Abstract
Intramembrane protein–protein interactions (PPIs) are involved in transmembrane signal transduction mediated by cell surface receptors and play an important role in health and disease. Recently, receptor-specific modulatory peptides rationally designed using a general platform of transmembrane signaling, the signaling chain homooligomerization (SCHOOL) model, have been proposed to therapeutically target these interactions in a variety of serious diseases with unmet needs including cancer, sepsis, arthritis, retinopathy, and thrombosis. These peptide drug candidates use ligand-independent mechanisms of action (SCHOOL mechanisms) and demonstrate potent efficacy in vitro and in vivo. Recent studies surprisingly revealed that in order to modify and/or escape the host immune response, human viruses use similar mechanisms and modulate cell surface receptors by targeting intramembrane PPIs in a ligand-independent manner. Here, I review these intriguing mechanistic similarities and discuss how the viral strategies optimized over a billion years of the coevolution of viruses and their hosts can help to revolutionize drug discovery science and develop new, disruptive therapies. Examples are given.
Keywords: Cell receptors, Transmembrane signal transduction, Protein–protein interactions, Signaling chain homooligomerization model, SCHOOL model, Viral pathogenesis, Evolution of the immune response, Human disease, Therapeutic peptide inhibitors, Novel therapies
1. Introduction
Protein–protein interactions (PPIs) are involved in the vast majority of biological processes (Fletcher and Hamilton, 2006, Fry, 2006, Ryan and Matthews, 2005, Toogood, 2002, Zinzalla and Thurston, 2009). Nearly every important pathway in health and disease includes and is critically influenced by PPIs (Fry, 2006). In this context, the specific and controlled modulation (inhibition) of PPIs provides a promising avenue for rational drug design, as revealed by recent progress in the design of inhibitory antibodies, peptides, and small molecules (Berg, 2008, Che et al., 2006, Fry, 2006, Pagliaro et al., 2004, Robinson, 2009, Sillerud and Larson, 2005, Veselovsky and Archakov, 2007).
Cell surface receptors are integral membrane proteins that translate extracellular information into intracellular signaling sequences and further into physiological cell response in the complex fundamental process called transmembrane signal transduction. This process plays a crucial role in health and disease (Kadmiel and Cidlowski, 2013, Rudd, 2006, Sabroe et al., 2003, Sigalov, 2008a, Sigalov, 2010g), which makes therapeutic control of PPIs involved in this process of both fundamental and clinical importance. However, until recently, the lack of a mechanistic understanding of what PPIs are actually taking place during transmembrane signal transduction has significantly impeded progress not only in fundamental studies in biology and life sciences but also in molecular target-driven drug discovery. The result of this lack of understanding is that the most widely used therapeutic strategies of receptor modulation attempt to prevent binding of receptors to their cognate ligands using clinically relevant antibodies (Hansel, Kropshofer, Singer, Mitchell, & George, 2010) or soluble receptor domains (Bouchon et al., 2001, Molloy et al., 2005). However, these large protein entities possess severe disadvantages including a long and costly development process, low activity per mass, manufacturing difficulties, unintended immune response, side effects, low organ and tissue penetration, and a lack of oral bioavailability (Hansel et al., 2010).
As integral membrane proteins, cell surface receptors consist of three domains: (1) extracellular ligand-binding domains that function to detect and bind external stimuli, (2) transmembrane domains that function as anchors to the cell membrane and mediators of signal transduction, and (3) cytoplasmic signaling domains that initiate an intracellular signal transduction cascades. Depending on whether extracellular and intracellular domains are located on the same or separate protein chains, unrelated and functionally diverse cell receptors can be divided into two main structural families: single-chain and multichain receptors (SRs and MRs), respectively (Fig. 1 ) (Keegan and Paul, 1992, Sigalov, 2004, Sigalov, 2010c). MRs most commonly represent immune receptors and are often referred to as multichain immune recognition receptors (MIRRs) (Keegan and Paul, 1992, Sigalov, 2004). The signature feature of MIRRs is the presence of one or more copies of the immunoreceptor tyrosine-based activation motif (ITAM) regions (Reth, 1989) or the YxxM motif (Wu, Cherwinski, Spies, Phillips, & Lanier, 2000) in their cytoplasmic signaling domains. Upon receptor triggering, tyrosine residues of the ITAM/YxxM regions are phosphorylated in an early and obligatory event in the signaling cascade.
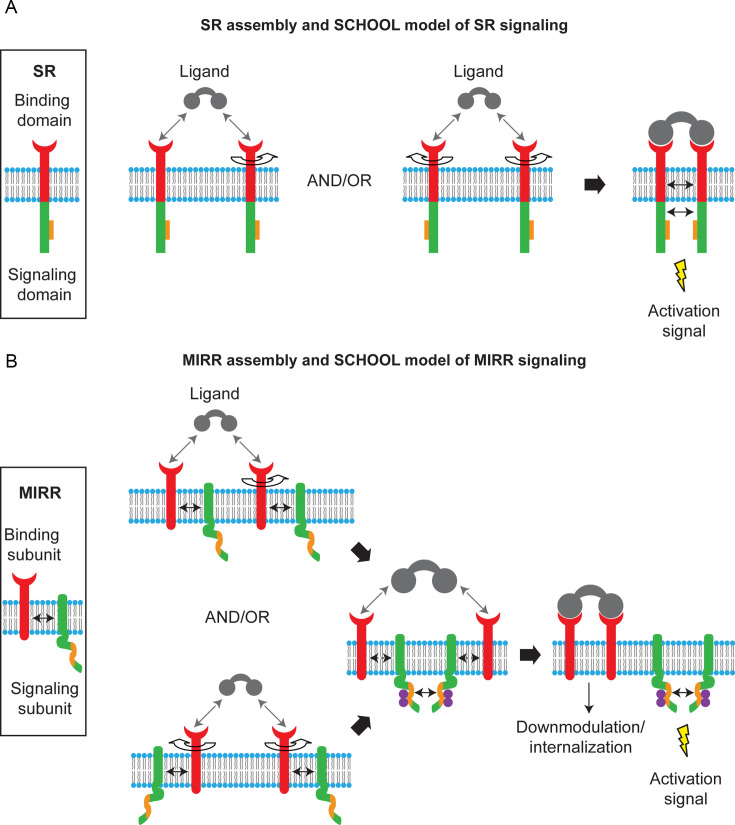
Fig. 1.
Single and multichain activating receptor assembly and signaling. (A) In single-chain receptors (SRs), extracellular recognition domain (red), and intracellular signaling domain (green) with a signaling sequence (orange rectangles) are located on the same protein chain. The signaling chain homooligomerization (SCHOOL) model of SR signaling model proposes that receptor homooligomerization in the cytoplasmic milieu plays a central role in triggering SRs. Ligand-induced SR clustering and reorientation (and/or receptor reorientation in preexisting SR clusters) results in SR oligomerization mediated by transmembrane interactions between charged residues. In these oligomers, receptors are in sufficient proximity and adopt a correct interreceptor relative orientation and geometry to promote homointeractions between cytoplasmic domains. Formation of competent signaling oligomers results in generation of the activation signal (for receptor tyrosine kinases, this means transautophosphorylation of Tyr residues in cytoplasmic signaling sequences) and thus triggers downstream signaling pathways. Protein–protein interactions are shown by solid black arrows. (B) In multichain immune recognition receptors (MIRRs), the extracellular recognition domains and intracellular ITAM-containing signaling domains are located on separate subunits bound together by noncovalent intramembrane interactions (solid arrow). ITAMs are colored orange. The signaling chain homooligomerization (SCHOOL) model of MIRR signaling. The model proposes that the homooligomerization of signaling subunits in the cytoplasmic milieu plays a key role in triggering MIRRs. Ligand-induced MIRR clustering and reorientation (and/or receptor reorientation in preexisting MIRR clusters) lead to formation of a dimeric/oligomeric intermediate. In this intermediate, receptors are in sufficient proximity and adopt the correct relative orientation and geometry to promote transhomointeractions between cytoplasmic domains of signaling subunits resulting in formation of competent signaling oligomers. In these oligomers, protein tyrosine kinases phosphorylate the ITAM tyrosine residues, leading to the generation of activation signal(s), dissociation of signaling oligomers, and internalization of the engaged MIRR ligand-binding subunits. Circular arrows indicate ligand-induced receptor reorientation. All interchain interactions in a dimeric intermediate are shown by dotted black arrows reflecting their transition state. Curved lines depict disorder of the cytoplasmic domains of MIRR signaling subunits. Phosphate groups are shown as purple circles. Abbreviations: ITAM, immunoreceptor tyrosine-based activation motif.
A general platform for receptor-mediated transmembrane signal transduction, the signaling chain homooligomerization (SCHOOL) platform (Sigalov, 2004, Sigalov, 2006, Sigalov, 2008a, Sigalov, 2008b, Sigalov, 2008c, Sigalov, 2010b, Sigalov, 2010c) uncovers for the first time the major mechanisms coupling recognition and signaling functions. Based on the unusual biophysical phenomenon—the ability of the intrinsically disordered cytoplasmic domains of MIRR signaling subunits to form specific homodimers and higher homooligomers (Sigalov et al., 2004, Sigalov et al., 2007), this platform was originally suggested for MIRRs (Sigalov, 2004) and then—as a general platform for transmembrane signaling mediated by MIRRs and SRs (Sigalov, 2008a, Sigalov, 2010c). Conceptually, the SCHOOL platform suggests that the similar architecture of the receptors dictates similar mechanisms of receptor triggering. Mechanistically, according to the platform, multivalent ligand binding outside the cell induces (or tunes) receptor oligomerization (clustering) which is then translated across the membrane into formation of competent signaling homooligomers in the cytoplasmic milieu—a force that drives transmembrane signaling and is necessary and sufficient to trigger receptor activation (Fig. 1). This provides the similarity of the targets revealed at the level of specific PPIs—biochemical processes that can be influenced and controlled for therapeutic purposes (Sigalov, 2004, Sigalov, 2008a, Sigalov, 2010b, Sigalov, 2010c).
2. PPIs in Transmembrane Signaling
The SCHOOL platform considers homooligomerization of cytoplasmic signaling domains of cell surface receptors (both SRs and MIRRs) as a driving force of transmembrane signaling and suggests that bringing these domains in the correct orientation and close enough proximity to one other to promote homotypic PPIs between them is an obligatory step to trigger the receptor (Sigalov, 2004, Sigalov, 2010c). This defines the process of ligand-induced receptor triggering and activation as an outcome of the interplay between three major driving forces: extracellular PPIs between receptors and their cognate ligands, inter- (SR) and intrareceptor (MIRR) PPIs in the cell membrane, and interreceptor homotypic PPIs in the cytoplasmic milieu. Interestingly, in transmembrane signaling mediated by receptor tyrosine kinases (RTKs), members of the SR family, a weak dimerization propensity for all RTK transmembrane domains allows for a tight control of the ratio between receptor monomers and dimers (Finger et al., 2009, Li and Hristova, 2006). In MIRR-mediated signaling, three major PPIs indicated earlier all are characterized by micromolar affinity and relatively rapid kinetics (Bormann and Engelman, 1992, Davis et al., 1998, Finger et al., 2006, Sigalov et al., 2004). This conjugated and well-balanced system of PPIs involved in receptor triggering explains the molecular mechanisms underlying the ability of MIRRs to transduce the diverse recognition (discrimination) information across the cell membrane and translate it into different signaling cascades, thus triggering different intracellular pathways and providing different cell responses. In the immune defense, this system of PPIs explains mechanistically how immune cells can have high specificity, selectivity, and sensitivity in the recognition and discrimination of different antigens (ligands) and how this recognition (discrimination) results in different functional outcomes.
In contrast to well-established strategies that target interaction of receptors with their ligands in order to modulate receptor signaling, the SCHOOL strategy is to target other PPIs involved in receptor-mediated transmembrane signal transduction—intramembrane and cytoplasmic homotypic PPIs. This “Freedom to Bind not to Signal” strategy allows for effective and selective therapeutic targeting using small molecule inhibitors and modulatory peptides and peptidomimetics.
2.1. Intramembrane and Cytoplasmic Interactions
2.1.1. Single-Chain Receptors
According to the SCHOOL platform (Sigalov, 2004, Sigalov, 2010c), upon binding to multivalent ligand, intramembrane PPIs between SRs mediate dimerization (oligomerization) of SRs to bring the receptors into close proximity and the correct interreceptor geometry to promote cytoplasmic homotypic PPIs and thus triggers the receptor (Fig. 1A). Interestingly, RTKs and some other SRs such as members of the tumor necrosis factor (TNF) receptor superfamily (Chan, 2007, Chan et al., 2000) can exist as preassembled dimers (oligomers) on the surface of resting cells. In this scenario, the platform suggests that binding to multivalent ligand leads to reorientation of receptors in these dimers (oligomers) to adopt the geometry competent to promote dimerization (oligomerization) of cytoplasmic signaling domains that trigger the receptor (Fig. 1A). Indeed, in RTK-mediated signaling, multivalent ligand binding results in a conformational change in the receptor extracellular domain, leading to the rotation of the whole receptor (Jiang and Hunter, 1999, Moriki et al., 2001). Triggering of the Neu RTK occurs only for a specific transmembrane dimer interface, the rotation of which leads to periodic oscillations in kinase activity (Bell et al., 2000), further confirming the necessity of the correct interreceptor orientation in the receptor dimers (oligomers) formed upon binding to multivalent ligand. In addition, the rotation of the RTK domain with respect to its transmembrane domain by inserting residues into the C-terminal transmembrane flanking region restores the RTK activity. Furthermore, upon binding of the epidermal growth factor receptor (EGFR) to its cognate ligand, the EGFR extracellular domain rotates and this rotation is transmitted to the receptor transmembrane domain (Moriki et al., 2001). Interreceptor orientation in receptor dimers plays a critical role in erythropoietin receptor-mediated transmembrane signaling (Livnah et al., 1998, Syed et al., 1998), suggesting a tight coupling of the extracellular domain orientation to the cytoplasmic signaling events. Furthermore, signal transduction via interleukin (IL)-6 requires not only gp130 homodimerization but also the correct relative orientation of the gp130 cytoplasmic domains in a ligand-specific receptor dimer, suggesting that subtle changes in the orientation of the receptor chains relative to each other might result in very different responses (Greiser, Stross, Heinrich, Behrmann, & Hermanns, 2002). Dimerization of the cytokine receptors by monoclonal antibodies is in most cases not enough to induce signal transduction (Autissier et al., 1998). Formation of homooligomers of Fas cytoplasmic tails plays an important role in receptor triggering (Siegel et al., 2004). Similarly, cytoplasmic domain-mediated dimerization of toll-like receptor-4 (TLR-4) is necessary for TLR-4 triggering and to activate signaling cascades (Lee, Dunzendorfer, & Tobias, 2004).
Thus, these and other studies of SRs strongly support the general SCHOOL platform concept and demonstrate that ligand-induced receptor dimerization is translated into protein dimerization in the cell membrane milieu and that the receptor dimer intramembrane interface contains the critical structural information that positions the receptor cytoplasmic signaling domains in a way competent for oligomerization and receptor triggering (Fig. 1A).
2.1.2. Multichain Receptors
Transmembrane signaling mediated by MIRRs can be roughly divided into four stages: (1) recognition and binding of the receptor to its multivalent cognate ligand outside the cell, (2) transmembrane transduction of this information to the cytoplasmic milieu, (3) phosphorylation of the ITAM or YxxM tyrosine residues by protein tyrosine kinases followed by activation of specific intracellular signaling cascades, and (4) activation of genes in the nucleus. While the molecular mechanisms underlying the stages 1, 3, and 4 are understood in significant detail, the mechanisms by which the MIRRs transduce recognition (discrimination) information via receptor transmembrane and juxtamembrane regions into intracellular biochemical events (stage 2) have been a long-standing mystery. It was also not clear how this putative mechanism could explain the intriguing ability of immune cells to discern and differentially respond to slightly different ligands in the immune defense. Ultimately, the specific PPIs involved in this process have remained largely unknown preventing the development of novel therapeutic approaches.
By uncovering a crucial physiological role of homotypic interactions between intrinsically disordered cytoplasmic domains of MIRR signaling subunits (Sigalov, 2011c, Sigalov et al., 2004, Sigalov et al., 2007), the biophysically unique phenomenon that still remains a matter of debate whether it exists or not (Nourse & Mittag, 2014; Sigalov, 2016; Uversky & Dunker, 2013), the SCHOOL model suggests that formation of competent MIRR signaling subunit oligomers is necessary and sufficient to trigger the receptor and induce downstream signaling cascades (Fig. 1B) (Sigalov, 2004, Sigalov, 2005, Sigalov, 2006, Sigalov, 2008c, Sigalov, 2010b). This is consistent with the structural hypothesis of cross-phosphorylation (Ortega, 1995, Pribluda et al., 1994) that assumes that the kinase(s) responsible for catalyzing ITAM Tyr residue phosphorylation exist associated with the receptors, and that for steric reasons, these kinases cannot phosphorylate tyrosine residues on ITAM-containing signaling chains of the same receptor complex. In contrast, in MIRR dimers (oligomers) formed upon binding to their multivalent cognate ligands, the kinases phosphorylate the tyrosines of a distinct receptor complex (cross-phosphorylation or transphosphorylation), thus triggering the receptor (Ortega, 1995).
In unstimulated (resting) cells, intramembrane PPIs between transmembrane helices of recognition and signaling MIRR subunits maintain receptor integrity and determine the relative positions of these subunits in the receptor complex (angles, distances, etc.), thus dictating the overall geometry and topology of MIRRs (Biassoni et al., 2000, Call et al., 2002, Daeron, 1997, DeFranco et al., 1994, Kim et al., 2003, Klesney-Tait et al., 2006, Moroi and Jung, 2004, Sigalov, 2004, Sigalov, 2005, Sigalov, 2008c). The SCHOOL platform suggests that MIRR engagement by multivalent ligand or anti-MIRR antibodies (e.g., antibodies against CD3ɛ and T cell receptor β (TCRβ) chains of TCR or anti-Igβ antibodies for B cell receptor) leads to receptor dimerization (oligomerization) coupled with a multistep structural reorganization aiming to promote homotypic PPIs between MIRR signaling subunits in the cytoplasmic milieu that trigger the receptor and initiate downstream signaling cascades (Fig. 1B). According to the SCHOOL model, sufficiently close interreceptor proximity and correct relative orientation in MIRR dimers (oligomers) as well as long enough duration of the MIRR–ligand interaction and sufficient lifetime of an individual receptor in these MIRR clusters all play important roles for productive MIRR triggering as strongly supported by a growing body of evidence (Carreno et al., 2006, Holler et al., 2001, Holowka et al., 2007, Kiessling et al., 2006, Klemm et al., 1998, Metzger, 2004, Miura et al., 2002, Ortega et al., 1988, Posner et al., 2002, Puffer et al., 2007, Radaev and Sun, 2003, Reth and Wienands, 1997, Schweitzer-Stenner et al., 1997, Tamir and Cambier, 1998, Yamasaki et al., 2004).
Some MIRRs such as TCR and major platelet collagen receptor, glycoprotein VI (GPVI), can exist as preassembled oligomers on the surface of resting cells (Berlanga et al., 2007, Jung et al., 2009, Schamel et al., 2005). In this scenario, similarly to that of SRs, the SCHOOL model suggests reorientation of MIRRs in these dimers (oligomers) to adopt the geometry competent to promote homotypic PPIs between cytoplasmic signaling domains that trigger the receptor.
In the immune defense, the SCHOOL mechanisms suggest that the diversity of the immune cell response is largely provided by the combinatorial nature of MIRR-mediated signaling. More specifically, signal diversification may be achieved through different patterns of MIRR signaling subunit oligomerization (Sigalov, 2004, Sigalov, 2005, Sigalov, 2006) in combination with distinct activation signals provided by different MIRR signaling modules (Jensen et al., 1997, Pike et al., 2004, Pitcher and van Oers, 2003, Sanchez-Mejorada and Rosales, 1998) and/or different ITAMs located on the same signaling module (e.g., TCRζ chain) (Chae et al., 2004). Thus, according to the model, the diversity of cell functional outcomes in response to stimulation by different ligands is increasing with the number of different signaling subunits that the MIRR complex has.
2.2. Transmembrane Signaling and Viral Evasion Strategies: Evolutionary and Molecular Aspects
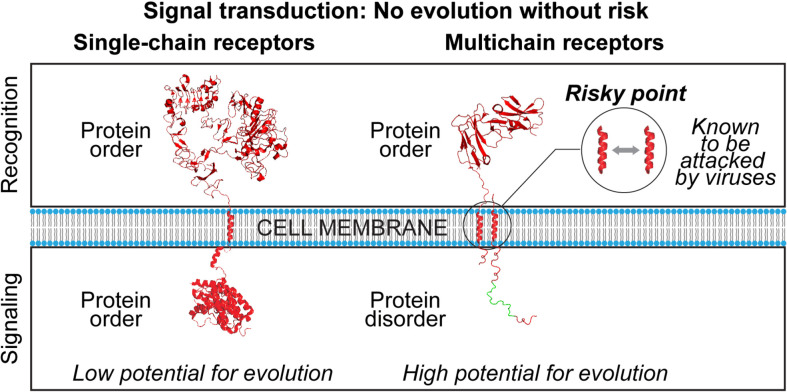
Structural consideration of transmembrane signaling mediated by a variety of functionally unrelated receptors expressed on various cells leads us to the important observation that recognition and signaling functions can be combined on one protein chain (SRs) or separated between different protein chains (MIRRs) (Fig. 1). Another observation is that while extracellular (ligand recognition), transmembrane and cytoplasmic (signaling) domains of SRs are all well structured (Sigalov, 2010c, Sigalov, 2011a, Sigalov, 2012b), cytoplasmic domains of MIRR signaling subunits are all intrinsically disordered (i.e., these domains lack a well-defined ordered structure under physiological conditions in vitro) in contrast to the well-structured MIRR extracellular (ligand recognition) and transmembrane domains (Sigalov, 2011a, Sigalov, 2011c, Sigalov, 2012b, Sigalov et al., 2004, Sigalov et al., 2007, Sigalov et al., 2006). Ultimately, these observations raise two intriguing questions (Sigalov, 2011a, Sigalov, 2012a). First, why did nature separate recognition and signaling functions for MIRRs, thereby increasing the risk of malfunction and potential attack by pathogens, and second, why did nature select protein disorder for MIRRs to translate recognition of distinct ligands into appropriate activation signals that would induce distinct specific functional outcomes (Fig. 2 )? Answering the first question, one can suggest that the modular assembly of MIRRs brings multiple benefits the most important of which is the capability to diversify and vary signal transduction (Sigalov, 2010c, Sigalov, 2010d, Sigalov, 2011b, Sigalov, 2012a). Ultimately, this provides the mechanistic basis for the diversity and variability of the immune response. According to Charles Darwin, diversity and variability are at the very core of evolutionary processes (Darwin, 1861). One can conclude that nature takes the risks associated with separation of functions in MIRRs in exchange for high potential for evolution of signal transduction (Fig. 2, Fig. 3 ). In the context of immune signaling, this is of great importance in the development and evolution of the immune defense. Protein disorder contributes to the diversity and variability of signal transduction without compromising efficiency and specificity of signaling (Sigalov, 2010c, Sigalov, 2010d, Sigalov, 2011b, Sigalov, 2012a, Sigalov, 2012b). Thus, evolutionary and developmental benefits of immune signaling-related protein disorder outweigh attendant disadvantages which are largely compensated for by increasing the host defense.
Fig. 2.
Evolutionary and molecular aspects of transmembrane signal transduction mediated by single- and multichain cell surface receptors. Images were created using PyMol (www.pymol.org) from Protein Data Bank entries 1NQL and 3GOP for the EGFR extra- and intracellular (juxtamembrane and kinase) domains, respectively (shown as an example of structure of a single-chain receptor), and entry 1UCT for the FcαRI extracellular domain (shown as an example of structure of a multichain receptor recognition subunit). For illustrative purposes, the cytoplasmic domain of a multichain receptor-associated signaling subunit is shown as a monomer and using arbitrary idealized structural elements to represent the ensemble of unfolded conformations of an IDR. The immunoreceptor tyrosine-based activation motif (ITAM) of multichain receptors is depicted in green. Intramembrane interactions between recognition and signaling subunits of multichain receptor are shown by a gray arrow. Abbreviations: EGFR, epidermal growth factor receptor; FcαRI, Fc receptor I for IgA.
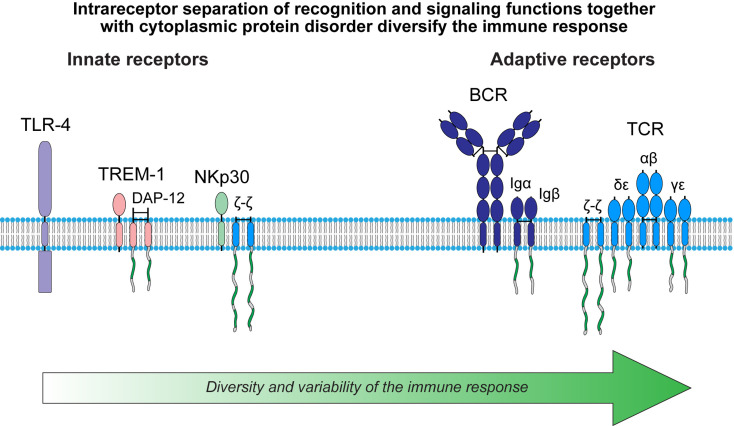
Fig. 3.
Diversity and variability of the immune response. The immunoreceptor tyrosine-based activation motif (ITAM) is shown in green. Curved lines depict intrinsic disorder of the cytoplasmic domains of MIRR signaling subunits. Abbreviations: BCR, B cell receptor; DAP-12, DNAX adapter protein of 12 kDa; Ig, immunoglobulin; MIRR, multichain immune recognition receptor; NK, natural killer cell; TCR, T cell receptor; TLR-4, toll-like receptor 4; TREM-1, triggering receptor expressed on myeloid cells 1.
There exist two types of immune receptors: the innate receptors, the germ line-encoded receptors that detect a limited set of conserved antigens; and the adaptive receptors, the somatically generated antigen receptors of the T and B cells (TCR and BCR, respectively) (Table 1 ). From the evolutionary standpoint, the adaptive immune response evolved long after the innate mechanisms of self-defense and provided significant added value in promoting survival (Cooper and Alder, 2006, Kimbrell and Beutler, 2001, Litman and Cooper, 2007). Structurally, the adaptive receptors belong to the MIRR family with two (BCR) and four (TCR) signaling chains (Fig. 3). In contrast, the innate receptors belong to the SR family (e.g., TLRs) or represent MIRRs that contain only one signaling chain (e.g., triggering receptors expressed on myeloid cells, TREMs, or natural killer cell receptors, NK receptors) (Fig. 3). Importantly, functions of the ITAM modules, including those located on different (Jensen et al., 1997, Pike et al., 2004, Pitcher and van Oers, 2003, Sanchez-Mejorada and Rosales, 1998, Sigalov, 2004, Sigalov, 2006, Sigalov, 2010c, Sigalov, 2011a, Sigalov, 2012b) or the same (e.g., three different ITAMs on the ζ chain) (Chae et al., 2004) signaling chains and those located on the same chain are rather distinct instead of redundant. This provides a molecular explanation for significantly higher diversity and variability of the adaptive response as compared to the innate response. Interestingly, following this logic, one can conclude that the extent of diversity and variability of NKp30-mediated signaling should be an intermediate between the innate (e.g., TLRs) and adaptive receptors (Fig. 3). This is indeed consistent with the idea that NK cells represent an “evolutionary bridge” between innate and adaptive immunity (Sun & Lanier, 2009).
Table 1.
The Characteristics of the Innate and Adaptive Receptors
| Innate Receptors | Adaptive Receptors |
|---|---|
| Germline encoded | Encoded in multiple gene segments |
| Nonclonal distribution | Clonal distribution |
| Do not require gene rearrangement | Require gene rearrangement |
| Trigger immediate response | Trigger delayed response |
| Broad specificity: recognize pathogen-associated molecular patterns | Narrow specificity: recognize a particular epitope |
As biochemical processes that can be influenced and controlled (Archakov et al., 2003, Arkin and Wells, 2004, Berg, 2003, Fletcher and Hamilton, 2007, Pagliaro et al., 2004), intramembrane PPIs between MIRR recognition and signaling subunits as well as homotypic interactions between cytoplasmic domains of MIRR signaling subunits represent potential points of attack by pathogens in order to modulate the MIRR-mediated signaling and thus to modulate the immune response (Sigalov, 2009, Sigalov, 2010f, Sigalov, 2012a).
Examples are viruses that in the billion-years-long struggle for their existence, in order to establish a successful infection, replicate, and persist in the host, have evolved numerous strategies to counter and evade host antiviral immune responses as well as to exploit them for productive viral replication. Several different viruses such as human immunodeficiency virus (HIV), cytomegalovirus (CMV), severe acute respiratory syndrome coronavirus (SARS-CoV), and human herpesvirus 6, that are pathogenic for humans uniformly target members of the MIRR family, including innate and adaptive receptors. Intriguingly, these viruses use either a modular assembly of MIRRs to disrupt receptor-mediated signaling or cytoplasmic protein disorder of MIRRs to surprisingly augment cell activation as required for self-preservation (Cohen et al., 2010, Shen and Sigalov, 2016, Sigalov, 2007a, Sigalov, 2008a, Sigalov, 2009, Sigalov, 2010f, Sigalov, 2012a). The example of the coevolution of viruses and their hosts confirms that there is no evolution and development without risks. Although we cannot avoid these risks, we can turn this lemon into lemonade and learn from nature how to efficiently target the immune system for therapeutic purposes (Kim and Sigalov, 2008, Shen and Sigalov, 2016, Sigalov, 2010a, Sigalov, 2010f). This will be further illustrated in the next section on the example of intramembrane PPIs as a therapeutic target.
3. Intramembrane PPIs as a Therapeutic Target
Although there is a growing line of experimental evidence indicating that targeting intramembrane PPIs to inhibit (modulate) SR-mediated cell activation might represent a promising therapeutic approach (Bennasroune et al., 2004, Bennasroune et al., 2005, Hebert et al., 1996, Sigalov, 2010a, Sigalov, 2010e, Smith et al., 2002, Tarasova et al., 1999), in this work, I will focus on therapeutic intramembrane PPIs using short synthetic peptides (SCHOOL peptides) in order to inhibit MIRR-mediated transmembrane signaling for therapeutic purposes.
Importantly, the receptor-specific SCHOOL peptides employ the molecular mechanisms of receptor inhibition that are not dependent upon binding of the receptor to its cognate ligand. This is especially important in those situations when ligands are still unknown. Example is TREM-1 receptor. Despite some recent evidence that peptidoglycan (PGN) recognition protein 1 (PGLYRP1) may potentially act as a ligand for TREM-1 (Read et al., 2015), the actual nature of the TREM-1 ligand(s) is still not yet well understood, impeding the development of clinically relevant inhibitors of TREM-1. This receptor functions as a potent amplifier of inflammation and mediates production of proinflammatory cytokines such as TNFα, IL-1β, and IL-6 (Bouchon et al., 2001, Murakami et al., 2009, Schenk et al., 2007). Importantly, TREM-1 activation preferentially induces expression of functional macrophage colony-stimulating factor (M-CSF, also known as CSF-1) (Dower, Ellis, Saraf, Jelinsky, & Lin, 2008) and in contrast to cytokine blockers, blockade of TREM-1 can blunt excessive inflammation while preserving the capacity for microbial control (Weber et al., 2014). Collectively, these findings implicate TREM-1 as a promising therapeutic target in a variety of inflammation-associated diseases (Bosco et al., 2016, Ford and McVicar, 2009, Tammaro et al., 2017).
3.1. Cancer
3.1.1. Nonsmall Cell Lung Cancer
Lung cancer is the leading cause of cancer deaths worldwide. There are two types of this cancer—nonsmall cell lung cancer (NSCLC) and small cell lung cancer. Despite advances made in chemotherapy (Felip, Santarpia, & Rosell, 2007), NSCLC kills over 1.1 million people annually worldwide, and the 5-year survival rate for patients with NSCLC is only 15% (Jemal et al., 2011). Similar to most solid tumors, the infiltrate of NSCLC, contains tumor-associated macrophages (TAMs) (Solinas, Germano, Mantovani, & Allavena, 2009). By secreting a variety of growth factors, cytokines, chemokines, and enzymes, TAMs regulate tumor growth, angiogenesis, invasion, and metastasis (Shih, Yuan, Chen, & Yang, 2006). High TAM content correlates with the promotion of tumor growth and metastasis (Solinas et al., 2009) and is associated with poor prognosis in NSCLC (Welsh et al., 2005). TAM recruitment, activation, growth, and differentiation are regulated by M-CSF (Elgert, Alleva, & Mullins, 1998). Increased pretreatment serum M-CSF level is a significant independent predictor of poor survival in patients with NSCLC (Kaminska et al., 2006).
In patients with NSCLC, TREM-1 expression on TAMs is associated with cancer recurrence and poor survival: patients with low TREM-1 expression have a 4-year survival rate of over 60%, compared with less than 20% for patients with high TREM-1 expression (Ho et al., 2008), which suggests inhibition of the TREM-1 pathway may be a promising target for the development of a targeted anticancer therapy.
Considering the unknown nature of TREM-1 ligand(s), in our recent study (Sigalov, 2014), we used the SCHOOL platform to design a novel, ligand-independent TREM-1 inhibitory peptide sequence GF9, and demonstrated that the GF9 peptide specifically blocks TREM-1-mediated transmembrane signaling in vitro and in vivo. Utilizing two human NSCLC xenograft nude mouse models (H292 and A549), we demonstrated for the first time that blockade of TREM-1 function using GF9 substantially decreases cytokine production in vitro and significantly delays tumor growth in vivo. We also showed that the SCHOOL peptide inhibitor of TREM-1 GF9 can be formulated into self-assembling macrophage-specific lipopeptide and lipoprotein complexes that mimic human high density lipoproteins (HDLs) for peptide half-life extension and targeted delivery (Sigalov, 2014). This incorporation significantly (up to 10-fold) increases GF9 therapeutic efficacy (Sigalov, 2014).
3.1.2. Pancreatic Cancer
Pancreatic cancer (PC, 85% of which are pancreatic ductal adenocarcinomas) is the fourth leading cause of cancer-related mortality across the world with very poor clinical outcome (Hariharan, Saied, & Kocher, 2008). Current treatments of PC all only marginally prolong survival or relieve symptoms in patients with PC (Schneider, Siveke, Eckel, & Schmid, 2005). There has been no significant progress in the field of targeted therapy for PC (Walker & Ko, 2014) and despite tremendous efforts, the 5-year survival rate remains less than 5%.
As lung cancer and many other solid tumors, PC is characterized by a marked infiltration of macrophages into the stromal compartment (Shih et al., 2006, Solinas et al., 2009). In patients with PC, macrophage infiltration begins during the preinvasive stage of the disease and increases progressively (Clark et al., 2007). The number of TAMs is significantly higher in patients with metastases (Gardian, Janczewska, Olszewski, & Durlik, 2012). Presence of TAMs in the PC stroma correlates with increased angiogenesis (Esposito et al., 2004), a known predictor of poor prognosis (Kuwahara et al., 2003). Similar to NSCLC, high pretreatment serum M-CSF is a strong independent predictor of poor survival in PC patients (Groblewska et al., 2007). Interestingly, M-CSF blockade not only suppresses tumor angiogenesis and lymphangiogenesis (Kubota et al., 2009) but also upregulates T cell checkpoint molecules, including programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte antigen-4 (CTLA-4), thereby overcoming limitations of PD-1 and CTLA-4 antagonists to achieve regression in even well-established tumors (Zhu et al., 2014). Importantly, continuous M-CSF inhibition affects only pathological angiogenesis but not healthy vascular and lymphatic systems outside tumors (Kubota et al., 2009). In contrast to blockade of vascular endothelial growth factor (VEGF), interruption of M-CSF inhibition does not promote rapid vascular regrowth (Kubota et al., 2009). TREM-1 activation is known to enhance release of most cytokines that are increased in patients with PC (Tjomsland et al., 2011, Yako et al., 2016) and play a vital role in creating and sustaining inflammation in the tumor favorable microenvironment, thus affecting patient survival. These include monocyte chemoattractant protein-1 (MCP-1), TNFα, IL-1α, IL-1β, IL-6, and M-CSF (Lagler et al., 2009, Schenk et al., 2007, Sigalov, 2014). Collectively, these findings suggest that a therapy that targets TREM-1-mediated intratumoral inflammation, angiogenesis, and vascularization can represent a promising strategy for treating PC.
Recently, we tested the therapeutic potential of TREM-1 inhibitory GF9 peptide sequences in three human PC xenograft mouse models (AsPC-1, BxPC-3, and Capan-1) (Zu T. Shen and Alexander B. Sigalov, unpublished). Administration of these SCHOOL peptides resulted in a strong antitumor effect achieving an optimal-treated/control (T/C) value of 19% depending on the xenograft and formulation used and persisting even after treatment was halted. The effect correlated significantly with increased survival, reduced angiogenesis, and suppressed TAM infiltration. The peptides were well tolerated when deployed in either free form or formulated into HDL-mimicking macrophage-targeted lipopeptide complexes. Further, blockade of TREM-1 significantly reduced serum levels of IL-1α, IL-6, and M-CSF, but not VEGF, suggesting an M-CSF-dependent inhibitory effect on angiogenesis.
Taken together with our previous study (Sigalov, 2014), these data suggest that SCHOOL TREM-1-specific peptide inhibitors have a cancer type independent, therapeutically beneficial antitumor activity and can be potentially used as a stand-alone therapy or as a component of combinational therapy for PC, NSCLC, and other solid tumors.
3.2. Autoimmune Diseases
Autoimmune diseases are a fascinating but poorly understood group of diseases that are characterized by a specific adaptive immune response that is mounted against self-antigens (Davidson & Diamond, 2001). As a result, the effector pathways of immunity cause chronic inflammatory injury to tissues, which may often prove lethal. Examples are rheumatoid arthritis (RA), systemic lupus erythematosus, inflammatory bowel disease, multiple sclerosis, atopic dermatitis (AD), and other disorders. T cells and macrophages, two main subtypes of immune cells, are known to be implicated in mediating many aspects of autoimmune inflammation.
RA is a chronic, systemic inflammatory disorder that causes chronic inflammation of the joints and affects about 1% of the world's population, with women affected three times more often than men. The economic burden created by RA is enormous with annual direct and indirect costs per patient of $2500–14,500 and $1500–45,000, respectively, explaining the high demand for new therapies (Kukar, Petryna, & Efthimiou, 2009). Typically, new approaches to RA involve the use of monoclonal antibodies, peptides, antigen mimetics, T cell vaccinations, infusion/injection of antiinflammatory cytokines, or cytokine inhibitors (Kukar et al., 2009, Kurosaka et al., 2007a). However, current RA treatments including TNF blockers such as Humira, all have multiple shortcomings including a high level of serious side effects and insufficient efficacy, thus highlighting the need for new and improved treatments. The pathogenesis of RA suggests a key role for aberrant pathways of T cell activation in the initiation and/or perpetuation of disease (Cope et al., 2007, Sakaguchi et al., 2012). This makes inhibition of pathways involved in T cell activation including TCR-mediated cell activation a promising therapeutic strategy for novel RA therapies (Lorenz, 2003, Won and Lee, 2008).
AD is an inflammatory skin autoimmune disease characterized by impaired epidermal barrier function and cutaneous inflammation. The prevalence of AD has steadily increased during the past few decades. AD affects 1%–3% of adults and up to 20% of children, in 85% of them the disease onset is before the age of 5 years (Novak, 2009). Novel therapeutic approaches are required, as most of the treatments of AD are limited to symptomatic therapies. Activation and skin-selective homing of peripheral blood T cells, and effector functions in the skin, are involved in the pathogenesis of AD (Akdis, Akdis, Trautmann, & Blaser, 2000). Numerous studies have pointed to the role of activated CD4 + T cells in AD (Nomura & Kabashima, 2016). Topical immunomodulators that affect T cells and block TCR-mediated cell activation may therefore have a role in the treatment of AD (Nomura & Kabashima, 2016).
Furthermore, macrophages are central to the pathogenesis of autoimmune diseases including RA and AD (Kasraie and Werfel, 2013, Kinne et al., 2000). The abundance and activation of macrophages in the inflamed synovial membrane significantly correlates with the severity of RA (Kinne et al., 2000, Mulherin et al., 1996). In order to be clinically effective, RA therapies should reduce the number of synovial sublining macrophages (Bresnihan et al., 2007). Major macrophage-related targets in RA and AD include TNFα, IL-1, IL-6, and M-CSF (Li, Hsu, & Mountz, 2012). M-CSF-dependent cells are known to be essential for collagen-induced arthritis (CIA) development (Campbell, Rich, Bischof, & Hamilton, 2000). These and other findings (Colonna and Facchetti, 2003, Genua et al., 2014, Kasraie and Werfel, 2013, Kuai et al., 2009) suggest targeting macrophage activation including TREM-1-mediated cell activation as another promising strategy to treat RA and AD.
3.2.1. Targeting T Cell Receptor
Structurally, TCR is a member of the MIRR family and has α and β antigen-binding subunits (TCRα and TCRβ, respectively) that are bound by electrostatic intramembrane PPIs with three signaling homo- and heterodimers: ζζ, CD3ɛδ, and CD3ɛγ (Fig. 3). Short synthetic peptides capable of inhibiting TCR-mediated cell activation in a ligand-independent manner are known since 1997 (Manolios et al., 1997) when TCR-targeted inhibitory activity was first reported for a synthetic peptide corresponding to the sequence of the TCRα transmembrane domain (the so-called core peptide, CP). In the TCR complex, this domain is known to interact with the transmembrane domains of CD3ɛδ and ζ (Call et al., 2002, Manolios et al., 1990). Later, the TCRα CP has been shown to reduce inflammation and inhibits the progression of experimental arthritis (Amon et al., 2006, Kurosaka et al., 2007a). In patients with AD, psoriasis, or lichen planus, a daily topical application of this peptide over three consecutive days has been demonstrated to inhibit inflammation and ameliorate the disease equally as well as betamethasone (Enk and Knop, 2000, Gollner et al., 2000).
Interestingly, similar ligand-independent TCR inhibitory activity was later reported for HIV fusion peptide (FP) found in the N terminus of the HIV envelope glycoprotein 41 (gp41) (Bloch et al., 2007, Quintana et al., 2005). The patterns of inhibition of TCR-mediated cell activation exhibited by TCR CP and HIV gp41 FP were intriguingly similar: both peptides inhibit antigen—but not anti-CD3-stimulated T cell activation (Quintana et al., 2005, Wang et al., 2002b). Similar to TCRα CP, HIV gp41 FP was shown to reduce inflammation and ameliorate T cell-mediated autoimmune arthritis in animal models (Quintana et al., 2005).
However, despite extensive studies (Ali et al., 2006, Amon et al., 2006, Bloch et al., 2007, Collier et al., 2006, Kurosaka et al., 2007b, Manolios et al., 1997, Quintana et al., 2005, Wang et al., 2002a), the mode of action of these clinically relevant peptides was enigmatic until the SCHOOL model was first introduced and applied to this field (Sigalov, 2004, Sigalov, 2006, Sigalov, 2007a). According to the model (Sigalov, 2004, Sigalov, 2005, Sigalov, 2006, Sigalov, 2010c, Sigalov, 2010e), TCRα CP and HIV gp41 both compete with the TCRα chain for binding to CD3δɛ and ζζ and disrupt the corresponding intramembrane PPIs. This results in disconnection/predissociation of the affected signaling subunits from the remaining receptor complex and upon stimulation by multivalent antigen, leads to inhibition of antigen—but not antibody-mediated TCR triggering and cell activation. Importantly, TCR assembly and cell surface expression are not affected by treatment with TCRα CP (Kurosaka, Bolte, et al., 2007). This finding as well as colocalization of TCR CP with TCR in the membrane of resting cells (Wang, Djordjevic, Bender, et al., 2002) directly prove the hypothesis about “pre-” rather than full dissociation state of the unstimulated TCR complex in the presence of TCRα CP, whereas upon stimulation, the affected signaling subunits, ζζ and CD3ɛδ, become physically disconnected from the remaining receptor complex. It should be noted that the proposed SCHOOL mechanism is the only mechanism consistent with all experimental and clinical data reported up to date for transmembrane peptides of TCR and other MIRRs as well as for lipid and/or sugar conjugates of these peptides (Ali et al., 2005, Ali et al., 2002, Amon et al., 2006, Amon et al., 2008, Bender et al., 2004, Collier et al., 2006, Enk and Knop, 2000, Gerber et al., 2005, Gollner et al., 2000, Huynh et al., 2003, Kurosaka et al., 2007b, Manolios et al., 2002, Sigalov, 2007b, Sigalov, 2008a, Wang et al., 2002a, Wang et al., 2002b).
Later, the use of the SCHOOL model and comparative primary sequence analysis of proven and predicted immunomodulatory sequences of viral fusion protein regions allowed not only to suggest the specific molecular mechanisms of inhibition of TCR-mediated cell activation by TCRα CP and HIV gp41 FP (Sigalov, 2007a, Sigalov, 2008b, Sigalov, 2009) but also to predict similar immunomodulatory activity for other viral FPs such as SARS-CoV FP (Sigalov, 2009). Our recent study (Shen & Sigalov, 2016) provided compelling experimental in vivo evidence in support of this hypothesis. As demonstrated, a synthetic 11 amino acid-long peptide-derived from SARS CoV FP predicted using the SCHOOL model to disrupt intramembrane PPIs in the TCR complex was able to reduce inflammation in DBA/1J mice with CIA and protect mice against bone and cartilage damage (Shen & Sigalov, 2016). Formulation of the peptide into self-assembling macrophage-targeted lipopeptide nanoparticles that mimic native human HDLs significantly increased peptide dosage efficacy (Shen & Sigalov, 2016). Collectively, these findings further confirm that viral immune evasion strategies evolved during the host–virus coevolution can be transferred to therapeutic strategies that require similar functionalities (e.g., in the treatment of autoimmune diseases).
3.2.2. Targeting TREM-1
TREM-1 expressed on macrophages is a promising target for the development of new rational therapies for autoimmune diseases including RA (Kuai et al., 2009). In addition, targeted delivery of antirheumatic drugs to macrophages is another highly desirable strategy for the treatment of RA because it would not only strike the cells that mediate or amplify most of the permanent tissue destruction but also spare other cells that do not affect joint damage (Garrood and Pitzalis, 2006, Kinne et al., 2007).
Recently, we demonstrated that a TREM-1-specific inhibitory nonapeptide GF9 designed using the SCHOOL model and capable of inhibiting TREM-1-mediated macrophage activation in a ligand-independent manner, suppresses release of proinflammatory cytokines and M-CSF, decreases inflammation and protects against bone and cartilage destruction in experimental arthritis (Shen & Sigalov, 2017). Administration of GF9 reduced the plasma levels of M-CSF, TNFα, IL-1, and IL-6 (Shen & Sigalov, 2017). Interestingly, 31-mer peptides with sequences from GF9 and helices 4 (GE31) and 6 (GA31) of the major HDL protein, apolipoprotein A–I, were able to perform three functions: assist in the self-assembly of GA/E31-HDL, target these particles to macrophages, and block TREM-1 signaling (Shen & Sigalov, 2017). Similar to our cancer studies (Sigalov, 2014), formulation of GF9 alone or as a part of GE31 and GA31 peptides into HDL significantly increased its therapeutic efficacy. Colocalization of GF9 with TREM-1 in the macrophage membrane observed using confocal microscopy indicates that GF9 self-inserts into the cell membrane and disrupts intramembrane PPIs between TREM-1 and its signaling partner, DAP-12, further confirming the SCHOOL mechanisms of action of this peptide (Shen & Sigalov, 2017). Collectively, these findings suggest that TREM-1 inhibitory SCHOOL sequences may be promising alternatives for the treatment of RA and possibly, other TREM-1-mediated autoimmune diseases.
3.3. Sepsis
Septic shock is a complex clinical syndrome that results from the systemic response to infection and is characterized by overwhelming production of proinflammatory cytokines that leads not only to tissue damage, but also to hemodynamic changes, multiple organ failure, and ultimately death (Cohen, 2002). Despite the use of potent antibiotics and advanced resuscitative equipment costing $17 billion annually, sepsis still kills about 375,000 Americans each year and mortality rates for patients with septic shock are nearly 50% (Martin, Mannino, Eaton, & Moss, 2003). The only approved sepsis drug, Xigris by Ely Lilly and Company, has been withdrawn from all markets in 2011 and currently, no approved drugs are available. In addition, over 30 drug candidates have failed late-stage clinical trials. Together, this highlights an urgent unmet medical need for new sepsis treatments.
Macrophages are known to secrete high levels of TNFα, IL-6, and IL-1 in septic mice (Ayala & Chaudry, 1996). Further, in patients with sepsis, M-CSF is overproduced (Oren, Duman, Abacioglu, Ozkan, & Irken, 2001). Furthermore, elevated levels of TNFα along with other proinflammatory cytokines are closely linked with poor patient outcome (Gogos, Drosou, Bassaris, & Skoutelis, 2000). Importantly, in patients with sepsis, TREM-1 expression on macrophages is markedly increased (Gibot, 2005, van Bremen et al., 2013). Initial findings established TREM-1 as an amplifier of the systemic inflammatory response syndrome associated with sepsis (Bouchon et al., 2000, Bouchon et al., 2001). Blockade of TREM-1 in septic mice lowers expression levels of TNF-α and IL-6 and increases survival from 5% to 10% in control animals to 70%–80% in treated animals (Bouchon et al., 2001, Gibot et al., 2007, Wang et al., 2012). Taken together, this suggests TREM-1-mediated macrophage activation as a promising therapeutic target for this syndrome.
However, as discussed earlier, the development of conventional inhibitors of TREM-1 that attempt to block binding of TREM-1 to its ligand (Bouchon et al., 2001, Gibot et al., 2007, Wang et al., 2012) is significantly complicated by the unknown nature of TREM-1 ligand(s) and hence the increased risk of failure of these approaches in clinical development. In this context, TREM-1-specific inhibitory SCHOOL peptide sequences that employ ligand-independent mechanism of receptor inhibition represent a promising alternative to conventional approaches. Ligand-independent blockade of the TREM-1 signaling pathway in mice with experimental lipopolysaccharide (LPS)-induced septic shock using TREM-1 inhibitory SCHOOL peptide GF9 was shown to substantially suppress proinflammatory cytokine production and prolong survival of septic mice (Sigalov, 2014). Targeted delivery of GF9 to macrophages using self-assembling lipopeptide complexes significantly increased peptide half-life and therapeutic efficacy (Sigalov, 2014). These data strongly support the hypothesis that ligand-independent modulation of TREM-1 function using small synthetic peptides might be a suitable treatment for sepsis.
3.4. Thrombosis
Damage to the integrity of the vessel triggers platelet adhesion and aggregation resulting in the formation of a thrombus, which prevents blood loss at sites of injury or leads to occlusion and irreversible tissue damage or infarction in diseased vessels (Leslie, 2010, Nieswandt et al., 2001). Despite intensive research and recent advances in antithrombotic drug discovery and development (Angiolillo, Bhatt, Gurbel, & Jennings, 2009), uncontrolled hemorrhage still remains the most common side effect associated with antithrombotic drugs that are currently in use on the about $11 billion market.
Engagement and clustering of GPVI, the major collagen receptor on platelets, play a crucial role in platelet adhesion, aggregation, and activation induced by collagen (Jung & Moroi, 2008). The selective inhibition of GPVI is believed to inhibit thrombosis without affecting hemostatic plug formation, thus providing new therapeutic strategies to fight platelet-mediated diseases (Li et al., 2007, Lockyer et al., 2006). Thus, in contrast to current drugs, GPVI-specific inhibitors may represent an ideal class of clinically suitable antithrombotics.
Despite intensive studies (Farndale, 2006, Gawaz, 2004, Gibbins et al., 1997, Moroi and Jung, 2004), the molecular mechanisms underlying GPVI triggering and platelet activation were not known until recently when the SCHOOL model was introduced and applied to GPVI triggering and transmembrane signal transduction (Sigalov, 2004, Sigalov, 2005, Sigalov, 2006, Sigalov, 2007b, Sigalov, 2007c, Sigalov, 2008a). This resulted in the development of a novel concept of platelet inhibition and the invention of new platelet inhibitors that employ the ligand-independent SCHOOL mechanisms of GPVI inhibition (Sigalov, 2007a, Sigalov, 2007b, Sigalov, 2008a).
Structurally, GPVI is a member of the MIRR family and signals through its signaling partner, the FcRγ chain (Moroi & Jung, 2004). Uncovering the molecular mechanisms of collagen-stimulated triggering of the GPVI-mediated signal cascade, the SCHOOL model reveals GPVI-FcRγ intramembrane PPIs as a novel therapeutic target for the prevention and treatment of platelet-mediated thrombotic events (Sigalov, 2006, Sigalov, 2007b, Sigalov, 2007c, Sigalov, 2008a, Sigalov, 2010a). Specific blockade or disruption of these PPIs causes a physical and functional disconnection of the GPVI and FcRγ subunits. Experimental data obtained using the GPVI-specific inhibitory SCHOOL peptide GV11 (Sigalov, 2007a, Sigalov, 2007b, Sigalov, 2008a) provided support for this novel concept of platelet inhibition and demonstrated that depending on the donor, incubation of whole blood samples with GV11 prior to addition of collagen (10 and 20 μg/mL) or convulxin (10 ng/mL) leads to a 30%–60% reduction in both the percentage of P-selectin-positive platelets and the expression of the platelet activation markers, P-selectin and PAC-1. This effect is specific: platelet activation via ADP (20 μM) is not affected by the peptide. Collectively, these findings suggest that targeting intramembrane PPIs using the SCHOOL technology opens exciting new avenues in innovative antithrombotic drug discovery and development.
3.5. Retinopathy
Pathologic retinal neovascularization (RNV) causes an angiogenesis-related vision impairment in retinopathy of prematurity (ROP), diabetic retinopathy (DR), and retinal vein occlusion (RVO), which are the most common causes of vision loss and blindness in each age group (Jo and Kim, 2010, Laouri et al., 2011, Mutlu and Sarici, 2013). In premature infants, normal retinal vascular development is interrupted resulting in retinal ischemia and invasion of the vitreous by abnormal neovessels. In addition, vitreoretinal neovascularization can promote traction retinal detachment, leading to blindness (Al-Shabrawey et al., 2013). In the United States, 14,000–16,000 premature infants are affected by ROP annually and about 4.1 million adults over 40 years have DR (Bashinsky, 2017, Hartnett, 2017). Complications of conventional therapeutic options including laser ablation (corneal edema, anterior chamber reaction, intraocular hemorrhage, cataract formation, and intraocular pressure changes) (Mutlu & Sarici, 2013) and anti-VEGF therapy (damage of healthy vessels, potential side effects on neurons, rapid vascular regrowth upon interrupting the VEGF blockade, and limited effectiveness in some patients) (Maharaj et al., 2008, Mancuso et al., 2006, Pieramici and Rabena, 2008, Verheul and Pinedo, 2007) highlight an unmet need for new targeted therapies that can better address the pathogenesis of neovascular retinal diseases and improve their treatment. In addition, drug delivery to the retina via systemic administration is ideal but it is still a challenge due to the blood–retinal barrier (BRB) (Gaudana, Ananthula, Parenky, & Mitra, 2010).
Retinal microglia and blood-derived macrophages (BDM) that regulate angiogenesis are critically involved in the pathogenesis of RNV diseases (Checchin et al., 2006, Espinosa-Heidmann et al., 2003, Kubota et al., 2009). In ROP, the retina is infiltrated by activated leukocytes and macrophages (Davies, Eubanks, & Powers, 2006). While retinal microglia can become activated in response to retinal pathologies (Penfold, Madigan, Gillies, & Provis, 2001), infiltration and activation of BDM may provide a major contribution in retinal degeneration (Caicedo, Espinosa-Heidmann, Pina, Hernandez, & Cousins, 2005). In mice with oxygen-reduced retinopathy (OIR), macrophages promote the development of pathological RNV (Gao et al., 2016). Macrophages induce angiogenesis by secreting multiple proangiogenic factors, including VEGF (Liu et al., 2013), MCP-1 (Niu, Azfer, Zhelyabovska, Fatma, & Kolattukudy, 2008) and proinflammatory cytokines, such as TNFα and IL-1 (Nagai et al., 2001). Inhibition of MCP-1 suppresses RNV (Kim et al., 2005, Yoshida et al., 2003). M-CSF that acts as an “angiogenic switch” (Lin et al., 2006) is involved in the differentiation of tissue macrophages and microglia during postnatal development (Cecchini et al., 1994). In contrast to VEGF blockade, interruption of M-CSF inhibition does not promote rapid vascular regrowth (Kubota et al., 2009). In the OIR mice, M-CSF is required for pathological RNV but not for the recovery of normal vasculature (Kubota et al., 2009). In the retinas of diabetic rats and in the vitreous of patients with proliferative DR, M-CSF levels are significantly higher compared to control subjects (Liu et al., 2009, Yoshida et al., 2015). This suggests targeting M-CSF either directly or via the specific inflammatory signaling pathway as a highly promising strategy for treating ocular neovascular diseases including ROP.
TREM-1 is upregulated under a variety of inflammatory conditions (Pelham & Agrawal, 2014). Upon activation, TREM-1 enhances the production of multiple cytokines and growth factors including MCP-1, TNFα, IL-1α, IL-1β, IL-6, and M-CSF (Dower et al., 2008, Lagler et al., 2009, Schenk et al., 2007, Shen and Sigalov, 2017, Sigalov, 2014). However, despite the recent identification of TREM-1 as a novel hypoxic marker in vivo and in vitro (Bosco et al., 2011), the role of TREM-1 in RNV diseases including ROP remained unclear. Recently, we found that TREM-1 is highly overexpressed in the retina of the OIR mice compared to controls (Modesto A. Rojas, Zu T. Shen, Ruth B. Caldwell, and Alexander B. Sigalov; unpublished data). Blockade of TREM-1 using the TREM-1 inhibitory SCHOOL GF9 peptide sequences substantially suppressed retinal protein levels of TREM-1 and M-CSF, suggesting an M-CSF-dependent inhibitory effect on angiogenesis, and significantly (up to 90%) reduced the area of vitreoretinal neovascularization (Modesto A. Rojas, Zu T. Shen, Ruth B. Caldwell, and Alexander B. Sigalov, unpublished data). Collectively, these promising data suggest that targeting intramembrane PPIs in the TREM-1/DAP-12 receptor complex using TREM-1-specific SCHOOL peptide inhibitors represent a novel strategy to treat RNV diseases including ROP.
4. Conclusions
There is a growing interest in therapeutic targeting specific PPIs involved in receptor-mediated transmembrane signal transduction and our improved understanding of the molecular mechanisms underlying this process can significantly contribute into the development of novel pharmacological approaches to a variety of diseases.
Example is the SCHOOL platform that by uncovering the molecular mechanisms of transmembrane signaling mediated by unrelated and functionally diversed surface receptors expressed on various cells, revealed the specific PPIs involved in receptor triggering as key points of therapeutic control. Importantly, the SCHOOL platform suggests that within the SR and MIRR families, the similar structural architecture of the receptors dictates similar mechanisms of receptor triggering. This, in turn, suggests similarity of therapeutic targets in seemingly unrelated diseases, which makes possible the development of global pharmacological approaches as well as the transfer of our clinical knowledge, experience, and therapeutic strategies between these diseases. Successful application of the SCHOOL strategy to target in vivo intramembrane PPIs involved in triggering of such unrelated receptors as TCR, TREM-1, and GPVI expressed on T cells, macrophages, and platelets, respectively, provided compelling evidence to support this hypothesis and resulted in the discovery of new drug candidates for a variety of diseases including cancer, sepsis, RA, AD, and retinopathy.
Further, the SCHOOL platform significantly improved our understanding of the immunomodulatory activity of many human viruses. It appears that different viruses use their fusogenic peptides not only to fuse their membranes to their target host cells but also to disarm the immune system using the SCHOOL mechanisms of receptor inhibition and to escape the host immune response. This gives an interesting example of how our advances in mechanistic understanding of the fundamental natural processes and their interactions can elucidate the billions years old strategies nature uses for combinatorial control and optimization at different scales, from evolution to organismal function.
Acknowledgments
This work was partially supported by the Defense Advanced Research Projects Agency (DARPA)/US Army Research Office (contract no. W911NF-12-C-0003), the National Cancer Institute of the National Institutes of Health (grant numbers R43CA167865 and R43CA195684), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (grant number R43AR066376). The additional funding has come from SignaBlok, Inc. The author thanks Dr. Zu Shen for his critical reading of the manuscript and excellent help with figures.
References
- Akdis C.A., Akdis M., Trautmann A., Blaser K. Immune regulation in atopic dermatitis. Current Opinion in Immunology. 2000;12(6):641–646. doi: 10.1016/s0952-7915(00)00156-4. [DOI] [PubMed] [Google Scholar]
- Ali M., Amon M., Bender V., Manolios N. Hydrophobic transmembrane-peptide lipid conjugations enhance membrane binding and functional activity in T-cells. Bioconjugate Chemistry. 2005;16(6):1556–1563. doi: 10.1021/bc050127j. [DOI] [PubMed] [Google Scholar]
- Ali M., De Planque M.R.R., Huynh N.T., Manolios N., Separovic F. Biophysical studies of a transmembrane peptide derived from the T cell antigen receptor. Letters in Peptide Science. 2002;8(3–5):227–233. [Google Scholar]
- Ali M., Salam N.K., Amon M., Bender V., Hibbs D.E., Manolios N. T-cell antigen receptor-alpha chain transmembrane peptides: Correlation between structure and function. International Journal of Peptide Research and Therapeutics. 2006;12(3):261–267. [Google Scholar]
- Al-Shabrawey M., Elsherbiny M., Nussbaum J., Othman A., Megyerdi S., Tawfik A. Targeting neovascularization in ischemic retinopathy: Recent advances. Expert Review of Ophthalmology. 2013;8(3):267–286. doi: 10.1586/eop.13.17. https://doi.org/10.1586/eop.13.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon M.A., Ali M., Bender V., Chan Y.N., Toth I., Manolios N. Lipidation and glycosylation of a T cell antigen receptor (TCR) transmembrane hydrophobic peptide dramatically enhances in vitro and in vivo function. Biochimica et Biophysica Acta. 2006;1763(8):879–888. doi: 10.1016/j.bbamcr.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Amon M.A., Ali M., Bender V., Hall K., Aguilar M.I., Aldrich-Wright J. Kinetic and conformational properties of a novel T-cell antigen receptor transmembrane peptide in model membranes. Journal of Peptide Science. 2008;14(6):714–724. doi: 10.1002/psc.987. [DOI] [PubMed] [Google Scholar]
- Angiolillo D.J., Bhatt D.L., Gurbel P.A., Jennings L.K. Advances in antiplatelet therapy: Agents in clinical development. The American Journal of Cardiology. 2009;103(3 Suppl):40A–51A. doi: 10.1016/j.amjcard.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Archakov A.I., Govorun V.M., Dubanov A.V., Ivanov Y.D., Veselovsky A.V., Lewi P. Protein–protein interactions as a target for drugs in proteomics. Proteomics. 2003;3(4):380–391. doi: 10.1002/pmic.200390053. [DOI] [PubMed] [Google Scholar]
- Arkin M.R., Wells J.A. Small-molecule inhibitors of protein–protein interactions: Progressing towards the dream. Nature Reviews. Drug Discovery. 2004;3(4):301–317. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- Autissier P., De Vos J., Liautard J., Tupitsyn N., Jacquet C., Chavdia N. Dimerization and activation of the common transducing chain (gp130) of the cytokines of the IL-6 family by mAb. International Immunology. 1998;10(12):1881–1889. doi: 10.1093/intimm/10.12.1881. [DOI] [PubMed] [Google Scholar]
- Ayala A., Chaudry I.H. Immune dysfunction in murine polymicrobial sepsis: Mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6(Suppl. 1):S27–S38. [PubMed] [Google Scholar]
- Bashinsky A.L. Retinopathy of prematurity. North Carolina Medical Journal. 2017;78(2):124–128. doi: 10.18043/ncm.78.2.124. https://doi.org/10.18043/ncm.78.2.124 [DOI] [PubMed] [Google Scholar]
- Bell C.A., Tynan J.A., Hart K.C., Meyer A.N., Robertson S.C., Donoghue D.J. Rotational coupling of the transmembrane and kinase domains of the Neu receptor tyrosine kinase. Molecular Biology of the Cell. 2000;11(10):3589–3599. doi: 10.1091/mbc.11.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender V., Ali M., Amon M., Diefenbach E., Manolios N. T cell antigen receptor peptide–lipid membrane interactions using surface plasmon resonance. The Journal of Biological Chemistry. 2004;279(52):54002–54007. doi: 10.1074/jbc.M403909200. [DOI] [PubMed] [Google Scholar]
- Bennasroune A., Fickova M., Gardin A., Dirrig-Grosch S., Aunis D., Cremel G. Transmembrane peptides as inhibitors of ErbB receptor signaling. Molecular Biology of the Cell. 2004;15(7):3464–3474. doi: 10.1091/mbc.E03-10-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennasroune A., Gardin A., Auzan C., Clauser E., Dirrig-Grosch S., Meira M. Inhibition by transmembrane peptides of chimeric insulin receptors. Cellular and Molecular Life Sciences. 2005;62(18):2124–2131. doi: 10.1007/s00018-005-5226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T. Modulation of protein–protein interactions with small organic molecules. Angewandte Chemie (International Ed. in English) 2003;42(22):2462–2481. doi: 10.1002/anie.200200558. [DOI] [PubMed] [Google Scholar]
- Berg T. Small-molecule inhibitors of protein–protein interactions. Current Opinion in Drug Discovery & Development. 2008;11(5):666–674. [PubMed] [Google Scholar]
- Berlanga O., Bori-Sanz T., James J.R., Frampton J., Davis S.J., Tomlinson M.G. Glycoprotein VI oligomerization in cell lines and platelets. Journal of Thrombosis and Haemostasis. 2007;5(5):1026–1033. doi: 10.1111/j.1538-7836.2007.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biassoni R., Cantoni C., Falco M., Pende D., Millo R., Moretta L. Human natural killer cell activating receptors. Molecular Immunology. 2000;37(17):1015–1024. doi: 10.1016/s0161-5890(01)00018-9. [DOI] [PubMed] [Google Scholar]
- Bloch I., Quintana F.J., Gerber D., Cohen T., Cohen I.R., Shai Y. T-cell inactivation and immunosuppressive activity induced by HIV gp41 via novel interacting motif. The FASEB Journal. 2007;21(2):393–401. doi: 10.1096/fj.06-7061com. [DOI] [PubMed] [Google Scholar]
- Bormann B.J., Engelman D.M. Intramembrane helix–helix association in oligomerization and transmembrane signaling. Annual Review of Biophysics and Biomolecular Structure. 1992;21:223–242. doi: 10.1146/annurev.bb.21.060192.001255. [DOI] [PubMed] [Google Scholar]
- Bosco M.C., Pierobon D., Blengio F., Raggi F., Vanni C., Gattorno M. Hypoxia modulates the gene expression profile of immunoregulatory receptors in human mature dendritic cells: Identification of TREM-1 as a novel hypoxic marker in vitro and in vivo. Blood. 2011;117(9):2625–2639. doi: 10.1182/blood-2010-06-292136. https://doi.org/10.1182/blood-2010-06-292136 [DOI] [PubMed] [Google Scholar]
- Bosco M.C., Raggi F., Varesio L. Therapeutic potential of targeting TREM-1 in inflammatory diseases and cancer. Current Pharmaceutical Design. 2016;22(41):6209–6233. doi: 10.2174/1381612822666160826110539. https://doi.org/10.2174/1381612822666160826110539 [DOI] [PubMed] [Google Scholar]
- Bouchon A., Dietrich J., Colonna M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. Journal of Immunology. 2000;164(10):4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- Bouchon A., Facchetti F., Weigand M.A., Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410(6832):1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Gerlag D.M., Rooney T., Smeets T.J., Wijbrandts C.A., Boyle D. Synovial macrophages as a biomarker of response to therapeutic intervention in rheumatoid arthritis: Standardization and consistency across centers. The Journal of Rheumatology. 2007;34(3):620–622. [PubMed] [Google Scholar]
- Caicedo A., Espinosa-Heidmann D.G., Pina Y., Hernandez E.P., Cousins S.W. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Experimental Eye Research. 2005;81(1):38–47. doi: 10.1016/j.exer.2005.01.013. https://doi.org/10.1016/j.exer.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Call M.E., Pyrdol J., Wiedmann M., Wucherpfennig K.W. The organizing principle in the formation of the T cell receptor-CD3 complex. Cell. 2002;111(7):967–979. doi: 10.1016/s0092-8674(02)01194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I.K., Rich M.J., Bischof R.J., Hamilton J.A. The colony-stimulating factors and collagen-induced arthritis: Exacerbation of disease by M-CSF and G-CSF and requirement for endogenous M-CSF. Journal of Leukocyte Biology. 2000;68(1):144–150. [PubMed] [Google Scholar]
- Carreno L.J., Gonzalez P.A., Kalergis A.M. Modulation of T cell function by TCR/pMHC binding kinetics. Immunobiology. 2006;211(1–2):47–64. doi: 10.1016/j.imbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Cecchini M.G., Dominguez M.G., Mocci S., Wetterwald A., Felix R., Fleisch H. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120(6):1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- Chae W.J., Lee H.K., Han J.H., Kim S.W., Bothwell A.L., Morio T. Qualitatively differential regulation of T cell activation and apoptosis by T cell receptor zeta chain ITAMs and their tyrosine residues. International Immunology. 2004;16(9):1225–1236. doi: 10.1093/intimm/dxh120. [DOI] [PubMed] [Google Scholar]
- Chan F.K. Three is better than one: Pre-ligand receptor assembly in the regulation of TNF receptor signaling. Cytokine. 2007;37(2):101–107. doi: 10.1016/j.cyto.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.F., Siegel M.R., Lenardo J.M. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity. 2000;13(4):419–422. doi: 10.1016/s1074-7613(00)00041-8. [DOI] [PubMed] [Google Scholar]
- Che Y., Brooks B.R., Marshall G.R. Development of small molecules designed to modulate protein–protein interactions. Journal of Computer-Aided Molecular Design. 2006;20(2):109–130. doi: 10.1007/s10822-006-9040-8. [DOI] [PubMed] [Google Scholar]
- Checchin D., Sennlaub F., Levavasseur E., Leduc M., Chemtob S. Potential role of microglia in retinal blood vessel formation. Investigative Ophthalmology and Visual Science. 2006;47(8):3595–3602. doi: 10.1167/iovs.05-1522. https://doi.org/10.1167/iovs.05-1522 [DOI] [PubMed] [Google Scholar]
- Clark C.E., Hingorani S.R., Mick R., Combs C., Tuveson D.A., Vonderheide R.H. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Research. 2007;67(19):9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. https://doi.org/10.1158/0008-5472.CAN-07-0175 [DOI] [PubMed] [Google Scholar]
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326. https://doi.org/10.1038/nature01326 [DOI] [PubMed] [Google Scholar]
- Cohen T., Cohen S.J., Antonovsky N., Cohen I.R., Shai Y. HIV-1 gp41 and TCRalpha trans-membrane domains share a motif exploited by the HIV virus to modulate T-cell proliferation. PLoS Pathogens. 2010;6(9) doi: 10.1371/journal.ppat.1001085. https://doi.org/10.1371/journal.ppat.1001085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S., Bolte A., Manolios N. Discrepancy in CD3-transmembrane peptide activity between in vitro and in vivo T-cell inhibition. Scandinavian Journal of Immunology. 2006;64(4):388–391. doi: 10.1111/j.1365-3083.2006.01806.x. [DOI] [PubMed] [Google Scholar]
- Colonna M., Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): A new player in acute inflammatory responses. The Journal of Infectious Diseases. 2003;187(Suppl. 2):S397–S401. doi: 10.1086/374754. [DOI] [PubMed] [Google Scholar]
- Cooper M.D., Alder M.N. The evolution of adaptive immune systems. Cell. 2006;124(4):815–822. doi: 10.1016/j.cell.2006.02.001. https://doi.org/10.1016/j.cell.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Cope, A. P., Schulze-Koops, H., & Aringer, M. (2007). The central role of T cells in rheumatoid arthritis. Clinical and Experimental Rheumatology, 25(5 Suppl. 46), S4–11. https://doi.org/2142 (pii) [PubMed]
- Daeron M. Fc receptor biology. Annual Review of Immunology. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- Darwin C.R. John Murray; London: 1861. On the origin of species by means of natural selection or the preservation of favoured races in the struggle for life. [PMC free article] [PubMed] [Google Scholar]
- Davidson A., Diamond B. Autoimmune diseases. The New England Journal of Medicine. 2001;345(5):340–350. doi: 10.1056/NEJM200108023450506. https://doi.org/10.1056/NEJM200108023450506 [DOI] [PubMed] [Google Scholar]
- Davies M.H., Eubanks J.P., Powers M.R. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Molecular Vision. 2006;12:467–477. [PubMed] [Google Scholar]
- Davis M.M., Boniface J.J., Reich Z., Lyons D., Hampl J., Arden B. Ligand recognition by alpha beta T cell receptors. Annual Review of Immunology. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- DeFranco A.L., Mittelstadt P.R., Blum J.H., Stevens T.L., Law D.A., Chan V.W. Mechanism of B cell antigen receptor function: Transmembrane signaling and triggering of apoptosis. Advances in Experimental Medicine and Biology. 1994;365:9–22. doi: 10.1007/978-1-4899-0987-9_2. [DOI] [PubMed] [Google Scholar]
- Dower K., Ellis D.K., Saraf K., Jelinsky S.A., Lin L.L. Innate immune responses to TREM-1 activation: Overlap, divergence, and positive and negative cross-talk with bacterial lipopolysaccharide. Journal of Immunology. 2008;180(5):3520–3534. doi: 10.4049/jimmunol.180.5.3520. [DOI] [PubMed] [Google Scholar]
- Elgert K.D., Alleva D.G., Mullins D.W. Tumor-induced immune dysfunction: The macrophage connection. Journal of Leukocyte Biology. 1998;64(3):275–290. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- Enk A.H., Knop J. T cell receptor mimic peptides and their potential application in T-cell-mediated disease. International Archives of Allergy and Immunology. 2000;123(4):275–281. doi: 10.1159/000053639. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann D.G., Suner I.J., Hernandez E.P., Monroy D., Csaky K.G., Cousins S.W. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Investigative Ophthalmology & Visual Science. 2003;44(8):3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- Esposito I., Menicagli M., Funel N., Bergmann F., Boggi U., Mosca F. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. Journal of Clinical Pathology. 2004;57(6):630–636. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale R.W. Collagen-induced platelet activation. Blood Cells, Molecules & Diseases. 2006;36(2):162–165. doi: 10.1016/j.bcmd.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Felip E., Santarpia M., Rosell R. Emerging drugs for non-small-cell lung cancer. Expert Opinion on Emerging Drugs. 2007;12(3):449–460. doi: 10.1517/14728214.12.3.449. [DOI] [PubMed] [Google Scholar]
- Finger C., Escher C., Schneider D. The single transmembrane domains of human receptor tyrosine kinases encode self-interactions. Science Signaling. 2009;2(89) doi: 10.1126/scisignal.2000547. ra56. [DOI] [PubMed] [Google Scholar]
- Finger C., Volkmer T., Prodohl A., Otzen D.E., Engelman D.M., Schneider D. The stability of transmembrane helix interactions measured in a biological membrane. Journal of Molecular Biology. 2006;358(5):1221–1228. doi: 10.1016/j.jmb.2006.02.065. [DOI] [PubMed] [Google Scholar]
- Fletcher S., Hamilton A.D. Targeting protein–protein interactions by rational design: Mimicry of protein surfaces. Journal of the Royal Society Interface. 2006;3(7):215–233. doi: 10.1098/rsif.2006.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher S., Hamilton A.D. Protein–protein interaction inhibitors: Small molecules from screening techniques. Current Topics in Medicinal Chemistry. 2007;7(10):922–927. doi: 10.2174/156802607780906735. [DOI] [PubMed] [Google Scholar]
- Ford J.W., McVicar D.W. TREM and TREM-like receptors in inflammation and disease. Current Opinion in Immunology. 2009;21(1):38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D.C. Protein–protein interactions as targets for small molecule drug discovery. Biopolymers. 2006;84(6):535–552. doi: 10.1002/bip.20608. [DOI] [PubMed] [Google Scholar]
- Gao X., Wang Y.S., Li X.Q., Hou H.Y., Su J.B., Yao L.B. Macrophages promote vasculogenesis of retinal neovascularization in an oxygen-induced retinopathy model in mice. Cell and Tissue Research. 2016;364(3):599–610. doi: 10.1007/s00441-015-2353-y. https://doi.org/10.1007/s00441-015-2353-y [DOI] [PubMed] [Google Scholar]
- Gardian K., Janczewska S., Olszewski W.L., Durlik M. Analysis of pancreatic cancer microenvironment: Role of macrophage infiltrates and growth factors expression. Journal of Cancer. 2012;3:285–291. doi: 10.7150/jca.4537. https://doi.org/10.7150/jca.4537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrood T., Pitzalis C. Targeting the inflamed synovium: The quest for specificity. Arthritis & Rhematology. 2006;54(4):1055–1060. doi: 10.1002/art.21720. https://doi.org/10.1002/art.21720 [DOI] [PubMed] [Google Scholar]
- Gaudana R., Ananthula H.K., Parenky A., Mitra A.K. Ocular drug delivery. The AAPS Journal. 2010;12(3):348–360. doi: 10.1208/s12248-010-9183-3. https://doi.org/10.1208/s12248-010-9183-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawaz M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovascular Research. 2004;61(3):498–511. doi: 10.1016/j.cardiores.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Genua M., Rutella S., Correale C., Danese S. The triggering receptor expressed on myeloid cells (TREM) in inflammatory bowel disease pathogenesis. Journal of Translational Medicine. 2014;12:293. doi: 10.1186/s12967-014-0293-z. https://doi.org/10.1186/s12967-014-0293-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber D., Quintana F.J., Bloch I., Cohen I.R., Shai Y. D-enantiomer peptide of the TCRalpha transmembrane domain inhibits T-cell activation in vitro and in vivo. The FASEB Journal. 2005;19(9):1190–1192. doi: 10.1096/fj.04-3498fje. [DOI] [PubMed] [Google Scholar]
- Gibbins J.M., Okuma M., Farndale R., Barnes M., Watson S.P. Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor gamma-chain. FEBS Letters. 1997;413(2):255–259. doi: 10.1016/s0014-5793(97)00926-5. [DOI] [PubMed] [Google Scholar]
- Gibot S. Clinical review: Role of triggering receptor expressed on myeloid cells-1 during sepsis. Critical Care. 2005;9(5):485–489. doi: 10.1186/cc3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibot S., Massin F., Marcou M., Taylor V., Stidwill R., Wilson P. TREM-1 promotes survival during septic shock in mice. European Journal of Immunology. 2007;37(2):456–466. doi: 10.1002/eji.200636387. https://doi.org/10.1002/eji.200636387 [DOI] [PubMed] [Google Scholar]
- Gogos C.A., Drosou E., Bassaris H.P., Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. The Journal of Infectious Diseases. 2000;181(1):176–180. doi: 10.1086/315214. https://doi.org/10.1086/315214 [DOI] [PubMed] [Google Scholar]
- Gollner G.P., Muller G., Alt R., Knop J., Enk A.H. Therapeutic application of T cell receptor mimic peptides or cDNA in the treatment of T cell-mediated skin diseases. Gene Therapy. 2000;7(12):1000–1004. doi: 10.1038/sj.gt.3301183. [DOI] [PubMed] [Google Scholar]
- Greiser J.S., Stross C., Heinrich P.C., Behrmann I., Hermanns H.M. Orientational constraints of the gp130 intracellular juxtamembrane domain for signaling. The Journal of Biological Chemistry. 2002;277(30):26959–26965. doi: 10.1074/jbc.M204113200. [DOI] [PubMed] [Google Scholar]
- Groblewska M., Mroczko B., Wereszczynska-Siemiatkowska U., Mysliwiec P., Kedra B., Szmitkowski M. Serum levels of granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) in pancreatic cancer patients. Clinical Chemistry and Laboratory Medicine. 2007;45(1):30–34. doi: 10.1515/CCLM.2007.025. https://doi.org/10.1515/CCLM.2007.025 [DOI] [PubMed] [Google Scholar]
- Hansel T.T., Kropshofer H., Singer T., Mitchell J.A., George A.J. The safety and side effects of monoclonal antibodies. Nature Reviews. Drug Discovery. 2010;9(4):325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- Hariharan D., Saied A., Kocher H.M. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008;10(1):58–62. doi: 10.1080/13651820701883148. https://doi.org/10.1080/13651820701883148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnett M.E. Advances in understanding and management of retinopathy of prematurity. Survey of Ophthalmology. 2017;62(3):257–276. doi: 10.1016/j.survophthal.2016.12.004. https://doi.org/10.1016/j.survophthal.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert T.E., Moffett S., Morello J.P., Loisel T.P., Bichet D.G., Barret C. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. The Journal of Biological Chemistry. 1996;271(27):16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- Ho C.C., Liao W.Y., Wang C.Y., Lu Y.H., Huang H.Y., Chen H.Y. TREM-1 expression in tumor-associated macrophages and clinical outcome in lung cancer. American Journal of Respiratory and Critical Care Medicine. 2008;177(7):763–770. doi: 10.1164/rccm.200704-641OC. [DOI] [PubMed] [Google Scholar]
- Holler P.D., Lim A.R., Cho B.K., Rund L.A., Kranz D.M. CD8(−) T cell transfectants that express a high affinity T cell receptor exhibit enhanced peptide-dependent activation. The Journal of Experimental Medicine. 2001;194(8):1043–1052. doi: 10.1084/jem.194.8.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowka D., Sil D., Torigoe C., Baird B. Insights into immunoglobulin E receptor signaling from structurally defined ligands. Immunological Reviews. 2007;217:269–279. doi: 10.1111/j.1600-065X.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- Huynh N.T., Ffrench R.A., Boadle R.A., Manolios N. Transmembrane T-cell receptor peptides inhibit B- and natural killer-cell function. Immunology. 2003;108(4):458–464. doi: 10.1046/j.1365-2567.2003.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. https://doi.org/10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- Jensen W.A., Pleiman C.M., Beaufils P., Wegener A.M., Malissen B., Cambier J.C. Qualitatively distinct signaling through T cell antigen receptor subunits. European Journal of Immunology. 1997;27(3):707–716. doi: 10.1002/eji.1830270320. [DOI] [PubMed] [Google Scholar]
- Jiang G., Hunter T. Receptor signaling: When dimerization is not enough. Current Biology. 1999;9(15):R568–R571. doi: 10.1016/s0960-9822(99)80357-1. [DOI] [PubMed] [Google Scholar]
- Jo D.H., Kim J.H. How to overcome retinal neuropathy: The fight against angiogenesis-related blindness. Archives of Pharmacal Research. 2010;33(10):1557–1565. doi: 10.1007/s12272-010-1007-6. https://doi.org/10.1007/s12272-010-1007-6 [DOI] [PubMed] [Google Scholar]
- Jung S.M., Moroi M. Platelet glycoprotein VI. Advances in Experimental Medicine and Biology. 2008;640:53–63. doi: 10.1007/978-0-387-09789-3_5. [DOI] [PubMed] [Google Scholar]
- Jung S.M., Tsuji K., Moroi M. Glycoprotein (GP) VI dimer as a major collagen-binding site of native platelets: Direct evidence obtained with dimeric GPVI-specific Fabs. Journal of Thrombosis and Haemostasis. 2009;7(8):1347–1355. doi: 10.1111/j.1538-7836.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- Kadmiel M., Cidlowski J.A. Glucocorticoid receptor signaling in health and disease. Trends in Pharmacological Sciences. 2013;34(9):518–530. doi: 10.1016/j.tips.2013.07.003. https://doi.org/10.1016/j.tips.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska J., Kowalska M., Kotowicz B., Fuksiewicz M., Glogowski M., Wojcik E. Pretreatment serum levels of cytokines and cytokine receptors in patients with non-small cell lung cancer, and correlations with clinicopathological features and prognosis. M-CSF—An independent prognostic factor. Oncology. 2006;70(2):115–125. doi: 10.1159/000093002. [DOI] [PubMed] [Google Scholar]
- Kasraie S., Werfel T. Role of macrophages in the pathogenesis of atopic dermatitis. Mediators of Inflammation. 2013;2013 doi: 10.1155/2013/942375. https://doi.org/10.1155/2013/942375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan A.D., Paul W.E. Multichain immune recognition receptors: Similarities in structure and signaling pathways. Immunology Today. 1992;13(2):63–68. doi: 10.1016/0167-5699(92)90136-U. [DOI] [PubMed] [Google Scholar]
- Kiessling L.L., Gestwicki J.E., Strong L.E. Synthetic multivalent ligands as probes of signal transduction. Angewandte Chemie (International Ed. in English) 2006;45(15):2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.Y., Byeon C.W., Hong K.H., Han K.H., Jeong S. Inhibition of the angiogenesis by the MCP-1 (monocyte chemoattractant protein-1) binding peptide. FEBS Letters. 2005;579(7):1597–1601. doi: 10.1016/j.febslet.2005.01.070. https://doi.org/10.1016/j.febslet.2005.01.070 [DOI] [PubMed] [Google Scholar]
- Kim M.K., Huang Z.Y., Hwang P.H., Jones B.A., Sato N., Hunter S. Fcgamma receptor transmembrane domains: Role in cell surface expression, gamma chain interaction, and phagocytosis. Blood. 2003;101(11):4479–4484. doi: 10.1182/blood.V101.11.4479. [DOI] [PubMed] [Google Scholar]
- Kim W.M., Sigalov A.B. Viral pathogenesis, modulation of immune receptor signaling and treatment. Advances in Experimental Medicine and Biology. 2008;640:325–349. doi: 10.1007/978-0-387-09789-3_22. https://doi.org/10.1007/978-0-387-09789-3_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrell D.A., Beutler B. The evolution and genetics of innate immunity. Nature Reviews. Genetics. 2001;2(4):256–267. doi: 10.1038/35066006. https://doi.org/10.1038/35066006 [DOI] [PubMed] [Google Scholar]
- Kinne R.W., Brauer R., Stuhlmuller B., Palombo-Kinne E., Burmester G.R. Macrophages in rheumatoid arthritis. Arthritis Research. 2000;2(3):189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne R.W., Stuhlmuller B., Burmester G.R. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Research & Therapy. 2007;9(6):224. doi: 10.1186/ar2333. https://doi.org/10.1186/ar2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm J.D., Schreiber S.L., Crabtree G.R. Dimerization as a regulatory mechanism in signal transduction. Annual Review of Immunology. 1998;16:569–592. doi: 10.1146/annurev.immunol.16.1.569. [DOI] [PubMed] [Google Scholar]
- Klesney-Tait J., Turnbull I.R., Colonna M. The TREM receptor family and signal integration. Nature Immunology. 2006;7(12):1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- Kuai J., Gregory B., Hill A., Pittman D.D., Feldman J.L., Brown T. TREM-1 expression is increased in the synovium of rheumatoid arthritis patients and induces the expression of pro-inflammatory cytokines. Rheumatology (Oxford) 2009;48(11):1352–1358. doi: 10.1093/rheumatology/kep235. https://doi.org/10.1093/rheumatology/kep235 [DOI] [PubMed] [Google Scholar]
- Kubota Y., Takubo K., Shimizu T., Ohno H., Kishi K., Shibuya M. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. The Journal of Experimental Medicine. 2009;206(5):1089–1102. doi: 10.1084/jem.20081605. https://doi.org/10.1084/jem.20081605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukar M., Petryna O., Efthimiou P. Biological targets in the treatment of rheumatoid arthritis: A comprehensive review of current and in-development biological disease modifying anti-rheumatic drugs. Biologics. 2009;3:443–457. [PMC free article] [PubMed] [Google Scholar]
- Kurosaka N., Ali M., Byth K., Manolios N. The mode of anti-arthritic peptide delivery impacts on the severity and outcome of adjuvant induced arthritis. APLAR Journal of Rheumatology. 2007;10(3):198–203. [Google Scholar]
- Kurosaka N., Bolte A., Ali M., Manolios N. T-cell antigen receptor assembly and cell surface expression is not affected by treatment with T-cell antigen receptor-alpha chain transmembrane peptide. Protein and Peptide Letters. 2007;14(3):299–303. doi: 10.2174/092986607780090865. [DOI] [PubMed] [Google Scholar]
- Kuwahara K., Sasaki T., Kuwada Y., Murakami M., Yamasaki S., Chayama K. Expressions of angiogenic factors in pancreatic ductal carcinoma: A correlative study with clinicopathologic parameters and patient survival. Pancreas. 2003;26(4):344–349. doi: 10.1097/00006676-200305000-00006. [DOI] [PubMed] [Google Scholar]
- Lagler H., Sharif O., Haslinger I., Matt U., Stich K., Furtner T. TREM-1 activation alters the dynamics of pulmonary IRAK-M expression in vivo and improves host defense during pneumococcal pneumonia. Journal of Immunology. 2009;183(3):2027–2036. doi: 10.4049/jimmunol.0803862. https://doi.org/10.4049/jimmunol.0803862 [DOI] [PubMed] [Google Scholar]
- Laouri M., Chen E., Looman M., Gallagher M. The burden of disease of retinal vein occlusion: Review of the literature. Eye (Lond) 2011;25(8):981–988. doi: 10.1038/eye.2011.92. https://doi.org/10.1038/eye.2011.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.K., Dunzendorfer S., Tobias P.S. Cytoplasmic domain-mediated dimerizations of toll-like receptor 4 observed by beta-lactamase enzyme fragment complementation. The Journal of Biological Chemistry. 2004;279(11):10564–10574. doi: 10.1074/jbc.M311564200. [DOI] [PubMed] [Google Scholar]
- Leslie M. Cell biology. Beyond clotting: The powers of platelets. Science. 2010;328(5978):562–564. doi: 10.1126/science.328.5978.562. [DOI] [PubMed] [Google Scholar]
- Li E., Hristova K. Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry. 2006;45(20):6241–6251. doi: 10.1021/bi060609y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hsu H.C., Mountz J.D. Managing macrophages in rheumatoid arthritis by reform or removal. Current Rheumatology Reports. 2012;14(5):445–454. doi: 10.1007/s11926-012-0272-4. https://doi.org/10.1007/s11926-012-0272-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Lockyer S., Concepcion A., Gong X., Takizawa H., Guertin M. The fab fragment of a novel anti-GPVI monoclonal antibody, OM4, reduces in vivo thrombosis without bleeding risk in rats. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(5):1199–1205. doi: 10.1161/ATVBAHA.107.140590. [DOI] [PubMed] [Google Scholar]
- Lin E.Y., Li J.F., Gnatovskiy L., Deng Y., Zhu L., Grzesik D.A. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Research. 2006;66(23):11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. https://doi.org/10.1158/0008-5472.CAN-06-1278 [DOI] [PubMed] [Google Scholar]
- Litman G.W., Cooper M.D. Why study the evolution of immunity? Nature Immunology. 2007;8(6):547–548. doi: 10.1038/ni0607-547. https://doi.org/10.1038/ni0607-547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Copland D.A., Horie S., Wu W.K., Chen M., Xu Y. Myeloid cells expressing VEGF and arginase-1 following uptake of damaged retinal pigment epithelium suggests potential mechanism that drives the onset of choroidal angiogenesis in mice. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072935. https://doi.org/10.1371/journal.pone.0072935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Xu G.Z., Jiang C.H., Da C.D. Expression of macrophage colony-stimulating factor (M-CSF) and its receptor in streptozotocin-induced diabetic rats. Current Eye Research. 2009;34(2):123–133. doi: 10.1080/02713680802650369. https://doi.org/10.1080/02713680802650369 [DOI] [PubMed] [Google Scholar]
- Livnah O., Johnson D.L., Stura E.A., Farrell F.X., Barbone F.P., You Y. An antagonist peptide–EPO receptor complex suggests that receptor dimerization is not sufficient for activation. Nature Structural Biology. 1998;5(11):993–1004. doi: 10.1038/2965. [DOI] [PubMed] [Google Scholar]
- Lockyer S., Okuyama K., Begum S., Le S., Sun B., Watanabe T. GPVI-deficient mice lack collagen responses and are protected against experimentally induced pulmonary thromboembolism. Thrombosis Research. 2006;118(3):371–380. doi: 10.1016/j.thromres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Lorenz H.M. T-cell-activation inhibitors in rheumatoid arthritis. BioDrugs. 2003;17(4):263–270. doi: 10.2165/00063030-200317040-00005. [DOI] [PubMed] [Google Scholar]
- Maharaj A.S., Walshe T.E., Saint-Geniez M., Venkatesha S., Maldonado A.E., Himes N.C. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. The Journal of Experimental Medicine. 2008;205(2):491–501. doi: 10.1084/jem.20072041. https://doi.org/10.1084/jem.20072041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso M.R., Davis R., Norberg S.M., O'Brien S., Sennino B., Nakahara T. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. The Journal of Clinical Investigation. 2006;116(10):2610–2621. doi: 10.1172/JCI24612. https://doi.org/10.1172/JCI24612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolios N., Bonifacino J.S., Klausner R.D. Transmembrane helical interactions and the assembly of the T cell receptor complex. Science. 1990;249(4966):274–277. doi: 10.1126/science.2142801. [DOI] [PubMed] [Google Scholar]
- Manolios N., Collier S., Taylor J., Pollard J., Harrison L.C., Bender V. T-cell antigen receptor transmembrane peptides modulate T-cell function and T cell-mediated disease. Nature Medicine. 1997;3(1):84–88. doi: 10.1038/nm0197-84. [DOI] [PubMed] [Google Scholar]
- Manolios N., Huynh N.T., Collier S. Peptides in the treatment of inflammatory skin disease. The Australasian Journal of Dermatology. 2002;43(3):226–227. doi: 10.1046/j.1440-0960.2002.00603.x. [DOI] [PubMed] [Google Scholar]
- Martin G.S., Mannino D.M., Eaton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. The New England Journal of Medicine. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. https://doi.org/10.1056/NEJMoa022139 [DOI] [PubMed] [Google Scholar]
- Metzger H. The high affinity receptor for IgE, FcepsilonRI. Novartis Foundation Symposium. 2004;257(59):51. discussion 59–64, 98–100, 276–185. [PubMed] [Google Scholar]
- Miura Y., Takahashi T., Jung S.M., Moroi M. Analysis of the interaction of platelet collagen receptor glycoprotein VI (GPVI) with collagen. A dimeric form of GPVI, but not the monomeric form, shows affinity to fibrous collagen. The Journal of Biological Chemistry. 2002;277(48):46197–46204. doi: 10.1074/jbc.M204029200. [DOI] [PubMed] [Google Scholar]
- Molloy P.E., Sewell A.K., Jakobsen B.K. Soluble T cell receptors: Novel immunotherapies. Current Opinion in Pharmacology. 2005;5(4):438–443. doi: 10.1016/j.coph.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Moriki T., Maruyama H., Maruyama I.N. Activation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domain. Journal of Molecular Biology. 2001;311(5):1011–1026. doi: 10.1006/jmbi.2001.4923. [DOI] [PubMed] [Google Scholar]
- Moroi M., Jung S.M. Platelet glycoprotein VI: Its structure and function. Thrombosis Research. 2004;114(4):221–233. doi: 10.1016/j.thromres.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Mulherin D., Fitzgerald O., Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis and Rheumatism. 1996;39(1):115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Akahoshi T., Aoki N., Toyomoto M., Miyasaka N., Kohsaka H. Intervention of an inflammation amplifier, triggering receptor expressed on myeloid cells 1, for treatment of autoimmune arthritis. Arthritis and Rheumatism. 2009;60(6):1615–1623. doi: 10.1002/art.24554. [DOI] [PubMed] [Google Scholar]
- Mutlu F.M., Sarici S.U. Treatment of retinopathy of prematurity: A review of conventional and promising new therapeutic options. International Journal of Ophthalmology. 2013;6(2):228–236. doi: 10.3980/j.issn.2222-3959.2013.02.23. https://doi.org/10.3980/j.issn.2222-3959.2013.02.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai A., Nakagawa E., Hatori K., Choi H.B., McLarnon J.G., Lee M.A. Generation and characterization of immortalized human microglial cell lines: Expression of cytokines and chemokines. Neurobiology of Disease. 2001;8(6):1057–1068. doi: 10.1006/nbdi.2001.0437. https://doi.org/10.1006/nbdi.2001.0437 [DOI] [PubMed] [Google Scholar]
- Nieswandt B., Brakebusch C., Bergmeier W., Schulte V., Bouvard D., Mokhtari-Nejad R. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. The EMBO Journal. 2001;20(9):2120–2130. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J., Azfer A., Zhelyabovska O., Fatma S., Kolattukudy P.E. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP) The Journal of Biological Chemistry. 2008;283(21):14542–14551. doi: 10.1074/jbc.M802139200. https://doi.org/10.1074/jbc.M802139200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Kabashima K. Advances in atopic dermatitis in 2015. The Journal of Allergy and Clinical Immunology. 2016;138(6):1548–1555. doi: 10.1016/j.jaci.2016.10.004. https://doi.org/10.1016/j.jaci.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Nourse A., Mittag T. The cytoplasmic domain of the T-cell receptor zeta subunit does not form disordered dimers. Journal of Molecular Biology. 2014;426(1):62–70. doi: 10.1016/j.jmb.2013.09.036. https://doi.org/10.1016/j.jmb.2013.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak N. New insights into the mechanism and management of allergic diseases: Atopic dermatitis. Allergy. 2009;64(2):265–275. doi: 10.1111/j.1398-9995.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- Oren H., Duman N., Abacioglu H., Ozkan H., Irken G. Association between serum macrophage colony-stimulating factor levels and monocyte and thrombocyte counts in healthy, hypoxic, and septic term neonates. Pediatrics. 2001;108(2):329–332. doi: 10.1542/peds.108.2.329. [DOI] [PubMed] [Google Scholar]
- Ortega E. How do multichain immune recognition receptors signal? A structural hypothesis. Molecular Immunology. 1995;32(13):941–945. doi: 10.1016/0161-5890(95)00070-u. [DOI] [PubMed] [Google Scholar]
- Ortega E., Schweitzer-Stenner R., Pecht I. Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast cells. The EMBO Journal. 1988;7(13):4101–4109. doi: 10.1002/j.1460-2075.1988.tb03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaro L., Felding J., Audouze K., Nielsen S.J., Terry R.B., Krog-Jensen C. Emerging classes of protein–protein interaction inhibitors and new tools for their development. Current Opinion in Chemical Biology. 2004;8(4):442–449. doi: 10.1016/j.cbpa.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Pelham C.J., Agrawal D.K. Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert Review of Clinical Immunology. 2014;10(2):243–256. doi: 10.1586/1744666X.2014.866519. https://doi.org/10.1586/1744666X.2014.866519 [DOI] [PubMed] [Google Scholar]
- Penfold P.L., Madigan M.C., Gillies M.C., Provis J.M. Immunological and aetiological aspects of macular degeneration. Progress in Retinal and Eye Research. 2001;20(3):385–414. doi: 10.1016/s1350-9462(00)00025-2. [DOI] [PubMed] [Google Scholar]
- Pieramici D.J., Rabena M.D. Anti-VEGF therapy: Comparison of current and future agents. Eye (Lond) 2008;22(10):1330–1336. doi: 10.1038/eye.2008.88. https://doi.org/10.1038/eye.2008.88 [DOI] [PubMed] [Google Scholar]
- Pike K.A., Baig E., Ratcliffe M.J. The avian B-cell receptor complex: Distinct roles of Igalpha and Igbeta in B-cell development. Immunological Reviews. 2004;197:10–25. doi: 10.1111/j.0105-2896.2004.0111.x. [DOI] [PubMed] [Google Scholar]
- Pitcher L.A., van Oers N.S. T-cell receptor signal transmission: Who gives an ITAM? Trends in Immunology. 2003;24(10):554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Posner R.G., Savage P.B., Peters A.S., Macias A., DelGado J., Zwartz G. A quantitative approach for studying IgE-FcepsilonRI aggregation. Molecular Immunology. 2002;38(16–18):1221–1228. doi: 10.1016/s0161-5890(02)00067-6. [DOI] [PubMed] [Google Scholar]
- Pribluda V.S., Pribluda C., Metzger H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(23):11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffer E.B., Pontrello J.K., Hollenbeck J.J., Kink J.A., Kiessling L.L. Activating B cell signaling with defined multivalent ligands. ACS Chemical Biology. 2007;2(4):252–262. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- Quintana F.J., Gerber D., Kent S.C., Cohen I.R., Shai Y. HIV-1 fusion peptide targets the TCR and inhibits antigen-specific T cell activation. The Journal of Clinical Investigation. 2005;115(8):2149–2158. doi: 10.1172/JCI23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaev S., Sun P.D. Structure and function of natural killer cell surface receptors. Annual Review of Biophysics and Biomolecular Structure. 2003;32:93–114. doi: 10.1146/annurev.biophys.32.110601.142347. [DOI] [PubMed] [Google Scholar]
- Read C.B., Kuijper J.L., Hjorth S.A., Heipel M.D., Tang X., Fleetwood A.J. Cutting Edge: Identification of neutrophil PGLYRP1 as a ligand for TREM-1. Journal of Immunology. 2015;194(4):1417–1421. doi: 10.4049/jimmunol.1402303. https://doi.org/10.4049/jimmunol.1402303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989;338(6214):383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Reth M., Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annual Review of Immunology. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- Robinson J.A. Design of protein–protein interaction inhibitors based on protein epitope mimetics. ChemBioChem. 2009;10(6):971–973. doi: 10.1002/cbic.200900055. [DOI] [PubMed] [Google Scholar]
- Rudd C.E. Disabled receptor signaling and new primary immunodeficiency disorders. The New England Journal of Medicine. 2006;354(18):1874–1877. doi: 10.1056/NEJMp068062. https://doi.org/10.1056/NEJMp068062 [DOI] [PubMed] [Google Scholar]
- Ryan D.P., Matthews J.M. Protein–protein interactions in human disease. Current Opinion in Structural Biology. 2005;15(4):441–446. doi: 10.1016/j.sbi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Sabroe I., Read R.C., Whyte M.K., Dockrell D.H., Vogel S.N., Dower S.K. Toll-like receptors in health and disease: Complex questions remain. Journal of Immunology. 2003;171(4):1630–1635. doi: 10.4049/jimmunol.171.4.1630. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Benham H., Cope A.P., Thomas R. T-cell receptor signaling and the pathogenesis of autoimmune arthritis: Insights from mouse and man. Immunology and Cell Biology. 2012;90(3):277–287. doi: 10.1038/icb.2012.4. https://doi.org/10.1038/icb.2012.4 [DOI] [PubMed] [Google Scholar]
- Sanchez-Mejorada G., Rosales C. Signal transduction by immunoglobulin fc receptors. Journal of Leukocyte Biology. 1998;63(5):521–533. doi: 10.1002/jlb.63.5.521. [DOI] [PubMed] [Google Scholar]
- Schamel W.W., Arechaga I., Risueno R.M., van Santen H.M., Cabezas P., Risco C. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. The Journal of Experimental Medicine. 2005;202(4):493–503. doi: 10.1084/jem.20042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk M., Bouchon A., Seibold F., Mueller C. TREM-1—Expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. The Journal of Clinical Investigation. 2007;117(10):3097–3106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G., Siveke J.T., Eckel F., Schmid R.M. Pancreatic cancer: Basic and clinical aspects. Gastroenterology. 2005;128(6):1606–1625. doi: 10.1053/j.gastro.2005.04.001. https://doi.org/S0016508505006323 [pii] [DOI] [PubMed] [Google Scholar]
- Schweitzer-Stenner R., Tamir I., Pecht I. Analysis of Fc(epsilon)RI-mediated mast cell stimulation by surface-carried antigens. Biophysical Journal. 1997;72(6):2470–2478. doi: 10.1016/S0006-3495(97)78891-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z.T., Sigalov A.B. SARS coronavirus fusion peptide-derived sequence suppresses collagen-induced arthritis in DBA/1J mice. Scientific Reports. 2016;6:28672. doi: 10.1038/srep28672. https://doi.org/10.1038/srep28672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z.T., Sigalov A.B. Rationally designed ligand-independent peptide inhibitors of TREM-1 ameliorate collagen-induced arthritis. J Cell Mol Med. 2017 doi: 10.1111/jcmm.13173. https://doi.org/10.1111/jcmm.13173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J.-Y., Yuan A., Chen J.J.-W., Yang P.-C. Tumor-associated macrophage: Its role in cancer invasion and metastasis. Journal of Cancer Molecules. 2006;2(3):101–106. [Google Scholar]
- Siegel R.M., Muppidi J.R., Sarker M., Lobito A., Jen M., Martin D. SPOTS: Signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. The Journal of Cell Biology. 2004;167(4):735–744. doi: 10.1083/jcb.200406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. Multichain immune recognition receptor signaling: Different players, same game? Trends in Immunology. 2004;25(11):583–589. doi: 10.1016/j.it.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Sigalov A. Multi-chain immune recognition receptors: Spatial organization and signal transduction. Seminars in Immunology. 2005;17(1):51–64. doi: 10.1016/j.smim.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Sigalov A.B. Immune cell signaling: A novel mechanistic model reveals new therapeutic targets. Trends in Pharmacological Sciences. 2006;27(10):518–524. doi: 10.1016/j.tips.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sigalov, A. B., inventor; University of Massachusetts, Boston, MA (US), assignee. (2007a). Inhibiting Collagen-induced Platelet Aggregation and Activation with Peptide Variants. US Patent US 8,278,271. 2012 Oct 2 (12/001,258).
- Sigalov A.B. Interaction between HIV gp41 fusion peptide and T cell receptor: Putting the puzzle pieces back together. The FASEB Journal. 2007;21(8):1633–1634. doi: 10.1096/fj.07-0603ltr. https://doi.org/10.1096/fj.07-0603ltr author reply 1635. [DOI] [PubMed] [Google Scholar]
- Sigalov A.B. More on: Glycoprotein VI oligomerization: A novel concept of platelet inhibition. Journal of Thrombosis and Haemostasis. 2007;5(11):2310–2312. doi: 10.1111/j.1538-7836.2007.02714.x. [DOI] [PubMed] [Google Scholar]
- Sigalov A.B., editor. Multichain immune recognition receptor signaling: From spatiotemporal organization to human disease. Springer-Verlag; New York: 2008. [PubMed] [Google Scholar]
- Sigalov A.B. Novel mechanistic concept of platelet inhibition. Expert Opinion on Therapeutic Targets. 2008;12(6):677–692. doi: 10.1517/14728222.12.6.677. [DOI] [PubMed] [Google Scholar]
- Sigalov A.B. SCHOOL model and new targeting strategies. Advances in Experimental Medicine and Biology. 2008;640:268–311. doi: 10.1007/978-0-387-09789-3_20. https://doi.org/10.1007/978-0-387-09789-3_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. Novel mechanistic insights into viral modulation of immune receptor signaling. PLoS Pathogens. 2009;5(7) doi: 10.1371/journal.ppat.1000404. https://doi.org/10.1371/journal.ppat.1000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. New therapeutic strategies targeting transmembrane signal transduction in the immune system. Cell Adhesion & Migration. 2010;4(2):255–267. doi: 10.4161/cam.4.2.10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. Protein intrinsic disorder and oligomericity in cell signaling. Molecular BioSystems. 2010;6(3):451–461. doi: 10.1039/B916030M. [DOI] [PubMed] [Google Scholar]
- Sigalov A.B. The SCHOOL of nature. I. Transmembrane signaling. Self Nonself. 2010;1(1):4–39. doi: 10.4161/self.1.1.10832. https://doi.org/10.4161/self.1.1.10832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. The SCHOOL of nature: II. Protein order, disorder and oligomericity in transmembrane signaling. Self Nonself. 2010;1(2):89–102. doi: 10.4161/self.1.2.11590. https://doi.org/10.4161/self.1.2.11590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. The SCHOOL of nature: III. From mechanistic understanding to novel therapies. Self Nonself. 2010;1(3):192–224. doi: 10.4161/self.1.3.12794. https://doi.org/10.4161/self.1.3.12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. The SCHOOL of nature: IV. Learning from viruses. Self Nonself. 2010;1(4):282–298. doi: 10.4161/self.1.4.13279. https://doi.org/10.4161/self.1.4.13279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. Transmembrane signaling: From understanding to action. Self Nonself. 2010;1(4):341–342. [Google Scholar]
- Sigalov A.B. Cells diversify transmembrane signaling through the controlled chaos of protein disorder. Self Nonself. 2011;2(2):75–79. doi: 10.4161/self.2.2.15756. https://doi.org/10.4161/self.2.2.15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. Cells diversify transmembrane signaling through the controlled chaos of protein disorder. Self Nonself. 2011;2(2) doi: 10.4161/self.2.2.15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. Uncoupled binding and folding of immune signaling-related intrinsically disordered proteins. Progress in Biophysics and Molecular Biology. 2011;106(3):525–536. doi: 10.1016/j.pbiomolbio.2011.08.005. https://doi.org/10.1016/j.pbiomolbio.2011.08.005 [DOI] [PubMed] [Google Scholar]
- Sigalov A.B. Evolution of immunity: No development without risk. Immunologic Research. 2012;52(3):176–181. doi: 10.1007/s12026-011-8256-4. https://doi.org/10.1007/s12026-011-8256-4 [DOI] [PubMed] [Google Scholar]
- Sigalov A.B. Interplay between protein order, disorder and oligomericity in receptor signaling. Advances in Experimental Medicine and Biology. 2012;725:50–73. doi: 10.1007/978-1-4614-0659-4_4. https://doi.org/10.1007/978-1-4614-0659-4_4 [DOI] [PubMed] [Google Scholar]
- Sigalov A.B. A novel ligand-independent peptide inhibitor of TREM-1 suppresses tumor growth in human lung cancer xenografts and prolongs survival of mice with lipopolysaccharide-induced septic shock. International Immunopharmacology. 2014;21(1):208–219. doi: 10.1016/j.intimp.2014.05.001. https://doi.org/10.1016/j.intimp.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B. Structural biology of intrinsically disordered proteins: Revisiting unsolved mysteries. Biochimie. 2016;125:112–118. doi: 10.1016/j.biochi.2016.03.006. https://doi.org/10.1016/j.biochi.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Sigalov A., Aivazian D., Stern L. Homooligomerization of the cytoplasmic domain of the T cell receptor zeta chain and of other proteins containing the immunoreceptor tyrosine-based activation motif. Biochemistry. 2004;43(7):2049–2061. doi: 10.1021/bi035900h. [DOI] [PubMed] [Google Scholar]
- Sigalov A.B., Aivazian D.A., Uversky V.N., Stern L.J. Lipid-binding activity of intrinsically unstructured cytoplasmic domains of multichain immune recognition receptor signaling subunits. Biochemistry. 2006;45(51):15731–15739. doi: 10.1021/bi061108f. https://doi.org/10.1021/bi061108f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov A.B., Zhuravleva A.V., Orekhov V.Y. Binding of intrinsically disordered proteins is not necessarily accompanied by a structural transition to a folded form. Biochimie. 2007;89(3):419–421. doi: 10.1016/j.biochi.2006.11.003. https://doi.org/10.1016/j.biochi.2006.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillerud L.O., Larson R.S. Design and structure of peptide and peptidomimetic antagonists of protein–protein interaction. Current Protein & Peptide Science. 2005;6(2):151–169. doi: 10.2174/1389203053545462. [DOI] [PubMed] [Google Scholar]
- Smith S.O., Smith C., Shekar S., Peersen O., Ziliox M., Aimoto S. Transmembrane interactions in the activation of the Neu receptor tyrosine kinase. Biochemistry. 2002;41(30):9321–9332. doi: 10.1021/bi012117l. [DOI] [PubMed] [Google Scholar]
- Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. Journal of Leukocyte Biology. 2009;86(5):1065–1073. doi: 10.1189/jlb.0609385. https://doi.org/10.1189/jlb.0609385 [DOI] [PubMed] [Google Scholar]
- Sun J.C., Lanier L.L. Natural killer cells remember: An evolutionary bridge between innate and adaptive immunity? European Journal of Immunology. 2009;39(8):2059–2064. doi: 10.1002/eji.200939435. https://doi.org/10.1002/eji.200939435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed R.S., Reid S.W., Li C., Cheetham J.C., Aoki K.H., Liu B. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395(6701):511–516. doi: 10.1038/26773. [DOI] [PubMed] [Google Scholar]
- Tamir I., Cambier J.C. Antigen receptor signaling: Integration of protein tyrosine kinase functions. Oncogene. 1998;17(11 Reviews):1353–1364. doi: 10.1038/sj.onc.1202187. [DOI] [PubMed] [Google Scholar]
- Tammaro A., Derive M., Gibot S., Leemans J.C., Florquin S., Dessing M.C. TREM-1 and its potential ligands in non-infectious diseases: From biology to clinical perspectives. Pharmacology & Therapeutics. 2017 doi: 10.1016/j.pharmthera.2017.02.043. https://doi.org/10.1016/j.pharmthera.2017.02.043 [DOI] [PubMed] [Google Scholar]
- Tarasova N.I., Rice W.G., Michejda C.J. Inhibition of G-protein-coupled receptor function by disruption of transmembrane domain interactions. The Journal of Biological Chemistry. 1999;274(49):34911–34915. doi: 10.1074/jbc.274.49.34911. [DOI] [PubMed] [Google Scholar]
- Tjomsland V., Spangeus A., Valila J., Sandstrom P., Borch K., Druid H. Interleukin 1alpha sustains the expression of inflammatory factors in human pancreatic cancer microenvironment by targeting cancer-associated fibroblasts. Neoplasia. 2011;13(8):664–675. doi: 10.1593/neo.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toogood P.L. Inhibition of protein–protein association by small molecules: Approaches and progress. Journal of Medicinal Chemistry. 2002;45(8):1543–1558. doi: 10.1021/jm010468s. [DOI] [PubMed] [Google Scholar]
- Uversky V.N., Dunker A.K. The case for intrinsically disordered proteins playing contributory roles in molecular recognition without a stable 3D structure. F1000 Biology Reports. 2013;5:1. doi: 10.3410/B5-1. https://doi.org/10.3410/B5-1 [1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bremen T., Dromann D., Luitjens K., Dodt C., Dalhoff K., Goldmann T. Triggering receptor expressed on myeloid cells-1 (Trem-1) on blood neutrophils is associated with cytokine inducibility in human E. coli sepsis. Diagnostic Pathology. 2013;8:24. doi: 10.1186/1746-1596-8-24. https://doi.org/10.1186/1746-1596-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul H.M., Pinedo H.M. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nature Reviews. Cancer. 2007;7(6):475–485. doi: 10.1038/nrc2152. https://doi.org/10.1038/nrc2152 [DOI] [PubMed] [Google Scholar]
- Veselovsky A.V., Archakov A.I. Inhibitors of protein–protein interactions as potential drugs. Current Computer-Aided Drug Design. 2007;3(1):51–58. [Google Scholar]
- Walker E.J., Ko A.H. Beyond first-line chemotherapy for advanced pancreatic cancer: An expanding array of therapeutic options? World Journal of Gastroenterology. 2014;20(9):2224–2236. doi: 10.3748/wjg.v20.i9.2224. https://doi.org/10.3748/wjg.v20.i9.2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.M., Djordjevic J.T., Bender V., Manolios N. T cell antigen receptor (TCR) transmembrane peptides colocalize with TCR, not lipid rafts, in surface membranes. Cellular Immunology. 2002;215(1):12–19. doi: 10.1016/s0008-8749(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Wang X.M., Djordjevic J.T., Kurosaka N., Schibeci S., Lee L., Williamson P. T-cell antigen receptor peptides inhibit signal transduction within the membrane bilayer. Clinical Immunology. 2002;105(2):199–207. doi: 10.1006/clim.2002.5270. [DOI] [PubMed] [Google Scholar]
- Wang F., Liu S., Wu S., Zhu Q., Ou G., Liu C. Blocking TREM-1 signaling prolongs survival of mice with Pseudomonas aeruginosa induced sepsis. Cellular Immunology. 2012;272(2):251–258. doi: 10.1016/j.cellimm.2011.10.006. https://doi.org/10.1016/j.cellimm.2011.10.006 [DOI] [PubMed] [Google Scholar]
- Weber B., Schuster S., Zysset D., Rihs S., Dickgreber N., Schurch C. TREM-1 deficiency can attenuate disease severity without affecting pathogen clearance. PLoS Pathogens. 2014;10(1) doi: 10.1371/journal.ppat.1003900. https://doi.org/10.1371/journal.ppat.1003900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh T.J., Green R.H., Richardson D., Waller D.A., O'Byrne K.J., Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. Journal of Clinical Oncology. 2005;23(35):8959–8967. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- Won J., Lee G.H. T-cell-targeted signaling inhibitors. International Reviews of Immunology. 2008;27(1–2):19–41. doi: 10.1080/08830180701798976. https://doi.org/10.1080/08830180701798976 [DOI] [PubMed] [Google Scholar]
- Wu J., Cherwinski H., Spies T., Phillips J.H., Lanier L.L. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells. The Journal of Experimental Medicine. 2000;192(7):1059–1068. doi: 10.1084/jem.192.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yako Y.Y., Kruger D., Smith M., Brand M. Cytokines as biomarkers of pancreatic ductal adenocarcinoma: A systematic review. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154016. https://doi.org/10.1371/journal.pone.0154016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S., Ishikawa E., Kohno M., Saito T. The quantity and duration of FcRgamma signals determine mast cell degranulation and survival. Blood. 2004;103(8):3093–3101. doi: 10.1182/blood-2003-08-2944. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kobayashi Y., Nakama T., Zhou Y., Ishikawa K., Arita R. Increased expression of M-CSF and IL-13 in vitreous of patients with proliferative diabetic retinopathy: Implications for M2 macrophage-involving fibrovascular membrane formation. The British Journal of Ophthalmology. 2015;99(5):629–634. doi: 10.1136/bjophthalmol-2014-305860. https://doi.org/10.1136/bjophthalmol-2014-305860 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Yoshida A., Ishibashi T., Elner S.G., Elner V.M. Role of MCP-1 and MIP-1alpha in retinal neovascularization during postischemic inflammation in a mouse model of retinal neovascularization. Journal of Leukocyte Biology. 2003;73(1):137–144. doi: 10.1189/jlb.0302117. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Knolhoff B.L., Meyer M.A., Nywening T.M., West B.L., Luo J. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Research. 2014;74(18):5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. https://doi.org/10.1158/0008-5472.CAN-13-3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzalla G., Thurston D.E. Targeting protein–protein interactions for therapeutic intervention: A challenge for the future. Future Medicinal Chemistry. 2009;1(1):65–93. doi: 10.4155/fmc.09.12. https://doi.org/10.4155/fmc.09.12 [DOI] [PubMed] [Google Scholar]
Further Reading
- Sigalov A.B. Transmembrane interactions as immunotherapeutic targets: Lessons from viral pathogenesis. Advances in Experimental Medicine and Biology. 2007;601:335–344. doi: 10.1007/978-0-387-72005-0_36. [DOI] [PubMed] [Google Scholar]
- Sigalov A.B. Signaling chain homooligomerization (SCHOOL) model. Advances in Experimental Medicine and Biology. 2008;640:121–163. doi: 10.1007/978-0-387-09789-3_12. [DOI] [PubMed] [Google Scholar]