Abstract
Antimicrobial stewardship program aims to reduce antibiotic use. Periodic measurement and monitoring of antibiotic use and comparison within the institution as well as with other organizations are important indicators. We analyzed antibiotic usage in a general hospital in Saudi Arabia. Antibiotic data were collected retrospectively for 2011 and from 2013 to 2015, and only adult patients (>15 year of age) were included in the study. Data were presented as days of therapy (DOT) and defined daily dose (DDD). DDD was adjusted per 100 bed-days and according to the case mix index (CMI). The total DDD was 37,557 in 2013, 36,550 in 2014 and 38,738 in 2015. The DDD per 100 patient-days was 90.7–94.5. There was a discordant findings of antibiotic measurements based on the DDD compared to DOT, and DDD/100 bed-days compared to DOT/100 bed-days. There was a negative correlation between CMI and DDD per 100 bed days (r −0.696), but a positive correlation of CMI with DOT (r +0.93). Adjusted DDD/100 bed-days showed decrease in the usage of antibiotics, reflecting activities of the antibiotic stewardship program. The increase in DOT/100 bed-days may indicate the favorable utilization of combination therapy. Antibiotic usage needs to be adjusted per 100 bed-days and correlated with CMI for better reflection of optimal antibiotic utilization, activities of the antibiotic stewardship program, and to allow benchmarking.
Keywords: DDD,; Antibiotic stewardship; Benchmarking; Days of therapy; DOT
Introduction
It is estimated that 20–50% of antibiotic use is inappropriate or unnecessary in acute care hospitals [1]. Inappropriate antimicrobial use has a negative impact on patients and the entire community [2], [3]. Misuse of antibiotics leads to an increase in antibiotic resistance and additional healthcare costs [1], [4]. Unfortunately, we are facing a dramatic increase in bacterial resistance with the obvious drop in the number of antibiotics discovered and approved each year [5], [6]. Therefore the antimicrobial stewardship program (ASP) was developed to ensure the proper use of antibiotics, reduce overutilization of antimicrobial agents, and halt the development of resistance [3], [4].
At Johns Hopkins Aramco Health (JHAH), we started an ASP in 2011 with an educational program of physicians and pharmacist. The program was enhanced mid-2012 with the following interventions: a re-designed antibiotic sensitivity report [2], intravenous to oral conversion program, vancomycin pharmacokinetic program [7], automatic renal dosing, antibiotic de-escalation, pre-operative antibiotic protocols utilizing adapted orders, and a multi-facteted approach to decrease antibiotics for respiratory tract infections [8]. The program incorporated “if you cannot measure it, you cannot improve it,” and thus included periodic measurement and monitoring of antibiotic use, and comparing data within the institution and with other institutions [1], [9].
To standardize the units for comparison, we used the most common definitions: defined daily dose (DDD), and days of therapy (DOT). DDD is as an average of the maintenance dose of a single antibiotic in its main indication for adults per day [10]. According to the World Health Organization (WHO), each drug has an anatomical therapeutics chemical (ATC) code and a DDD value in grams [10]. To define the exact consumption rate, it was recommended to express DDD per 100 bed-days in hospitals and DDDs per 1000 inhabitant-days for out-patients [11], [12], [13]. DOT is the number of days that a patient receives an antibiotic regardless of the dose [1]. DDD has better estimation than DOT, especially in patients receiving a combination of antibiotics or one dose only (e.g., surgical prophylaxis), and it can be calculated even in the absence of a computerized pharmacy system [1].
A better measure for comparing DDD with an external hospital, is the quantitative assessment of antibiotics use, with adjustment for severity of illness among hospitalized patients, using the case mix index (CMI) [11], [14]. There are limited studies of antibiotic utilization in Saudi Arabia [15]. In this study, we compare the DDD, DOT, DDD per 100 bed-days, and the adjusted DDD according to CMI.
Materials and methods
The study was carried out at Dhahran Health Center as a part of Johns Hopkins Aramco Healthcare (JHAH), which serves a population of approximately 370,000 patients [16]. Dhahran Health Center is the main general hospital with a 380-bed capacity and five intensive care units (Cardiac, medical, surgical, pediatric, and neonatal) [16]. The hospital provides acute, general medicine and surgery, intensive care services, and management of hematological and solid organ malignancies [16].
A computerized database was generated for all prescribed antibiotics. Antibiotic data were collected for 2011 to have a baseline, and then retrospectively for 2013–2015. All data were collected for the first 6 months of 2011, 2013–2015. To minimize the drawback of using DDD, only adult patients (above 15 years of age) were included in the study. The data were transferred to an Excel spreadsheet. The World Health Organization 2013 Guidelines for Anatomical Therapeutic Chemical/Defined Daily Dose (ATC/DDD) were utilized for the calculation of DDD. DDD for each antibiotic was calculated separately, the total number of grams administered for each antibiotic per year was divided by the WHO DDD in grams [10]. Thus, the DDD is an estimate of the number of days of antibiotic therapy. Data of day surgery was not included in calculating the bed days. Antibiotic usage was adjusted per 100 bed-days by dividing DDD by daily occupied beds and multiplied by 100. A direct measure of the number of days of therapy (DOTs) is a common method of antibiotic usage evaluation. DOT is simply the sum of the total number of days of all used antibiotics. Thus, when the same patient receives more than one antibiotic, more than one DOT was counted.
The case mix index (CMI), an economic surrogate marker, was calculated by dividing total cost weights for all inpatient in specific period by the number of admission [17], as provided by the hospital information department. CMI describes the average patients' morbidity of individual hospitals [17].
Results
Antibiotic data were collected for 2011 as a baseline, and for subsequent years 2013, 2014, and 2015. The collected data were expressed as DDD and DOT and were adjusted per 100 patient-days.
Defined daily dose (DDD) and days of therapy (DOT)
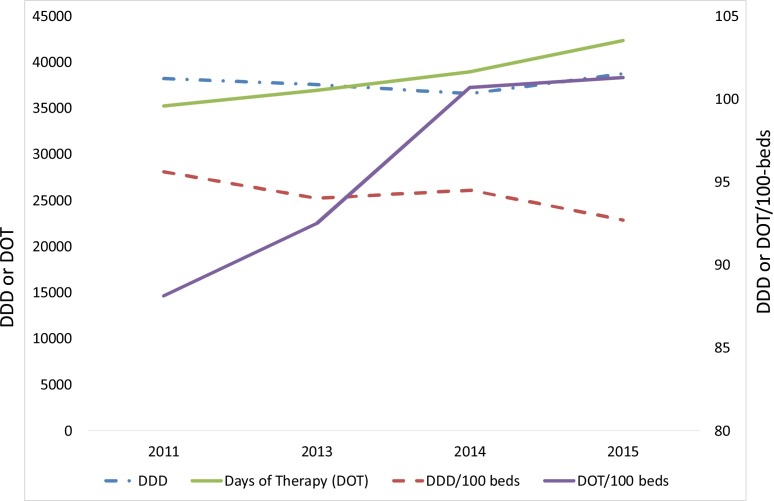
The total DDD was 38,270 in 2011, 37,557 in 2013, 36,550 in 2014, and 38,738 in 2015 (Fig. 1 ). However, there was no significant increase in the DDD overtime with an correlation coefficient (R2) trend of 0.006. A reduction in the DDD was more pronounced if we excluded antimicrobials given to suspected MERS-CoV patients during the 2014 outbreak [18] where the DDD decreased from 38,738 to 35,942. On the other hand, days of therapy (DOT) were 35,218, 36,958, 38,945 and 42,326 days at base line and in the years 2013–2015, respectively (Fig. 1) with a significant R2 trend of 0.973.
Fig. 1.
A line graph showing the DDD and DOT (left Y-axis) and the DDD/100 bed-days (right Y axis) plotted over the year.
DDD; Defined Daily Dose; DOT; Days Of Therapy
Adjusted DDD per 100 bed-days and based on case mix index (CMI)
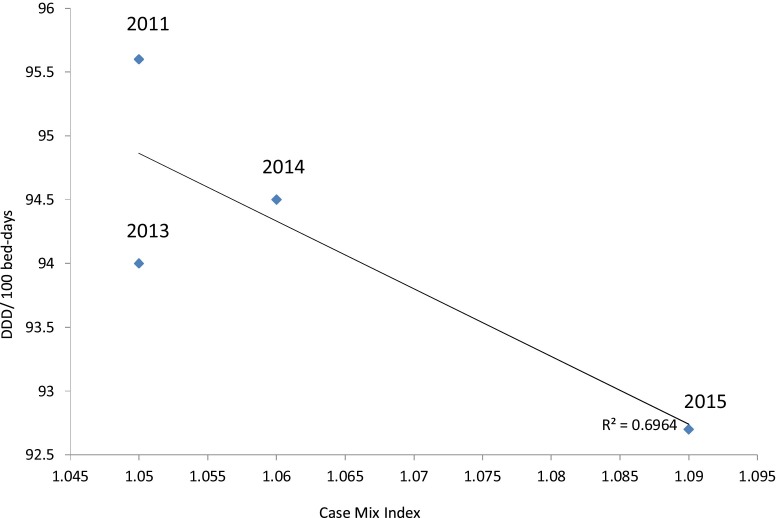
DDD showed a non-significant increase in 2015 compared to baseline. To avoid variation between different hospital settings and to allow comparison with similar hospitals, we reported DDD and DOT per 100 bed-days, as well as the adjustment of DDD/100 bed-days in relation to CMI. Data for DDD/100 bed-days showed a slight negative correlation with an R2 of −0.775 whereas there was a clear positive correlation in the case of DOT/100 bed-days with an R2 of 0.918. Adjusted for the bed-days and the CMI, the DDD/100 bed-days is shown in Fig. 2 . The correlation between DDD/100 bed-days and CMI was −0.696. A blot of the DDD/100 bed-days against the CMI showed that DDD/100 bed-days falls within the benchmark in relation to the CMI, based on data by Kuster et al. [17]. A total reduction in antibiotic usage after adjustment using 100 bed-days and CMI was 5.13% (from 95.6 to 90.7 between the baseline in 2011 and year 2015) (Fig. 2).
Fig. 2.
A plot graph showing risk adjustment of DDD/100 bed-days in relation to the chronic medical index (CMI).
Numbers on the graph (2011–2015) represent the year.
Discussion
Evaluation of antimicrobial usage is standardized using the DDD as a recommended strategy for comparison with similar hospitals [13]. The use of risk adjustment is needed to overcome the challenge of benchmarking. Two patient characteristics are associated with clinical outcomes and use of the healthcare system. These factors are: patient mix and severity of illness [19], [20]. Direct comparison of the quantity of antibiotic consumed between hospitals is flawed, due to multiple factors, such as severity of illness, structures, and missions [17]. We reported antibiotic consumption per 100 bed-days for inpatients, to overcome the variation in hospital size and occupancy rate. We also adjusted antibiotic usage using the case mix index (CMI), to reduce the variation of patient morbidity among hospitals. CMI is a tool for comparison and had been shown to have a moderate correlation with antibiotic use [17], [21].
The use of DDD estimates indirectly the actual DOT [19], [20]. The measurement of DOT is insensitive to the actual dosages administered. And DOT measurement favors those situations where broad spectrum monotherapy is used. One of the drawbacks of DDD measurement is related to patients with renal or hepatic dysfunction needing adjustment [22]. In this report, we observed a discordant findings of antibiotic measurements based on the DDD and DOT and a discordant findings of antibiotic measurements based on DDD/100 bed-days compared to DOT/100 bed-days. Similarly, previous studies showed discordant measurements between DDD and DOT [22], [23], [24], [25], [26]. The DDD method is useful for benchmarking, but not for evaluation of the number of DOTs or relative use for antibacterial agents [27]. And that DOT is more difficult to measure, but is a superior measurement methodology [27].
Reporting DDD data could be misleading and may not reflect the activity of the ASP, because other factors may interfere, such as hospital days and the morbidity of admitted patients. This findings is obvious in this report where DDD increased and the DDD per 100 bed days decreased in 2015 compared to the baseline. The adjusted DDD/100 bed-days showed a decrease in the usage of antibiotics, reflecting activities of the antibiotic stewardship program. Measuring DDDs per 1000 patient-days and DOTs per 1000 patient-days (or 100 patient-days) may be required especially in relation to antimicrobial resistance [27]. Further adjustment of DDD/100 bed-days based on CMI in our study showed a negative correlation with increasing CMI, reflecting the efficacy of ASP activities in our institute. The reduction of 5.13% of DDD adjusted to 100 bed-days, and correlation with CMI, gave us an indicator of the ASP’s success. Similarly, previous studies showed a strong correlation between CMI and antibacterial usage expressed as DDD/100 patient days [17]. However, increasing CMI may lead to increased combination therapy as shown by an increase in the DOT and DOT/100 bed-days. This increase in combination therapy could be also the target of future antimicrobial stewardship program, particularly targeting deescalation from combination therapy to monotherapy.
In conclusion, antibiotic usage needs to be adjusted per 100 bed-days, and correlated with CMI, for better reflection of ASP activities, and to allow benchmarking with other organizations. It is important to monitor DDD and the addition of DOT monitoring may also shed light on the pattern of antibiotic usage in the form of combination versus monotherapy.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Approved by the JHAH IRB.
References
- 1.Pollack L.A., Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis. 2014;59(Suppl. 3):S97–100. doi: 10.1093/cid/ciu542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Tawfiq J.A., Momattin H., Al-Habboubi F., Dancer S.J. Restrictive reporting of selected antimicrobial susceptibilities influences clinical prescribing. J Infect Public Health. 2015;8:234–241. doi: 10.1016/j.jiph.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Owens R.C. Antimicrobial stewardship: concepts and strategies in the 21st century. Diagn Microbiol Infect Dis. 2008;61:110–128. doi: 10.1016/j.diagmicrobio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 4.MacDougall C., Polk R.E. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18:638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Society for Healthcare Epidemiology of America N, Infectious Diseases Society of America, Pediatric Infectious Diseases Society Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS) Infect Control Hosp Epidemiol. 2012;33:322–327. doi: 10.1086/665010. [DOI] [PubMed] [Google Scholar]
- 6.Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 7.Momattin H., Zogheib M., Homoud A., Al-Tawfiq J.A. Safety and outcome of pharmacy-led vancomycin dosing and monitoring. Chemotherapy. 2016;61:3–7. doi: 10.1159/000440607. [DOI] [PubMed] [Google Scholar]
- 8.Al-Tawfiq J.A., Alawami A.H. A multifaceted approach to decrease inappropriate antibiotic use in a pediatric outpatient clinic. Ann Thorac Med. 2017;12:51–54. doi: 10.4103/1817-1737.197779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen I.-L., Lee C.-H., Su L.-H., Tang Y.-F., Chang S.-J., Liu J.-W. Antibiotic consumption and healthcare-associated infections caused by multidrug-resistant gram-negative bacilli at a large medical center in Taiwan from 2002 to 2009: implicating the importance of antibiotic stewardship. PLoS One. 2013;8:e65621. doi: 10.1371/journal.pone.0065621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . 2016. WHOCC — definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/ [Accessed 7 January 2017] [Google Scholar]
- 11.Hutchinson J.M., Patrick D.M., Marra F., Ng H., Bowie W.R., Heule L. Measurement of antibiotic consumption: a practical guide to the use of the Anatomical Thgerapeutic Chemical classification and Defined Daily Dose system methodology in Canada. Can J Infect Dis. 2004;15:29–35. doi: 10.1155/2004/389092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pope S.D., Dellit T.H., Owens R.C., Hooton T.M., Infectious Diseases Society of America, Society for Healthcare Epidemiology of America Results of survey on implementation of Infectious Diseases Society of America and Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Infect Control Hosp Epidemiol. 2009;30:97–98. doi: 10.1086/592979. [DOI] [PubMed] [Google Scholar]
- 13.Dellit T.H., Owens R.C., McGowan J.E., Gerding D.N., Weinstein R.A., Burke J.P. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 14.Kanerva M., Ollgren J., Lyytikainen O., Agthe N., Mottonen T., Kauppinen M. Benchmarking antibiotic use in Finnish acute care hospitals using patient case-mix adjustment. J Antimicrob Chemother. 2011;66:2651–2654. doi: 10.1093/jac/dkr333. [DOI] [PubMed] [Google Scholar]
- 15.Al-Tawfiq J.A. Changes in the pattern of hospital intravenous antimicrobial use in Saudi Arabia, 2006–2008. Ann Saudi Med. 2012;32:517–520. doi: 10.5144/0256-4947.2012.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Tawfiq J.A. Distribution and epidemiology of Candida species causing fungemia at a Saudi Arabian hospital, 1996–2004. Int J Infect Dis. 2007;11:239–244. doi: 10.1016/j.ijid.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Kuster S.P., Ruef C., Bollinger A.K., Ledergerber B., Hintermann A., Deplazes C. Correlation between case mix index and antibiotic use in hospitals. J Antimicrob Chemother. 2008;62:837–842. doi: 10.1093/jac/dkn275. [DOI] [PubMed] [Google Scholar]
- 18.Al-Tawfiq J.A., Hinedi K., Ghandour J., Khairalla H., Musleh S., Ujayli A. Middle East Respiratory Syndrome-Coronavirus (MERS-CoV): a case-control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim O.M., Polk R.E. Benchmarking antimicrobial drug use in hospitals. Expert Rev Anti Infect Ther. 2012;10:445–457. doi: 10.1586/eri.12.18. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim O.M., Polk R.E. Antimicrobial use metrics and benchmarking to improve stewardship outcomes. Infect Dis Clin North Am. 2014;28:195–214. doi: 10.1016/j.idc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Mylotte J.M., Keagle J. Benchmarks for antibiotic use and cost in long-term care. J Am Geriatr Soc. 2005;53:1117–1122. doi: 10.1111/j.1532-5415.2005.53351.x. [DOI] [PubMed] [Google Scholar]
- 22.Mandy B., Koutny E., Cornette C., Woronoff-Lemsi M.-C., Talon D. Methodological validation of monitoring indicators of antibiotics use in hospitals. Pharm World Sci. 2004;26:90–95. doi: 10.1023/b:phar.0000018595.78732.1c. [DOI] [PubMed] [Google Scholar]
- 23.Muller A., Monnet D.L., Talon D., Hénon T., Bertrand X. Discrepancies between prescribed daily doses and WHO defined daily doses of antibacterials at a university hospital. Br J Clin Pharmacol. 2006;61:585–591. doi: 10.1111/j.1365-2125.2006.02605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kern W.V., Steib-Bauert M., de With K., Reuter S., Bertz H., Frank U. Fluoroquinolone consumption and resistance in haematology–oncology patients: ecological analysis in two university hospitals 1999–2002. J Antimicrob Chemother. 2005;55:57–60. doi: 10.1093/jac/dkh510. [DOI] [PubMed] [Google Scholar]
- 25.Zagorski B.M., Trick W.E., Schwartz D.N., Wisniewski M.F., Hershow R.C., Fridkin S.K. The effect of renal dysfunction on antimicrobial use measurements. Clin Infect Dis. 2002;35:1491–1497. doi: 10.1086/344753. [DOI] [PubMed] [Google Scholar]
- 26.Shetka M., Pastor J., Phelps P. Evaluation of the defined daily dose method for estimating anti-infective use in a university hospital. Am J Health Syst Pharm. 2005;62:2288–2292. doi: 10.2146/ajhp050140. [DOI] [PubMed] [Google Scholar]
- 27.Polk R.E., Fox C., Mahoney A., Letcavage J., MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664–670. doi: 10.1086/511640. [DOI] [PubMed] [Google Scholar]