Highlights
► This paper examines the public health impact of recently emerged bat zoonotic viruses. ► A review is provided for the high impact viruses originated from bats. ► Potential drivers for emergence of each virus were comparatively reviewed. ► Risk factors, transmission routes and future research directions were discussed.
Abstract
Bats are being increasingly recognized as an important reservoir of zoonotic viruses of different families, including SARS coronavirus, Nipah virus, Hendra virus and Ebola virus. Several recent studies hypothesized that bats, an ancient group of flying mammals, are the major reservoir of several important RNA virus families from which other mammalian viruses of livestock and humans were derived. Although this hypothesis needs further investigation, the premise that bats carry a large number of viruses is commonly accepted. The question of whether bats have unique biological features making them ideal reservoir hosts has been the subject of several recent reviews. In this review, we will focus on the public health implications of bat derived zoonotic viral disease outbreaks, examine the drivers and risk factors of past disease outbreaks and outline research directions for better control of future disease events.
Current Opinion in Virology 2013, 3:84–91
This review comes from a themed issue on Environmental virology
Edited by Marion Koopmans
For a complete overview see the Issue and the Editorial
Available online 21st December 2012
1879-6257/$ – see front matter, Crown Copyright © 2012 Published by Elsevier Ltd. All rights reserved.
Introduction
Approximately 75% of emerging infectious diseases are zoonoses [1, 2]. The rate of emergence of zoonotic viruses appears to be increasing and/or our ability to detect new viruses is improving. Viruses are well adapted to their reservoir hosts and therefore exhibit stability within their host's cellular and ecological environments and display little or no clinical disease in these species. However, when a virus jumps the species barrier and spills over into humans, the effects can be devastating. Only when these viruses from wildlife spillover to humans or their domesticated animals and cause mortalities, does this become a significant concern for public health. High mortality rates often characterize these spillover events as do high economic losses. This is particularly true for the bat borne viruses that have emerged in the last 20 years such as Severe Acute Respiratory Syndrome coronavirus (SARS-CoV), Nipah virus (NiV) Hendra virus (HeV) and Ebola virus (EBOV). International travel has contributed to the transmission of many infectious diseases and the bat borne zoonotic viruses are no exception with the most notable being the transmission of SARS-CoV from the key port in Hong Kong to Canada, the Americas, Europe, Asia and Australasia resulting in 8422 cases with 916 deaths (10.9% case fatality) [3].
Bats, order Chiroptera, comprise greater than 20% of living mammalian species with more than 1100 species across 17 families [4]. They are among the most ancient of mammals, their extensive speciation occurred before development of most modern mammals, and they are the only mammals capable of powered flight. Bats in general are also very long-lived and more widely dispersed globally than other mammals; they play vital roles as pollinators of hundreds of species of plants and trees and in the control of arthropod populations. More recently, resurgence in interest in bat biology and ecology has been sparked by their recognition as the known reservoir hosts of some of the most deadly viral zoonoses [5], to which they appear resistant to any pathogenic effects. Whether bats are unique or special as a reservoir of viruses has been the subject of several recent publications [6, 7] and will not be covered in this review. This review will focus on the ecology, pathogen–host interface and public health considerations of selected bat zoonotic viruses.
Key examples of bat zoonotic viruses
Hendra virus (HeV)
HeV first emerged in 1994 in an outbreak of respiratory disease that infected 20 horses and two humans, resulting in the death of all horses and one of the humans [8, 9]. There have been a total of 39 spillover events identified resulting in the infection of 78 horses, one dog and seven humans. In humans the case fatality rate from HeV infection is 57%. The four species of flying fox, Pteropus poliocephalus, P. alecto, P. scapulatus and P. conspicillatus have been found to be seropositive for HeV antibodies [10] and all have detectable virus in their urine [11•, 12]. All human cases of HeV infection have been associated with veterinarians and the continual outbreaks have resulted in many veterinarians leaving the equine field due to fears of HeV infection and legal liability [13].
Nipah virus (NiV)
There have been two clusters of Nipah virus outbreaks detected since the end of the 1990s, one in Malaysia/Singapore and the other in Bangladesh/India. NiV first emerged in Malaysia, causing an outbreak of neurological and respiratory disease affecting pigs between September 1998 and June 1999. Transmission from pigs to humans resulted in 283 human cases and 109 deaths (39% case fatality) in Malaysia and 11 cases and one death in Singaporean abattoir workers [14, 15]. The outbreak was controlled by the slaughtering of over 1.1 million pigs [16]. The reservoir hosts of NiV have been identified as Pteropus vampyrus and Pteropus hypomenalus in Malaysia [17, 18, 19•] and P. giganteus in Bangladesh and India [20, 21].
Since 2001, outbreaks of NiV in Bangladesh and India have occurred almost on an annual basis with a total of 302 cases and 210 human deaths (69.5% case fatality) [22, 23]. In Bangladesh and India, NiV shedding in P. giganteus bats appears to result in direct transmission to humans via consumption of raw date palm sap contaminated with bat excreta (saliva, urine, faeces) without transmission through an intermediate host [24••, 25]. Person to person transmission has also been observed [26, 27].
Severe Acute Respiratory Syndrome coronavirus (SARS-CoV)
In 2003, a global outbreak of SARS started in southern China and Hong Kong leading to 8422 cases and 916 human deaths (10.9% case fatality) worldwide [28]. For the transmission of SARS-CoV to humans, the virus required rapid adaption through the intermediary host which was thought to be the palm civet (Paguma sp.) where adaption allowed for transmission of the sufficiently fit virus to humans [29••]. The cave dwelling fruit bat Rousettus leschenaultia was identified as the reservoir host of SARS-like coronaviruses [29••]. The SARS-CoV outbreak was estimated to have cost $US54 billion globally [30].
Ebola virus (EBOV)
The transmission of EBOV to humans has usually occurred through the capture and slaughtering of animals, commonly non-human primates, for ‘bush meat’ [31]. The fruit bats Hypsignathus monstrosus, Epomops franqueti and Myonycteris torquata are possible reservoirs for EBOV in Africa based on serological surveys [32••]. The initial outbreaks of EBOV in 1976 in Sudan and Zaire were exacerbated by the reuse of contaminated needles [33, 34]. Person to person transmission occurs through contact with bodily fluids (blood, semen, organs, urine, faeces and secretions) [35] including contact with cadavers [36].
Initially identified in imported macaques in the USA, Ebola Reston has recently emerged in pigs in the Philippines and poses a concern for Public Health and agriculture. Of particular significance was the co-infection of the pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Serological studies identified six people that had seroconverted to Ebola Reston virus, all of which has contact with sick pigs [37]. Importantly, experimental infection with Ebola Reston virus found that the disease was asymptomatic in pigs despite shedding, indicating a risk for farm and abattoir workers [38]. Antibodies to Ebola Reston virus were detected in Rousettus amplexicaudatus bats implicating this bat as a potential reservoir host [39].
In addition to the ‘high impact’ bat zoonotic viruses discussed above, there are other bat viruses which have caused zoonotic infections. See Table 1 for a summary list of major bat zoonotic viruses recently emerged.
Table 1.
Comparison of drivers and risk factors for selective emerging bat zoonotic viruses
| Virus | Drivers | Risk factors/modes of transmission | References |
|---|---|---|---|
| SARS-CoV | Economic growth Desire for game meat Live wild animal trading in wet markets International travel |
Slaughtering Social/cultural practices Farming of wild animals Laboratory acquired infection |
[29••, 54, 55, 93] |
| Ebola virus | Desire for game meat (Bush meat) Live wild animal trading Burial practices |
Slaughtering/hunters Social/cultural Practices Poor health care practices |
[31] |
| Marburg virus | Infected monkeys used for research Mining Tourism |
Laboratory acquired infection Caves (eco-tourism) Mining |
[84, 94, 95, 96] |
| Hendra virus | Population growth/urbanization/human encroachment/synanthrophy Climate change Starvation Reproductive stress |
Inadequate PPE for veterinarians Intermit contact with horses (cuts, abrasions, respiratory secretions) For example performing necropies |
[13, 46, 62] |
| Nipah virus (Bangladesh) | Date palm juice Cultural tradition |
Drinking date palm juice Caring for infected patients |
[26, 27, 90, 97, 98] |
| Nipah virus (Malaysia) | Agricultural intensification (dual land use) Encroachment into forested areas Movement of pigs to grower piggeries within Malaysia Food processing in Singapore Trade Habitat destruction Stress |
Piggery workers (aerosols, husbandry practices) Abbatoir workers (slaughtering) |
[53, 99, 100] |
| Reoviruses (Melaka virus and related viruses) | Urbanization Tourism |
Close proximity to bats Eco-tourism |
[101, 102] |
| Menangle virus | Agricultural practices (transportation of pigs within farm) Movement of pigs to grower piggeries Close proximity to flying fox colony |
Piggery workers (husbandry practices — birthing and necropies without PPE) | [47] |
| Lyssaviruses for example Rabies, Australian bat lyssavirus (ABLV), European bat lyssaviruses (EBLV) 1 and 2, Lagos bat virus, Duvenhage virus | Urbanization Deforestation Synanthrophy |
Bat carers Cohabitation with bats in houses |
[42, 44•] |
Understanding the ecology of disease emergence
There are many potential drivers that can contribute to a spillover of zoonotic bat-borne disease (Table 1 and Figure 1 ). These factors can be extrinsic and/or intrinsic. Extrinsic factors such as environmental and anthropogenic stressors can have an effect on the ecology of disease in bats. Environmental stressors such as climatic events (typhoons/cyclones and droughts) that destroy habitat and food resources have been hypothesized to have an impact on the health of bats [40, 41, 42, 43, 44•]. In addition, human activities are selecting for some species of bats that are synanthrophic, and so are benefiting from living close to humans, thereby increasing their numbers and the risk of transmission of disease to humans [45]. Human activities are artificially increasing animal densities by changing the land use and this is increasing the contact between humans, domesticated animals and bats [46]. In the cases of Hendra virus, Nipah virus and Menangle virus, domesticated animals are the amplifying hosts of these zoonotic viruses [15, 47, 48]. Habitat change such as deforestation force changes in roosting sites and can lead to alterations in the population density and the migratory patterns of bats. These anthropogenic activities may be impacting our ecosystem in such a way that the equilibrium is disturbed and spillover of zoonotic viruses readily occur [49, 50, 51].
Figure 1.
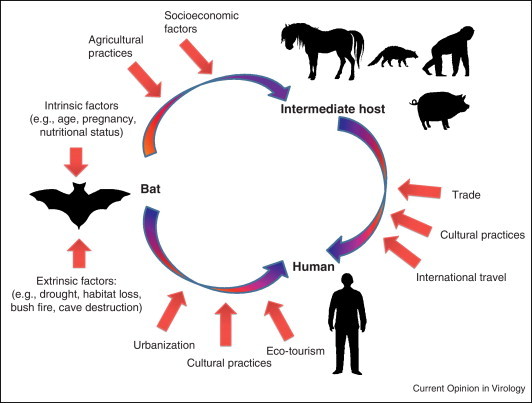
Examples of drivers responsible for zoonotic virus spillover from bats.
In the case of the NiV outbreak in Malaysia, the drivers of the outbreak were primarily agricultural intensification and more specifically the co-location of pig farms and fruit orchards. It is believed that the fruiting trees overhanging the intensive pig farms attracted flying foxes, leading to NiV spillover into pigs [52]. It has been hypothesized that the Nipah virus outbreak in pigs was contributed by the El Nino Southern Oscillation induced drought and subsequent forest fires in Indonesia that produced a smoke haze that led to the migration of flying foxes into Malaysia and the subsequent spillover of NiV into pigs [52]. However, this hypothesis was refuted by a later study which suggested that there were multiple introductions of NiV into the piggery. It was thought that repeated introductions of NiV allowed for viral persistence to be established within the pig population which in turn resulted in transmission of NiV to humans [53]. NiV underwent a host shift from fruit bats into pigs that allowed for high levels of virus to be shed and persist within a new host. Management practices of relocating pigs to grower farms further spread the virus. Trade allowed for further NiV transmission with pigs being transported to Singapore for processing increasing the spread of the outbreak [14].
The drivers for transmission of NiV in Bangladesh differed to those in Malaysia. In Bangladesh, cultural practices of consumption and trade of date palm sap have allowed for the transmission of NiV from bats to humans [24••, 25]. Traditional social practices of family members caring for the sick in the absence of barrier nursing have resulted in person-to-person transmission further contributing to outbreaks of disease [26, 27].
Similarly, cultural traditions of eating wild animal meat or ‘bush meat’ in Africa and Asia have led to outbreaks of EBOV and SARS-CoV. In the case of EBOV, the transmission has been further exacerbated by the lack of barrier nursing and the use of traditional burial practices where mourners make contact with the deceased [36]. Desire for wild animal meat including bats, and trade in live wet markets have allowed susceptible animals to come into contact with bats enabling the subsequent transmission of SARS-CoV into humans [54, 55]. Once established, person-to-person transmission occurred and the virus was widely spread by international travellers [56]. Importantly, international collaboration allowed for the aetiology of the outbreak to be rapidly determined. This in turn allowed for the development of diagnostic assays for the detection of the virus and greatly assisted in the control of the outbreak [3]. Recently, a novel coronavirus isolated from a Saudi make patient suffering pneumonia and renal failure was determined to be most similar to bat coronaviruses through an international collaboration [57, 58•]. Following the posting on Promed of this case [57], another coronavirus with 99.5% similarity to the virus was identified as the causative agent of a Saudi man from Qatar (and hospitalized in London) suffering from a severe respiratory illness [59]. This allowed the development of real-time molecular assays for the rapid detection of these viruses [60]. More recently, another Saudi man has been diagnosed with a similar coronavirus infection [61].
Overall, ecology-based management of drivers that can lead to spillover are likely to be more effective than movement or culling, as they have the potential to reduce disease susceptibility in the reservoir host and opportunities for transmission [42, 44•].
Understanding the virus–bat interface
Our understanding of virus–host interactions is in its infancy. The virus–bat interface is impacted by the extrinsic factors mentioned above, as well as viral and host factors (intrinsic factors). There are multiple intrinsic factors of the reservoir host such as age, body condition, reproductive status, sex and social status that are impacted by stress. For instance, stresses due to starvation and the breeding season for males may play an important role in the epidemiology of disease as stress caused by these factors may dampen the immune response making individuals more susceptible to infection [62]. In the case of EBOV, it is thought that starvation leads to fruit bats and primates coming into close proximity during their quest for food, facilitating spillover [63]. Transmission of viruses could also occur following changes in the hierarchy of the colony that lead to fighting for dominance, and during courtship and mating via grooming and biting [7]. Seasonal periods when juveniles have waning maternal antibody and are therefore susceptible to virus infection have been associated with increased transmission of Marburg virus to humans [64••] and have been thought to have a role in increasing the incidence of transmission within bat colonies for HeV as well as Marburg virus [46, 62, 64••]. Bats live on average 3.5 times longer than a mammal of similar size [65], hence longevity in bats promotes persistence of viruses in the reservoir host, while the ability to fly allows long-distance dispersal of the infectious agent. Aerosols, direct contact and arthropods could also serve as vectors for transmission within the colony or to other species.
Little is known about the diversity of viruses, the amount of virus present, the mechanisms of shedding, the incidence of supershedders or the contact rates between infectious and susceptible individuals. From metagenomic analyses, bats harbour a range of viruses and there is a possibility of multiple viral infections in bats spilling over [66, 67, 68]. Notably, all of the zoonotic viruses of bat origin so far identified are RNA viruses. Many of these highly pathogenic viruses display a broad cell tropism, being able to infect a wide range of cells and hosts (HeV, NiV, EBOV, SARS-CoV) [69, 70, 71, 72]. Viruses such as HeV, lyssaviruses and NiV show high genome conservation within their bat hosts, suggesting that they are under strong selective constraints [11•, 73, 74, 75, 76].
Public health considerations: prevention and control strategies
Understanding the ecology of bat-borne viral pathogens and identifying the triggers of an outbreak will assist in the control or reduction of emerging zoonotic disease outbreaks. By understanding the mechanisms of emergence, outbreak management plans can be developed and risk mitigation processes can be implemented. Once identified, risk reduction strategies can be implemented through education of the general public, doctors, veterinarians and policy makers [77]. Measures such as the wearing of appropriate protective equipment (PPE) when caring for patients or animals and restriction on the sale and consumption of game meat would reduce the risks of transmission of bat-borne viruses.
Following outbreaks of disease, public health measures implemented have included enhanced surveillance and increased infection control, whether it is in hospitals in the case of SARS-CoV, EBOV and NiV, or during veterinary procedures in the case of HeV. Quarantine and contact tracing to limit the spread of viruses have also implemented in outbreaks [16, 78, 79••, 80, 81, 82, 83, 84]. A communication strategy is implemented to inform the public aims to reduce further spread by avoidance of risky activities or alteration of activities. For example, following the recognition of SARS, response teams were formed and communication to the public was instigated including global alerts from the World Health Organization [85].
Implementation of prevention and control measures can be carried out at many different levels. At the farming level, changing agricultural practices by the creation of buffers between fruiting trees and domesticated animals would significantly reduce the transmission for HeV and NiV, which is believed to have already played an important role in preventing further NiV outbreaks in Malaysia [86, 87]. The introduction of biocontainment measures within piggeries, including surveillance of pigs being transferred between farms and sent to abattoirs, is another effective approach which can be applied at the farming and trade level [87, 88, 89]. Since some of these zoonotic agents involve an intermediate host of livestock importance, it is highly important that veterinarians wear appropriate PPE when performing procedures on animals to minimize the risk of transmission of zoonotic diseases.
In addition to the above general strategies, some disease-specific prevention and control measures can also be applied. In Bangladesh, the installation of barriers on the date palms that prevent the bats from accessing the collection vessels is a simple strategy currently being investigated to control transmission of NiV. This strategy also improves the quality of sap and therefore results in a higher price [90, 91, 92••]. For prevention of HeV infection in humans, a One Health approach is being adopted. This involved the development of a recombinant protein-based vaccination program for horses in high-risk areas. The vaccination aims to achieve two purposes: prevent horses from HeV infection and, more importantly, block the horse-to-human transmission. With the recent release of a vaccine against HeV in early November 2012, it will be interesting to see whether this One Health strategy is effective in interrupting the zoonotic transmission cycle of HeV.
Strategies for the development of surveillance for new and emerging diseases and the management of bats need to be developed. With increased interaction between humans and their domesticated animals and bats, increasing rates of infections will continue to occur. In the past, there has been passive surveillance on dead animals, and it is time to form an international consortium for active surveillance of different bat populations to detect potential zoonotic agents as well as unknown viruses of low pathogenicity that could combine with other viruses to be pathogenic [42]. The use of new technologies such as high throughput sequencing and multiplex serological tests should be an integral part of this effort to increase our ability for pre-emergence monitoring of potential zoonotic pathogens.
Future directions
With human activity increasingly overlapping the habitats of bats, there is no doubt that zoonotic viruses will continue to emerge from these species. In order to help predict and prevent the emergence of viruses, we need a greater understanding of the infection dynamics within their hosts and to understand the impact of human changes to the environment on the potential for virus spillover. A fully integrated One Health approach with international scientists, ecologists, veterinarians, health professionals, social scientists and politicians working together is required to minimize the impact of bat borne zoonotic diseases. It is critical that we are able to coexist with bats as these unique creatures are vital to our ecosystem.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgments
We thank Gary Crameri and Michelle Baker for critical reading of the manuscript.
References
- 1.Taylor L.H., Latham S.M., Woolhouse M.E. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . The World Health Report 2003. 2004. SARS: lessons from a new disease; pp. 71–82. [Google Scholar]
- 4.Nowak R. The Johns Hopkins University Press; 1994. Walker's Bats of the World. [Google Scholar]
- 5.Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L.F., Walker P.J., Poon L.L. Mass extinctions, biodiversity and mitochondrial function: are bats “special” as reservoirs for emerging viruses? Curr Opin Virol. 2011;1:649–657. doi: 10.1016/j.coviro.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong S., Lau S., Woo P., Yuen K.Y. Bats as a continuing source of emerging infections in humans. Rev Med Virol. 2007;17:67–91. doi: 10.1002/rmv.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray K., Rogers R.J., Selvey L.A., Selleck P., Hyatt A.D., Gould A.R., Gleeson L.J., Hooper P.T., Westbury H. A novel morbillivirus pneumonia of horses and its transmission to humans. Emerg Infect Dis. 1995;1:31–33. doi: 10.3201/eid0101.950107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvey L.A., Wells R.M., McCormack J.G., Ansford A.J., Murray K., Rogers R.J., Lavercombe P.S., Selleck P., Sheridan J.W. Infection of humans and horses by a newly described morbillivirus. Med J Aust. 1995;162:642–645. doi: 10.5694/j.1326-5377.1995.tb126050.x. [DOI] [PubMed] [Google Scholar]
- 10.Young P.L., Halpin K., Selleck P.W., Field H., Gravel J.L., Kelly M.A., Mackenzie J.S. Serologic evidence for the presence in Pteropus bats of a paramyxovirus related to equine morbillivirus. Emerg Infect Dis. 1996;2:239–240. doi: 10.3201/eid0203.960315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Smith I., Broos A., de Jong C., Zeddeman A., Smith C., Smith G., Moore F., Barr J., Crameri G., Marsh G. Identifying Hendra virus diversity in pteropid bats. PLoS One. 2011;6:e25275. doi: 10.1371/journal.pone.0025275. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report of the isolation of HeV from the urine of naturally infected flying foxes, and reported that the genome is highly conserved between bat, horses and human isolates
- 12.Field H., de Jong C., Melville D., Smith C., Smith I., Broos A., Kung Y.H., McLaughlin A., Zeddeman A. Hendra virus infection dynamics in Australian fruit bats. PLoS One. 2011;6:e28678. doi: 10.1371/journal.pone.0028678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendez D.H., Judd J., Speare R. Unexpected result of Hendra virus outbreaks for veterinarians, Queensland, Australia. Emerg Infect Dis. 2012;18:83–85. doi: 10.3201/eid1801.111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paton N.I., Leo Y.S., Zaki S.R., Auchus A.P., Lee K.E., Ling A.E., Chew S.K., Ang B., Rollin P.E., Umapathi T. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet. 1999;354:1253–1256. doi: 10.1016/S0140-6736(99)04379-2. [DOI] [PubMed] [Google Scholar]
- 15.Chua K.B., Bellini W.J., Rota P.A., Harcourt B.H., Tamin A., Lam S.K., Ksiazek T.G., Rollin P.E., Zaki S.R., Shieh W. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 16.Field H., Young P., Yob J.M., Mills J., Hall L., Mackenzie J. The natural history of Hendra and Nipah viruses. Microbes Infect. 2001;3:307–314. doi: 10.1016/s1286-4579(01)01384-3. [DOI] [PubMed] [Google Scholar]
- 17.Yob J.M., Field H., Rashdi A.M., Morrissy C., van der Heide B., Rota P., bin Adzhar A., White J., Daniels P., Jamaluddin A. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg Infect Dis. 2001;7:439–441. doi: 10.3201/eid0703.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chua K.B., Koh C.L., Hooi P.S., Wee K.F., Khong J.H., Chua B.H., Chan Y.P., Lim M.E., Lam S.K. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 2002;4:145–151. doi: 10.1016/s1286-4579(01)01522-2. [DOI] [PubMed] [Google Scholar]
- 19•.Chua K.B. A novel approach for collecting samples from fruit bats for isolation of infectious agents. Microbes Infect. 2003;5:487–490. doi: 10.1016/s1286-4579(03)00067-4. [DOI] [PubMed] [Google Scholar]; Description of the successful isolation of NiV, other paramyxoviruses and bacteria using a method that did not involve capture of bats for sampling
- 20.Epstein J.H., Prakash V., Smith C.S., Daszak P., McLaughlin A.B., Meehan G., Field H.E., Cunningham A.A. Henipavirus infection in fruit bats (Pteropus giganteus), India. Emerg Infect Dis. 2008;14:1309–1311. doi: 10.3201/eid1408.071492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yadav P.D., Raut C.G., Shete A.M., Mishra A.C., Towner J.S., Nichol S.T., Mourya D.T., Jonathan S. Short report: detection of Nipah virus RNA in fruit bat (Pteropus giganteus) from India. Am J Trop Med Hyg. 2012;87:576–578. doi: 10.4269/ajtmh.2012.11-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization Regional Office for South East Asia: Nipah virus infection 2009.
- 23.World Health Organization Regional Office for South East Asia: Nipah virus outbreaks in the WHO South-East Asia Region 2012.
- 24••.Luby S.P., Rahman M., Hossain M.J., Blum L.S., Husain M.M., Gurley E., Khan R., Ahmed B.N., Rahman S., Nahar N. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006;12:14–16. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recognized Date palm sap as a vehicle for transmission of NiV which is important for the control of transmission of the virus
- 25.Rahman M.M.A., Hossain M.J., Sultana S., Homaira N., Khan S.U., Gurley E.S., Rollin P.E., Lo M.K., Comer J.A., Lowe L. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis. 2012;12:65–72. doi: 10.1089/vbz.2011.0656. [DOI] [PubMed] [Google Scholar]
- 26.Gurley E.S., Montgomery J.M., Hossain M.J., Bell M., Azad A.K., Islam M.R., Molla M.A., Carroll D.S., Ksiazek T.G., Rota P.A. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis. 2007;13:1031–1037. doi: 10.3201/eid1307.061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum L.S., Khan R., Nahar N., Breiman R.F. In-depth assessment of an outbreak of Nipah encephalitis with person-to-person transmission in Bangladesh: implications for prevention and control strategies. Am J Trop Med Hyg. 2009;80:96–102. [PubMed] [Google Scholar]
- 28.World Health Organization: Summary table of SARS cases by country, 1 November 2002-7 August 2003 2003.
- 29••.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]; Identification of bats as the reservoir host of SARS-like coronaviruses
- 30.Lee J.-W., McKibbon W. Estimating the global cost of SARS. In: Knobler S., Mahmoud A., Lemon S., Mack A., Sivitz L., Oberholtzer K., editors. Learning from SARS: Preparing for the Next Disease Outbreak — Workshop Summary. National Academies Press; 2004. pp. 92–109. [PubMed] [Google Scholar]
- 31.Leroy E.M., Epelboin A., Mondonge V., Pourrut X., Gonzalez J.-P., Muyembe-Tamfum J.-J., Formenty P. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009;9:723–728. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- 32••.Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P., Délicat A., Paweska J.T., Gonzalez J.-P., Swanepoel R. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]; Detection of antibodies against EBOV in bats confirming them as a reservoir host
- 33.World Health Organization Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56:271–293. [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization Ebola haemorrhagic fever in Sudan, 1976. Bull World Health Organ. 1978;56:247–270. [PMC free article] [PubMed] [Google Scholar]
- 35.Bausch D.G., Towner J.S., Dowell S.F., Kaducu F., Lukwiya M., Sanchez A., Nichol S.T., Ksiazek T.G., Rollin P.E. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(Suppl):S142–S147. doi: 10.1086/520545. [DOI] [PubMed] [Google Scholar]
- 36.Dowell S.F., Mukunu R., Ksiazek T.G., Khan A.S., Rollin P.E., Peters C.J. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1995;179:S87–S91. doi: 10.1086/514284. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization: Ebola Reston in pigs and humans in the Philippines 2009. [PubMed]
- 38.Marsh G.A., Haining J., Robinson R., Foord A., Yamada M., Barr J.A., Payne J., White J., Yu M., Bingham J. Ebola Reston virus infection of pigs: clinical significance and transmission potential. J Infect Dis. 2011;204:S804–S809. doi: 10.1093/infdis/jir300. [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi S., Watanabe S., Masangkay J.S., Omatsu T., Ikegami T., Alviola P., Ueda N., Iha K., Fujii H., Ishii Y. Reston Ebolavirus antibodies in bats, the Philippines. Emerg Infect Dis. 2011;17:1559–1560. doi: 10.3201/eid1708.101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Field H.E. Bats and emerging zoonoses: henipaviruses and SARS. Zoonoses Public Health. 2009;56:278–284. doi: 10.1111/j.1863-2378.2008.01218.x. [DOI] [PubMed] [Google Scholar]
- 41.Wood J.L.N., Leach M., Waldman L., Macgregor H., Fooks A.R., Jones K., Restif O., Dechmann D., Hayman D.T.S., Baker K.S. A framework for the study of zoonotic disease emergence and its drivers: spillover of bat pathogens as a case study. Philos Trans R Soc Lond B Biol Sci. 2012;367:2881–2892. doi: 10.1098/rstb.2012.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathews F. Advances in Parasitology. Elsevier Ltd.; 2009. Zoonoses in wildlife integrating ecology into management. 185–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daszak P., Cunningham A.A., Hyatt A.D. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001;78:103–116. doi: 10.1016/s0001-706x(00)00179-0. [DOI] [PubMed] [Google Scholar]
- 44•.Streicker D.G., Recuenco S., Valderrama W., Gomez Benavides J., Vargas I., Pacheco V., Condori Condori R.E., Montgomery J., Rupprecht C.E., Rohani P. Ecological and anthropogenic drivers of rabies exposure in vampire bats: implications for transmission and control. Proc R Soc Biol Sci. 2012;279:3384–3392. doi: 10.1098/rspb.2012.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]; A good example of how culling is not the answer to controlling bat borne viruses
- 45.McFarlane R., Sleigh A., McMichael T. Synanthropy of wild mammals as a determinant of emerging infectious diseases in the Asian-Australasian region. EcoHealth. 2012;9:24–35. doi: 10.1007/s10393-012-0763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plowright R.K., Foley P., Field H.E., Dobson A.P., Foley J.E., Eby P., Daszak P. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.) Proc Biol Sci. 2011;278:3703–3712. doi: 10.1098/rspb.2011.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philbey A., Kirkland P.D., Ross A.D., Davis R.J., Gleeson A., Love R.J., Daniels P.W., Gould A., Hyatt A. An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats. Emerg Infect Dis. 1998;4:269–271. doi: 10.3201/eid0402.980214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray K., Selleck P., Hooper P., Hyatt A., Gould A.R., Gleeson L.J., Westbury H., Hiley L., Selvey L.A., Rodwell B. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- 49.Wolfe N.D., Daszak P., Kilpatrick A.M., Burke D.S. Deforestation, and prediction of zoonotic disease emergence. Emerg Infect Dis. 2005;11:1822–1827. doi: 10.3201/eid1112.040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morse S.S. Factors and determinants of disease emergence. Rev Sci Tech. 2004;23:443–451. doi: 10.20506/rst.23.2.1494. [DOI] [PubMed] [Google Scholar]
- 51.Field H.E., Mackenzie J.S., Daszak P. Henipaviruses: emerging paramyxoviruses associated with fruit bats. Curr Top Microbiol Immunol. 2007;315:133–159. doi: 10.1007/978-3-540-70962-6_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chua K.B., Chua B.H., Wang C.W. Anthropogenic deforestation, El Nino and the emergence of Nipah virus in Malaysia. Malays J Pathol. 2002;24:15–21. [PubMed] [Google Scholar]
- 53.Pulliam J.R.C., Epstein J.H., Dushoff J., Rahman S.A., Bunning M., Jamaluddin A.A., Hyatt A.D., Field H.E., Dobson A.P., Daszak P. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J R Soc Interface. 2012;9:89–101. doi: 10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webster R.G. Rapid review. Wet markets — a continuing source of severe acute respiratory syndrome and influenza? Lancet. 2004;363:234–236. doi: 10.1016/S0140-6736(03)15329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 56.Lam W.K., Zhong N.S., Tan W.C. Overview on SARS in Asia and the world. Respirology. 2003;8(Suppl):S2–S5. doi: 10.1046/j.1440-1843.2003.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Promed, Novel coronavirus — Saudi Arabia: human isolate. Archive Number: 20120920.1302733 2012.
- 58•.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.a.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012 doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]; A recent emergence of a coronavirus that has caused mortalities that phylogenetically is closely related to bat coronaviruses. Another example of international collaboration to rapidly identify a virus
- 59.Bermingham A., Chand M., Brown C., Aarons E., Tong C., Langrish C., Hoschler K., Brown K., Galiano M., Myers R. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:1–5. [PubMed] [Google Scholar]
- 60.Corman V., Eckerle I., Bleicker T., Zaki A., Landt O., Eschbach-Bludau M., van Boheemen S., Gopal R., Ballhause M., Bestebroer T. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17:1–6. doi: 10.2807/ese.17.39.20285-en. [DOI] [PubMed] [Google Scholar]
- 61.Promed: Novel coronavirus — Saudi Arabia (15): new case. Archive Number: 20121104.1391285 2012.
- 62.Plowright R.K., Field H.E., Smith C., Divljan A., Palmer C., Tabor G., Daszak P., Foley J.E. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus) Proc Biol Sci. 2008;275:861–869. doi: 10.1098/rspb.2007.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez J.P., Pourrut X., Leroy E. Ebolavirus and other filoviruses. Curr Top Microbiol Immunol. 2007;315:363–387. doi: 10.1007/978-3-540-70962-6_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Amman B.R., Carroll S.A., Reed Z.D., Sealy T.K., Balinandi S., Swanepoel R., Kemp A., Erickson B.R., Comer J.A., Campbell S. Seasonal pulses of marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 2012;8:e1002877. doi: 10.1371/journal.ppat.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]; Extensive study of Marburg virus in bats and the linking of virus shedding to waning antibody levels in juveniles and therefore increased risk to humans during this time
- 65.Wilkinson G.S., South J.M. Life history, ecology and longevity in bats. Aging Cell. 2002;1:124–131. doi: 10.1046/j.1474-9728.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 66.Li L., Victoria J.G., Wang C., Jones M., Fellers G.M., Kunz T.H., Delwart E. Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J Virol. 2010;84:6955–6965. doi: 10.1128/JVI.00501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ge X., Li Y., Yang X., Zhang H., Zhou P., Zhang Y., Shi Z. Metagenomic analysis of viruses from bat fecal samples reveals many novel viruses in insectivorous bats in China. J Virol. 2012;86:4620–4630. doi: 10.1128/JVI.06671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donaldson E.F., Haskew A.N., Gates J.E., Huynh J., Moore C.J., Frieman M.B. Metagenomic analysis of the viromes of three North American bat species: viral diversity among different bat species that share a common habitat. J Virol. 2010;84:13004–13018. doi: 10.1128/JVI.01255-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaye M. SARS-associated coronavirus replication in cell lines. Emerg Infect Dis. 2006;12:128–133. doi: 10.3201/eid1201.050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aljofan M., Saubern S., Meyer A.G., Marsh G., Meers J., Mungall B.A. Characteristics of Nipah virus and Hendra virus replication in different cell lines and their suitability for antiviral screening. Virus Res. 2009;142:92–99. doi: 10.1016/j.virusres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Westbury H.A., Hooper P.T., Selleck P.W., Murray P.K. Equine morbillivirus pneumonia: susceptibility of laboratory animals to the virus. Aust Vet J. 1995;72:278–279. doi: 10.1111/j.1751-0813.1995.tb03549.x. [DOI] [PubMed] [Google Scholar]
- 72.Van der Groen G., Webb P., Johnson K., Lange J., Linsday H., Eliott L. Ebola virus Haemorrhagic Fever. Elsevier/North-Holland Biomedical Press; Amsterdam: 1978. Growth of Lassa and Ebola viruses in different cell lines. 255–260. [Google Scholar]
- 73.Guyatt K.J., Twin J., Davis P., Holmes E.C., Smith G.A., Smith I.L., Mackenzie J.S., Young P.L. A molecular epidemiological study of Australian bat lyssavirus. J Gen Virol. 2003;84:485–496. doi: 10.1099/vir.0.18652-0. [DOI] [PubMed] [Google Scholar]
- 74.Arankalle V.A., Bandyopadhyay B.T., Ramdasi A.Y., Jadi R., Patil D.R., Rahman M., Majumdar M., Banerjee P.S., Hati A.K., Goswami R.P. Genomic characterization of Nipah virus, West Bengal, India. Emerg Infect Dis. 2011;17:907–909. doi: 10.3201/eid1705.100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marsh G.A., Todd S., Foord A., Hansson E., Davies K., Wright L., Morrissy C., Halpin K., Middleton D., Field H.E. Genome sequence conservation of Hendra virus isolates during spillover to horses, Australia. Emerg Infect Dis. 2010;16:1767–1769. doi: 10.3201/eid1611.100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Streicker D.G., Turmelle A.S., Vonhof M.J., Kuzmin I.V., McCracken G.F., Rupprecht C.E. Host phylogeny constrains cross-species emergence and establishment of rabies virus in bats. Science. 2010;329:676–679. doi: 10.1126/science.1188836. [DOI] [PubMed] [Google Scholar]
- 77.Brown C. Emerging zoonoses and pathogens of public health significance — an overview. Rev Sci Tech. 2004;23:435–442. doi: 10.20506/rst.23.2.1495. [DOI] [PubMed] [Google Scholar]
- 78.Svoboda T., Henry B., Shulman L., Kennedy E., Rea E., Ng W., Wallington T., Yaffe B., Gournis E., Vicencio E. Public health measures to control the spread of the severe acute respiratory syndrome during the outbreak in Toronto. N Engl J Med. 2004;350:2352–2361. doi: 10.1056/NEJMoa032111. [DOI] [PubMed] [Google Scholar]
- 79••.Knobler S., Mahmoud A., Lemon S., Mack A., Sivitz L., Oberholtzer K. National Academies Press; 2004. Learning from SARS: Preparing for the Next Disease Outbreak-Workshop Summary. [PubMed] [Google Scholar]; A good summary of the SARS outbreak and all the factors involved in the spread of the virus and its control
- 80.Francesconi P., Yoti Z., Declich S., Onek P.A., Fabiani M., Olango J., Andraghetti R., Rollin P.E., Opira C., Greco D. Ebola hemorrhagic fever transmission and risk factors of contacts, Uganda. Emerg Infect Dis. 2003;9:1430–1437. doi: 10.3201/eid0911.030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsang T., Lam T.H. SARS: public health measures in Hong Kong. Respirology. 2003;8(Suppl):S46–S48. doi: 10.1046/j.1440-1843.2003.00524.x. [DOI] [PubMed] [Google Scholar]
- 82.Mahalingam S., Herrero L.J., Playford E.G., Spann K., Herring B., Rolph M.S., Middleton D., McCall B., Field H., Wang L.-F. Hendra virus: an emerging paramyxovirus in Australia. Lancet Infect Dis. 2012;3099:1–9. doi: 10.1016/S1473-3099(12)70158-5. [DOI] [PubMed] [Google Scholar]
- 83.World Health Organization: Severe acute respiratory syndrome (SARS): status of the outbreak and lessons for the immediate future 2003.
- 84.Timen A., Koopmans M., Vossen A., van Doornum G., Gunther S., van den Berkmortel F., Verduin K., Dittrich S., Emmerich P., Osterhaus A. Response to imported case of Marburg hemorrhagic fever, the Netherlands. Emerg Infect Dis. 2009;15:1171–1175. doi: 10.3201/eid1508.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.World Health Organization: The World Health Report 2003. Shaping the future 2003.
- 86.Field H., Mackenzie J., Daszak P. Novel viral encephalitides associated with bats (Chiroptera) — host management strategies. Arch Virol Suppl. 2004;S18:113–121. doi: 10.1007/978-3-7091-0572-6_9. [DOI] [PubMed] [Google Scholar]
- 87.Daszak P., Plowright R.K., Epstein J.H., Pulliam J.R., Abdul Rahman S., Field H.E., Jamaluddin A., Sharifah S.H., Smith C.S., Olival K.J. The emergence of Nipah and Hendra virus: pathogen dynamics across wildlife livestock human continuum. In: Collinge S., Ray C., editors. Disease Ecology: Community Structure and Pathogen Dynamics. Oxford University Press; Oxford, United Kingdom: 2006. pp. 186–201. [Google Scholar]
- 88.Daniels P., Ksiazek T.G., Eaton B.T. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect. 2001;3:289–295. doi: 10.1016/s1286-4579(01)01382-x. [DOI] [PubMed] [Google Scholar]
- 89.Chua K.B. Epidemiology, surveillance and control of Nipah virus infections in Malaysia. Malays J Pathol. 2010;32:69–73. [PubMed] [Google Scholar]
- 90.Nahar N., Sultana R., Gurley E.S., Hossain M.J., Luby S.P. Date palm sap collection: exploring opportunities to prevent Nipah transmission. EcoHealth. 2010;7:196–203. doi: 10.1007/s10393-010-0320-3. [DOI] [PubMed] [Google Scholar]
- 91.Khan S.U., Gurley E.S., Hossain M.J., Nahar N., Sharker M.A.Y., Luby S.P. A randomized controlled trial of interventions to impede date palm sap contamination by bats to prevent Nipah virus transmission in Bangladesh. PLoS One. 2012;7:e42689. doi: 10.1371/journal.pone.0042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92••.Nahar N., Mondal U.K., Sultana R., Hossain M.J., Khan M.S., Gurley E.S., Oliveras E., Luby S.P. Piloting the use of indigenous methods to prevent Nipah virus infection by interrupting bats’ access to date palm sap in Bangladesh. Health Promot Int. 2012 doi: 10.1093/heapro/das020. [DOI] [PubMed] [Google Scholar]; Describes the implementation of practical solution to prevent the transmission of NiV in Bangladesh from contaminated date palm sap
- 93.Lim P.L., Kurup A., Gopalakrishna G., Chan K.P., Wong C.W., Ng L.C., Se-Thoe S.Y., Oon L., Bai X., Stanton L.W. Laboratory-acquired severe acute respiratory syndrome. N Engl J Med. 2004;350:1740–1745. doi: 10.1056/NEJMoa032565. [DOI] [PubMed] [Google Scholar]
- 94.Adjemian J., Farnon E.C., Tschioko F., Wamala J.F., Byaruhanga E., Bwire G.S., Kansiime E., Kagirita A., Ahimbisibwe S., Katunguka F. Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007. J Infect Dis. 2011;204(Suppl):S796–S799. doi: 10.1093/infdis/jir312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bausch D.G., Borchert M., Grein T., Roth C., Swanepoel R., Libande M.L., Talarmin A., Bertherat E., Muyembe-Tamfum J.-J., Tugume B. Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo. Emerg Infect Dis. 2003;9:1531–1537. doi: 10.3201/eid0912.030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beer B., Kurth R., Bukreyev A. Characteristics of Filoviridae: Marburg and Ebola viruses. Naturwissenschaften. 1999;86:8–17. doi: 10.1007/s001140050562. [DOI] [PubMed] [Google Scholar]
- 97.Luby S.P., Gurley E.S., Hossain M.J. Transmission of human infection with Nipah virus. Clin Infect Dis. 2009;49:1743–1748. doi: 10.1086/647951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Homaira N., Rahman M., Hossain M.J., Epstein J.H., Sultana R., Khan M.S.U., Podder G., Nahar K., Ahmed B., Gurley E.S. Nipah virus outbreak with person-to-person transmission in a district of Bangladesh, 2007. Epidemiol Infect. 2010;138:1630–1636. doi: 10.1017/S0950268810000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chua K.B., Lam S.K., Goh K.J., Hooi P.S., Ksiazek T.G., Kamarulzaman A., Olson J., Tan C.T. The presence of Nipah virus in respiratory secretions and urine of patients during an outbreak of Nipah virus encephalitis in Malaysia. J Infect. 2001;42:40–43. doi: 10.1053/jinf.2000.0782. [DOI] [PubMed] [Google Scholar]
- 100.Chua K.B. Nipah virus outbreak in Malaysia. J Clin Virol. 2003;26:265–275. doi: 10.1016/s1386-6532(02)00268-8. [DOI] [PubMed] [Google Scholar]
- 101.Chua K.B., Crameri G., Hyatt A., Yu M., Tompang M.R., Rosli J., McEachern J., Crameri S., Kumarasamy V., Eaton B.T. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc Natl Acad Sci U S A. 2007;104:11424–11429. doi: 10.1073/pnas.0701372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chua K.B., Voon K., Yu M., Keniscope C., Abdul Rasid K., Wang L.-F. Investigation of a potential zoonotic transmission of orthoreovirus associated with acute influenza-like illness in an adult patient. PLoS One. 2011;6:e25434. doi: 10.1371/journal.pone.0025434. [DOI] [PMC free article] [PubMed] [Google Scholar]


