Abstract
Background
MERS-CoV emerged as a zoonotic disease in Saudi Arabia with 1437 cases as of July 2016. This study aimed at describing the epidemiology of MERS-CoV infection, clinical aspects of the disease and the determinants of survival.
Methods
The medical records of Prince Mohamed Bin Abdulaziz Hospital were reviewed between April 2014 and December 2015 to identify admission and discharge with MERS-CoV. Patient’s characteristics, epidemiologic and clinical data and laboratory results were extracted and described. Logistic regression analyses were used to model the determinants of the survival of these patients. Significance of the results were judged at the 5% level.
Results
249 confirmed cases were admitted mostly in August (20.48%) and September (14.86%) of the year 2015. A third (39.36%) reported contact with an index case, developed the disease after 6.2 days and continued to shed the virus for 13.17 days on average. The case fatality rate was 20.08%. Independent predictors of being discharged alive among confirmed cases were younger age (ORA = 0.953), breathing ambient air (ORA = 8.981), not being transferred to the ICU (ORA = 24.240) and not receiving renal replacement therapy (ORA = 8.342). These variables explain 63.9% of the variability of patients’ status at discharge.
Conclusion
MERS-CoV spread from human-to-human as community acquired and nosocomial infection. The study identified high risk patients in need for special medical attention in order to improve patients’ outcome.
Keywords: MERS-CoV, Epidemiology, Determinants of survival, Saudi Arabia
Introduction
Middle East Respiratory Syndrome Coronavirus (MERS-CoV) emerged as a fatal respiratory infection with epidemic potentials. Since the identification of the first case in June 2012, Saudi Arabia continues to bear the greatest burden of the disease. As of July 2016, 1437 confirmed cases were reported from Saudi Arabia which represent 85% of the global reported cases [1].
MERS-CoV is a known zoonosis with evidence supporting the role of bats and camels as reservoir of infection [2]. Previous studies suggest that MERS-CoV has been circulating for decades as an endemic disease in dromedary camels without large scale fatalities among humans in countries of the Horn of Africa [3], [4] and the Middle East [5], [6], [7], [8] including Saudi Arabia [5], [6]. There is a strong belief that MERS-CoV originated in bats as it is closely related to other β coronaviruses affecting bats [9]. The fact that bats exposed to MERS-CoV through intranasal and intraperitoneal routes shed the virus in respiratory secretions without developing signs of the disease [10] provide evidence of bats being the natural reservoir of infection [2].
The similarity of the genomic sequence of dromedary MERS-CoV and that of the virus affecting humans provides enough evidence that dromedaries are the natural host of the virus [11]. However, most of MERS-CoV infections have occurred through human-to-human contact [12], [13] among household contacts in close association with cases [14], [15], [16] and health workers caring for patients [14], [16], [17] without any evidence of spread into the community [16].
The circumstances and modes of transmission resulting in the spread of MERS-CoV to human population in 2012 have not been fully investigated [18] and the World Health Organization urged affected countries to launch epidemiologic investigations for better understanding of the patterns of transmission. This study was conducted to describe the epidemiology of MERS-CoV and to identify the rate and determinants of survival among confirmed cases.
Methods
Research setting
The study was conducted in Prince Mohamed Bin Abdulaziz Hospital (PMAH) affiliated to the Ministry of Health. It is a tertiary hospital in Riyadh city designated as a referral site and a center of excellence for the management of cases of MERS-CoV in Riyadh region since April 2014. Suspected cases are received in a MERS-CoV triage area in the emergency department from where individuals with suggestive signs and symptoms are channeled to an isolation room with respiratory precautions then to an isolation ward or intensive care unit with negative pressure.
Participants and data extraction
The electronic medical records (CERNER) has been reviewed between April 2014 and December 2015 to identify all admission and discharge diagnosis with “MERS-CoV”. Data have been extracted and transferred to an electronic transfer sheet designed using the Epidemiologic Statistics for Public Health Software (Epi-Info ver.7) including patient’s demographic characteristics, epidemiologic and clinical data as well as laboratory results.
A “confirmed case” is a patient in whom the virus has been identified by Polymerase Chain Reaction (PCR) in a specimen obtained from the nasopharyngeal, throat or endotracheal tube aspirate. A “suspected case” is a patient presenting with acute febrile respiratory illness with clinical, radiological, or histopathological evidence of pulmonary parenchymal disease including pneumonia or acute respiratory distress syndrome; and epidemiologically linked to a confirmed MERS-CoV case; and the testing for MERS-CoV is unavailable, negative on a single inadequate specimen or inconclusive [19]. Suspected cases were excluded from this study.
Data analysis
Data have been transferred from the Epidemiologic Statistics for Public Health Software (Epi-Info ver.7.1.5.0) to the Statistical Package for Social Sciences (SPSS, ver. 21) for analysis. Data have been summarized using number and percentage as well as mean and standard deviation and presented in suitable tables. Univariate and multivariate logistic regression analyses were used to model the determinants of survival of confirmed cases as a function of epidemiologic and clinical data. Significance of the obtained results was judged at the 5% level.
Ethical considerations
Ethical approval is weaved as the study doesn’t involve obtaining data directly from human subjects. Approval for reviewing hospital records and retrieving patients’ data has been obtained from Ministry of Health and the director of PMAH. A unique identifier (ID) has been assigned to each patient to ensure anonymity. Data have been stored in a secure manner accessible only to the research team.
Results
Epidemiology of MERS-CoV: person, place and time
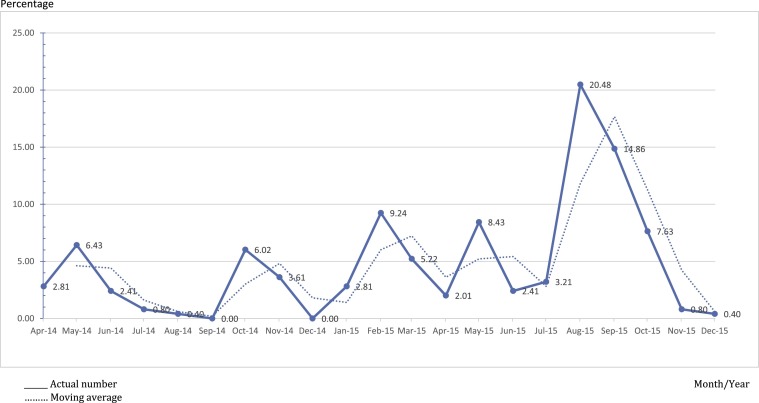
Between April 2014 and December 2015, MERS-COV was confirmed among 30.63% (n = 249) of the patients with an admission diagnosis of MERS-CoV (N = 813 patients). Almost a quarter of confirmed cases (22.49%) were admitted to PMAH between April and December 2014, mostly in May (6.43%) and October (6.02%). During the year 2015, the largest proportions of cases were admitted in August (20.48%) and September (14.86%) then decline to reach 0.40% in December of the same year (Fig. 1 ).
Fig. 1.
Percentage of confirmed cases of MERS-CoV admitted to PMAH between April 2014 and December 2015.
The majority of confirmed MERS-CoV cases were non-healthcare workers (85.14%) and dwellers of Riyadh region (96.39%). More than half of these cases were male (57.03%) and Saudi nationals (58.63%). One third of non-Saudi patients (n = 35; 33.98%) were from Philippines. The age of the patients ranged from 9 months to 93 years (46.71 ± 17.92 years). The highest proportion of cases were in the age group of 50 to less than 60 years (22.09%) followed by those in the age group of 20 to less than 30 years (18.07%) and 30 to less than 40 years (17.27%). About two thirds (64.26%) of the patients reported co-morbidities. Most of these co-morbidities were cardiovascular diseases (85.62%), diabetes mellitus (61.25%), lung diseases (16.25%), chronic renal diseases (11.25%) in addition to less frequent medical conditions.
History of exposure and development of symptoms
Exposure to a potential human source of infection was given by 39.36% (n = 98) of the cases including the exposure to confirmed MERS-CoV cases in the household or in the workplace (n = 71; 72.45%) or in the healthcare setting (n = 44; 44.90%). Only 5 patients reported contact with livestock; 3 were in contact with camels while 2 were in contact with goats or sheep.
Information on the time between exposure to a potential human source of infection and the development of the first symptoms was available for 18 out of the 98 cases who reported an exposure to confirmed or suspected cases of MERS-CoV. These cases reported the occurrence of the first symptom after a period ranging from 1 to 14 days with an average of 6.27 + 4.35 days. The time between the development of symptoms and a negative swab for the virus was available for 167 cases. A negative swab was obtained after a period ranging from 2 to 40 days of the symptoms with a mean of 13.17 + 8.06 days.
Clinical profile
Table 1 shows that 83.53% of the patients reported symptoms of the disease, the commonest were fever (84.62%), cough (73.08%) and shortness of breath (50.96%). Nearly three quarters of the patients (73.89%) visited healthcare facilities for their symptoms and 55.82% were admitted to other hospitals before presenting to PMAH.
Table 1.
Distribution of confirmed MERS-CoV cases admitted to PMAH (2014–2015) according to the reported symptoms, respiratory rate, oxygen saturation and creatinine level.
| Symptoms, respiration and creatinine level | No. | % |
|---|---|---|
| Reported symptoms | ||
| No | 41 | 9.64 |
| Yesa | 208 | 83.53 |
| Fever | 176 | 84.62 |
| Cough | 152 | 73.08 |
| Shortness of breath | 106 | 50.96 |
| Sore throat | 36 | 17.31 |
| Diarrhea | 33 | 15.87 |
| Head and body aches | 23 | 11.06 |
| Vomiting | 19 | 9.13 |
| Chest pain/tightness | 10 | 4.81 |
| Running nose | 10 | 4.81 |
| Altered conscious/confusion | 6 | 2.88 |
| Sweating | 6 | 2.88 |
| Abdominal pain | 5 | 2.40 |
| Weakness/fatigue | 3 | 1.44 |
| Dizziness | 3 | 1.44 |
| Loss of appetite | 2 | 0.96 |
| Shivering | 1 | 0.48 |
| Othersb | 6 | 2.88 |
| Respiratory rate (breath/minute)c | ||
| Within normal range | 12 | 4.82 |
| Above normal range | 237 | 95.18 |
| Oxygen saturation (%)c | ||
| Within normal range | 160 | 64.26 |
| Below normal range | 89 | 35.74 |
| Creatinine (umol/L)c | ||
| Within normal range | 161 | 64.66 |
| Below normal range | 31 | 12.45 |
| Above normal range | 57 | 22.89 |
Categories are not mutually exclusive.
Others include seizures, dysuria, epistaxis and cyanosis.
Normal range based on the provided laboratory figures.
The majority of patients (95.18%) had a respiratory rate above the normal range and 35.74% had oxygen saturation below the normal range. Creatinine level was above the normal range for 22.89% of patients. Chest radiography was performed to 96.79% of the patients and abnormalities were detected among 63.07% of them including consolidation (n = 138; 90.79%), atelectasis (n = 7; 4.61%) and pleural effusion (n = 7; 4.61%) (Table 1).
In the emergency room, more than two thirds of the patients were in a conscious and stable condition (71.08%). More than half of the patients were breathing room air (55.42%) while 30.52% were on oxygen mask and 14.06% were on mechanical ventilator (Table 2 ).
Table 2.
Distribution of confirmed MERS-CoV cases admitted to PMAH (2014–2015) according to their general condition and care provided.
| Condition and care provided | No. | % |
|---|---|---|
| General condition | ||
| Conscious and stable | 177 | 71.08 |
| Conscious and unstable | 37 | 14.86 |
| On ventilator and stable | 27 | 10.84 |
| On ventilator and unstable | 8 | 3.21 |
| Mode of respiration | ||
| Ambient air | 138 | 55.42 |
| Oxygen mask | 76 | 30.52 |
| Mechanical ventilator | 35 | 14.06 |
| Chest radiography | ||
| Not performed | 8 | 3.21 |
| Performed | 241 | 96.79 |
| Normal | 89 | 36.93 |
| Abnormality detected | 152 | 63.07 |
| Transferred to ICU | ||
| No | 140 | 56.22 |
| Yes | 109 | 43.78 |
| Placed on mechanical ventilator in ICU | ||
| No | 173 | 69.48 |
| Yes | 76 | 30.52 |
| Renal problems due to MERS-CoV | ||
| No | 219 | 87.95 |
| Yes | 30 | 12.05 |
| Receiving continuous renal replacement therapy (CRRT) | ||
| No | 216 | 86.75 |
| Yes | 33 | 13.25 |
Less than half of the patients (43.78%) were transferred to the intensive care unit (ICU) and stayed for a duration ranging from 1 to 64 days with a mean of 15.60 ± 15.30 days. Only 30.52% of those patients admitted to ICU were placed on mechanical ventilator. The duration of stay on mechanical ventilator ranged from 0.5 to 104 days with a mean of 18.48 ± 18.51 days. Two patients received extracorporeal membrane oxygenation (ECMO); one for 5 days and the second for 21 days. Only 12.05% of confirmed MERS-CoV developed secondary renal problems and 13.25% of the patients were on continuous renal replacement therapy (CRRT) for an average duration of 11.48 ± 11.48 days (Table 2).
Most of the patients (89.96%) who were presented to the emergency room were admitted on the same day. Only 10.04% were managed in the emergency room for a day (72.00%) or two (12.00%) or longer (16.00%). The duration of hospital stay ranged from 0.5 to 90 days with a mean of 15.21 ± 14.24 days. One third of the patients (33.87%) stayed between 8 to 14 days.
More than two thirds (67.47%) of the confirmed MERS-CoV cases were discharged alive with a full recovery, few were discharged alive with tracheostomy, renal or pulmonary problems (9.24%) or against medical advice (3.21%) while 20.08% were discharged dead.
Determinants of being discharged alive among patients with confirmed MERS-CoV infection
The likelihood of being discharged alive was significantly higher among non-Saudi patients (OR = 2.350; 95%CI = 1.178, 4.689), those with no co-morbidities (OR = 6.574; 95%CI = 2.503, 17.269) and those working in healthcare setting (OR = 10.822; 95%CI = 1.447, 80.970). On the other hand, older patients were less likely to be discharged alive relative to younger ones (OR = 0.959; 95%CI = 0.941, 0.978) (Table 3 ).
Table 3.
Probability of being discharged alive among confirmed MERS-CoV cases admitted to PMAH (2014–2015) in relation to their demographic characteristics and clinical status.
| Demographic characteristic and clinical status | Discharge status |
Odds ratio | 95% CI | |||
|---|---|---|---|---|---|---|
| Alive (n = 199) |
Dead (n = 50) |
|||||
| No | % | No | % | |||
| Sex | ||||||
| Malea | 108 | 54.27 | 34 | 68.00 | 1.789 | 0.929–3.448 |
| Female | 91 | 45.73 | 16 | 32.00 | ||
| Nationality | ||||||
| Saudia | 109 | 54.77 | 37 | 74.00 | 2.350 | 1.178–4.689 |
| Non-Saudi | 90 | 45.23 | 13 | 26.00 | ||
| Working in healthcare setting | ||||||
| Noa | 163 | 81.91 | 49 | 98.00 | 10.822 | 1.447–80.970 |
| Yes | 36 | 18.09 | 1 | 2.00 | ||
| Presence of co-morbidities | ||||||
| Yesa | 115 | 57.79 | 45 | 90.00 | 6.574 | 2.503–17.269 |
| No | 84 | 42.21 | 5 | 10.00 | ||
| Previous visit to health facility | ||||||
| Yesa | 138 | 69.35 | 46 | 92.00 | 5.083 | 1.752–14.749 |
| No | 61 | 30.65 | 4 | 8.00 | ||
| Prior hospital admission | ||||||
| Yesa | 96 | 48.24 | 43 | 86.00 | 6.591 | 2.829–15.356 |
| No | 103 | 51.76 | 7 | 14.00 | ||
| General condition in the ER | ||||||
| On ventilator (stable/unstable)a | 14 | 7.04 | 21 | 42.00 | 1 | |
| Conscious and unstable | 19 | 9.55 | 18 | 36.00 | 1.583 | 0.622–4.030 |
| Conscious and stable | 166 | 83.42 | 11 | 22.00 | 22.636 | 9.104–56.286 |
| Age in years | ||||||
| ± s | 44.26 ± 17.22 | 56.68 ± 17.36 | 0.959 | 0.941–0.978 | ||
| Min–max | 0.75–85 | 21–93 | ||||
| Median | 44 | 57 | ||||
| Duration of hospital stay (days) | ||||||
| ± s | 14.60 ± 13.76 | 17.60 ± 15.81 | 0.987 | 0.968–1.006 | ||
| Min–max | 2–90 | 1–72 | ||||
| Median | 11 | 14 | ||||
| Mode of respiration | ||||||
| Mechanical ventilatora | 14 | 7.04 | 21 | 42.00 | 1 | |
| Oxygen mask | 50 | 25.13 | 26 | 52.00 | 2.885 | 1.263–6.588 |
| Ambient air | 135 | 67.84 | 3 | 6.00 | 67.500 | 17.869–254.973 |
| Oxygen saturation (%) | ||||||
| Below normal rangea | 59 | 29.65 | 30 | 60.00 | 3.559 | 1.872–6.766 |
| Within normal range | 140 | 70.35 | 20 | 40.00 | ||
| Respiratory rate (breath/min) | ||||||
| Above normal rangea | 192 | 96.48 | 45 | 90.00 | 0.328 | 0.099–1.081 |
| Within normal range | 7 | 3.52 | 5 | 10.00 | ||
| Creatinine (umol/L) | ||||||
| Above normal rangea | 31 | 15.58 | 26 | 52.00 | 1 | |
| Below normal range | 25 | 12.56 | 6 | 12.00 | 3.495 | 1.245–9.811 |
| Within normal range | 143 | 71.86 | 18 | 36.00 | 6.663 | 3.258–13.627 |
| Chest radiography | ||||||
| Abnormality detecteda | 107 | 53.77 | 45 | 90.00 | 7.738 | 2.948–20.312 |
| No abnormality detected | 92 | 46.23 | 5 | 10.00 | ||
| Transferred to ICU | ||||||
| Yesa | 60 | 30.15 | 49 | 98.00 | 113.517 | 15.319–841.198 |
| No | 139 | 69.85 | 1 | 2.00 | ||
| Placed on mechanical ventilator | ||||||
| Yesa | 29 | 14.57 | 47 | 94.00 | 91.840 | 26.800–314.762 |
| No | 170 | 85.43 | 3 | 6.00 | ||
| Receiving continuous renal replacement therapy (CRRT) | ||||||
| Yesa | 8 | 4.02 | 25 | 50.00 | 23.880 | 9.720–58.645 |
| No | 191 | 95.98 | 25 | 50.00 | ||
| Renal problems due to MERS-CoV | ||||||
| Yesa | 9 | 4.52 | 21 | 42.00 | 15.290 | 6.385–36.606 |
| No | 190 | 95.48 | 29 | 58.00 | ||
Reference category.
The likelihood of being discharged alive was significantly higher among patients who neither visited a health facility (OR = 5.083; 95%CI = 1.752, 14.749) nor had a prior hospital admission (OR = 6.591; 95%CI = 2.829, 15.356) before presenting to PMAH (Table 3).
The probability of being discharged alive was significantly higher among patients who were conscious and stable (OR = 22.636; 95%CI = 9.104, 56.286). The likelihood of being discharged alive was significantly higher among patients who were breathing ambient room air (OR = 67.500; 95%CI = 17.869, 254.973), those on oxygen mask (OR = 2.885; 95%CI = 1.263, 6.588) as well as those with oxygen saturation within the normal range (OR = 3.559; 95%CI = 1.872, 6.766) (Table 3).
Patients who had a creatinine level within the normal range had significantly higher likelihood of being discharged alive (OR = 6.663; 95%CI = 3.258, 13.627). The likelihood of being discharged alive was higher for patients with no abnormalities detected in chest radiography (OR = 7.738; 95%CI = 2.948, 20.312) (Table 3).
Patients who were not transferred to the ICU were significantly more likely to be discharged alive (OR = 113.517; 95%CI = 15.319, 841.198). Similarly, those who were not placed on mechanical ventilator were significantly more likely to be discharged alive relative to those who were on mechanical ventilator (OR = 91.840; 95%CI = 26.800, 314.762). Patients who did not develop renal problems due to MERS-CoV infection (OR = 15.290; 95%CI = 6.385, 36.606) and those who did not receive continuous renal replacement therapy (OR = 23.880; 95%CI = 9.720, 58.645) were significantly more likely to be discharged alive (Table 3).
The status of being discharged alive was modelled as a function of the significant predictors obtained by the univariate analysis with the exclusion of one variable namely being on mechanical ventilator as it was correlated with the mode of respiration. The constructed model had an overall sensitivity of 90.0%; it classified correctly 95% of patients who were discharged alive and 70% of the patients who were discharged dead.
The independent predictors of being discharged alive following MERS-CoV infection were younger age, breathing ambient air, not being transferred to the ICU and not receiving continuous renal replacement therapy. The model explained 63.9% (Nagelkerke R2 = 0.639) of the variability of patients’ status at discharge (Table 4 ).
Table 4.
Independent predictors of being discharged alive among confirmed MERS-CoV cases admitted to PMAH (2014–2015).
| Independent predictors | B | P-value | Adjusted OR | 95% CI |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age (years) | −0.48 | 0.001 | 0.953 | 0.927 | 0.980 |
| Mode of respiration | |||||
| Mechanical ventilatora | |||||
| Ambient air | 2.195 | 0.006 | 8.981 | 1.901 | 42.419 |
| Transferred to the ICU | |||||
| Yesa | |||||
| No | 3.188 | 0.003 | 24.240 | 2.909 | 201.975 |
| Receiving continuous renal replacement therapy (CRRT) | |||||
| Yesa | |||||
| No | 2.121 | 0.000 | 8.342 | 2.874 | 24.217 |
| Constant | 0.790 | 0.315 | 2.203 | ||
Nagelkerke R square = 0.639.
Reference category.
Discussion
In response to the fatal MERS-CoV epidemic, all cases with a sudden onset of respiratory or gastrointestinal symptoms in Riyadh region were referred to PMAH for laboratory confirmation and management. As a result, only 30.63% of patients with an admission diagnosis of MERS-CoV between April 2014 and December 2015 tested positive for the virus by PCR. Previous studies reported that cases of MERS-CoV could present with respiratory symptoms [20], [21] or gastrointestinal symptoms [20], [22]. Most of the confirmed cases in this study presented with symptoms of respiratory tract infection including fever, cough and shortness of breath rather than gastrointestinal symptoms such as diarrhea, vomiting and abdominal pain. Almost all admitted patients were isolated in a room with negative pressure (93.17%) or in a single room (5.22%) to reduce the risk of nosocomial infection. Among cases in this study, only 14.86% were healthcare workers which can be attributed to the stringent infection control procedures applied in all healthcare facilities in response to the MERS-CoV epidemic in Saudi Arabia.
The analysis of the rate of admission of confirmed cases in relation to the month of the year did not reveal any seasonal pattern. However, the rates of admitted cases were the lowest during December 2014 and 2015 while the highest were in August 2015. Probably the high ambient temperature and low relative humidity during the month of August have enhanced the spread of the infection among the population as postulated by Alghamdi et al. [23].
It has been postulated that the behavior of MERS-CoV changed due to mutation with subsequent ability to spread from human-to-human [14], [15], [17]. In fact, several investigations reported the contact of confirmed MERS-CoV cases with an index case [12], [13], [16], [24]. In this study, 39.36% of the cases reported being in contact with a confirmed or suspected case of MERS-CoV either inside or outside the healthcare facilities and in 72.45% of these instances, the contact was with a confirmed MERS-CoV case. Despite the mounting evidence of the role of camels in the infectious cycle of MERS-CoV [6], [7], [8], [11], Saad et al. [25] reported rare contact with animals among MERS-CoV cases in Saudi Arabia. In this study, only 5 cases reported contact with farm animals and history of contact with camels was given by three of them. However, the importance of camels in the spread of the infection cannot be ruled out in view of the missed information for almost half of the cases.
MERS-CoV cases who reported contact with a confirmed or suspected MERS-CoV case developed the disease after an average duration of 6.2 days which is similar to the average incubation period of 6 days reported by a previous study [26]. The range of incubation period of 1–14 days observed among cases in this study is similar to the one reported by the Centers for Disease Control and prevention [27] and the longest incubation period of 14 days is the duration recommended by the World Health Organization to monitor travelers returning from the Middle East [28]. In line with previous report [29], cases in this study were able to transmit the infection for an average duration of 13.17 days which is extended to 40 days in some cases.
Among patients in this study, the case fatality rate of 20.08% was lower than the 29.8% reported from Saudi Arabia between 2014 and 2016 [30] as well as the global reported rate of 35% [1], [31]. Ahmed [30] noted the recent decrease in mortality rate among confirmed MERS-CoV cases in Saudi Arabia and attributed the improvement of patients’ outcome to the adopted prevention and control policies as well as the attention given to case management. The severity of MRS-CoV ranges from mild to fatal illness [20] and can progress rapidly to severe respiratory distress and death [32], [33]. Respiratory distress was noted in a proportion of MERS-CoV patients at the time they presented to the emergency department; 14.06% were on mechanical ventilation and 30.52% were breathing through oxygen mask. Condition of 43.78% of the patients required care in intensive care unit and 30.52% of them required mechanical ventilation. Lung lesions were detected in the chest radiography of nearly two-thirds of the patients, the commonest was pulmonary consolidation. Moreover, 12.05% developed renal problems that required continuous renal replacement therapy. All these factors were taken as a proxy of disease severity and was found to affect significantly the probability of these patients to be discharged alive. Most probably patients who did not visit any health facility and those who were not hospitalized before being admitted to PMAH had milder form of illness and consequently higher probability of being discharged alive.
In this study, the need for mechanical ventilation independently predicted mortality which is in accordance with previous reports [20], [34]. Furthermore, patients with normal oxygen saturation levels were more likely to be discharged alive. Only two of the patients in this study required extracorporeal membrane oxygenation (ECMO) which is similar to the number of patients in the study of Al-Hameed et al. [32]. Arabi et al. [35] reported that, the presence of co-morbidities may prevent the use of extracorporeal membrane oxygenation (ECMO) though needed by the patients.
Co-morbid conditions were documented in the records of 64.26% of MERS-COV patients enrolled in this study. These diseases tend to affect older patients resulting in lower immunity which in turn leads to severe MERS-CoV infection and lower likelihood of survival as shown by this study and previous ones [23], [36]. In fact, younger age independently predicted survival after controlling for the effect of co-morbidities as well as other determinants of survival. The higher probability of survival among younger patients was also reported by Saad et al. [25] and others [22], [23]. Previous studies attributed the low risk of mortality from MERS-CoV among healthcare workers to the fact of being younger [32] and healthy with no history of any co-morbid conditions [32]. Also, their awareness of the risk of exposure may have prompted them to seek early diagnosis and treatment.
Of all studies conducted in Saudi Arabia, the number of confirmed cases of MERS-CoV included in this study is the largest. The accumulation of MERS-CoV cases over the period of 21 months provided a unique opportunity to describe the epidemiologic pattern, clinical aspects and outcome of the disease in Riyadh region. The data compiled from the electronic medical records was used as well to estimate the case fatality rate and to identify the predictors of survival. This information may be used by healthcare providers to identify high risk patients in need for special medical attention in order to improve patients’ outcome and survival. Nonetheless, the study has a number of limitation inherent to the retrospective charts review. As hospital charts are mainly used for the clinical management and follow up of cases, epidemiologic data necessary for the description of cases in relation to person, place and time were either missing or incomplete. The data provided incomplete information pertinent to the exposure to potential human and animal source of infection, incubation period of the disease as well as period of communicability.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgments
The authors are grateful to the Deanship of Scientific Research, king Saud University for funding through Vice Deanship of Scientific Research Chairs.
References
- 1.World Health Organization . WHO; 2015. Middle East respiratory syndrome coronavirus (MERS-CoV). Fact sheet N°401. Available from: http://www.who.int/mediacentre/factsheets/mers-cov/en/. [Last Accessed 12 August 2016] [Google Scholar]
- 2.Han H.J., Yu H., Yu X.J. Evidence for zoonotic origins of Middle East respiratory syndrome coronavirus. J Gen Virol. 2016;97(2):274–280. doi: 10.1099/jgv.0.000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller M.A., Corman V.M., Jores J., Meyer B., Younan M., Liljander A. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983–1997. Emerg Infect Dis. 2014;20(12):2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reusken C.B., Messadi L., Feyisa A., Ularamu H., Godeke G.J., Danmarwa A. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg Infect Dis. 2014;20(8):1370–1374. doi: 10.3201/eid2008.140590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemida M.G., Perera R.A., Al Jassim R.A., Kayali G., Siu L.Y., Wang P. Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveill. 2014;19(23) doi: 10.2807/1560-7917.es2014.19.23.20828. PMC4674219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5(2) doi: 10.1128/mBio.00884-14. e00884–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reusken C.B., Haagmans B.L., Muller M.A., Gutierrez C., Godeke G.J., Meyer B. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farag E.A., Reusken C.B., Haagmans B.L., Mohran K.A., Stalin Raj V., Pas S.D. High proportion of MERS-CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect Ecol Eepidemiol. 2015;5:28305. doi: 10.3402/iee.v5.28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohd H.A., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir. Virol J. 2016;13(1) doi: 10.1186/s12985-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munster V.J., Adney D.R., van Doremalen N., Brown V.R., Miazgowicz K.L., Milne-Price S. Replication and shedding of MERS-CoV in Jamaican fruit bats (Artibeus jamaicensis) Sci Rep. 2016;6 doi: 10.1038/srep21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemida M.G., Chu D.K., Poon L.L., Perera R.A., Alhammadi M.A., Ng H.Y. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis. 2014;20(7):1231–1234. doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arwady M.A., Alraddadi B., Basler C., Azhar E.I., Abuelzein E., Sindy A.I. Middle East respiratory syndrome coronavirus transmission in extended family, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22(8):1395–1402. doi: 10.3201/eid2208.152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balkhy H.H., Alenazi T.H., Alshamrani M.M., Baffoe-Bonnie H., Al-Abdely H.M., El-Saed A. Notes from the field: nosocomial outbreak of Middle East respiratory syndrome in a large tertiary care hospital—Riyadh, Saudi Arabia, 2015. MMWR. 2016;65(6):163–164. doi: 10.15585/mmwr.mm6506a5. [DOI] [PubMed] [Google Scholar]
- 14.Oboho I.K., Tomczyk S.M., Al-Asmari A.M., Banjar A.A., Al-Mugti H., Aloraini M.S. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. N Engl J Med. 2015;372(9):846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drosten C., Meyer B., Muller M.A., Corman V.M., Al-Masri M., Hossain R. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371(9):828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 16.WHO MERS-CoV Research Group State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013;5 doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben Embarek P.K., Van Kerkhove M.D. Middle East respiratory syndrome coronavirus (MERS-CoV): current situation 3 years after the virus was first identified. Weekly Epidemiol Rec/Health Sect Secretariat League Nations. 2015;90(20):245–250. [PubMed] [Google Scholar]
- 19.Command & Control Center . 3rd edition. Ministry of Health; Kingdom of Saudi Arabia: 2015. Infection prevention and control guidelines for Middle East respiratory syndome coronavirus (MERS-CoV) infection. Available from: http://www.moh.gov.sa/en/CCC/Regulations/2015%20update.pdf. [Last Accessed 12 August 2016] [Google Scholar]
- 20.Sherbini N., Iskandrani A., Kharaba A., Khalid G., Abduljawad M., Al-Jahdali H. Middle East respiratory syndrome coronavirus in Medinah City, Saudi Arabia: demographic, clinical and survival data. J Epidemiol Glob Health. 2017:29–36. doi: 10.1016/j.jegh.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Memish Z.A., Al-Tawfiq J.A., Alhakeem R.F., Assiri A., Alharby K.D., Almahallawi M.S. Middle East respiratory syndrome coronavirus (MERS-CoV): a cluster analysis with implications for global management of suspected cases. Travel Med Infect Dis. 2015;13(4):311–314. doi: 10.1016/j.tmaid.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alghamdi I.G., Hussain I.I., Almalki S.S., Alghamdi M.S., Alghamdi M.M., El-Sheemy M.A. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Health Protection Agency UKNCIt Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill. 2013;18(11):20427. doi: 10.2807/ese.18.11.20427-en. [DOI] [PubMed] [Google Scholar]
- 25.Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virlogeux V., Park M., Wu J.T., Cowling B.J. Association between severity of MERS-CoV infection and incubation period. Emerg Infect Dis. 2016;22(3):526–528. doi: 10.3201/eid2203.151437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention . CDC; Atlanta: 2016. People who may be at increased risk for MERS. Available from: http://www.cdc.gov/coronavirus/mers/risk.html. [Last Accessed 12 August 2016] [Google Scholar]
- 28.World Health Organization. Laboratory testing for Middle East Respiratory Syndrome Coronavirus. Available from: http://www.who.int/csr/disease/coronavirus_infections/mers-laboratory-testing/en/. [Last Accessed 12 August 2016].
- 29.Memish Z.A., Assiri A.M., Al-Tawfiq J.A. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014;29:307–308. doi: 10.1016/j.ijid.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed A.E. The predictors of 3- and 30-day mortality in 660 MERS-CoV patients. BMC Infect Dis. 2017;17:615. doi: 10.1186/s12879-017-2712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention . CDC; Atlanta: 2016. Middle East respiratory syndrome (MERS), symptoms & complications. Available from: http://www.cdc.gov/coronavirus/mers/about/symptoms.html. [Last Accessed 12 August 2016] [Google Scholar]
- 32.Al-Hameed F., Wahla A.S., Siddiqui S., Ghabashi A., Al-Shomrani M., Al-Thaqafi A. Characteristics and outcomes of middle east respiratory syndrome coronavirus patients admitted to an intensive care unit in Jeddah, Saudi Arabia. J Intensive Care Med. 2016;31(5):344–348. doi: 10.1177/0885066615579858. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention . CDC; Atlanta: 2015. MERS clinical features. Available from: http://www.cdc.gov/coronavirus/mers/clinical-features.html. [Last Accessed 18 August 2016] [Google Scholar]
- 34.Al-Abdallat M.M., Payne D.C., Alqasrawi S., Rha B., Tohme R.A., Abedi G.R. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59(9):1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arabi Y.M., Arifi A.A., Balkhy H.H., Najm H., Aldawood A.S., Ghabashi A. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 36.Senga M., Arabi Y.M., Fowler R.A. Clinical spectrum of the Middle East respiratory syndrome coronavirus (MERS-CoV) J Infect Public Health. 2016 doi: 10.1016/j.jiph.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]