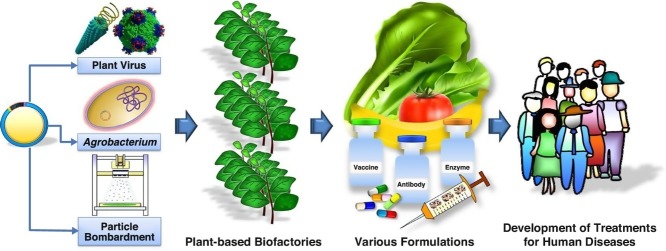
Graphical abstract
Highlights
-
•
Concept of plant-based biofactories for therapeutics and biologics.
-
•
Industrial preference of transient expression system — agroinfiltration.
-
•
Advancement of virus-like particles from epitope presentation to nanomedicine.
-
•
Recent progress of plant-made therapeutics and biologics against human diseases.
Abstract
Production of proteins in plants for human health applications has become an attractive strategy attributed by their potentials for low-cost production, increased safety due to the lack of human or animal pathogens, scalability and ability to produce complex proteins. A major milestone for plant-based protein production for use in human health was achieved when Protalix BioTherapeutics produced taliglucerase alfa (Elelyso®) in suspension cultures of a transgenic carrot cell line for the treatment of patients with Gaucher's disease, was approved by the USA Food and Drug Administration in 2012. In this review, we are highlighting various approaches for plant-based production of proteins and recent progress in the development of plant-made therapeutics and biologics for the prevention and treatment of human diseases.
Current Opinion in Virology 2017, 26:81–89
This review comes from a themed issue on Engineering for viral resistance
Edited by John Carr and Peter Palukaitis
For a complete overview see the Issue and the Editorial
Available online 8th August 2017
http://dx.doi.org/10.1016/j.coviro.2017.07.019
1879-6257/© 2017 Elsevier B.V. All rights reserved.
Introduction
Infectious diseases remain as one of the leading causes of mortality and morbidity in developing countries and are exacerbated by the lack of resources and infrastructure to prevent, treat and control diseases. Therefore, emerging and re-emerging pathogens have frequently resulted in epidemics in these countries. Over the past several decades, production of proteins in plants has been shown to be a promising approach for the manufacture of targets for human health applications. Plants, when compared to other production systems, offer some advantages, including ease of scaling and lack of human and animal pathogens [1, 2, 3] (Table 1 ).
Table 1.
General comparison of expression hosts for the production of heterologous proteins for medical and pharmaceutical applications
| Expression host | Expression level | Production lead time | Production cost | Storage and distribution cost | Scale-up capacity | Glycosylation pattern | Risk of contamination |
|---|---|---|---|---|---|---|---|
| Bacterium | Medium — high | Short | Low | Moderate | High | None | High: endotoxins |
| Yeast | Low — high | Medium | Medium | Moderate | High | Incorrect: higher manosylation | Low |
| Insect cell culture | Low — high | Medium | High | Expensive | Medium | Incorrect: higher manosylation | High: baculovirus, mammalian viruses |
| Mammalian cell culture | Low — medium | Long | High | Expensive | Very low | Correct | High: mammalian viruses, prions, oncogenic DNA |
| Animal | Medium — high | Very long | High | Expensive | Low | Correct | High: mammalian viruses, prions, oncogenic DNA |
| Plant cell culture | Medium — high | Short | Low | Moderate | High | Minor difference | Low |
| Plant | Medium — high | Medium (transienta) Long (stableb) |
Very low | Inexpensive | Very high | Minor difference | Low |
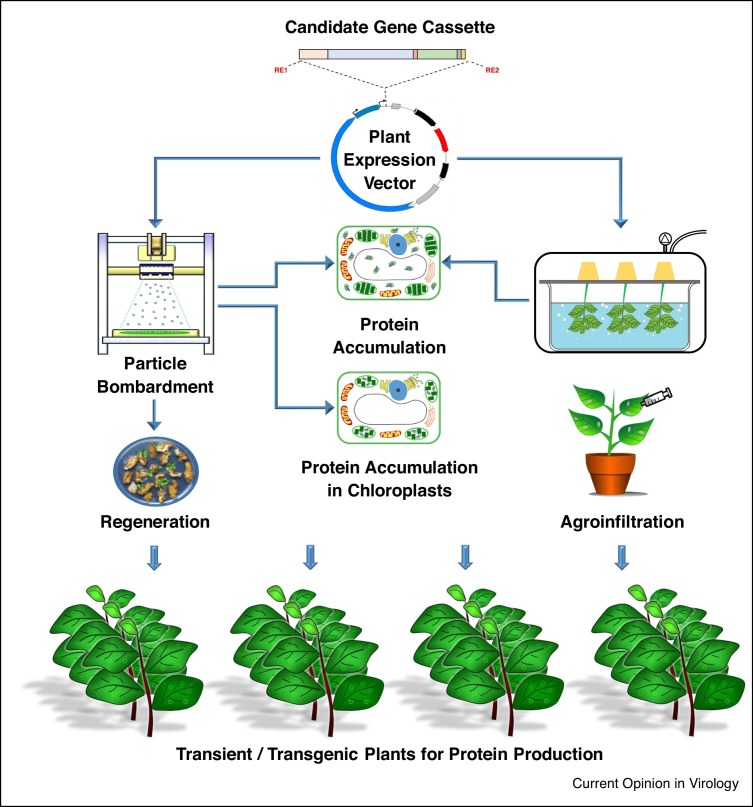
This review focuses on several approaches that have been used to produce proteins in plants for prophylactic and therapeutic applications to combat human disease conditions. The various approaches for plant-based production of proteins are illustrated in Figure 1 .
Figure 1.
Schematic illustration of the production of proteins in plants using transient expression (agroinfiltration) and transgenic (stable nuclear and chloroplast transformation) strategies.
Transgenic plants
Stable nuclear and chloroplast transformations are the two approaches utilized to express heterologous recombinant proteins in plants. Agrobacterium-mediated stable transformation has a long history in plant genetic manipulation, and is achieved by stable integration of T-DNA into plant nuclear genome [4]. However, the approach is time consuming, with a lead time ranging from 12 to 18 months and typically has low levels of the target protein expressed [5]. Stable introduction of target genes into chloroplast genome, that is, chloroplast transformation or transplastomics, however, allows for higher levels of target expression as compared to nuclear transformation, largely due to the lack of gene silencing and high gene copy number [6], but it is technically difficult, lacks most post-translational modifications and has only been successful in a limited number of plant species.
Transient expression in plants
Transient expression of target proteins in plants using modified plant viruses or viral vectors integrated into binary vectors delivered via Agrobacterium [7, 8••] is often considered a more robust approach when compared to stable transformation, due to its rapid production capabilities and relatively high protein expression [8••]. The majority of plant viral vectors used to date are based on single-stranded RNA viruses, such as tobacco mosaic virus, potato virus X and cowpea mosaic virus (CPMV), which encode for at least three proteins with functions in viral replication (replicase), encapsidation (coat protein) and movement from cell-to-cell (movement protein) [9]. The initial strategy involved production of recombinant proteins using plant viruses by exploiting their natural ability to infect (full virus) plants. However, this approach generally failed due to instability of viral genome modified by the introduction of large target genes [7]. This issue was largely resolved by using Agrobacterium-mediated gene delivery or agroinfiltration. The target gene can either be directly cloned into an Agrobacterium vector or through a modified plant viral vector which has been integrated into an Agrobacterium binary plasmid, and delivered into the plant tissues by infiltration with the transformed Agrobacterium [7, 8••]. Agroinfiltration allows for high levels of target protein expression with the potential for cost-effective production [5, 10]. The peak protein expression is typically observed in less than 7 days postinfiltration which is significantly faster when compared to the full virus strategy which requires more than 2 weeks in order to generate a systemic infection for expression. The promise of this platform has been evidenced in numerous successful clinical trials, which demonstrated safety and efficacy of plant-made protein therapeutics and biologics [11•]. For example, in responding to the H1N1 influenza virus pandemic that occurred in 2009, Medicago, a Canadian company, reported producing the vaccine candidate, hemagglutinin in 19 days in Nicotiana benthamiana [10]. As such, agroinfiltration provides a rapid response capability and is currently the preferred approach for the production of proteins in plants.
Prophylactic and therapeutic applications of plant-made proteins
Numerous examples of plant-produced proteins targeting prophylactic and therapeutic applications (subsectioned as vaccines, antibodies and other biopharmaceuticals) in preclinical development are shown in Table 2 . Several lead candidates have gone through clinical trials (Table 3 ) and have been comprehensively reviewed [12, 13•].
Table 2.
Recent examples of plant-derived vaccines, antibodies and other biopharmaceuticals for the prevention and treatment of human diseases
| Target protein | Indication/disease | Plant host/expression strategy | Functionality evaluation | Reference |
|---|---|---|---|---|
| Vaccines | ||||
| Anthrax protective antigen 83 (PA83) | SUV against Anthrax (Bacillus anthracis) | Nicotiana benthamiana/transient | • Detection of high-titer toxin-neutralizing antibodies. • 100% survival of immunized rabbits (IM) against lethal Anthrax challenge. |
[29] |
| Anthrax PA83 | SUV against B. anthracis | Brassica juncea (mustard)/transgenic (nuclear) | • Detection of systemic and mucosal immune responses. • 60% survival of orally immunized mice against lethal Anthrax challenge. |
[30] |
| Anthrax PA83 | SUV against B. anthracis | Nicotiana tabacum (tobacco)/transgenic (chloroplast) | • Detection of systemic and mucosal immune responses. • 80% survival of orally immunized mice against lethal Anthrax challenge. |
[30] |
| Dengue consensus domain III of envelope glycoprotein (cEDIII) in hybrid with 6D8 anti-Ebola IgG | Recombinant immune complex vaccine against dengue virus (DENV) serotypes | N. benthamiana/transient | • Detection of virus-neutralizing specific anti-cEDIII humoral immune response in immunized mice (SC). | [31] |
| Ebola glycoprotein (GP) in fusion with 6D8 anti-Ebola IgG (6D8 IgG-GP1) | Antigen-antibody fusion vaccine against Ebola virus (EBOV) | N. benthamiana/transient | • Detection of humoral immune responses. • 80% survival of immunized mice (SC) against lethal EBV challenge. |
[32] |
| EBOV GP1 in fusion with E. coli heat-labile enterotoxin B subunit (LTB-EBOV) | SUV against EBOV | Tobacco/transgenic (nuclear) | • Detection of serum IgG in immunized mice (SC) and fecal IgA in immunized mice via oral administration. | [33•] |
| Hepatitis B virus (HBV) small surface antigen (S-HBsAg) | eVLP vaccine against HBV | Lactuca sativa (lettuce)/transgenic (nuclear) | • Detection of serum IgG in immunized mice via oral administration. | [34] |
| HBV surface antigen (HBsAg) | SUV against HBV | Solanum tuberosum (potato)/transgenic (nuclear) | • Induction of serum antibodies and stable immunological memory in immunized mice fed with transgenic potato tubers. | [35] |
| Human immunodeficiency virus (HIV) gp120 multi-epitopic envelope protein (C4(V3)6) | SUV against multiple HIV strains | Lettuce/transgenic (nuclear) | • Detection of cell-mediated and humoral immunities in immunized mice via oral administration. | [36] |
| HIV gp120 and gp41 multi-epitopic envelope proteins (Multi-HIV) | SUV against multiple HIV strains | Tobacco/transgenic (chloroplast) | • Detection of antibody and cellular responses as well as specific IFN-γ production in immunized mice via oral administration. | [37] |
| HIV-1 envelope proteins (Gag/Dgp41) | eVLPs vaccine against HIV-1 | N. benthamiana/transient | • Induced Gag-specific serum antibody and CD4 and CD8 T-cell responses in mice via systemic (IP) and mucosal (IN) immunizations. | [38] |
| Human papillomavirus type 16 (HPV-16) HPV-16L1 | SUV against HPV-16 | Tobacco/transgenic (nuclear) | • Detection of cell-mediated and humoral immunities in immunized mice via oral administration. | [39] |
| HPV-16 E6 and E7 fusion (HPV-16L1 E6/E7) | cVLP vaccine against HPV-16 | Solanum lycopersicum (tomato)/transgenic (nuclear) | • Detection of persistent neutralizing antibodies and 57% tumor reduction in immunized mice via oral administration. | [40] |
| Influenza H1N1 trimeric HA from A/California/04/09 strain (tHA-BC) | SUV against H1N1 influenza virus | N. benthamiana/transient | • Detection of serum HI antibody responses. • 100% survival of immunized mice (IM) against lethal H1N1 challenge. |
[41] |
| Influenza H1N1 HA from A/California/04/09 strain (HAC-VLPs) | eVLP vaccine against H1N1 influenza virus | N. benthamiana/transient | • Detection of serum HI antibody responses in immunized mice (IM). | [42••] |
| Influenza H3N2 nucleoprotein | SUV against H3N2 influenza virus | Zea mays (maize)/transgenic (nuclear) | • Detection of humoral immune responses in immunized mice via oral administration. | [43] |
| Influenza H5N1 HA1 domain (HA1-MY) | SUV against H5N1 influenza virus | N. benthamiana/transient | • Detection of serum HI antibody responses in immunized mice (IM). | [44] |
| Rabies virus glycoprotein in fusion with ricin toxin B chain (RGB-RTB) | SUV against rabies virus | Solanum lycopersicum (tomato) hairy roots/transgenic (nuclear) | • Detection of serum IgG and Th2 lymphocyte responses in immunized mice via intra-mucosal administration. | [45] |
| Severe acute respiratory syndrome coronavirus (SARS-CoV) nucleocapsid (N) protein | SUV against SARS-CoV | N. benthamiana/transient | • Recognition of SARS patient sera by purified N protein. | [46] |
| Antibodies | ||||
| Anthrax PA83 full size PANG MAb (non-glycosylated) | MAb therapy for B. anthracis infection | N. benthamiana/transient | • 100% survival of treated mice (IP) and non-human primates (IV) against lethal Anthrax challenges. | [47] |
| Ebola GP triple cocktail (13C6, 13F6, 6D8) MAb (humanized and glycoengineered) (MB-003) | MAb therapy for Ebola virus infection | N. benthamiana/transient | • 43–100% survival of treated rhesus macaques (IV) depending on the treatment time postinfection of EBOV. | [48] |
| Ebola GP triple cocktail (13C6, 2G4, 4G7) MAb (humanized and glycoengineered) (ZMapp) | MAb therapy for Ebola virus infection | N. benthamiana/transient | • 100% survival of treated rhesus macaques (IV) at 5 days postinfection of EBOV. | [16] |
| West Nile E DIII (hE16) MAbs: humanized, glycoengineered, full-size hE16 and scFv-CH fusion | MAb therapy for West Nile virus (WNV) infection | N. benthamiana/transient | • Detection of enhanced in vitro WNV neutralization activity. • 70–100% survival of treated mice (IP) depending on prophylactic or therapeutic regimen. |
[49] |
| West Nile E DIII (hE16) MAbs: full size hE16; humanized monomeric scFv-CH fusion and tetravalent scFv-CH/scFv-CL fusion (Tetra-hE16) | MAb therapy for WNV infection | N. benthamiana/transient | • No detection of ADE activity. • 70–90% survival of treated mice (IP) depending on prophylactic or therapeutic regimen. |
[50, 51] |
| Other biopharmaceuticals | ||||
| Hemophilia A coagulation factor VIII (FVIII) heavy chain (HC) and C2 domain in fusion with cholera toxin B (CTB-HC and CTB-C2) | Coagulation factor VIII replacement therapy for hemophilia A | Tobacco/transgenic (chloroplast) | • Oral delivery of bioencapsulated CTB-HC and CTB-C2 antigens substantially suppressed T helper cell responses and inhibitors formation against FVIII in hemophilia A mice. | [52] |
| Hemophilia B coagulation factor IX (FIX) in fusion with CTB (CTB-FIX) | Coagulation factor IX replacement therapy for hemophilia B | Lettuce/transgenic (chloroplast) | • Oral feeding of CTB-FIX in hemophilia B mice could efficiently reach to the gut immune system and suppressed IgE (inhibitor) formation and anaphylaxis against FIX. | [53] |
| Pompe acid alpha glucosidase (GAA) in fusion with CTB (CTB-GAA) | Enzyme replacement therapy for GAA deficiency in Pompe disease | Tobacco/transgenic (chloroplast) | • Bioencapsulated GAA suppressed the specific IgG1 and IgG2a inhibitory antibody formation in Pompe mice via oral administration. | [54•] |
| Type II diabetes dipeptidyl peptidase IV (DPP-IV) resistant glucagon like peptide (GLP-1) analog – exendin-4 (EX4) in fusion with CTB (CTB-EX4) | Peptide hormone replacement therapy to increase insulin secretion for type II diabetes | Tobacco/transgenic (chloroplast) | • Purified CTB-EX4 increased level of insulin secretion from pancreatic cells. • Oral feeding of lyophilized CTB-EX4 lowered blood glucose level in mice. |
[55] |
Keys for abbreviations: ADE, antibody-dependent enhancement; CH, constant domains of immunoglobulin heavy chain; CL, constant domain of immunoglobulin light chain; CTB, cholera toxin B; cVLP, chimeric virus-like particle; DIII, domain III; DPP, dipeptidyl peptidase; E, envelope; eVLP, enveloped virus-like particle; EX, exendin; F, coagulation factor; GAA, acid alpha glucosidase; GLP, glucagon like peptide; GP, glycoprotein; HA, hemagglutinin; HI, hemagglutination-inhibition; HC, heavy chain; Ig, immunoglobulin; LTB, heat-labile enterotoxin B subunit; IM, intramuscular; IN, intranasal; IP, intraperitoneal; IV, intravenous; MAb, monoclonal antibody; N, nucleocapsid; PA, protective antigen; RTB, ricin toxin B; sAg, surface antigen; SC, subcutaneous; scFv, single-chain variable fragment of immunoglobulin; SUV, subunit vaccine; VLP, virus-like particle.
Table 3.
Examples of plant-based vaccines, antibodies and other biopharmaceuticals at various stages of clinical trials
| Product | Plant host | Application | Clinicaltrials.gov identifier | Status | Company (sponsora) |
|---|---|---|---|---|---|
| Vaccines | |||||
| Pfs25 VLP-FhCMB | N. benthamiana | Malaria transmission blocking vaccine against Plasmodium falciparum | NCT02013687 | Phase 1 (completed in 2015) | FhCMB, USA |
| PA83-FhCMB | N. benthamiana | SUV against Anthrax (Bacillus anthracis) | NCT02239172 | Phase 1 (completed in 2015) | FhCMB, USA |
| HAC1 | N. benthamiana | SUV against H1N1 seasonal influenza virus | NCT01177202 | Phase 1 (completed in 2012) | FhCMB, USA |
| HAI-05 | N. benthamiana | SUV against H5N1 pandemic influenza virus | NCT01250795 | Phase 1 (completed in 2011) | FhCMB, USA |
| H1 VLP | N. benthamiana | eVLP vaccine against H1N1 seasonal influenza virus | NCT01302990 | Phase 1 (completed in 2011) | Medicago, Canada |
| Quadrivalent VLP | N. benthamiana | Quadrivalent eVLP vaccine against H1N1, H3N2, seasonal influenza B viruses | NCT01991587 | Phases 1 and 2 (completed in 2014) | Medicago, Canada |
| Quadrivalent VLP | N. benthamiana | Quadrivalent eVLP vaccine against H1N1, H3N2, seasonal influenza B viruses | NCT02233816 | Phase 2 (ongoing, not recruiting) | Medicago, Canada |
| Quadrivalent VLP | N. benthamiana | Quadrivalent eVLP vaccine against H1N1, H3N2, seasonal influenza B viruses | NCT02236052 | Phase 2 (ongoing, not recruiting) | Medicago, Canada |
| H5 VLP | N. benthamiana | eVLP vaccine against H5N1 pandemic influenza virus | NCT00984945 | Phase 1 (completed in 2010) | Medicago, Canada |
| H5 VLP | N. benthamiana | eVLP vaccine against H5N1 pandemic influenza virus | NCT01244867 | Phase 2 (completed in 2011) | Medicago, Canada |
| H5 VLP | N. benthamiana | eVLP vaccine against H5N1 pandemic influenza virus | NCT01991561 | Phase 2 (completed in 2014) | Medicago, Canada |
| H5-VLP + GLA-AF | N. benthamiana | eVLP vaccine against H5N1 pandemic influenza virus | NCT01657929 | Phase 1 (completed in 2014) | Medicago (IDRI), Canada |
| H7 VLP | N. benthamiana | eVLP vaccine against H7N9 pandemic influenza virus | NCT02022163 | Phase 1 (completed in 2014) | Medicago, Canada |
| Autologous FL vaccine | N. benthamiana | Full-idiotype vaccine against follicular lymphoma (non-Hodgkin's lymphoma) | NCT01022255 | Phase 1 (completed in 2013) | Icon Genetics GmbH, Germany |
| Antibodies | |||||
| P2G12 | N. tabacum (tobacco) | MAb therapy for HIV-1 infection | NCT02923999 | Phase 1 (not yet recruiting) | St George's University of London, UK |
| P2G12 | Tobacco | MAb therapy for HIV-1 infection | NCT01403792 | Phase 1 (completed in 2011) | University of Surrey, UK |
| ZMapp | N. benthamiana | MAb therapy for Ebola virus infection | NCT02363322 | Phases 1 and 2 (ongoing; not recruiting) | LeafBio (NIAID), Canada |
| ZMapp | N. benthamiana | MAb therapy for Ebola virus infection | NCT02389192 | Phase 1 (recruiting) | LeafBio (NIAID), Canada |
| Other biopharmaceuticals | |||||
| Taliglucerase Alfa (Human Glucocerebrosidase, prGCD) | Daucus carota (carrot) cell culture | ERT for Gaucher's disease | NCT00376168 | Phase 3 (completed in 2012); FDA (approved in 2012) | Protalix BioTherapeutics, Israel |
| Moss-aGal (Human Apha-galactosidase A) | Physcomitrella patens (moss) | ERT for Fabry disease | NCT02995993 | Phase 1 (recruiting) | Greenovation Biotech GmbH, Germany |
| PRX-102 (Human Alpha-galactosidase A) | Tobacco cell culture | ERT for Fabry disease | NCT01769001 | Phases 1 and 2 (ongoing; enrolling by invitation) | Protalix BioTherapeutics, Israel |
| Recombinant Human Intrinsic Factor | Arabidopsis thaliana | Dietary supplement for vitamin B12 deficiency | NCT00279552 | Phase 2 (completed in 2006) | University in Aarhus, Denmark |
| Recombinant Lactoferrin | Oryza sativa (rice) | Anti-inflammation treatment for HIV patients | NCT01830595 | Phase 2 (completed in 2006) | Jason Baker (MMRF), USA |
| rhLactoferrin | Rice | Treatment for chronic inflammation in the elderly | NCT02968992 | Phase 2 (ongoing, not recruiting) | Johns Hopkins University, USA |
| Locteron (Controlled-release Interferon Alpha 2b) | Lemna minor (duckweed) | Antiviral treatment for hepatitis C virus infection | NCT00593151 | Phases 1 and 2 (completed in 2009) | Biolex Therapeutics, USA |
Examples of clinical studies that are registered at https://clinicaltrials.gov showing a status as accessed in 31st March 2017.
Keys for abbreviations: ERT, enzyme replacement therapy; eVLP, enveloped virus-like particle; FDA, Food and Drug Administration; FhCMB, Fraunhofer USA Center for Molecular Biotechnology; HIV-1, Human immunodeficiency virus type 1; IDRI, Infectious Disease Research Institute; MAb, monoclonal antibody; MMRF, Minneapolis Medical Research Foundation; NIAID, National Institute of Allergy and Infectious Diseases; SUV, subunit vaccine.
Sponsor which is not from the same company.
Vaccines are highly effective tools for the prevention of infections. Over the last three decades, plant-produced antigens targeting various pathogens have been shown to be effective in animal models (Table 2). Several of these candidates have progressed into early stage clinical development and were evaluated in Phase 1–2 human clinical trials (Table 3) with safety demonstrated. To date, there are no plant-based vaccines approved for human use. In fact, a purified injectable Newcastle disease virus vaccine for poultry produced in a suspension cell culture of transgenic tobacco by Dow AgroSciences had been approved by US Department of Agriculture in 2006 [14], but the company has no intention to market the product.
The first plant-derived antibody produced under good manufacturing practices to undergo clinical testing in Europe was the human P2G12 which was produced in stably transformed tobacco against HIV-1. P2G12 has been shown to be safe and well-tolerated in healthy women based on intravaginal administration [15••]. Another example of plant-produced antibodies is the triple cocktail (13C6, 2G4, 4G7) directed against the surface glycoprotein of Ebola, ZMapp, produced in N. benthamiana. ZMapp treatment was able to reverse Ebola infection in 100% of the infected Rhesus macaques that received a live virus challenge [16]. ZMapp was administered to several Ebola patients as an investigational postexposure therapy during the Ebola outbreak in West Africa that occurred in 2014 even though the drug had not been approved by Food and Drug Administration (FDA). Though a limited number of people were treated, ZMapp along with medical care successfully saved several patients from death. In early 2015, ZMapp received an approval from FDA as an investigational new drug, allowing the start of clinical trials in Liberia (Table 3).
Planet Biotechnology (Hayward, CA) produced the world's first plant-derived clinically tested secretory IgA monoclonal antibody which recognizes the surface antigen I/II of Streptococcus mutans (CaroRx™) that predominantly causes dental caries. Following the successful demonstration of safety and efficacy in a Phase 2 clinical trial, CaroRx™ has been licensed in Europe in a medical device category [17, 18] and applied as an oral topical solution to prevent tooth decay.
In 1986, the recombinant human growth hormone was the first plant-based biopharmaceutical protein produced in plants [19]. Then over two decades later, the FDA in May 2012 approved ELELYSO® (human recombinant taliglucerase alfa or glucocerebrosidase), an enzyme produced in genetically engineered carrot cells for treating type 1 Gaucher's disease (GD) by Protalix BioTherapeutics and its partner, Pfizer [20]. GD is a lysosomal storage disorder caused by a hereditary deficiency of the enzyme, glucocerebrosidase (GCD). GD is currently treated by enzyme replacement therapy using this recombinant GCD that is administered intravenously every 2 weeks [21].
Virus-like particles (VLPs) as nanomedicines
In addition to offering a versatile production platform for numerous plant-made proteins, plant viruses have been engineered to provide medical applications in other ways [22]. VLPs offer advantages over recombinant protein vaccines as they tend to elicit a higher immune response [23]. Virus nanoparticles have also been developed for the targeted delivery for disease treatment and diagnostic purposes. For example, CPMV represents an icosahedral nanoparticle with its capsid surface displaying 300 accessible lysine residues; each of these can be conjugated to various chemical moieties like fluorescent dyes/arrays, polyethylene glycol polymers and subcellular targeting molecules [24, 25]. The use of this technology includes the construction of CPMV nanoparticles displaying gastrin-releasing peptide receptors that are overexpressed in human prostate cancers [26]. Another example, cowpea chlorotic mottle virus can stably assemble in vitro and package the RNA derived from sindbis virus, a mammalian virus. These hybrid cowpea chlorotic mottle virus-based VLPs were shown to protect against RNA degradation by cellular nucleases and were able to deliver and release their RNA contents within the cytoplasm of mammalian cells. Moreover, these hybrid VLPs with the fusion of subcellular targeting moieties could be directed toward distinct sites within the cell [27] and potentially applied as a medical targeted delivery tool. Plant viruses have also been engineered to act as adjuvants to elicit an immune response that is more potent and effective. The rod-shaped papaya mosaic virus nanoparticles have been engineered to express an influenza epitope on their surface, and mice and ferrets immunized with these recombinant nanoparticles exhibited an increase in robust humoral response to influenza virus infection [28].
Conclusions
There is growing evidence that plants are capable of making proteins with desired quality to address a range of human health-related issues. Plant production platforms for protein therapeutics and biologics, in particular the transient agroinfiltration approach, have demonstrated the ability to be used for broad research and development, as well as commercial needs. It has been extensively discussed that the transient agroinfiltration approach is the ideal platform for fast and scalable production in response to new outbreaks of highly infectious diseases and has been demonstrated under various programs. The success of Protalix Biotherapeutics in gaining FDA approval for the therapeutic enzyme, ELELYSO® for human use was a significant milestone for the plant molecular pharming field. More importantly, the primary benefits of plant-made protein therapeutics and biologics in terms of product safety and potential cost-effectiveness will further contribute to global public health in both developed and developing nations.
Conflict of interest
The authors declare no conflict of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The authors would like to thank Dr Stephen Streatfield (FhCMB) for editorial assistance.
References
- 1.Ma J.K.C., Drake P.M.W., Christou P. The production of recombinant pharmaceutical proteins in plants. Nature. 2003;4:749–805. doi: 10.1038/nrg1177. [DOI] [PubMed] [Google Scholar]
- 2.Floss D.M., Falkenburg D., Conrad U. Production of vaccines and therapeutic antibodies for veterinary applications in transgenic plants: an overview. Transgenic Res. 2007;16:315–332. doi: 10.1007/s11248-007-9095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merlin M., Gecchele E., Capaldi S., Pezzotti M., Avesani L. Comparative evaluation of recombinant protein production in different biofactories: the green perspective. Biomed Res Int. 2014:136419. doi: 10.1155/2014/136419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tinland B. The integration of T-DNA into plant genome. Trends Plant Sci. 1996;1:178–184. [Google Scholar]
- 5.Gleba Y., Klimyuk V., Marillonnet S. Magnifection — a new platform for expressing recombinant vaccines in plants. Vaccine. 2005;23:2042–2048. doi: 10.1016/j.vaccine.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S., Daniell H. Engineering the chloroplast genome for hyperexpression of human therapeutic proteins and vaccine antigens. Methods Mol Biol. 2004;267:365–383. doi: 10.1385/1-59259-774-2:365. [DOI] [PubMed] [Google Scholar]
- 7.Musiychuk K., Stephenson N., Bi H., Farrance C.E., Orozovic G., Brodelius M., Brodelius P., Horsey A., Ugulava N., Shamloul A.M. A launch vector for the production of vaccine antigens in plants. Influenza Other Respir Viruses. 2007;1:19–25. doi: 10.1111/j.1750-2659.2006.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Peyret H., Lomonossoff G.P. When plant virology met Agrobacterium: the rise of the deconstructed clones. Plant Biotechnol J. 2015;13:1121–1135. doi: 10.1111/pbi.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reviews the development of technologies used in the stable transformation and transient expression systems. Particular focus is placed on the development of modern plant virus-based overexpression vectors in combination with Agrobacterium-mediated gene transfer which has been proven extremely effective for the production of numerous pharmaceutical proteins.
- 9.Gergerich R.C., Dolja V.V. Introduction to plant viruses, the invisible foe. Plant Health Instruct. 2006 doi: 10.1094/PHI-I-2006-0414-01. [DOI] [Google Scholar]
- 10.D’Aoust M.A., Couture M.M., Charland N., Trépanier S., Landry N., Ors F., Vézina L.P. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J. 2010;8:607–619. doi: 10.1111/j.1467-7652.2009.00496.x. [DOI] [PubMed] [Google Scholar]
- 11•.Chen Q., Lai H. Gene delivery into plant cells for recombinant protein production. Biomed Res Int. 2015:932161. doi: 10.1155/2015/932161. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the general gene delivery methodologies in plants, and then focuses on the recent progress of agroinfiltration methods in the contexts of their applications and scalability involving several expression vectors and plant species for large-scale manufacturing of recombinant proteins.
- 12.Yusibov V., Streatfield S.J., Kushnir N. Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Hum Vaccines. 2011;7:313–321. doi: 10.4161/hv.7.3.14207. [DOI] [PubMed] [Google Scholar]
- 13•.Yao J., Weng Y.Q., Dickey A., Wang Y.J. Plants as factories for human pharmaceuticals: applications and challenges. Int J Mol Sci. 2015;16:28549–28565. doi: 10.3390/ijms161226122. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper illustrates the plant molecular farming or pharming concept in relation to human pharmaceutical applications. Several types of plant-based production platforms are described. The challenges such as plant-type glycosylations, downstream bioprocesses and biosafety concerns are also discussed. Besides, some of the preclinical and clinical studies that have been enlisted in our review paper are elaborated here.
- 14.Rybicki E.P. Plant-made vaccines for humans and animals. Plant Biotechnol J. 2010;8:620–637. doi: 10.1111/j.1467-7652.2010.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Ma J.K.C., Drossard J., Lewis D., Altmann F., Boyle J., Christou P., Cole T., Dale P., van Dolleweerd C.J., Isitt V. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol J. 2015;13:1106–1120. doi: 10.1111/pbi.12416. [DOI] [PubMed] [Google Scholar]; This is an excellent case study to learn about the historical development of plant-derived HIV-neutralizing monoclonal antibody (P2G12) which has been entering into the clinical phases. The clinical evaluation proved that P2G12 was safe and well tolerated in healthy women when administered intravaginally.
- 16.Qiu X., Wong G., Audet J., Bello A., Fernando L., Alimonti J.B., Fausther-Bovendo H., Wei H., Aviles J., Hiatt E. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larrick J.W., Yu L., Naftzger C., Jaiswal S., Wycoff K. Production of secretory IgA antibodies in plants. Biomol Eng. 2001;18:87–94. doi: 10.1016/s1389-0344(01)00102-2. [DOI] [PubMed] [Google Scholar]
- 18.De Muynck B., Navarre C., Boutry M. Production of antibodies in plants: status after twenty years. Plant Biotechnol J. 2010;8:529–563. doi: 10.1111/j.1467-7652.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 19.Barta A., Sommergruber K., Thompson D., Hartmuth K., Matzke M.A., Matzke A.J.M. The expression of a nopaline synthase — human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant Mol Biol. 1986;6:347–357. doi: 10.1007/BF00034942. [DOI] [PubMed] [Google Scholar]
- 20.Fox J.L. First plant-made biologic approved. Nat Biotechnol. 2012;30:472. [Google Scholar]
- 21.Shaaltiel Y., Gingis-Velitski S., Tzaban S., Fiks N., Tekoah Y., Aviezer D. Plant-based oral delivery of β-glucocerebrosidase as an enzyme replacement therapy for Gaucher's disease. Plant Biotechnol J. 2015;13:1033–1040. doi: 10.1111/pbi.12366. [DOI] [PubMed] [Google Scholar]
- 22.Hefferon K. Plant virus expression vector development: new perspectives. Biomed Res Int. 2014:785382. doi: 10.1155/2014/785382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kushnir N., Streatfield S.J., Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31:58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinmetz N.F., Calder G., Lomonossoff G.P., Evans D.J. Plant viral capsids as nanobuilding blocks: construction of arrays on solid supports. Langmuir. 2006;22:10032–10037. doi: 10.1021/la0621362. [DOI] [PubMed] [Google Scholar]
- 25.Steinmetz N.F., Cho C.F., Ablack A., Lewis J.D., Manchester M. Cowpea mosaic virus nanoparticles target surface vimentin on cancer cells. Nanomedicine. 2011;6:351–364. doi: 10.2217/nnm.10.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinmetz N.F., Ablack A.L., Hickey J.L., Ablack J., Manocha B., Mymryk J.S., Luyt L.G., Lewis J.D. Intravital imaging of human prostate cancer using viral nanoparticles targeted to gastrin-releasing peptide receptors. Small. 2011;7:1664–1672. doi: 10.1002/smll.201000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azizgolshani O., Garmann R.F., Cadena-Nava R., Knobler C.M., Gelbart W.M. Reconstituted plant viral capsids can release genes to mammalian cells. Virology. 2013;441:12–17. doi: 10.1016/j.virol.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Savard C., Guerin A., Drouin K., Bolduc M., Laliberté-Gagné M., Dumas M.C., Majeau N., Leclerc D. Improvement of the trivalent inactivated flu vaccine using PapMV nanoparticles. PLoS ONE. 2011;6:e21522. doi: 10.1371/journal.pone.0021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chichester J.A., Manceva S.D., Rhee A., Coffin M.V., Musiychuk K., Mett V., Shamloul M., Norikane J., Streatfield S.J., Yusibov V. A plant-produced protective antigen vaccine confers protection in rabbits against a lethal aerosolized challenge with Bacillus anthracis Ames spores. Hum Vaccines Immunother. 2013;9:544–552. doi: 10.4161/hv.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorantala J., Grover S., Rahi A., Chaudhary P., Rajwanshi R., Sarin N.B., Bhatnagar R. Generation of protective immune response against anthrax by oral immunization with protective antigen plant-based vaccine. J Biotechnol. 2014;176:1–10. doi: 10.1016/j.jbiotec.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Kim M.Y., Reljic R., Kilbourne J., Ceballos-Olvera I., Yang M.S., Reyes-Del Valle J., Mason H.S. Novel vaccination approach for dengue infection based on recombinant immune complex universal platform. Vaccine. 2015;33:1830–1880. doi: 10.1016/j.vaccine.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 32.Phoolcharoen W., Bhoo S.H., Lai H., Ma J., Arntzen C.J., Chen Q., Mason H.S. Expression of an immunogenic Ebola immune complex in Nicotiana benthamiana. Plant Biotechnol J. 2011;9:807–816. doi: 10.1111/j.1467-7652.2011.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Ríos-Huerta R., Monreal-Escalante E., Govea-Alonso D.O., Angulo C., Rosales-Mendoza S. Expression of an immunogenic LTB-based chimeric protein targeting Zaire ebolavirus epitopes from GP1 in plant cells. Plant Cell Rep. 2017;36:355–365. doi: 10.1007/s00299-016-2088-6. [DOI] [PubMed] [Google Scholar]; Brief background about Ebola virus is mentioned in this paper. Development of plant-based vaccine against Ebola virus is in fact scarcely reported till date. The authors have demonstrated the successful expression of the chimeric protein, LTB-EBOV in plant cells and its immunogenicity in BALB/c mice via subcutaneous and oral administrations.
- 34.Czyz M., Dembczynski R., Marecik R., Wojas-Turek J., Milczarek M., Pajtasz-Piasecka E., Wietrzyk J., Pniewski T. Freeze-drying of plant tissue containing HBV surface antigen for the oral vaccine against hepatitis B. Biomed Res Int. 2014:485689. doi: 10.1155/2014/485689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rukavtsova E.B., Rudenko N.V., Puchko E.N., Zakharchenko N.S., Buryanov Y.I. Study of the immunogenicity of hepatitis B surface antigen synthesized in transgenic potato plants with increased biosafety. J Biotechnol. 2015;203:84–88. doi: 10.1016/j.jbiotec.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Govea-Alonso D.O., Rubio-Infante N., Garcia-Hernandez A.L., Varona-Santos J.T., Korban S.S., Moreno-Fierros L., Rosales-Mendoza S. Immunogenic properties of a lettuce-derived C4(V3)6 multiepitopic HIV protein. Planta. 2013;238:785–792. doi: 10.1007/s00425-013-1932-y. [DOI] [PubMed] [Google Scholar]
- 37.Rubio-Infante N., Govea-Alonso D.O., Romero-Maldonado A., Garcia-Hernández A.L., Ilhuicatzi-Alvarado D., Salazar-González J.A., Korban S.S., Rosales-Mendoza S., Moreno-Fierros L. A plant-derived multi-HIV antigen induces broad immune responses in orally immunized mice. Mol Biotechnol. 2015;57:662–674. doi: 10.1007/s12033-015-9856-3. [DOI] [PubMed] [Google Scholar]
- 38.Kessans S.A., Linhart M.D., Meador L.R., Kilbourne J., Hogue B.G., Fromme P., Matoba N., Mor T.S. Immunological characterization of plant-based HIV-1 Gag/Dgp41 virus-like particles. PLOS ONE. 2016;11:e0151842. doi: 10.1371/journal.pone.0151842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H., Li X., Lei T., Li W., Si L., Zheng J. Transgenic tobacco expressed HPV16-L1 and LT-B combined immunization induces strong mucosal and systemic immune responses in mice. Hum Vaccines Immunother. 2013;9:83–89. doi: 10.4161/hv.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monroy-Garcia A., Gomez-Lim M.A., Weiss-Steider B., Hernandez-Montes J., Huerta-Yepez S., Rangel-Santiago J.F., Santiago-Osorio E., de Mora Garcia M.L. Immunization with an HPV-16 L1-based chimeric virus-like particle containing HPV-16 E6 and E7 epitopes elicits long-lasting prophylactic and therapeutic efficacy in an HPV-16 tumor mice model. Arch Virol. 2014;159:291–305. doi: 10.1007/s00705-013-1819-z. [DOI] [PubMed] [Google Scholar]
- 41.Shoji Y., Jones R.M., Mett V., Chichester J.A., Musiychuk K., Sun X., Tumpey T.M., Green B.J., Shamloul M., Norikane J. A plant produced H1N1 trimeric hemagglutinin protects mice from a lethal influenza virus challenge. Hum Vaccines Immunother. 2013;9:553–560. doi: 10.4161/hv.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Shoji Y., Prokhnevsky A., Leffet B., Vetter N., Tottey S., Satinover S., Musiychuk K., Shamloul M., Norikane J., Jones R.M. Immunogenicity of H1N1 influenza virus-like particles produced in Nicotiana benthamiana. Hum Vaccines Immunother. 2015;11:118–123. doi: 10.4161/hv.34365. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors have developed the recombinant hemagglutinin from the A/California/04/09 strain of H1N1 influenza A virus in the form of enveloped VLPs (HAC-VLPs) in plants. HAC-VLPs resemble the influenza A virus by morphology. This paper suggests that VLPs have an enhanced immunogenicity over soluble antigen.
- 43.Nahampun H.N., Bosworth B., Cunnick J., Mogler M., Wang K. Expression of H3N2 nucleoprotein in maize seeds and immunogenicity in mice. Plant Cell Rep. 2015;34:969–980. doi: 10.1007/s00299-015-1758-0. [DOI] [PubMed] [Google Scholar]
- 44.Pua T.L., Chan X.Y., Loh H.S., Omar A.R., Yusibov V., Musiychuk K., Hall A.C., Coffin M.V., Shoji Y., Chichester J.A. Purification and immunogenicity of hemagglutinin from highly pathogenic avian influenza virus H5N1 expressed in Nicotiana benthamiana. Hum Vaccines Immunother. 2017;13:306–313. doi: 10.1080/21645515.2017.1264783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh A., Srivastava S., Chouksey A., Panwar B.S., Verma P.C., Roy S., Singh P.K., Saxena G., Tuli R. Expression of rabies glycoprotein and ricin toxin B chain (RGP-RTB) fusion protein in tomato hairy roots: a step towards oral vaccination for rabies. Mol Biotechnol. 2015;57:359–370. doi: 10.1007/s12033-014-9829-y. [DOI] [PubMed] [Google Scholar]
- 46.Demurtas O.C., Massa S., Illiano E., De Martinis D., Chan P.K.S., Di Bonito P., Franconi R. Antigen production in plant to tackle infectious diseases flare up: the case of SARS. Front Plant Sci. 2016;7:54. doi: 10.3389/fpls.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mett V., Chichester J.A., Stewart M.L., Musiychuk K., Bi H., Reifsnyder C.J., Hull A.K., Albrecht M.T., Goldman S., Baillie L.W. A non-glycosylated, plant-produced human monoclonal antibody against anthrax protective antigen protects mice and non-human primates from B. anthracis spore challenge. Hum Vaccines. 2011;7:183–190. doi: 10.4161/hv.7.0.14586. [DOI] [PubMed] [Google Scholar]
- 48.Pettitt J., Zeitlin L., Kim D.H., Working C., Johnson J.C., Bohorov O., Bratcher B., Hiatt E., Hume S.D., Johnson A.K. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci Transl Med. 2013;5:199ra113. doi: 10.1126/scitranslmed.3006608. [DOI] [PubMed] [Google Scholar]
- 49.Lai H., He J., Hurtado J., Stahnke J., Fuchs A., Mehlhop E., Gorlatov S., Loos A., Diamond M.S., Chen Q. Structural and functional characterization of an anti-West Nile virus monoclonal antibody and its single-chain variant produced in glycoengineered plants. Plant Biotechnol J. 2014;12:1098–1107. doi: 10.1111/pbi.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He J., Lai H., Engle M., Gorlatov S., Gruber C., Steinkellner H., Diamond M.S., Chen Q. Generation and analysis of novel plant-derived antibody based therapeutic molecules against West Nile virus. PLOS ONE. 2014;9:e93541. doi: 10.1371/journal.pone.0093541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He J., Peng L., Lai H., Hurtado J., Stahnke J., Chen Q. A plant produced antigen elicits potent immune responses against West Nile virus in mice. Biomed Res Int. 2014:952865. doi: 10.1155/2014/952865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherman A., Su J., Lin S., Wang X., Herzog R.W., Daniell H. Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood. 2014;124:1659–1668. doi: 10.1182/blood-2013-10-528737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su J., Zhu L., Sherman A., Wang X., Lin S., Kamesh A., Norikane J.H., Streatfield S.J., Herzog R.W., Daniell H. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials. 2015;70:84–93. doi: 10.1016/j.biomaterials.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Su J., Sherman A., Doerfler P.A., Byrne B.J., Herzog R.W., Daniell H. Oral delivery of acid alpha glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol J. 2015;13:1023–1032. doi: 10.1111/pbi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors have demonstrated the successful expression of acid alpha glucosidase (GAA) in fusion with a transmucosal carrier, Cholera toxin B in chloroplasts. By using oral administration of GAA bioencapsulated in plant cells, an induction of oral tolerance and significant suppression of GAA-specific inhibitory antibody (which will jeopardize the enzyme replacement therapy) were evidenced in Pompe mice.
- 55.Kwon K.C., Nityanandam R., New J.S., Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol J. 2013;11:77–86. doi: 10.1111/pbi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]