenting trimeric HIV-1 gp140 spikes constitutively expressed in stable double transfected insect cell line. Vaccine. 2011;29:4913–4922. doi: 10.1016/j.vaccine.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Rogo L.D., Mokhtari-Azad T., Kabir M.H., Rezaei F. Human parvoviru B19: a review. Acta Virol. 2014;60:49–54. doi: 10.4149/av. [DOI] [PubMed] [Google Scholar]
- 66.Herbst-Kralovetz M., Mason H.S., Chen Q. Norwalk virus-like particles as vaccines. Expert Rev Vaccines. 2010;9:299–307. doi: 10.1586/erv.09.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho Y., Lin P.-H., Liu C.Y.Y., Lee S.-P., Chao Y.-C. Assembly of human severe acute respiratory syndrome coronavirus-like particles. Biochem Biophys Res Commun. 2004;318:833–838. doi: 10.1016/j.bbrc.2004.04.111. [DOI] [PMC free article] [PubMed] [Google Scholar]