Highlights
► A number of respiratory viruses are emergent and re-emergent pathogens in humans. ► Influenza, human metapneumovirus, coronaviruses and enteroviruses are key examples. ► Despite viral diversity and unpredictability, common response strategies are possible. ► Surveillance and early public health interventions support clinical countermeasures. ► Pandemics and severe epidemics enable policies to be tested and gaps identified.
Abstract
Respiratory viruses have emerged and re-emerged in humans for hundreds of years. In the recent past avian and animal influenza viruses have caused human disease ranging from conjunctivitis to respiratory illnesses, including the 2009–10 A(H1N1)pdm09 pandemic. Coronaviruses, human metapneumovirus (hMPV) and enteroviruses have also impacted humans globally. Since the likely public health impacts are common, plans and policies for intervention strategies can be developed, encompassing early detection through surveillance and diagnostics, as well as treatment and prevention through clinical and non-clinical interventions. The global comprehensiveness of these varies according to differing resources, competing health priorities and the causative agent, yet, irrespective of this, activities must be proportional to the threat. Pandemics and severe epidemics enable policies to be tested and gaps identified.
Current Opinion in Virology 2013, 3:192–198
This review comes from a themed issue on Emerging viruses
Edited by Ian Lipkin and Ab Osterhaus
For a complete overview see the Issue and the Editorial
Available online 9th March 2013
1879-6257/$ – see front matter, © 2013 Elsevier B.V. All rights reserved.
Introduction
Over the last 15 years, a large proportion of emergent, re-emergent or newly recognised pathogens in human have been respiratory viruses.
Influenza viruses
In 1997, 18 human cases of severe infection associated with Highly Pathogenic Avian Influenza (HPAI) A(H5N1) caused significant international concern about the possibility of an influenza pandemic with its epicentre in Hong Kong. However the virus failed to transmit from person-to-person and the efficient and widespread culling of poultry by the Hong Kong authorities interrupted spread from birds to humans [1]. Shortly afterwards, in 1998–99 cases of Low Pathogenic Avian Influenza (LPAI) A(H9N2) were described in humans, associated with influenza-like illness (ILI) [2]; and a further human case occurred in 2003 [3]. In the same year, a large outbreak of HPAI A(H7N7) in poultry and swine in the Netherlands resulted in 89 human cases of conjunctivitis, and 13 cases of respiratory illness, including 1 death [4, 5]. Of far greater potential significance, HPAI A(H5N1) also re-emerged in humans in 2003. Its endemicity in poultry in many parts of the world, and the continuing occurrence of low numbers of severe, often lethal, infections in humans reinforce its ongoing pandemic threat [6]. In 2004 and 2006 cases of human infection with LPAI A(H7N3) were documented in British Columbia and England causing both ILI and conjunctivitis [7, 8]; two further cases occurred in Mexico in 2012 [9]. In addition, in 2007, an LPAIV A(H7N2) outbreak in poultry produced at least four cases of human infection in England and Wales, manifesting as ILI, of which three were hospitalised [10]; low-level person-to-person transmission was suspected but never proven [11]. These events all served to emphasise the potential pandemic threat from avian influenza, most notably subtypes H5, H7 and H9. However, the biggest impact of emerging influenza viruses of the last 15 years has been the A(H1N1)pdm09 pandemic in 2009–10. This emerged from an unpredicted epicentre in Mexico rather than southeast Asia as widely predicted; and it was an unanticipated virus (not derived from HPAI A(H5N1) as widely predicted), albeit containing genetic material from both avian and swine influenza viruses [12]. The 2009–10 pandemic has substantially informed and modified thinking about intervention strategies for emerging respiratory virus infections. But its occurrence has neither increased nor diminished the ongoing threat from other avian influenza viruses.
Coronaviruses
In late 2002, clusters of atypical pneumonia in southern China led to the eventual discovery of SARS-CoV associated with Severe Acute Respiratory Syndrome (SARS) [13, 14, 15]. This new pathogen provided a rather different perspective from A(H5N1), with severe illness, efficient person–person transmission, and rapid international spread via the medium of air travel [16]. In early 2003 SARS spread from Hong Kong to Vietnam, Singapore and Canada in just three weeks, demonstrating its massive pandemic potential. In the first three months of circulation, over 8000 cases and 774 deaths occurred worldwide across 29 countries (http://www.who.int/csr/sars/country/table2004_04_21/en/index.html). In retrospect, a SARS pandemic was averted because persons infected were maximally infectious several days after symptom onset; this allowed effective quarantine and infection control measures to be instituted in a way that would not be possible for influenza. Had this not been so, the outcome would have been a highly lethal pandemic with few options for definitive treatment or prevention by vaccination. In 2004, two novel human coronaviruses, NL63 and HKU1 were identified in humans, being associated with typical acute respiratory illness and pneumonia respectively [17, 18]. In late 2012, the detection of a novel betacoronavirus (HCoV-EMC) in a small number of humans with severe respiratory infection in Saudi Arabia, Qatar and Jordan has reinforced the pandemic potential of coronaviruses [19, 20]; it is at present unclear what the future of HCoV-EMC in humans will be.
Other respiratory viruses
In 2001 human metapneumovirus (hMPV) was first identified in humans [21], and is now recognised to be responsible for around 10% of respiratory infections, especially in children under five years of age [22]. In fact this pathogen had probably circulated for at least two decades. Although ubiquitous and with an epidemic pattern of occurrence, it was nevertheless clear that hMPV was simply one of a large number of respiratory pathogens producing the symptoms of acute respiratory infection (ARI) in humans [22]. Since 2009–10 the human enterovirus EV-D68, hitherto rarely recognised in humans, has emerged worldwide as a cause of ARI in humans [23, 24] (Table 1 ).
Table 1.
Summary of emergent, re-emergent and newly identified respirator virus threats in humans since 1997
| Year | Virus | Status | Pandemic potential |
|---|---|---|---|
| 1997 | HPAI A(H5N1) | • Bird-to-human transmission resulting in severe respiratory infection in humans. • New emergence. |
Yes |
| 1998–99 | LPAI A(H9N2) | • Small numbers of cases of low severity ILI after contact with infected poultry. • Possibly newly identified, rather than truly emergent. |
Yes |
| 2001–present | hMPV | • Mainly mild respiratory illness but widespread epidemic occurrence. • Clear person-to-person transmission. • Almost certainly newly identified but not genuinely emergent. |
No |
| 2003 | LPAI A(H9N2) | • Further human case associated with mild ILI. | Yes |
| 2003 | HPAI A(H7N7) | • Wide spectrum of disease from conjunctivitis to severe respiratory infection after contact with infected animals/birds. • No clear evidence of person-to-person transmission. • New emergence. |
Yes |
| 2003 | SARS-CoV | • Severe respiratory infections. Rapid global spread and person-to-person transmission. • New emergence. |
Yes |
| 2003–present | HPAI A(H5N1) | • Substantial re-emergence of human disease related to contact with infected poultry. • No evidence of sustained person-to-person transmission. |
Yes |
| 2004 and 2006 | LPAI A(H7N3) | • Mild respiratory infections and/or conjunctivitis after contact with infected poultry. • No person-to-person transmission. • Probable new emergence. |
Yes |
| 2004 | HCoV-NL63 | • Mainly mild respiratory illness in humans. • Readily transmitted person-to-person. • Uncertain whether new emergence or new recognition. |
No |
| 2004 | HCoV-HKU1 | • Associated with wide spectrum of acute respiratory illness including pneumonia. • Readily transmitted person-to-person. • Uncertain whether new emergence or new recognition. |
No |
| 2007 | LPAI A(H7N2) | • Small human outbreak associated with contact with infected poultry. • Spectrum of disease unclear but some moderate-severe ILI. • Low-level person-to-person transmission suspected, not proven. • Probably new emergence. |
Yes |
| 2009 | Pandemic influenza A(H1N1)pdm09 | • Mild global pandemic. • New emergence. |
Yes |
| 2009–10 | EV-D68 | • Broad spectrum of disease in humans. • Clearly transmissible person-to-person. • Probable re-emergence with further diversification. |
No |
| 2012 | HCoV-EMC | • Small numbers of cases of severe respiratory infection, often fatal. • Evidence of limited person-to-person transmission. • New emergence, evolving situation. |
Yes |
Although the diversity of respiratory virus threats is considerable, many have pandemic potential and the strategies required for preparedness and response are remarkably similar because of shared characteristics in terms of their potential impact on human populations, notably:
-
•
Rapid emergence
-
•
Unpredictability of timing and duration
-
•
Unpredictability of epicentre
-
•
Rapid global spread, once person-to-person transmission is established
-
•
Impact on health systems
-
•
Wider impacts on society if severe in nature
In the broadest sense and from a public health perspective, ‘intervention’ strategies therefore encompass measures aimed at early detection as well as treatment and prevention
Surveillance and diagnostics
If future emerging respiratory virus infections are to be detected rapidly, surveillance systems must be in place that are capable of detecting unusual syndromic patterns. That such systems are linked to diagnostic sampling is equally important to enable known pathogens to be recognised and ruled out, so that disease related to novel pathogens will stand out. Whilst it is unrealistic to expect all countries of the world, irrespective of resources, to have fully comprehensive surveillance systems, it is equally unacceptable that there are very large gaps in coverage at the present time, especially in resource poor settings. For example, it is highly relevant to question how rapidly the novel, but somewhat mild A(H1N1)pdm09 pandemic virus might have been detected had its epicentre been in sub-Saharan Africa rather than in Mexico.
Whilst surveillance is important for the detection of novel threats, in most of the modern settings it has equal value in quantifying the public health impact of an emerging problem over time, and at different levels of the healthcare system, so that resources may be moved around and focused on where they are most needed. One large problem, obvious during the 2009 pandemic, was the dearth of surveillance systems geared towards secondary care. Whilst many countries could track syndromic respiratory illness in primary care settings quite effectively (and some coupled this to virological surveillance), at the start of the pandemic, few had systems capable of doing much more than counting the number of hospital admissions due to respiratory illness. Ad hoc systems were devised in haste, but detailed clinical and epidemiological information on severe acute respiratory illness (SARI) was initially hard to locate. In the aftermath of the 2009 pandemic it has been recognised that SARI surveillance systems are critically important but mainly underdeveloped; and that their usefulness will extend over a broad range of acute respiratory pathogens, known and unknown. For example, a sudden upsurge in HCoV-EMC might well be detected by monitoring an unexplained increase in the requirement for intensive care for acute respiratory cases.
During the early stages of the 2009 pandemic, epidemiological information was sparse and it took some time before a clear enough picture emerged from Mexico and North America. Other countries still without pandemic cases were dependent upon such early information for fine-tuning their operational response plans. Hitherto, this inter-dependency had not been fully recognised. Some countries had enacted earlier plans for an extremely detailed epidemiological examination of the first few hundred (FF100) cases of novel infection. In the UK the FF100 system was highly successful in yielding useful early information [25•].
Public health measures
Public health measures are interventions that can be enacted by individuals (but applied collectively) or prescribed by state authorities, to prevent or reduce the impact of a communicable disease threat and which do not involve pharmaceutical products or vaccines [26••]. In that context, it should be noted that for non-influenza threats, notably those posed by novel coronaviruses, the possibilities for effective licensed vaccines within realistic timeframes and in meaningful quantities are almost nil. Likewise, other than neuraminidase inhibitors (NAIs) for influenza, the available therapeutic options are supportive rather than curative. As a consequence, for pandemic threats such as SARS-CoV and even for influenza in resource poor settings where drugs and vaccines are largely unaffordable, public health measures may be the only feasible option.
It is important to recognise that the underlying purpose of public health measures is not to prevent or stop a pandemic or epidemic as much as to slow down transmission. If applied successfully, the national epidemic curve changes to become broader (of longer duration) but flatter (of lower peak severity); and the timing of peak activity may also be delayed somewhat (Figure 1 ) [26••]. It is well recognised that health services cannot cope with extreme surges in demand; thus to be less busy for longer is preferable than to be overwhelmed in a short time frame. Likewise, it is now well recognised, and subsequently proven by the experiences of 2009–10 that vaccine manufacture takes time; and delaying of peak disease activity may well encroach into the window of vaccine availability, with positive public health consequences [26••].
Figure 1.
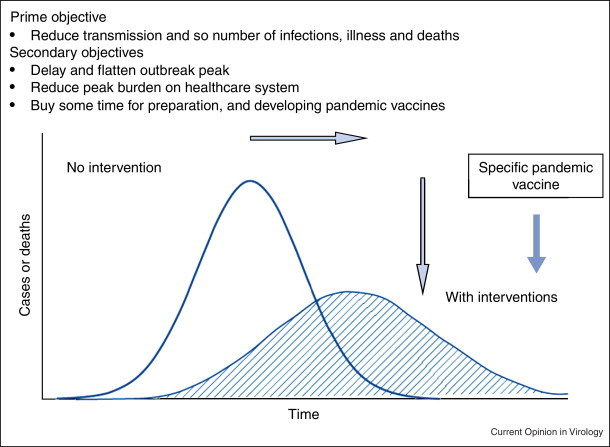
Objectives of applying public health measures against major (pandemic) respiratory virus threats.
Adapted with permission from [26••].
An illustrative list of possible public health measures is provided in Table 2 . It is important to recognise that although a detailed examination of the evidence base for each is outside the scope of this paper, the evidence for a great many is scanty or non-existent. Given the doubts about the effectiveness of some individual measures, it is generally recognised that packages of multiple measures (known as ‘layered interventions’ or ‘defence in depth’) are the most appropriate implementation strategy. Even so, decisions associated with optimal timing (commencement and cessation), public acceptance and the avoidance of public fatigue are complex and require careful thought.
Table 2.
Illustrative summary of non-pharmaceutical, non-vaccine, public health measures to counter widespread emerging respiratory virus threats
| Category | Specific intervention | Evidence base and consequences |
|---|---|---|
| International travel measures | Travel advice | • Minimal evidence |
| Entry screening | • Minimal evidence — moderately disruptive | |
| Border closures | • Minimally effective unless 100% complete • Unsustainable until vaccine is available • Very disruptive to society |
|
| Personal protective measures | Regular hand washing | • Effective to some extent |
| Good respiratory hygiene | • Uncertain (presumed effective) | |
| General mask-wearing outside the home | • Uncertain | |
| Mask-wearing in high-risk situations | • Uncertain • Possibly partially effective in healthcare settings |
|
| Mask-wearing by people with respiratory infection | • Effective to some extent | |
| Early self-isolation | • Uncertain but presumed effective | |
| Quarantine measures | • Ineffective for influenza; more effective for SARS-CoV | |
| Social distancing measures | Internal travel restrictions | • Possibly minor delaying effect • Disruptive |
| Reactive school closures | • Effective to some extent • Potentially disruptive secondary effects • Difficult to gauge when to re-open |
|
| Proactive school closures | • Effective to some extent • Very substantial secondary effects • Difficult to gauge when to re-open |
|
| Reactive workplace closures | • Uncertain | |
| Home working and reducing meetings | • Uncertain | |
| Cancellation of public gatherings | • Uncertain • Potentially disruptive |
|
Adapted with permission from [26••].
Notwithstanding the availability of evidence to support many public health measures, it should be remembered that many are intuitive for governments to apply although, with the exception of respiratory hygiene and hand washing, few are without substantial economic costs or consequences [26••]. These secondary effects can be considerable, for example in the case of severe international travel restrictions (leading to economic losses) and school closures (leading to increased parental workplace absenteeism). The emerging thinking after the 2009–10 pandemic has therefore tended to be about recognising the proportionality of public health measures in relation to the severity of the threat. Put simply, severe public health measures would be more justifiable and better understood by the public for an A(H5N1) or SARS-CoV scenario than for a mild A(H1N1)pdm09-like one.
Vaccines
The mainstay of seasonal influenza control is the vaccination of vulnerable individuals. In the context of emerging respiratory virus threats, vaccination as a public health measure can only be considered realistic in the context of influenza where the infrastructure and global production capacity for the manufacture of seasonal vaccines already exists and was rapidly redeployed in early 2009 towards the manufacture of pandemic vaccines. Even so, the 2009–10 pandemic revealed that many issues remain.
Global manufacturing capacity for influenza vaccines at the beginning of the 2009 was for 900 million doses per annum, representing an almost trebling of capacity over the last decade. Even so the estimated global requirement for pandemic vaccine stood at up to 7 billion doses (14 billion if two doses required) — a considerable shortfall [27••]. The vaccine industry had always made it clear to public health authorities and governments that it would take some five to six months for the first commercial batches of vaccine to become available, even with innovative regulatory solutions for licensure; this turned out to be the case with supplies available from October 2009 after the emergence of the pandemic virus in April. Nevertheless this contrasted sharply with the reality of disease incidence in countries of the southern hemisphere, which suffered first pandemic waves in summer 2009 along with substantial disease activity in Mexico, north America and the UK. Even among well-resourced countries in Europe, pandemic vaccines were introduced gradually throughout autumn 2009 as supplies became available coinciding with major autumn pandemic waves rather than the ideal of vaccination campaigns having been completed, before they began. In resource poor countries, which relied on donated supplies, pandemic vaccine distribution only occurred from early 2010 onwards [27••]. Such dissonance in the timing of disease activity and the availability of supplies, coupled to generally mild disease meant that the public's perception and receptivity towards vaccine were ultimately rather mixed.
The above statements about vaccine supply and logistics should not serve to undermine the very substantial effectiveness of the products supplied. Many studies now confirm that pandemic vaccines were highly effective in protecting recipients against infection (typically 80% effectiveness against confirmed infection in field studies) [28•]. However, it is clear that first, the public health effectiveness of vaccines depends crucially upon the timing of availability in relation to disease activity and second, that countries should recognise that unless manufacturing technologies or approaches change radically there will be considerable reliance on public health measures and antiviral drugs for the early stages of a future influenza pandemic, as in 2009–10. In circumstances where the world is confronted by a non-influenza respiratory pandemic, these issues would of course be compounded.
Antiviral drugs and antibiotics
Specifically in the context of pandemic influenza, many countries developed strategies for the use of NAIs. These varied from no use at all (usually due to affordability issues), through restricted use in high-risk patients (the majority approach), to widespread availability for all patients with qualifying symptoms (UK). All the policies were based upon evidence from the study of seasonal influenza suggesting that NAIs reduce symptom duration and severity by a modest amount, but may also reduce complications, hospitalisations and mortality; as the outcome became more serious the evidence became weaker [29]. The UK also stands out in having pursued an extended policy of household post-exposure prophylaxis with antiviral drugs to reduce the incidence of secondary cases. The evidence suggests that this approach was highly effective at the household level [30•], but it is impossible to say with any certainty whether this affected the course of the pandemic in the UK overall or bought much time compared with countries that did not implement such a policy. Other data are emerging which suggest that a very small proportion of patients in the UK with A(H1N1)pdm09 infection received NAIs despite enhanced accessibility via Internet algorithms and by telephone (A Hayward, personal communication, 2013). In the context of respiratory virus infections that might lead to bacterial secondary infections, the stockpiling of antibiotics and potentially extended pre-pandemic use of pneumococcal vaccines might also be important policy considerations [31, 32].
The biggest question to be asked about NAIs in the post-pandemic era is whether they proved to be an effective measure in 2009–10 in terms of their impact on outcomes of public health importance. Mytton and colleagues have suggested that the higher mortality due to A(H1N1)pdm09 noted in the UK's third pandemic wave in winter 2010–11 might relate to the restricted availability of NAIS in 2010–11 as compared with the first and second pandemic waves in 2009–10 [33]. An important meta-analysis of studies undertaken during the 2009–10 pandemic period certainly suggests that mortality was reduced when NAI treatment was commenced early (within 48 hours of symptom onset) as compared with late commencement of treatment [34]; but this effect might be confounded by late treatment of very severe cases that were unlikely to survive whatever therapy was given. However a comparison of early treatment vs. none might be confounded in the opposite direction (to underestimate effectiveness); this too showed that NAIs were associated with a 65% reduction in mortality [34••]. Further confirmatory data are required which, if consistent, would then justify the replenishment of NAI stockpiles in readiness for a future influenza pandemic.
Over recent years, emergent and re-emergent respiratory viruses have presented many challenges to the global human population. These include outbreaks, global pandemics and ‘everyday’ mild respiratory illness. Despite the different natures of these pathogens, there are many commonalties in the public health countermeasures that contribute to response strategies. Specific clinical countermeasures require further research and development efforts to ensure optimum effectiveness against the target pathogen.
Conflicts of interest
JSN-V-T has received funding to attend influenza related meetings, lecture and consultancy fees and research funding from several influenza antiviral drug and vaccine manufacturers (Baxter AG, Novartis, Sanofi Pasteur MSD, GlaxoSmithKline, Solvay and F.Hoffmann-La Roche). Research funding from GlaxoSmithKline, Astra-Zeneca and F Hoffmann-La Roche is ongoing; all forms of personal remuneration ceased in September 2010. He is a former employee of SmithKline Beecham plc. (now GlaxoSmithKline), Roche Products Ltd (UK) and Aventis-Pasteur MSD (now Sanofi-Pasteur MSD), all before 2005, with no remaining pecuniary interests by way of share holdings, share options and accrued pension rights. CS has received lecture fees from Baxter AG.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1.Webster R.G., Hay A.J. The H5N1 influenza outbreak in Hong Kong: a test of pandemic preparedness. In: Nicholson K.G., Webster R.G., Hay A.J., editors. Textbook of Influenza. Blackwell Science; 1998. pp. 561–565. [Google Scholar]
- 2.Peiris M., Yuen K.Y., Leung C.W., Chan K.H., Ip P.L., Lai R.W., Orr W.K., Shortridge K.F. Human infection with influenza H9N2. Lancet. 1999;354:916–917. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 3.Butt K.M., Smith G.J., Chen H., Zhang L.J., Leung Y.H., Xu K.M., Lim W., Webster R.G., Yuen K.Y., Peiris J.S. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol. 2005;43:5760–5767. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fouchier R.A., Schneeberger P.M., Rozendaal F.W., Broekman J.M., Kemink S.A., Munster V., Kuiken T., Rimmelzwaan G.F., Schutten M., Van Doornum Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koopmans M., Wilbrink B., Conyn M., Natrop G., van der Nat H., Vennema H., Meijer A., van Steenbergen J., Fouchier R., Osterhaus A. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 6.Peiris J.S., Yu W.C., Leung C.W., Cheung C.Y., Ng W.F., Nicholls J.M., Ng T.K., Chan K.H., Lai S.T., Lim W.L. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tweed S.A., Skowronski D.M., David S.T., Larder A., Petric M., Lees W., Li Y., Katz J., Krajden M., Tellier R. Human illness from avian influenza H7N3, British Columbia. Emerg Infect Dis. 2004;10:2196–2199. doi: 10.3201/eid1012.040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen-Van-Tam J.S., Nair P., Acheson P., Baker A., Barker M., Bracebridge S., Croft J., Ellis J., Gelletlie R., Gent N. Outbreak of low pathogenicity H7N3 avian influenza in UK, including associated case of human conjunctivitis. Euro Surveill. 2006;11 doi: 10.2807/esw.11.18.02952-en. E060504.2. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Notes from the field: highly pathogenic avian influenza A (H7N3) virus infection in two poultry workers — Jalisco, Mexico, July 2012. MMWR Morb Mortal Wkly Rep. 2012;61:726–727. [PubMed] [Google Scholar]
- 10.Avian influenza A/(H7N2) outbreak in the United Kingdom. Euro Surveill. 2007;12 E070531.2. [PubMed] [Google Scholar]
- 11.Eames K.T., Webb C., Thomas K., Smith J., Salmon R., Temple M. Assessing the role of contact tracing in a suspected H7N2 influenza A outbreak in humans in Wales. BMC Infect Dis. 2010;10:141. doi: 10.1186/1471-2334-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood F.S., Jain S., Finelli L., Shaw M.W., Lindstrom S., Garten R.J., Gubareva L.V., Xu X., Bridges C.B. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 13.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 14.Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 15.Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 17.van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C., Wertheim-van Dillen P.M., Kaandorp J., Spaargaren J., Berkhout B. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fouchier R.A., Hartwig N.G., Bestebroer T.M., Niemeyer B., de Jong J.C., Simon J.H., Osterhaus A.D. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci U S A. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 20.Anderson L.J., Baric R.S. Emerging human coronaviruses — disease potential and preparedness. N Engl J Med. 2012;367:1850–1852. doi: 10.1056/NEJMe1212300. [DOI] [PubMed] [Google Scholar]
- 21.van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A., Osterhaus A.D. A. newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn J.S. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–557. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Clusters of acute respiratory illness associated with human enterovirus 68 — Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1301–1304. [PubMed] [Google Scholar]
- 24.Tokarz R., Firth C., Madhi S.A., Howie S.R., Wu W., Sall A.A., Haq S., Briese T., Lipkin W.I. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol. 2012;93(Pt 9):1952–1958. doi: 10.1099/vir.0.043935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.McLean E., Pebody R.G., Campbell C., Chamberland M., Hawkins C., Nguyen-Van-Tam J.S., Oliver I., Smith G.E., Ihekweazu C., Bracebridge S. Pandemic (H1N1) 2009 influenza in the UK: clinical and epidemiological findings from the first few hundred (FF100) cases. Epidemiol Infect. 2010;138:1531–1541. doi: 10.1017/S0950268810001366. [DOI] [PubMed] [Google Scholar]; This paper clearly describes the ethos and outputs arising from detailed study of the first few hundred cases of pandemic influenza in the UK, demonstrating the potential usefulness of such an approach during future pandemic and the importance of replicating this system internationally.
- 26••.Nicoll A., Lopez Chavarrias V. National and international public health measures. In: Van-Tam J., Sellwood C., editors. Pandemic Influenza. edn 2. CABI; 2012. pp. 152–163. [Google Scholar]; This textbook is the only one of its kind in the world focussing on pandemic preparedness and response. The chapter on public health measures offers a synoptic insight into a highly complex subject.
- 27••.Carrasco P., Leroux-Roels G. Pandemic Vaccines. In: Van-Tam J., Sellwood C., editors. Pandemic Influenza. edn 2. CABI; 2012. pp. 139–151. [Google Scholar]; This textbook is the only one of its kind in the world focussing on pandemic preparedness and response. The chapter on vaccines describes the science as well as the considerable policy, logistic and communication difficulties encountered in 2009–10.
- 28•.Yin J.K., Chow M.Y., Khandaker G., King C., Richmond P., Heron L., Booy R. Impacts on influenza A(H1N1)pdm09 infection from cross-protection of seasonal trivalent influenza vaccines and A(H1N1)pdm09 vaccines: systematic review and meta-analyses. Vaccine. 2012;30:3209–3222. doi: 10.1016/j.vaccine.2012.02.048. [DOI] [PubMed] [Google Scholar]; This is the first published meta-analysis of the effectiveness of pandemic A(H1N1) vaccines; it also examines possible cross-protection conferred by seasonal H1N1 vaccination.
- 29.Beck C.R., Sokal R., Arunachalam N., Puleston R., Cichowska A., Kessel A., Zambon M., Nguyen-Van-Tam J.S., on behalf of the UK Antiviral Effectiveness Review Group Neuraminidase inhibitors for influenza: a review and public health perspective in the aftermath of the 2009 pandemic. Influenza Other Respi Viruses. 2013;7:14–24. doi: 10.1111/irv.12048. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Pebody R.G., Harris R., Kafatos G., Chamberland M., Campbell C., Nguyen-Van-Tam J.S., McLean E., Andrews N., White P.J., Wynne-Evans E. Use of antiviral drugs to reduce household transmission of pandemic (H1N1) 2009, United Kingdom. Emerg Infect Dis. 2011;17:990–999. doi: 10.3201/eid1706.101161. [DOI] [PMC free article] [PubMed] [Google Scholar]; The UK pursued an almost unique policy of widespread and sustained post-exposure prophylaxis in households between May and July 2009. This paper demonstrates the impact of that policy on the incidence of secondary cases in households.
- 31.Gupta R.K., George R., Nguyen-Van-Tam J.S. Bacterial pneumonia and pandemic influenza planning. Emerg Infect Dis. 2008;14:1187–1192. doi: 10.3201/eid1408.070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van-Tam J., Lim W.S. Pharmaceutical interventions. In: Van-Tam J., Sellwood C., editors. Pandemic Influenza. edn 2. CABI; 2012. pp. 122–138. [Google Scholar]
- 33.Mytton O.T., Rutter P.D., Donaldson L.J. Influenza A(H1N1)pdm09 in England, 2009 to 2011: a greater burden of severe illness in the year after the pandemic than in the pandemic year. Euro Surveill. 2012;17 [PubMed] [Google Scholar]
- 34••.Muthuri S.G., Myles P.R., Venkatesan S., Leonardi-Bee J., Nguyen-Van-Tam J.S. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009–2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J Infect Dis. 2013;207:553–563. doi: 10.1093/infdis/jis726. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first meta-analysis of observational data on antiviral effectiveness pertaining specifically to the 2009–10 pandemic period. It provides important clues about whether the public health strategy of using antiviral drugs against pandemic influenza impacted on public health outcomes such as mortality.


