Abstract
Background
Respiratory viral infections (RVI) are a leading cause of mortality worldwide. We compared the epidemiology and severity of RVI in Ecuador during 2009–2016.
Methods
Respiratory specimens collected within the national surveillance system were tested for influenza viruses, respiratory syncytial virus (RSV), adenovirus, parainfluenza virus, and human metapneumovirus. Overall and virus-specific positive detection rate (PDR) were calculated and compared the timing of epidemics caused by the different viruses. Logistic regression models were used to compare the age distribution and risk of death across respiratory viruses.
Results
A total of 41,172 specimens were analyzed: influenza (PDR = 14.3%) and respiratory syncytial virus (RSV) (PDR = 9.5%) were the most frequently detected viruses. Influenza epidemics typically peaked in December–January and RSV epidemics in March; seasonality was less evident for the other viruses. Compared to adults, children were more frequently infected with RSV, adenovirus, parainfluenza, and influenza B, while the elderly were less frequently infected with influenza A(H1N1)p. The age-adjusted risk of death was highest for A(H1N1)p (odds ratio [OR] 1.73, 95% confidence intervals [CI] 1.38–2.17), and lowest for RSV (OR 0.75, 95%CI 0.57–0.98).
Conclusions
Whilst influenza and RSV were the most frequently detected pathogens, the risk of death differed by RVI, being highest for pandemic influenza and lowest for RSV.
Abbreviations: BRaVe, Battle against Respiratory Viruses; CFR, Case-fatality ratio; ILI, Influenza-like illness; INSPI, National institute of public health research; PDR, Positive detection rate; RRR, Relative risk ratio; RSV, Respiratory syncytial virus; RT-PCR, Real-time polymerase chain reaction; RVI, Respiratory viral infections; SARI, Severe acute respiratory infection; CDC, Centers for disease control and prevention; WHO, World Health Organization
Keywords: Respiratory viral infections, Epidemiology, Age distribution, Case-fatality ratio, Ecuador
Introduction
Respiratory viral infections (RVIs) are usually mild and tend to resolve spontaneously without treatment, but they can be exacerbated by superimposed bacterial pneumonia, bronchitis, otitis media and other severe complications, and can require hospitalization and be life-threatening, especially among the very young, the elderly, and subjects with underlying medical conditions [1], [3]. Because of their widespread diffusion and potentially severe complications, RVIs are a leading cause of mortality and disability worldwide, particularly in developing countries and among children under five years of age [3], [4].
The causal agents of RVIs include influenza viruses, respiratory syncytial virus (RSV), parainfluenza viruses, adenovirus, and others [1], [2], [3]. Most RVIs typically present with nonspecific symptoms like cough, fever and rhinorrhoea, but the causal viruses may largely differ between one another in terms of epidemiology, temporal appearance throughout the year, age distribution, and severity [5]. In addition, while influenza can be prevented by vaccination and treated with antivirals, there are currently no vaccines or therapeutics of proven efficacy for most other respiratory viruses [6], [7], [8].
In 2012, the World Health Organization (WHO) launched the Battle against Respiratory Viruses (BRaVe) initiative [3], [6] with the aim of tackling the burden of disease of RVIs globally. The BRaVe initiative identified priority research tracks and topic areas for future RVIs studies, and stressed the need to better define the epidemiology and burden of disease of specific RVIs across age spectrum and settings as a pre-requisite for implementing effective public health interventions [3]. This is particularly important in low- and middle-income tropical countries, where RVIs place a massive burden on relatively fragile healthcare systems and have the highest socioeconomic impact.
Ecuador is a tropical country located in Southern America with a total population of about 16.3 million inhabitants. Influenza epidemiology in Ecuador has been previously described [9], [10], but a comparative analysis of RVIs caused by different viruses is still lacking. Consistent with the BRaVE research line, we presented here a comparative study of the epidemiology, age distribution and severity of acute RVIs in Ecuador during 2009–2016.
Materials and methods
Ecuador is crossed by the equator and divided into four regions: the Coast, Sierra and Oriente in the mainland, and the Galápagos Islands in the Pacific. Most of Ecuador’s population lives in the Coast (52.6%) and Sierra (42.1%) regions; the median age is 27.7 years [11].
Surveillance of respiratory viral infections in Ecuador
Respiratory virus surveillance data from 2009 to 2016 were available for the analysis. There were two major changes in the surveillance system over that period. Data were initially (weeks 1–19 in 2009) collected from influenza-like illness (ILI) or severe acute respiratory infection (SARI) patients swabbed in only four provinces in the country. Because of the short duration, limited geographical coverage, and small sample size (679 specimens overall), data from this period were not used in the analyses. From week 20/2009 to week 9/2011, a universal surveillance scheme was implemented whereby all ILI and SARI patients presenting at all hospitals, local health units, airports and harbours in the country were swabbed. Finally, from week 10/2011 until the end of the study period, a sentinel surveillance scheme for SARI was in place that included selected hospitals and local health units in the whole country.
During 2011–2016 an event-based surveillance system was also in place that collected information on “unusual” respiratory events posing a potential risk to public health [13]. These were defined as hospitalized or deceased SARI cases matching at least one of the following criteria: aged 5–64 years with no comorbidities; healthcare workers; swine and poultry workers; recent travels to sites with transmission of highly pathogenic respiratory agents; or belonging to a SARI cluster. These “unusual” SARI cases were left in the database since they provided <4% of all specimens and their exclusion did not alter the results.
An ILI case was defined as a person with sudden onset of fever (>38 °C), and cough or sore throat, in the absence of an alternative cause. SARI cases were defined as subjects aged ≥5 years with sudden onset of fever (>38 °C), any of cough, sore throat, or difficulty breathing, and requiring hospital admission; or children aged <5 years with suspected severe pneumonia and requiring hospitalization [12].
Laboratory methods
The following respiratory viruses were searched: influenza (pandemic AH1N1, AH3N2, B), RSV, adenovirus, parainfluenza virus (type 1, 2 and 3), and (since 2014) human metapneumovirus (hMPV). The detection of respiratory viruses was made using nasal swabs. Laboratory tests were conducted at the National Influenza Reference Laboratory in Guayaquil and in the two regional laboratories in Quito and Cuenca.
The detection of influenza viruses was performed using real-time polymerase chain reaction (RT-PCR) in accordance to the protocol of the US Centers for Disease Control and Prevention [14]. The viral RNA was extracted using commercially available kits (Quick-RNA™ Viral, Zymo Research, and QIAamp Viral RNA Mini Kit, QIAGEN) according to the instructions provided by the manufacturers. Specimens whose crossing point in the amplification curve was between 16 and 37 were considered as positive. The detection of respiratory viruses other than influenza was made using direct immunofluorescence assays (Light Diagnostics™ Respiratory Viral Screen and Identification DFA Kit, Millipore; and D3 Ultra™ DFA Respiratory Virus Screening and ID Kit, Diagnostic Hybrids).
Statistical analysis
We retrospectively analysed data collected during 2009–2016 within the nationwide surveillance system for respiratory viral infections in Ecuador. Different statistical approaches were adopted in order to answer specific study questions.
The overall and virus-specific positive detection rate (PDR) were defined as the proportion of specimens testing positive on at least one or, respectively, each given respiratory virus. Patients infected with parainfluenza virus type 1, 2 and 3 were analyzed together due to limited numbers. Overall and virus-specific PDR were calculated overall and separately by: surveillance scheme, patients’ age group (0–1, 2–5, and 6–11 months; 1–4, 5–17, 18–39, 40–64 and ≥65 years), gender, and region (Coast and Sierra; Oriente and Galápagos Islands were not considered because of the limited number of samples).
Average monthly virus-specific PDR were calculated by averaging over the years 2010–2016 (data from 2009 were not used for this analysis because of the AH1N1 influenza pandemic) in order to compare the timing of epidemics caused by each respiratory virus (except of adenovirus and hMPV because of the limited number of cases).
A multinomial logistic regression model was fitted to compare the age distribution of patients infected with each respiratory viruses. The multinomial logistic regression model generalizes binary logistic regression to problems where the dependent variable is nominal with more than two values. Here, the dependent variable was the result of the diagnostic test (with “no virus detected” as the reference), and the patient’s age was the independent variable; the model was further adjusted for year, type of surveillance scheme, region, and gender. The distribution of the dependent variable was compared across age groups using patients aged 18–39 years (young adults) as the reference group. The regression coefficients are expressed as relative risk ratio (RRR), which should be interpreted as follows: a RRR equal to 1.43 for influenza B among patients aged 5–17 years means that their relative risk of testing positive for influenza B (instead of testing negative) is 43% higher than among young adults.
Multivariable logistic regression models were used to evaluate the risk of death among patients infected with each respiratory virus, using test-negative patients as reference. Odds ratio were adjusted by age group and gender, year, type of surveillance scheme, and region. This analysis used data from 2011 to 2016 only as information on outcome was not available for the previous period.
Statistical analyses were conducted using Stata version 14 (Stata Corp, College Station, TX). All tests were two-sided; a test with a p-value below 0.05 was considered as statistically significant.
Ethical aspects and consent
An oral informed consent is obtained from each patient at the moment of swab taking. In accordance with applicable laws and regulations, no clearance of an Ethics Committee is required in Ecuador for the retrospective analysis of anonymised data collected within routine influenza surveillance schemes.
Results
A total of 41,172 specimens were collected, of which 12,765 (31.0%) were from ILI and SARI patients sampled between weeks 20/2009 and 09/2011 (“universal” surveillance scheme), and 28,407 (69.0%) from SARI patients sampled between week 10/2011 and week 52/2016 (“sentinel” surveillance scheme) (Table 1 ). In terms of age distribution, 27.7% and 31.3% of specimens were from children aged less than 1 year or 1–4 years, respectively; whereas 8.3% of specimens were taken among patients aged ≥65 years. Males and females provided 54.1% and 45.9% of all specimens, respectively. Almost all of the specimens were collected in the Sierra (51.5%) and in the Coast (47.3%) regions.
Table 1.
Viral aetiology of respiratory infections according to type of surveillance scheme, country region, and patients’ age group and gender. Ecuador, 2009–2016.
| No. specimens | A(H1N1)p | A(H3N2) | A other/not subtyped | B | RSV | Parainfluenza | Adenovirus | hMPVa | Co-infectionsb | No virus | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 41,172 | 9.8% (9.5–10.1%) | 2.6% (2.5–2.8%) | 0.7% (0.6–0.8%) | 1.2% (1.1–1.3%) | 9.5% (9.2–9.8%) | 1.8% (1.7–1.9%) | 0.6% (0.5–0.7%) | 0.5% (0.4–0.6%) | 0.3% (0.2–0.4%) | 73.2% (72.8–73.6%) |
| Surveillance scheme and regionc | |||||||||||
| Universal surveillanced | 12,765 | 20.3% | 3.0% | 1.8% | 0.1% | 4.4% | 1.0% | 0.4% | – | 0.1% | 68.7% |
| Coast | 8138 | 13.2% | 3.4% | 2.6% | 0.2% | 6.9% | 1.5% | 0.7% | – | 0.2% | 71.3% |
| Sierra | 4251 | 33.1% | 2.3% | 0.3% | 0.0% | 0.1% | 0.1% | 0.0% | – | 0.0% | 64.1% |
| Sentinel surveillanced | 28,407 | 5.1% | 2.4% | 0.1% | 1.7% | 11.7% | 2.2% | 0.6% | 0.6% | 0.3% | 75.2% |
| Coast | 11,317 | 2.7% | 1.7% | 0.2% | 1.0% | 14.0% | 2.5% | 0.5% | 0.2% | 0.3% | 77.0% |
| Sierra | 16,935 | 6.7% | 3.0% | 0.1% | 2.2% | 10.3% | 2.0% | 0.7% | 0.9% | 0.2% | 73.9% |
| Gendere | |||||||||||
| Male | 22,228 | 9.6% | 2.5% | 0.7% | 1.2% | 10.1% | 1.9% | 0.6% | 0.5% | 0.3% | 72.6% |
| Female | 18,880 | 9.9% | 2.7% | 0.6% | 1.2% | 8.8% | 1.7% | 0.5% | 0.4% | 0.2% | 73.9% |
| Age groupf | |||||||||||
| 0–1 months | 2653 | 0.8% | 1.2% | 0.4% | 0.4% | 22.5% | 2.1% | 0.2% | 0.2% | 0.3% | 72.0% |
| 2–5 months | 4440 | 1.1% | 1.0% | 0.1% | 1.0% | 18.9% | 2.7% | 0.5% | 0.5% | 0.4% | 73.7% |
| 6–11 months | 4314 | 2.4% | 2.3% | 0.1% | 1.1% | 18.4% | 3.2% | 0.7% | 0.6% | 0.5% | 70.7% |
| 1–4 years | 12,884 | 5.4% | 2.7% | 0.9% | 1.1% | 11.7% | 2.4% | 1.0% | 0.7% | 0.3% | 73.8% |
| 5–17 years | 4603 | 19.4% | 3.0% | 1.1% | 1.8% | 2.2% | 1.1% | 0.5% | 0.2% | 0.1% | 70.5% |
| 18–39 years | 4726 | 24.1% | 3.0% | 0.8% | 1.1% | 0.4% | 0.4% | 0.0% | 0.1% | 0.0% | 70.0% |
| 40–64 years | 4115 | 21.8% | 3.2% | 0.7% | 1.3% | 0.5% | 0.4% | 0.1% | 0.3% | 0.0% | 71.7% |
| 65 + years | 3424 | 7.2% | 4.0% | 0.4% | 1.8% | 1.0% | 1.0% | 0.3% | 0.1% | 0.1% | 84.2% |
RSV: Respiratory syncytial virus. hMPV: Human metapneumovirus.
hMPV was searched for only in 2014–2016.
It includes specimens that tested positive to more than one respiratory virus.
Not shown because of limited numbers: 406 specimens from the Oriente region and 93 specimens from the Galápagos Islands. Information on the region was not available for 32 specimens.
“Universal” surveillance scheme: ILI and SARI patients seen at all hospitals, local health units, airports and harbours in the country from week 20/2009 to week 09/2011. “Sentinel” surveillance scheme: SARI patients seen at selected hospitals and health units from week 10/2011 to week 52/2016. See text for detail.
Information on patient’s gender was not available for 64 specimens.
Information on patient’s age was not available for 58 specimens.
The overall PDR during the study period was 26.8%: influenza (14.3%) and RSV (9.5%) were the most frequent causes of RVI, while parainfluenza virus, adenovirus and hMPV were respectively detected in 1.8%, 0.6% and 0.5% of respiratory specimens (Table 1). The most frequently detected influenza virus (sub)type was A(H1N1)p (9.5%), followed by A(H3N2) (2.6%) and B (1.2%). Circulation of viruses varied by period: during 2009–11, the overall PDR was 31.3% and the 2009 influenza pandemic strain was found in 20.3% of specimens, while during 2011–16, the overall PDR was 24.8% and RSV accounted for nearly half of positive specimens (11.7%). Differences emerged also by region, as the most frequently detected virus was RSV in the Coast (11.0% of all specimens) and the pandemic strain in the Sierra (12.0%). In contrast, there were no differences in the overall and virus-specific PDR by gender.
Temporal patterns of RVIs epidemics
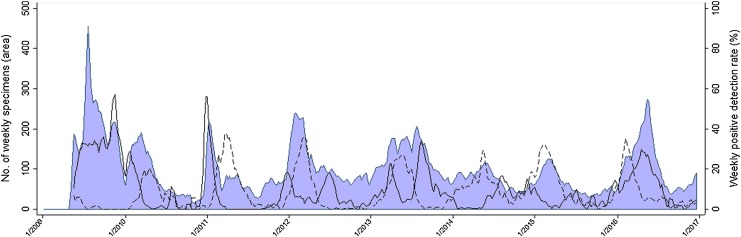
The weekly number of specimens, and the PDR for influenza (any type) and RSV, which were the two most frequently detected respiratory viruses, are shown in Fig. 1 . There appeared to be interference between the epidemics caused by influenza and RSV, as the epidemics caused by those two viruses never peaked in the same period, RSV epidemics often started when the number of influenza cases had begun to decline, and vice versa. For instance, the peak in RSV-specific PDR occurring in early March 2012 was preceded and followed by influenza epidemics, that peaked in January and June of the same year, and were caused by A(H3N2) and B influenza virus, respectively.
Fig. 1.
Number of weekly respiratory specimens (blue area) and positive detection rate for influenza (solid line) and respiratory syncytial virus (dashed line). Ecuador, 2009–2016.
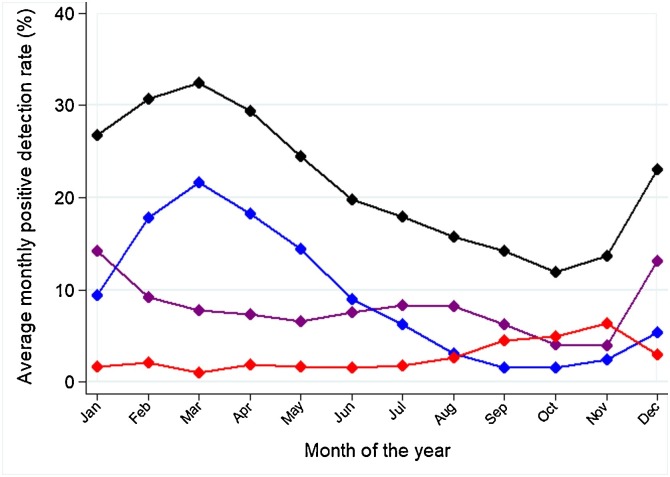
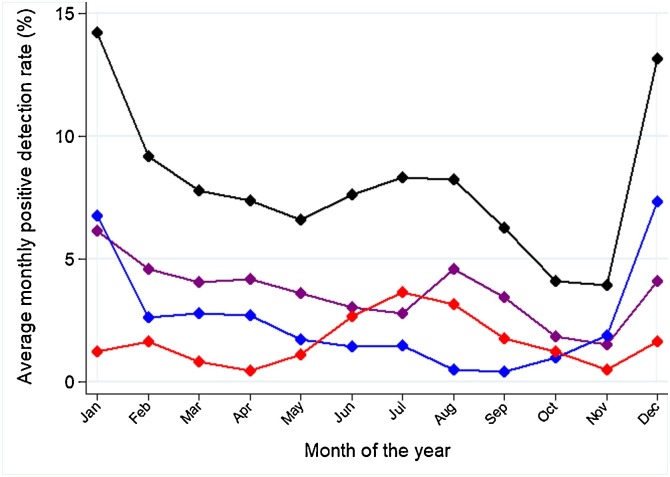
The average monthly PDR differed between respiratory viruses (Fig. 2 ). The most distinct seasonality was for RSV, whose average monthly PDR peaked in March (21.7%) and had its trough in September-October (1.6% in both months). The period of the year with the most intense influenza activity embraced December (PDR 13.2%) and January (PDR 14.2%); a milder (PDR 8.2%–8.3%), yet longer peak of activity was in June-August. The peak of influenza activity in December and January was mainly driven by the A(H1N1)p and A(H3N2) virus subtypes, while the peak of activity in June-August was caused by influenza A(H1N1)p and B (Fig. 3 ). There was weak evidence of intra-annual variation in the proportion of respiratory specimens testing positive for parainfluenza viruses: the PDR ranged between 6.3% in November, and 1.1% in March (Fig. 2).
Fig. 2.
Average monthly positive detection rate, overall (black), and for influenza (purple), respiratory syncytial virus (blue), and parainfluenza (red). Ecuador, 2010–2016 (data from 2009 were not included because of the AH1N1 influenza pandemic).
Fig. 3.
Average monthly positive detection rate for influenza virus: overall (black), A(H1N1)p (purple), A(H3N2) (blue), and B (red). Ecuador, 2010–2016 (data from 2009 were not included because of the AH1N1 pandemic).
Age distribution of patients infected with different respiratory viruses
The overall PDR ranged within a narrow interval (26.2% and 30.0%) in all age groups except the elderly (≥65 years), where it was 15.8% (Table 1). The virus-specific PDR was dependent on age for most respiratory viruses. In the multinomial logistic regression, young children (<1 and 1–4 years) were significantly more likely to test positive for RSV, adenovirus, parainfluenza or hMPV, and less likely to test positive for influenza, than young adults (18–39 years) (Table 2 ). Instead, older children (5–17 years) were more likely to be detected with influenza B (in addition to RSV, adenovirus or parainfluenza) than young adults. There were no significant differences between young (18–39 years) and older (40–64) adults. The elderly were less likely to test positive for the A(H1N1)p influenza, and more likely to be detected with adenovirus, than young adults.
Table 2.
Relationship of patient’s age to viral aetiology. Multinomial logistic regression model adjusted for year, surveillance scheme, region of sampling, and patient’s gender. Ecuador, 2009–2016.
| <1 year |
1–4 years |
5–17 years |
18–39 years |
40–64 years |
65 + years |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RRR | 95%CI | p | RRR | 95%CI | p | RRR | 95%CI | p | Reference category | RRR | 95%CI | p | RRR | 95%CI | p | |
| Influenza A(H1N1)p | 0.19 | (0.16–0.23) | <0.001 | 0.39 | (0.34–0.43) | <0.001 | 0.96 | (0.86–1.07) | 0.467 | 1.00 | 1.02 | (0.92–1.14) | 0.694 | 0.43 | (0.36–0.50) | <0.001 |
| Influenza A(H3N2) | 0.46 | (0.36–0.59) | <0.001 | 0.77 | (0.62–0.96) | 0.018 | 1.08 | (0.84–1.39) | 0.525 | 1.00 | 0.93 | (0.72–1.19) | 0.548 | 1.00 | (0.77–1.28) | 0.971 |
| Influenza B | 0.41 | (0.29–0.58) | <0.001 | 0.73 | (0.53–1.02) | 0.065 | 1.43 | (1.00–2.05) | 0.050 | 1.00 | 0.87 | (0.59–1.30) | 0.503 | 0.72 | (0.49–1.06) | 0.094 |
| RSV | 34.18 | (21.65–53.98) | <0.001 | 21.60 | (13.69–34.06) | <0.001 | 5.01 | (3.06–8.20) | <0.001 | 1.00 | 0.98 | (0.52–1.86) | 0.955 | 1.15 | (0.63–2.10) | 0.653 |
| Adenovirus | 9.02 | (2.18–37.29) | 0.002 | 17.18 | (4.23–69.71) | <0.001 | 10.98 | (2.58–46.66) | 0.001 | 1.00 | 2.87 | (0.58–14.27) | 0.196 | 4.45 | (0.97–20.39) | 0.054 |
| Parainfluenza | 4.63 | (2.88–7.44) | <0.001 | 4.25 | (2.66–6.80) | <0.001 | 2.51 | (1.48–4.28) | 0.001 | 1.00 | 0.84 | (0.43–1.64) | 0.606 | 1.54 | (0.87–2.73) | 0.137 |
| hMPV | 2.66 | (1.05–6.74) | 0.039 | 5.31 | (2.14–13.17) | <0.001 | 1.69 | (0.58–4.90) | 0.335 | 1.00 | 1.94 | (0.69–5.48) | 0.211 | 0.37 | (0.09–1.56) | 0.177 |
RRR: relative risk ratio. RSV: respiratory syncytial virus. hMPV: human metapneumovirus.
Case-fatality ratio (CFR) by respiratory virus and patient’s age
Information of final outcome of respiratory infection was available for 84.9% of patients during 2011-2016. The overall CFR was 4.0%: this varied by causal agents (from 2.0% for RSV to 11.0% for the 2009 pandemic strain) and age group (from 1.6% among children aged 1–4 years to 13.9% among the elderly), while there were no differences by gender (Table 3 ). In the multivariate logistic regression, the odds ratio of dying was significantly increased among patients who tested positive for A(H1N1)p (1.73, 95%CI 1.38–2.17, p < 0.001), and significantly decreased for those testing positive for RSV (0.75, 95%CI 0.57–0.98, p = 0.033), compared to test-negative patients. Compared to young adults, the risk of dying was significantly reduced for children of any age, with the lowest OR for those aged 1–4 years (0.13, 95%CI 0.10–0.17, p < 0.001), and significantly increased among the elderly (OR 1.58, 95%CI 1.29–1.93, p < 0.001).
Table 3.
Risk of death among patients with respiratory viral infections according to virus type and patient’s age. Logistic regression models adjusted by year, type of surveillance scheme, country region, and patient’s gender. Ecuador, 2011–2016.
| No. | Case-fatality ratio (%) | Univariate logistic regression |
Multivariate logistic regression |
|||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Respiratory virus | ||||||||
| Negative | 19,126 | 4.8% | 1.00 | 1.00 | ||||
| Influenza A(H1N1)p | 1023 | 11.0% | 2.44 | 1.98–3.00 | <0.001 | 1.73 | 1.38–2.17 | <0.001 |
| Influenza A(H3N2) | 626 | 5.3% | 1.09 | 0.76–1.56 | 0.627 | 0.99 | 0.69–1.44 | 0.974 |
| Influenza B | 411 | 5.8% | 1.22 | 0.80–1.85 | 0.356 | 1.17 | 0.76–1.80 | 0.482 |
| RSV | 3153 | 2.0% | 0.41 | 0.31–0.53 | <0.001 | 0.75 | 0.57–0.98 | 0.033 |
| Parainfluenza | 571 | 2.8% | 0.57 | 0.34–0.93 | 0.026 | 0.79 | 0.48–1.32 | 0.377 |
| Adenovirus | 149 | 4.7% | 0.97 | 0.45–2.07 | 0.933 | 1.69 | 0.77–3.72 | 0.190 |
| hMPV | 158 | 2.5% | 0.51 | 0.19–1.38 | 0.185 | 1.23 | 0.44–3.41 | 0.694 |
| Other/multiple | 96 | 3.1% | 0.63 | 0.20–2.00 | 0.437 | 0.85 | 0.26–2.74 | 0.784 |
| Age group | ||||||||
| <1 year | 10,376 | 2.9% | 0.25 | 0.20–0.30 | <0.001 | 0.23 | 0.18–0.28 | <0.001 |
| 1–4 years | 7319 | 1.6% | 0.13 | 0.10–0.17 | <0.001 | 0.13 | 0.10–0.17 | <0.001 |
| 5–17 years | 1865 | 2.9% | 0.25 | 0.18–0.34 | <0.001 | 0.27 | 0.20–0.37 | <0.001 |
| 18–39 years | 1515 | 10.8% | 1.00 | 1.00 | ||||
| 40–64 years | 1828 | 12.1% | 1.15 | 0.93–1.42 | 0.212 | 1.19 | 0.95–1.47 | 0.126 |
| ≥65 years | 2409 | 13.9% | 1.34 | 1.10–1.64 | 0.004 | 1.58 | 1.29-1.93 | <0.001 |
OR: Odds ratio. CI: Confidence intervals. RSV: Respiratory syncytial virus. hMPV: Human metapneumovirus.
Discussion
We conducted a retrospective comparative analysis of syndromic surveillance data from Ecuador (2009–2016), using different methodological approaches to compare the epidemiology, age distribution, and CFR of RVIs caused by different viruses types and subtypes. The overall PDR was 26.8%, with influenza and RSV accounting for most test-positive specimens. Comparing the overall and virus-specific PDR between studies is made problematic by the variability of factors like the demographic structure of the country, the surveillance scheme and the clinical features of patients being sampled, the sampling techniques being used [15], and the laboratory procedures and assays (which may vary in terms of number of respiratory pathogens that can be detected) [16]. By and large, a viral aetiology could be established in a smaller number of patients compared to previous studies [5], [17], [18], [19], [20], which might be explained by the failure to detect common causes of RVIs like rhinovirus, coronavirus, and others [21].
Viral interference between epidemics of influenza and RSV was observed previously [22], [23]. This phenomenon seems to affect other respiratory viruses as well (like rhinovirus, coronavirus, adenovirus and others [22], [23], [24]), although it was not possible to verify this in the available data due to limited numbers. Viral interference is associated with prolonged shedding from the primary virus infection in ferret influenza models [25], and hypothesized mechanisms include the production of interferon and other cytokines by infected cells [26]. There was an evident, yet distinct seasonality for influenza (two main periods of activity, in December–January and June–August, consistently with previous reports [10], [27]) and RSV (in March), and a less evident pattern for parainfluenza. Ecuador is a geographically and climatically diverse country, and meteorological parameters like humidity, temperature, and rainfall are known to affect virus survival and transmissibility [18], [28]. Viral interference, diverse association with climatic parameters and other factors [29] may contribute to shape the seasonal cycles of RVIs in Ecuador, although their relative importance is difficult to establish.
Diversity in the age distribution of subjects infected with the different respiratory viruses can be explained by the variability between viruses in terms of factors that co-determine their transmission dynamics among humans, such as the reproductive number (R0), the rate of mutation accumulation, the duration of immunity, and the presence of cross-protection. RSV has two major antigenic group (A and B), but some antigenic variability may also occur within each group [30]; its reproduction number is high (between 5 and 7) [31] and immunity wanes over time [32]. Because of these features, RSV has the highest incidence among young children but causes repeat infections throughout life and represents a health threat for the elderly as well [33]. RSV infection can also induce cross-protection against human metapneumovirus and parainfluenza virus, affecting the epidemiological dynamics of those pathogens [34], [35]. Influenza viruses evolve via antigenic drift and (only for type A virus) antigenic shift, and novel variants are introduced constantly into the community. The A(H3N2) subtype has shown an accelerated mutation accumulation rate in recent years [36], [37], which may explain its higher incidence in the elderly compared to other (sub)types.
The relationship between one’s age and the risk of death was J-shaped: infants were more likely to die than older children, and the CFR was highest among the elderly. The high incidence among infants and the greater severity among the elderly explain why these population subgroups suffer the largest RVI burden of disease [1], [4]. After adjusting by age, patients infected with A(H1N1)p had a 73% higher risk of death compared to test-negative patients. This is consistent with recent literature reports and contrasts the common belief that the most clinically severe influenza virus subtype is A(H3N2) [38]. In contrast, RSV patients had a significantly reduced risk of death compared to test-negative patients. Given the high raw CFR (4.8%) of test-negative patients, a possible explanation is that the latter could actually be infected with untested viruses (e.g. coronaviruses, bocaviruses, or respiratory enteroviruses), bacteria (e.g. Mycoplasma pneumoniae, Legionella pneumophila, or Chlamydophila pneumoniae), or other micro-organisms [39], some of which may cause a more severe disease (i.e. with a higher CFR) than RSV.
The main strength of this paper is the availability of a very large sample of subjects of any age who were tested for multiple respiratory viruses within a surveillance system covering an entire country. The study has some limitations as well. The surveillance system underwent a major transition during the period covered by the study, and patients with different syndromes were sampled in different sub-periods: although all analyses were stratified or adjusted to account for this change, some residual confounding cannot be ruled out. No information was available on patients’ vaccination status, underlying conditions, and use of antivirals and antibiotics. While influenza viruses were searched by RT-PCR throughout the study period, the identification of the other respiratory viruses was performed using direct immunofluorescence, which is a less sensitive technique [40]. Therefore, the PDR for viruses other than influenza may be underestimated.
Conclusions
In conclusion, our findings showed that the epidemiology (including the temporal occurrence of epidemics), age distribution, and severity of respiratory infections in Ecuador vary greatly across viruses. Influenza was the most frequent cause of RVIs in 2011–16, and the A(H1N1)p subtype was that associated with the highest CFR. However, much of the burden of disease of respiratory infections is caused by pathogens other than influenza viruses (especially RSV), which represent a public health priority that needs to be addressed through adequate allocation of resources and coordinated research effort.
Funding
No funding Sources.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgments
We thank all healthcare professionals contributing to the Surveillance System for Acute Respiratory Infections, Ministry of Public Health, Ecuador.
References
- 1.Battle against Respiratory Viruses (BRaVe) initiative. Concept paper: addressing unmet needs. http://www.who.int/influenza/patient_care/clinical/BRAVE_Concept_Paper.pdf?ua=1. [Accessed 28 December 2017].
- 2.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legand A., Briand S., Shindo N., Brooks W.A., de Jong M.D., Farrar J. Addressing the public health burden of respiratory viruses: the Battle against Respiratory Viruses (BRaVe) initiative. Future Virol. 2013;8(10):953–968. [Google Scholar]
- 4.GBD 2015 LRI Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(11):1133–1161. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmerman R.K., Rinaldo C.R., Nowalk M.P., Gk B., Thompson M.G., Moehling K.K. Influenza and other respiratory virus infections in outpatients with medically attended acute respiratory infection during the 2011–12 influenza season. Influenza Other Respir Viruses. 2014;8(4):397–405. doi: 10.1111/irv.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO). Battle against Respiratory Viruses (BRaVe) initiative. http://www.who.int/influenza/patient_care/clinical/brave/en/. [Accessed 28 December 2017].
- 7.Hayden F.G. Advances in antivirals for non-influenza respiratory virus infections. Influenza Other Respir Viruses. 2013;7(Suppl. 3):36–43. doi: 10.1111/irv.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brendish N.J., Clark T.W. Antiviral treatment of severe non-influenza respiratory virus infection. Curr Opin Infect Dis. 2017;30(6):573–578. doi: 10.1097/QCO.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 9.Caini S., Andrade W., Badur S., Balmaseda A., Barakat A., Bella A. Global Influenza B Study. Temporal patterns of influenza A and B in tropical and temperate countries: what are the lessons for influenza vaccination? PLoS One. 2016;11(3):e0152310. doi: 10.1371/journal.pone.0152310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caini S., Alonso W.J., Balmaseda A., Bruno A., Bustos P., Castillo L. Global Influenza B Study group–Latin America. Characteristics of seasonal influenza A and B in Latin America: Influenza surveillance data from ten countries. PLoS One. 2017;12(3):e0174592. doi: 10.1371/journal.pone.0174592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Central Intelligence Agency. The World Factbook. https://www.cia.gov/library/publications/the-world-factbook/. [Accessed 3 May 2018].
- 12.Ministerio de Salud Pública del Ecuador/Instituto Nacional de Higiene y Medicina Tropical Leopoldo Izquieta Pérez. Plan nacional de contingencia para enfrentar posible pandemia de influenza en el Ecuador. Guía operativa de vigilancia epidemiológica de las Enfermedades Tipo Influenza (ETI) e Infecciones Respiratorias Agudas Graves (IRAG). Quito, Ecuador, May 2008. [in Spanish]. https://www.paho.org/ecu/index.php?option=com_docman&view=download&alias=61-guia-operativa-para-la-vigilancia-epidemiologica-de-las-enfermedades-tipo-influenza&category_slug=publications&Itemid=599. [Accessed 28 March 2018].
- 13.Ministerio de Salud Pública del Ecuador. Subsecretaría Nacional de Vigilancia de la Salud Pública. Dirección Nacional de Vigilancia Epidemiológica. Sistema Integrado de Vigilancia Epidemiológica (SIVE): Norma técnica. [in Spanish]. https://aplicaciones.msp.gob.ec/salud/archivosdigitales/documentosDirecciones/dnn/archivos/EDITOGRAN%20NORMA%20SIVE.pdf. [Accessed 28 March 2018].
- 14.US Centers for Disease Control and Prevention (CDC). Protocol of realtime RT-PCR for swine influenza A(H1N1). 28 April 2009, revision 1 30 April 2009. Spanish version. http://cidbimena.desastres.hn/docum/AH1N1/CDCrealtimeTRPCRprotoc_SPA20090430.pdf. [Accessed 28 March 2018].
- 15.Li L., Chen Q.Y., Li Y.Y., Wang Y.F., Yang Z.F., Zhong N.S. Comparison among nasopharyngeal swab, nasal wash, and oropharyngeal swab for respiratory virus detection in adults with acute pharyngitis. BMC Infect Dis. 2013;13:281. doi: 10.1186/1471-2334-13-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun S.G., Kim M.Y., Choi J.M., Lee C.K., Lim C.S., Cho Y. Comparison of three multiplex PCR assays for detection of respiratory viruses: Anyplex II RV16, AdvanSure RV, and Real-Q RV. J Clin Lab Anal. 2018;32(2):e22230. doi: 10.1002/jcla.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed J.A., Katz M.A., Auko E., Njenga M.K., Weinberg M., Kapella B.K. Epidemiology of respiratory viral infections in two long-term refugee camps in Kenya, 2007-2010. BMC Infect Dis. 2012;12:7. doi: 10.1186/1471-2334-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H., Wei Q., Tan A., Wang L. Epidemiological analysis of respiratory viral etiology for influenza-like illness during 2010 in Zhuhai, China. Virol J. 2013;10:143. doi: 10.1186/1743-422X-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva R.C., Mendes Gda S., Rojas M.A., Amorim A.R., Couceiro J.N., Lupi O. Frequency of viral etiology in symptomatic adult upper respiratory tract infections. Braz J Infect Dis. 2015;19(1):30–35. doi: 10.1016/j.bjid.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes-Matano L., Monroy-Muñoz I.E., Angeles-Martínez J., Sarquiz-Martinez B., Palomec-Nava I.D., Pardavé-Alejandre H.D. Prevalence of non-influenza respiratory viruses in acute respiratory infection cases in Mexico. PLoS One. 2017;12(5):e0176298. doi: 10.1371/journal.pone.0176298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Royston L., Tapparel C. Rhinoviruses and respiratory enteroviruses: not as simple as ABC. Viruses. 2016;8(1) doi: 10.3390/v8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Asten L., Bijkerk P., Fanoy E., van Ginkel A., Suijkerbuijk A., van der Hoek W. Early occurrence of influenza A epidemics coincided with changes in occurrence of other respiratory virus infections. Influenza Other Respir Viruses. 2016;10(1):14–26. doi: 10.1111/irv.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X., Song Z., Li Y., Zhang J., Wang X.L. Possible interference between seasonal epidemics of influenza and other respiratory viruses in Hong Kong, 2014–2017. BMC Infect Dis. 2017;17(1):772. doi: 10.1186/s12879-017-2888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karppinen S., Toivonen L., Schuez-Havupalo L., Waris M., Peltola V. Interference between respiratory syncytial virus and rhinovirus in respiratory tract infections in children. Clin Microbiol Infect. 2016;22(2):208.e1–208.e6. doi: 10.1016/j.cmi.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Laurie K.L., Guarnaccia T.A., Carolan L.A., Yan A.W., Aban M., Petrie S. Interval between infections and viral hierarchy are determinants of viral interference following influenza virus infection in a ferret model. J Infect Dis. 2015;212(11):1701–1710. doi: 10.1093/infdis/jiv260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linde A., Rotzén-Ostlund M., Zweygberg-Wirgart B., Rubinova S., Brytting M. Does viral interference affect spread of influenza? Euro Surveill. 2009;14(40) [PubMed] [Google Scholar]
- 27.Durand L.O., Cheng P.Y., Palekar R., Clara W., Jara J., Cerpa M. Timing of influenza epidemics and vaccines in the American tropics, 2002–2008, 2011–2014. Influenza Other Respir Viruses. 2016;10(3):170–175. doi: 10.1111/irv.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paynter S. Humidity and respiratory virus transmission in tropical and temperate settings. Epidemiol Infect. 2015;143(6):1110–1118. doi: 10.1017/S0950268814002702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sloan C., Moore M.L., Hartert T. Impact of pollution, climate, and sociodemographic factors on spatiotemporal dynamics of seasonal respiratory viruses. Clin Transl Sci. 2011;4(1):48–54. doi: 10.1111/j.1752-8062.2010.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullender W.M. Respiratory syncytial virus genetic and antigenic diversity. Clin Microbiol Rev. 2000;13(1):1–15. doi: 10.1128/cmr.13.1.1-15.2000. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber A., Weber M., Milligan P. Modeling epidemics caused by respiratory syncytial virus (RSV) Math Biosci. 2001;172(2):95–113. doi: 10.1016/s0025-5564(01)00066-9. [DOI] [PubMed] [Google Scholar]
- 32.Varga S.M., Braciale T.J. The adaptive immune response to respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:155–171. doi: 10.1007/978-3-642-38919-1_8. [DOI] [PubMed] [Google Scholar]
- 33.Hall C.B., Simőes E.A., Anderson L.J. Clinical and epidemiologic features of respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:39–57. doi: 10.1007/978-3-642-38919-1_2. [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharyya S., Gesteland P.H., Korgenski K., Bjørnstad O.N., Adler F.R. Cross-immunity between strains explains the dynamical pattern of paramyxoviruses. Proc Natl Acad Sci U S A. 2015;112(43):13396–13400. doi: 10.1073/pnas.1516698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen X., Mousa J.J., Bates J.T., Lamb R.A., Crowe J.E., Jr., Jardetzky T.S. Structural basis for antibody cross-neutralization of respiratory syncytial virus and human metapneumovirus. Nat Microbiol. 2017;2:16272. doi: 10.1038/nmicrobiol.2016.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein E.Y., Serohijos A.W., Choi J.M., Shakhnovich E.I., Pekosz A. Influenza A H1N1 pandemic strain evolution–divergence and the potential for antigenic drift variants. PLoS One. 2014;9(4):e93632. doi: 10.1371/journal.pone.0093632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tewawong N., Prachayangprecha S., Vichiwattana P., Korkong S., Klinfueng S., Vongpunsawad S. Assessing antigenic drift of seasonal influenza A(H3N2) and A(H1N1)pdm09 viruses. PLoS One. 2015;10(10):e0139958. doi: 10.1371/journal.pone.0139958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caini S., Kroneman M., Wiegers T., El Guerche-Séblain C., Paget J. Clinical characteristics and severity of influenza infections by virus type, subtype, and lineage: a systematic literature review. Influenza Other Respir Viruses. 2018 doi: 10.1111/irv.12575. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wertheim H.F., Nadjm B., Thomas S., Agustiningsih A., Malik S., Diep N.N. Viral and atypical bacterial aetiologies of infection in hospitalised patients admitted with clinical suspicion of influenza in Thailand, Vietnam and Indonesia. Influenza Other Respir Viruses. 2015;9(6):315–322. doi: 10.1111/irv.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuypers J., Wright N., Ferrenberg J., Huang M.L., Cent A., Corey L. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44(7):2382–2388. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]