Graphical abstract
Highlights
-
•
Three coronaviruses are emerging/reemerging in pigs.
-
•
The three porcine coronaviruses may have originated from other species.
-
•
The clinical signs and pathogenesis of the three viruses are similar.
-
•
No cross-protection among the three porcine coronaviruses.
-
•
Individual vaccines are needed for each virus for disease prevention and control.
Abstract
Porcine epidemic diarrhea virus (PEDV), porcine deltacoronavirus (PDCoV), and swine acute diarrhea syndrome-coronavirus (SADS-CoV) are emerging/reemerging coronaviruses (CoVs). They cause acute gastroenteritis in neonatal piglets. Sequence analyses suggest that PEDV and SADS-CoV may have originated from bat CoVs and PDCoV from a sparrow CoV, reaffirming the interspecies transmission of CoVs. The clinical signs and pathogenesis of the three viruses are similar. Necrosis of infected intestinal epithelial cells occurs, causing villous atrophy that results in malabsorptive diarrhea. The severe diarrhea and vomiting may lead to dehydration and death of piglets. Natural infection induces protective immunity, but there is no cross-protection among the three viruses. Besides strict biosecurity measures, individual vaccines are needed for each virus for disease prevention and control.
Current Opinion in Virology 2019, 34:39–49
This review comes from a themed issue on Emerging viruses: interspecies transmission
Edited by Adolfo García-Sastre and Juergen A Richt
For a complete overview see the Issue and the Editorial
Available online 14th January 2019
https://doi.org/10.1016/j.coviro.2018.12.001
1879-6257/© 2018 Elsevier B.V. All rights reserved.
Introduction
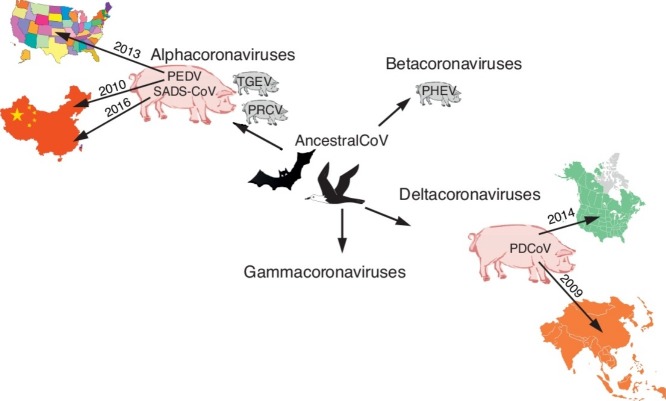
Coronaviruses (CoVs) are the largest positive-sense, single-stranded RNA (+RNA) viruses. They belong to the Coronaviridae family of the Nidovirales order. Four genera are established: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. Currently, six CoVs infect pigs, including four alphacoronaviruses [(transmissible gastroenteritis virus (TGEV), porcine respiratory coronavirus (PRCV), porcine epidemic diarrhea virus (PEDV), and swine acute diarrhea syndrome-coronavirus (SADS-CoV)], one betacoronavirus [porcine hemagglutinating encephalomyelitis virus (PHEV)], and a porcine deltacoronavirus (PDCoV) (Figure 1, Figure 2 ). Among them, TGEV, PRCV, and PHEV have been circulating in pigs for decades, whereas PEDV, PDCoV, and SADS-CoV are considered as emerging coronaviruses. Although classical PEDV was first discovered in Europe in the early 1970s [1], and it later spread to Asia in 1970s [2], a re-emerging highly virulent PEDV caused massive outbreaks, with high mortality in suckling piglets, in late 2010 [3]. In 2013–2014, PEDV caused nationwide outbreaks in the US, culminating in major losses to the pork industry. PDCoV was initially detected from pig fecal samples in 2009 in Asia, but its etiological role was not identified until 2014 when it caused diarrhea in pigs in the US [4•,5]. Most recently, another highly pathogenic enteric CoV, SADS-CoV, emerged in China in 2016 with high mortality among baby pigs [6,7,8••]. All three newly emerged porcine enteric CoVs were first detected in China. To date, the emerging PEDV strains have spread to other Asian and North American countries and to Europe. PEDV has become the second most important pathogen for swine in China (containing >50% of the world’s pig population), after porcine reproductive and respiratory syndrome virus (PRRSV). PDCoV has spread to the US, Canada, and other Asian countries [9]. No SADS-CoV has been reported from other countries besides China. In addition, recombinant viruses between TGEV and PEDV had been reported in Italy, Germany, and Slovakia [10, 11, 12]. These recombinant swine enteric coronaviruses (SeCoV), Italy/213306/2009 and GER/L00930/2012, share similar recombination pattern and 99.5% nucleotide identity and have potential parental strains TGEV H16 strain (major, backbone) and classical PEDV CV777 strain (minor, S gene). Because there were no more reports on SeCoV outbreaks, virulence, and tissue tropism, we will not discuss SeCoV further in this review.
Figure 1.
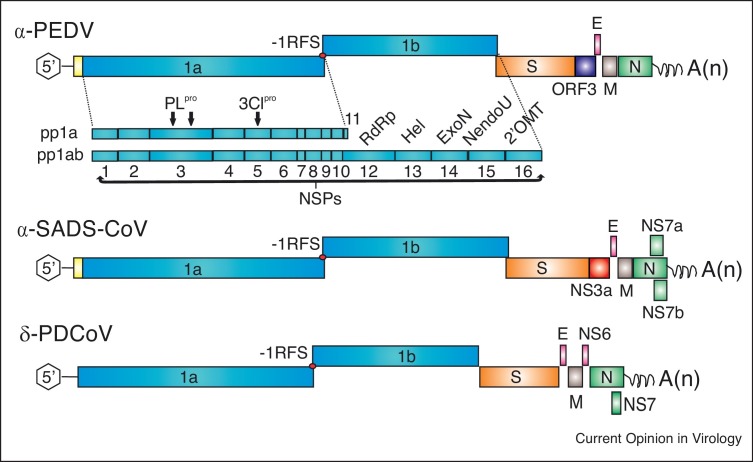
Genomic organization of alphacoronavirus porcine epidemic diarrhea virus (α-PEDV), swine acute diarrhea syndrome-coronavirus (α-SADS-CoV), and porcine deltacoronavirus (δ-PDCoV). Each genome is 5′capped and 3′ polyadenylated. Viral genomes are flanked by UTRs and are polycistronic encoding replicase ORFs1a and 1b followed by the genes encoding spike glycoprotein (S), envelope (E), membrane (M), and nucleocapsid (N) proteins. Among the structural protein genes, the genome also contains accessory genes: ORF3 of PEDV; NS3a, NS7a and NS7b of SADS-CoV; and NS6 and NS7 of PDCoV. Expression of ORF 1a and 1b yields 2 polyproteins (pp1a and pp1ab) through a -1 programmed ribosomal frameshift (-1RFS). The polyprotein is co-translationally or post-translationally processed into an estimated 14–16 nonstructural proteins (NSPs): PLpro, papain-like cysteine protease; 3CLpro, main 3C-like cysteine protease; RdRp, RNA-dependent RNA polymerase; Hel, helicase; ExoN, 3′-5′ exonuclease; NendoU, nidovirus uridylate-specific endoribonuclease; 2′OMT, ribose-2′-O-methyltransferase.
Figure 2.
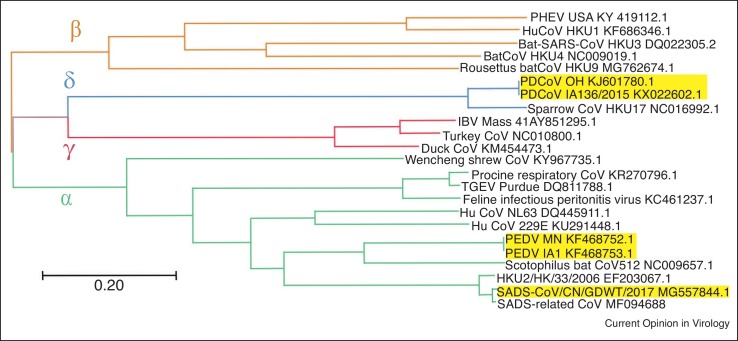
Phylogenetic comparison of the full-length genomes of PEDV, SADS-CoV, and PDCoV with other coronavirus species. Evolutionary history was inferred using the Maximum Likelihood method [112]. The tree with the highest log likelihood (−511751.49) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Joining and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 23 nucleotide sequences. There were a total of 32,538 positions in the final dataset. Evolutionary analyses were conducted in MEGA X [113].
The basic genomic organization is shared among CoVs [13]. The genome size is about 25–30 kb. It contains at least six open reading frames (ORFs) in the order: ORF1a, ORF1b, spike (S), envelope (E), membrane (M), and nucleocapsid (N) (Figure 1). Each virus contains one to several accessory genes, the ORF3 of PEDV, NS3a, NS7a and NS7b of SADS-CoV, and NS6 and NS7 of PDCoV. ORF1a and ORF1b are at the 5′-end and comprise two-thirds of the genome. They encode two non-structural polyproteins that are processed to generate an estimated 14–16 nonstructural proteins (NSPs) involved in proteolytic processing, genome replication and transcription [13]. Among the structural proteins, the S glycoprotein is most important to elicit virus neutralizing antibodies and is diverse among strains. The N protein, which is the most abundant structural protein, binds to the viral genomic RNA and is packed into the nucleocapsid, while the M and E are membrane proteins.
In this review, we will focus on the origin and potential for intra/interspecies transmission, host receptors for virus replication, antigenic relationships, comparative pathogenesis, and disease control and prevention of the three emerging porcine CoVs. For PEDV, we will focus on the highly virulent PEDV strains.
Origin and potential for intra/interspecies transmission
Coronaviruses, including emerging porcine CoVs of two different genera, alphacoronaviruses and deltacoronaviruses, exhibit an increased propensity for interspecies transmission [14,15]. Scattered genome-wide point mutations normally appear within host evolution of CoVs and are not associated with major changes in their biological functions [16,17]. In contrast, significant gene modifications (deletions or insertions in accessory or spike protein genes) are often involved in or occur following CoV host-shift or tissue tropism change events [18,19,20•,21,22, 23, 24]. However, in the case of PEDV, a large deletion (194-216-aa Del) in the N terminus of S protein results in partial attenuation of the virus but did not alter tissue tropism [25,26]. This differs from PRCV whereby the large S deletion (207-227-aa) changes the major tissue tropism from enteric to respiratory compared with the parental TGEV. This may reflect the different receptor usage of these two alphacoronaviruses. TGEV uses aminopeptidase N (APN) as the receptor, and APN is distributed ubiquitously in many tissues. PEDV does not use APN as the receptor, which was confirmed by the fact that PEDV infects APN-knockout pigs [27]. Also, the size and localization of the deletions in the spike gene are virus species-specific with variable biological outcomes [28]. Frequent host-shifting (animal-to-animal and occasionally animal-to-human) events are characteristic for CoV evolution, with severe acute respiratory syndrome (SARS)- and Middle East respiratory syndrome (MERS)-CoVs being the most prominent recent examples [29, 30, 31, 32]. While palm civets, raccoon dogs, Chinese ferret badgers, or dromedary camels were implicated as incidental hosts that facilitated SARS- or MERS-CoV spread to humans, respectively, bats are considered the natural reservoirs for these viruses [33,34]. Besides SARS- and MERS-CoVs, molecular analyses confirmed that numerous animal and human CoVs emerged as a result of recombination and interspecies transmission events. Those CoVs include alphacoronaviruses, betacoronaviruses, and deltacoronaviruses and are summarized below [14]. Canine CoV (CCoV) II has reportedly emerged via recombination between the original CCoV-I and an unknown CoV, while feline CoV (FCoV) II acquired the CCoV-II spike gene and the backbone from FCoV-I [20•]. Another alphacoronavirus, TGEV, is suggested to have originated from CCoV-II because of the high genetic relatedness between the two viruses and by the presence of ORF3 remnants in both genomes [20•,35].
Recent studies have further emphasized that bats are important reservoirs contributing to the immense diversity of human and animal CoVs. For example, it was demonstrated that Ghana bat CoV group I and HCoV-229E share a common ancestor [36,37]. Additionally, a recent transmission of bat-CoV-HKU10 from Leschenault’s rousettes to Pomona leaf-nosed bats has been reported [38]. Among betacoronaviruses, there are the two human CoVs, respiratory HCoV−OC43 associated with common colds and human enteric CoV (HECoV-4408) isolated from a child with diarrhea that are suggested to have been introduced into human population from bovine species [23,39]. Additionally, bovine-like CoVs were identified in captive wild ruminants and camelids such as waterbuck, sambar deer, white-tailed deer, elk, giraffe, sable antelope [18,40, 41, 42], alpaca, llama, and dromedary camel [43]. Deltacoronaviruses are genetically highly heterogenous and represent the only genus in the Coronaviridae family that infects avian and mammalian species with frequent host-shift events [44,45]. Recent molecular analysis of avian deltacoronaviruses in the Middle East provided long-sought evidence of interspecies transmission between falcons and their prey, houbara bustards and pigeons, as well as avian-to-swine transmission associated with recombination events in the spike gene [46].
There are no historic large-scale surveillance data that definitively establish that PEDV was introduced into the pig population from bats in the 1970s [47•]. However, recently, it was demonstrated that PEDV is genetically more closely related to BtCoV/512/2005 than to TGEV, leading to the hypothesis that PEDV may have originated from bats (Figure 2) [47•]. Additionally, PEDV infects cells from pigs, humans, monkeys, duck, and bats, further supporting the theory that PEDV had crossed the bat-swine interspecies barrier sometime in the past (Table 1 ) [48,49]. PEDV has been detected from feral pigs in Korea and the US [50,51•], with the latter study suggesting that PEDV may have spilled over from domestic to feral pigs. However, because there are no control measures to prevent PEDV circulation in feral pigs, they may represent a PEDV reservoir that can lead to PEDV outbreaks in the future.
Table 1.
Summary of the three emerging enteric coronaviruses in pigs
| Coronavirus (Genus) |
Year of emergence (re-emergence | Prototype (GenBank Accession no.) | Receptor | Mortality in neonatal piglets | Infect other animal species besides pigs | Cell lines for virus propagation | Potential ancestor (GenBank Accession no.) |
|---|---|---|---|---|---|---|---|
| PEDV (Alphacoronavirus) | 1971 (2010) | CV777 (NC_003436) | Unknown | Approaching 100% [71] | No | African green monkey kidney (Vero and MARC-145), swine intestine (IECs), bladder, kidney (PK15 and IB-RS-2), testis (ST), and alveolar macrophage (3D4), bat lung (Tb1-Lu),duck intestine (MK-DIEC), and human liver (HuH-7) and lung (MRC-5) [49] | CHN/BtCoV/512/2005 (DQ648858) [47•] |
| PDCoV (Deltacoronavirus) | 2009 | HKU15-44 (JQ065042) | APN; others? | Up to 40% [9] | Bovine [111] | Swine ST, LLC-PK1, IPEC-J2, PKFA, SK6, PD5, and PK15, galline hepatoma (LMH) and fibroblast (DF-1), human hepatoma (Huh7), A549, HeLa, and HRT-18 [53••,114,115] | Recombinant between Sparrow CoV HKU17/2007 (NC_016992) and bulbul CoV HKU11 (FJ376619) [4•,46] |
| SADS-CoV (Alphacoronavirus) | 2016 | GDS04 (MF167434); CH/GD-01/2017 (MF370205); CH/GDWT/2017 (MG557844) | Unknown | 90-100% in pigs ≤ 5 days of age and 5% in pigs > 8 days of age [8••,72] | unknown | Vero [7,8••,72] | BtCoV/HKU2/HK/33/2006 (EF203067); Bt/SADSr-CoV 162140/2016 (MF094688) [8••,52] |
The SADS-CoV genome was shown to be highly similar (95% nucleotide identity) to another bat CoV (HKU2-CoV) identified in 2007 (Figure 2) [6,7,8••,52]. However, the S gene sequence identity is only 86%, suggesting that HKU2-CoV may not be the direct progenitor of SADS-CoV, but that they may share a common ancestor. This prompted the search for new CoVs in bat specimens collected in Guangdong Province between 2013 and 2016. SADS-related CoVs (sharing 96–98% sequence identity with SADS-CoV) were identified in 9.8% of bats, predominantly in horseshoe bats (Rhinolophus spp.) that are known reservoirs of SARS-related CoV (Figure 2) [8••].
In contrast, molecular clock analyses suggest that PDCoV originated relatively recently from a host-switching event between avian and mammalian species (Figure 2) [4•,44]. Most recent phylogenetic analyses have confirmed that quail deltacoronavirus UAE-HKU30 belongs to the same deltacoronavirus species as porcine coronavirus HKU15 (Por CoV HKU15) and sparrow coronavirus HKU17 (SpCoV HKU17) [46]. In addition, PDCoV was shown to efficiently infect cells of broad host range, including swine, humans, and chickens (Table 1) [53••]. Accordingly, PDCoV S protein was found to target the phylogenetically conserved catalytic domain of APN [53••]. Moreover, transient expression of porcine, feline, human, and chicken APN renders cells susceptible to PDCoV infection [53••].
Host receptors
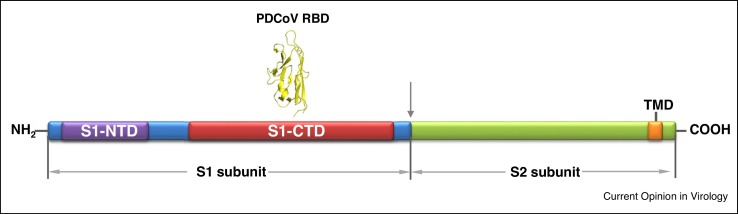
The major determinant for attachment and entry is binding of the S glycoprotein to receptors on the cell surface. This step is one of the key divergence points among CoVs, contributing significantly to species tropism, tissue tropism, the resultant pathogenesis in the host, and the ability to cross between species. The S protein is a type I glycoprotein consisting of S1 and S2 subunits present on the virion surface as a trimer (Figure 3 ) [54,55•]. The S1 is involved in receptor binding and contains N-terminal and C-terminal domains (S1-NTD and S1-CTD, respectively), both of which may serve as a receptor-binding domain (RBD) [56]. The S2 subunit forms the stalk of the spike trimer, involved in triggering the fusion of the viral envelope and target cell membrane [53••,55•]. Identification of specific viral receptors can often be difficult due to the S protein potentially binding to multiple cell receptors, the necessity for co-receptors, and the involvement of potential host receptor proteins in viral replication stages other than initial binding. In the case of PEDV, there appears to be promiscuous binding to cell receptors [48]. APN and sialic acid have both been suggested as binding receptors for PEDV [48,57]. Further research suggests that porcine APN (pAPN) is not a functional receptor, but rather it contributes to PEDV infection through its protease activity [27, 58•,59,60]. This is confirmed by the recent report that APN-knockout pigs became resistant to TGEV infection, but remained susceptible to PEDV infection [27]. Furthermore, the binding efficiency of PEDV to sialic acid appears to be strain-specific. Sialic acid binding is variably required for other CoV infections in cell culture with sialic acid serving as a primary receptor in some instances (A1 lineage betacoronaviruses) [57], as a potential secondary receptor (TGEV) [61,62,63•], or it may be dispensable for entry into cells as noted in cell culture (TGEV) [64]. A similar quandary exists for PDCoV where the S1 domain binds to APN of many different species, allowing functional infection of diverse cell types; however, removal of APN does not render cells completely resistant to infection [53••,65]. These findings suggest that PDCoV not only utilizes APN, but it can also utilize another unknown receptor(s) in the absence of APN. Less research has been done on the emerging SADS-CoV binding receptor due to its recent identification [6,7,8••].
Figure 3.
Schematic diagram of S protein of emerging porcine coronaviruses. S1-NTD – S1 subunit N terminal domain is shown in purple; S1-CTD – S1 subunit C terminal domain is shown in red; RBD – PDCoV receptor binding domain is shown in yellow; TMD – trans-membrane domain is shown in orange.
Antigenic relationships
The antigenic relationships among porcine CoVs are complex and include intergenus and intragenus cross-reactivity [66, 67, 68]. One-way cross-reactivity was observed between TGEV (Miller strain) and classical and current US PEDV strains. This cross-reactivity was due to at least one epitope on the N-terminal region of the N protein [67] and is similar to the N protein-mediated antigenic cross-reactivity between SARS-CoV and alphacoronaviruses of porcine, canine and feline origin reported previously [66,68]. Because it was confirmed that PEDV does not utilize pAPN as a cellular receptor [27,58•,59,60], whereas TGEV does, the biological relevance of the observed antigenic cross-reactivity between TGEV and PEDV needs to be evaluated further, but could interfere in diagnostic tests based on whole virus or N protein.
The fact that pAPN acts as a cross-genus CoV functional receptor for both the enteropathogenic PDCoV and alphacoronavirus (TGEV) suggests that some sequence/structural homology may exist to enable this functional interaction between pAPN and the PDCoV/TGEV S protein receptor binding domain region [53••]. The existence and extent of antigenic cross-reactivity between TGEV and PDCoV are unknown; however, N-protein mediated two-way cross-reactivity between PEDV and PDCoV was recently confirmed [69]. Thus, the above data suggest that receptor selection in the emergence of porcine CoVs is subject to certain restrictions and may lead to structural re-arrangements in the spike as well as other viral proteins, including the highly conserved (and therefore cross-reactive) N protein. Furthermore, recent experiments demonstrated that angiotensin-converting enzyme 2 (ACE2), pAPN and dipeptidyl peptidase 4 (DPP4) do not function as receptors for SADS-CoV [8••]. Thus, additional experimental work is needed to identify cellular receptors for PEDV and SADS-CoV, as well as to gain mechanistic understanding of antigenic interrelationships among emerging porcine CoVs. Specifically, additional information is needed to establish whether there is co-regulation between receptor usage and antigenic cross-reactivity among these CoVs.
Comparative pathogenesis
PEDV, PDCoV, and SADS-CoV are all enteric pathogens, causing indistinguishable acute gastroenteritis in all ages of pigs (Table 1). Clinical signs include mainly anorexia, diarrhea and vomiting that may lead to dehydration, loss of body weight, lethargy, and death. Those clinical signs generally do not last over 10 days. Disease is generally more severe and often lethal in neonatal piglets, especially those born from seronegative sows. In older pigs, such as weaned pigs and sows, morbidity is high but the mortality rate is low. In general, the emerging highly virulent PEDV is more virulent than PDCoV strains tested [9]. During the first year of outbreaks in the US, the pig morbidity rate due to PEDV and PDCoV was about 100% and 30%, respectively [70]. PEDV and PDCoV caused up to 100% and up to 40% mortality rates in neonatal piglets, respectively [9,71]. The mortality rates of SADS-CoV in piglets less than five days of age and older than eight days of age were 90–100% and 5%, respectively [8••,72]. Diseases caused by porcine enteric CoVs (TGEV, PEDV, SADS-CoV, and PDCoV) cannot be differentiated without laboratory diagnosis. Transmission is mainly via the fecal-oral route. Vehicles include contaminated feed/ingredients, transportation trucks/trailers, environment, etc. [71]. Airborne transmission was reported for both PEDV and PDCoV, probably via ingestion of aerosolized virus particles [71]. Recently, Li et al. [73] investigated the potential mechanisms of airborne transmission of PEDV. They concluded that the virions can replicate in the nasal epithelial cells, then are carried by dendritic cells (DCs) and passed to CD3+ T cells that transport PEDV to the gut to infect intestinal cells. However, such a conclusion cannot be made based on the data presented in the paper. The major concern is that the authors used extremely high inoculum dose (7 log10 PFU/pig) to intranasally inoculate five-day-old CDCD piglets. Considering the very low infectious dose for neonatal piglets of the emerging highly virulent PEDV, ingestion of even a very small portion (1/10 million, 1 PFU) of this inoculum, which was highly possible, can result in a 100% infection rate in piglets within 24 hpi, as described previously [74,75]. Therefore, although PEDV can replicate in nasal epithelial cells and be taken up by DCs and T cells, whether the virions carried by the DCs and T cells can cause intestinal infection and diarrhea should be investigated further. Also, PEDV is more prevalent than PDCoV in the field [76]. Infectious PEDV can be shed in the feces of infected pigs weeks after clinical signs cease, posing difficulties for disease management on farms [71]. Co-infections with more than one pig enteric pathogen are common and often more severe clinically [77,78].
The pathogenesis of PEDV and PDCoV has been studied in different ages of pigs [9,71,79,80]. Disease is more severe in younger pigs than in older pigs, which is called age-dependent resistance, probably due to the immature immune responses and lower rate of enterocyte regeneration in younger pigs [81•,82, 83, 84]. In general, pathological changes in young pigs are similar between these two viruses. Clinical signs appear at 1–3 days post-inoculation. Viral replication occurs quickly and mainly in the villous epithelial cells of the small intestine, resulting in villous atrophy. Therefore, diarrhea is probably a consequence of malabsorption due to massive loss of absorptive enterocytes. PEDV and PDCoV antigens also were detected occasionally in crypt cells, but may not contribute significantly to pathogenesis since enterocyte regeneration capacity was preserved [9,79]. No histopathologic changes have been observed in the caecum/colon, although PEDV and PDCoV antigens were detected in the epithelial cells of these intestinal sections. During the acute phase of virus infection, large number of viruses are shed in feces. Viral RNA was also detected transiently in the serum. Low levels of viral RNA were detected in multiple organs by real-time reverse transcription (RT)-PCR possibly due to viremia, since no viral antigen was detected. A recent study showed significantly lower levels of serotonin-secreting enteroendocrine cells in duodenum, mid-jejunum, ileum, or colon in PEDV-infected pigs that had vomiting than in negative control pigs, suggesting that serotonin secretion during PEDV infection might contribute to vomiting [85]. The pathogenesis of the recently emerging SADS-CoV was studied in piglets by three laboratories [7,8••,72]. Pan et al. [7] noted that SADS-CoV caused only mild-moderate intestinal lesions in three-day-old piglets. In the second study, SADS-CoV proteins were detected in only a few jejunal epithelial cells, which may not correlate with the 50% (3/6) mortality in the inoculated three-day-old piglets [8••]. In the most recent study Xu et al. [72] reported that SADS-CoV mainly infects the intestine, causing 100% (12/12) and 50% (2/4) mortality rates in five-day-old and contact control conventional piglets (infected at ˜7 days of age based on fecal shedding data), respectively. SADS-CoV RNA was also detected from the heart, liver, spleen, kidneys, stomach, and lungs, but not the blood of piglets euthanized at 7 dpi when pigs still had severe diarrhea. Therefore, more detailed pathogenesis studies are warranted to understand SADS-CoV replication in vivo, factors contributing to disease severity, tissue tropism, etc.
Immune responses and disease control and prevention
Immune responses have been studied for PEDV infection, but there are few reports for PDCoV and SADS-CoV [86, 87, 88, 89]. Serum PEDV IgG Ab responses were studied in seven-week-old conventional pigs inoculated with non-S INDEL PEDV via the oragastric route at −7 to 42 days post-inoculation (dpi). IgG antibodies were detected as early as 7 dpi for the S1 subunit of the S protein and 10 dpi for the N and M structural proteins and the whole virus particles [90•]. The antibody titers peaked at 14–17 dpi and remained detectable through 42 dpi. However, no E protein-specific IgG antibodies were detected throughout the study. Also, the PEDV S1 does not cross-react with IgG antibodies against the other porcine enteric coronaviruses, TGEV (and its variant PRCV) and PDCoV. Therefore, the S protein is the most sensitive and specific marker for PEDV infection. In 11-day-old conventional pigs, a strong positive correlation exists between protection against PEDV challenge and the levels of IgA and IgG antibody secreting cells (ASCs) in the gut associated lymphoid tissues (duodenum and ileum lamina propria and mesenteric lymph nodes) and the blood as well as between protection and serum IgG and IgA antibody titers [91,92]. In sows exposed to PEDV in the field, IgA and IgG PEDV ASCs were detected in the intestine of sows at one month post-PEDV exposure, then waned, but remained detectable at six months post-PEDV exposure [93]. Additionally, PEDV IgA antibody was detectable in oral fluids [93]. Several experimental challenge studies using naive animals of different ages demonstrated that serum viral neutralizing antibodies became detectable around 10 dpi, peaked around 18 dpi, decreased slightly around 21 dpi, and remained at high level through 42 dpi [88].
T cell immune responses may play an important role in inducing protective immunity because pigs infected with the attenuated and highly virulent PEDV PC22A strain generated similar level of humoral immune responses, but only pigs infected with the virulent PEDV were protected from challenge with homologous virulent virus [94]. However, limited experimental data are available on cellular immune responses, focusing mainly on cytokine responses [82]. PEDV-infected suckling pigs had significantly lower natural killer (NK) cell frequencies, undetectable NK cell activity and lower IFN-γ producing CD3−CD4−CD8+ NK cells in blood and ileum compared with PEDV-infected weaned pigs [82]. Deficiency in the innate immune function of neonatal NK cells may contribute to the more severe PEDV infection in suckling pigs compared with weaned pigs as also reported for TGEV infections [95]. Inflammatory responses play a significant role in the pathogenesis of enteric coronaviruses. Compared with suckling pigs, weaned pigs had a delayed pro-inflammatory cytokine induction that coincided with the delayed onset of infection, disease and shedding of PEDV RNA in feces [82]. Toll-like receptor 2 (TLR2), TLR3, and TLR9 may contribute to NF-κB activation in response to PEDV infection in small intestinal epithelial cells in vitro [96]. The viral proteins E and N upregulated IL-8 expression by inducing endoplasmic reticulum stress and subsequent activation of the NF-κB pathway [97,98]. PEDV can evade host innate immune responses via at least 11 proteins (nsp1, nsp3, nsp5, nsp7, nsp14, nsp15, nsp16, ORF3, E, M, and N) that function as interferon (IFN) antagonists [99, 100, 101, 102]. Identification of the virus-encoded IFN antagonists and understanding their mechanism of action may lead to novel therapeutic targets and more effective vaccines. In summary, infection of PEDV induces protection against homologous virus challenge in neonatal, growing and adult pigs [89,103,104]. Although there is only one serotype of PEDV, only incomplete cross-protection exists among different genetic clusters of PEDV variants [3,103]. Sows previously infected with PEDV can generate lactogenic immunity and passively protect nursing piglets from disease [86,104]. Mucosal IgA antibody levels correlate to protective viral neutralizing antibody levels and the neutralizing epitopes are mainly located in the S glycoprotein [105, 106, 107].
Rapid diagnosis is critical for the control of these diseases to prevent them from spreading. Diagnostic methods for PEDV and PDCoV for the detection of viral RNA, viral antigens, and antibodies have been reviewed [76,88]. No specific antiviral drugs are available to treat infected pigs. High level biosecurity and vaccines are still the best choice to prevent these enteric infections [104]. No cross-neutralization exists between antibodies to PEDV and PDCoV [69]. SADS-CoV outbreaks occurred at a swine farm that suffered from recent PEDV infections, suggesting no cross-protection between PEDV and SADS-CoV [8••]. Therefore, the prevention of these three emerging porcine CoV diseases will require the development of separate virus-specific vaccines. Although PED occurs in pigs of all ages, piglets up to one week of age experience high mortality and need to be protected by transfer of maternal antibodies, especially neutralizing antibodies, via colostrum and milk from immunized dams. The mechanisms of lactogenic protection described for TGEV and other enteric viral infections apply to PEDV as well [86,108]. Colostrum and milk IgA or neutralizing antibody titers are better markers for lactogenic immunity than antibodies in serum [109]. Live, inactivated, and subunit (S) PEDV vaccines targeting sows have been developed in China, Japan, Korea and the US [104,110]. However, their efficacy has not been sufficient to control PEDV outbreaks that still occur on farms with PEDV-vaccinated pigs. No reports are yet available on vaccine development for PDCoV or SADS-CoV. Some farms use feedback methods with intentional exposure of pregnant sows pre-farrowing to autogenous virus using minced intestines from acutely infected piglets [108]. The goal is to stimulate lactogenic immunity in sows and reduce mortality in nursing pigs. The problem with these methods is that PEDV infection may become persistent on farms and other enteric pathogens could be transmitted to the entire herd.
Conclusions
PDCoV, SADS-CoV, and highly virulent PEDV emerged or reemerged recently (Table 1). PEDV has spread to Asian, North American, and European countries, causing huge economic losses in the swine industry. Among the three enteric CoVs, PEDV has been studied more extensively and some vaccines have been developed, although their efficacy in the field is still questionable. For PDCoV and SADS-CoV, more research is needed to better understand their emergence, evolution, pathogenesis, and immune responses. Currently, disease control and prevention mainly depend on swine farm management, focusing on high biosecurity measures and disease containment within and among farms. Coronaviruses often spill over to other species: PEDV and PDCoV can infect cells from different species, and PDCoV can also infect calves [111]. Currently, these emerging porcine CoVs are only known to infect pigs, but not humans [48,53••].
Nevertheless, continued monitoring of these porcine CoVs is necessary for both swine and public health.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was partially supported by National Institute of Food and Agriculture, U.S. Department of Agriculture (USDA) [award number 2015-67015-23067, 2015] and USDA Agricultural Research Service [award number 58-3625-4-087, 2014]. Salaries and research support were provided by state and federal funds provided to the Ohio Agricultural Research and Development Center (OARDC), The Ohio State University.
References
- 1.Wood E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet Rec. 1977;100:243–244. doi: 10.1136/vr.100.12.243. [DOI] [PubMed] [Google Scholar]
- 2.Sun D., Wang X., Wei S., Chen J., Feng L. Epidemiology and vaccine of porcine epidemic diarrhea virus in China: a mini-review. J Vet Med Sci. 2016;78:355–363. doi: 10.1292/jvms.15-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun R.Q., Cai R.J., Chen Y.Q., Liang P.S., Chen D.K., Song C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg Infect Dis. 2012;18:161–163. doi: 10.3201/eid1801.111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]; It is a nice study on the evolution of coronaviruses.
- 5.Wang L., Byrum B., Zhang Y. Porcine coronavirus HKU15 detected in 9 US states, 2014. Emerg Infect Dis. 2014;20:1594–1595. doi: 10.3201/eid2009.140756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong L., Li J., Zhou Q., Xu Z., Chen L., Zhang Y., Xue C., Wen Z., Cao Y. A new Bat-HKU2-like Coronavirus in Swine, China, 2017. Emerg Infect Dis. 2017;23 doi: 10.3201/eid2309.170915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y., Tian X., Qin P., Wang B., Zhao P., Yang Y.L., Wang L.X., Wang D., Song Y., Zhang X. Discovery of a novel swine enteric alphacoronavirus (SeACoV) in southern China. Vet Microbio. 2017;211:15–21. doi: 10.1016/j.vetmic.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Zhou P., Fan H., Lan T., Yang X.L., Shi W.F., Zhang W., Zhu Y., Zhang Y.W., Xie Q.M., Mani S. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature. 2018;556:255–258. doi: 10.1038/s41586-018-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensively, investigation of a deadly pig diarrhea outbreak caused by a novel alphacoronavirus. They also identified SADS-related CoVs from bats, highlighting the interspecies transmission of bat CoVs to pigs.
- 9.Jung K., Hu H., Saif L.J. Porcine deltacoronavirus infection: etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus Res. 2016;226:50–59. doi: 10.1016/j.virusres.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akimkin V., Beer M., Blome S., Hanke D., Hoper D., Jenckel M., Pohlmann A. New Chimeric Porcine Coronavirus in Swine Feces, Germany, 2012. Emerg Infect Dis. 2016;22:1314–1315. doi: 10.3201/eid2207.160179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boniotti M.B., Papetti A., Lavazza A., Alborali G., Sozzi E., Chiapponi C., Faccini S., Bonilauri P., Cordioli P., Marthaler D. Porcine epidemic diarrhea virus and discovery of a recombinant swine enteric coronavirus, Italy. Emerg Infect Dis. 2016;22:83–87. doi: 10.3201/eid2201.150544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandelik R., Sarvas M., Jackova A., Salamunova S., Novotny J., Vilcek S. First outbreak with chimeric swine enteric coronavirus (SeCoV) on pig farms in Slovakia - lessons to learn. Acta Veterinaria Hungarica. 2018;66:488–492. doi: 10.1556/004.2018.043. [DOI] [PubMed] [Google Scholar]
- 13.Brian D.A., Baric R.S. Coronavirus genome structure and replication. Curr Topics Microbiol Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan J.F., To K.K., Tse H., Jin D.Y., Yuen K.Y. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21:544–555. doi: 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlasova A.N., Saif L.J. Biological aspects of the interspecies transmission of selected coronaviruses. In: Singh S.K., editor. Viral Infections and Global Change. Wiley; 2013. pp. 393–418. [Google Scholar]
- 16.Zhang C.Y., Wei J.F., He S.H. Adaptive evolution of the spike gene of SARS coronavirus: changes in positively selected sites in different epidemic groups. BMC Microbiol. 2006;6:88. doi: 10.1186/1471-2180-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X., Hasoksuz M., Spiro D., Halpin R., Wang S., Vlasova A., Janies D., Jones L.R., Ghedin E., Saif L.J. Quasispecies of bovine enteric and respiratory coronaviruses based on complete genome sequences and genetic changes after tissue culture adaptation. Virology. 2007;363:1–10. doi: 10.1016/j.virol.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasoksuz M., Alekseev K., Vlasova A., Zhang X., Spiro D., Halpin R., Wang S., Ghedin E., Saif L.J. Biologic, antigenic, and full-length genomic characterization of a bovine-like coronavirus isolated from a giraffe. J Virol. 2007;81:4981–4990. doi: 10.1128/JVI.02361-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laude H., Van Reeth K., Pensaert M. Porcine respiratory coronavirus: molecular features and virus-host interactions. Vet Res. 1993;24:125–150. [PubMed] [Google Scholar]
- 20•.Lorusso A., Decaro N., Schellen P., Rottier P.J., Buonavoglia C., Haijema B.J., de Groot R.J. Gain, preservation, and loss of a group 1a coronavirus accessory glycoprotein. J Virol. 2008;82:10312–10317. doi: 10.1128/JVI.01031-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; A nice study on the evolutionary history of alphacoronaviruses based on accessory gene ORF3.
- 21.Vaughn E.M., Halbur P.G., Paul P.S. Sequence comparison of porcine respiratory coronavirus isolates reveals heterogeneity in the S, 3, and 3-1 genes. J Virol. 1995;69:3176–3184. doi: 10.1128/jvi.69.5.3176-3184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vennema H., Poland A., Foley J., Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150–157. doi: 10.1006/viro.1998.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijgen L., Keyaerts E., Moes E., Thoelen I., Wollants E., Lemey P., Vandamme A.M., Van Ranst M. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J Virol. 2005;79:1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Hasoksuz M., Spiro D., Halpin R., Wang S., Stollar S., Janies D., Hadya N., Tang Y., Ghedin E. Complete genomic sequences, a key residue in the spike protein and deletions in nonstructural protein 3b of US strains of the virulent and attenuated coronaviruses, transmissible gastroenteritis virus and porcine respiratory coronavirus. Virology. 2007;358:424–435. doi: 10.1016/j.virol.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou Y., Lin C.M., Yokoyama M., Yount B.L., Marthaler D., Douglas A.L., Ghimire S., Qin Y., Baric R.S., Saif L.J. Deletion of a 197-amino-acid region in the N-terminal domain of spike protein attenuates porcine epidemic diarrhea virus in piglets. J Virol. 2017;91 doi: 10.1128/JVI.00227-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T., Shibahara T., Yamaguchi R., Nakade K., Yamamoto T., Miyazaki A., Ohashi S. Pig epidemic diarrhoea virus S gene variant with a large deletion non-lethal to colostrum-deprived newborn piglets. J Gen Virol. 2016;97:1823–1828. doi: 10.1099/jgv.0.000513. [DOI] [PubMed] [Google Scholar]
- 27.Whitworth K.M., Rowland R.R.R., Petrovan V., Sheahan M., Cino-Ozuna A.G., Fang Y., Hesse R., Mileham A., Samuel M.S., Wells K.D. Resistance to coronavirus infection in amino peptidase N-deficient pigs. Transgenic Res. 2018 doi: 10.1007/s11248-018-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terada Y., Shiozaki Y., Shimoda H., Mahmoud H.Y.A.H., Noguchi K., Nagao Y., Shimojima M., Iwata H., Mizuno T., Okuda M. Feline infectious peritonitis virus with a large deletion in the 5'-terminal region of the spike gene retains its virulence for cats. J Gen Virol. 2012;93:1930–1934. doi: 10.1099/vir.0.043992-0. [DOI] [PubMed] [Google Scholar]
- 29.Graham R.L., Baric R.S. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. J Virol. 2010;84:3134–3146. doi: 10.1128/JVI.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau S.K., Woo P.C., Li K.S., Huang Y., Tsoi H.W., Wong B.H., Wong S.S., Leung S.Y., Chan K.H., Yuen K.Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 32.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 33.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drexler J.F., Corman V.M., Drosten C. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res. 2014;101:45–56. doi: 10.1016/j.antiviral.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Decaro N., Mari V., Elia G., Addie D.D., Camero M., Lucente M.S., Martella V., Buonavoglia C. Recombinant canine coronaviruses in dogs, Europe. Emerg Infect Dis. 2010;16:41–47. doi: 10.3201/eid1601.090726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfefferle S., Oppong S., Drexler J.F., Gloza-Rausch F., Ipsen A., Seebens A., Muller M.A., Annan A., Vallo P., Adu-Sarkodie Y. Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerg Infect Dis. 2009;15:1377–1384. doi: 10.3201/eid1509.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corman V.M., Baldwin H.J., Tateno A.F., Zerbinati R.M., Annan A., Owusu M., Nkrumah E.E., Maganga G.D., Oppong S., Adu-Sarkodie Y. Evidence for an ancestral association of human coronavirus 229E with bats. J Virol. 2015;89:11858–11870. doi: 10.1128/JVI.01755-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau S.K., Li K.S., Tsang A.K., Shek C.T., Wang M., Choi G.K., Guo R., Wong B.H., Poon R.W. Recent transmission of a novel alphacoronavirus, bat coronavirus HKU10, from Leschenault’s rousettes to pomona leaf-nosed bats: first evidence of interspecies transmission of coronavirus between bats of different suborders. Lam C.S., editor. J Virol. 2012;86:11906–11918. doi: 10.1128/JVI.01305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han M.G., Cheon D.S., Zhang X., Saif L.J. Cross-protection against a human enteric coronavirus and a virulent bovine enteric coronavirus in gnotobiotic calves. J Virol. 2006;80:12350–12356. doi: 10.1128/JVI.00402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alekseev K.P., Vlasova A.N., Jung K., Hasoksuz M., Zhang X., Halpin R., Wang S., Ghedin E., Spiro D., Saif L.J. Bovine-like coronaviruses isolated from four species of captive wild ruminants are homologous to bovine coronaviruses, based on complete genomic sequences. J Virol. 2008;82:12422–12431. doi: 10.1128/JVI.01586-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsunemitsu H., el-Kanawati Z.R., Smith D.R., Reed H.H., Saif L.J. Isolation of coronaviruses antigenically indistinguishable from bovine coronavirus from wild ruminants with diarrhea. J Clin Microbiol. 1995;33:3264–3269. doi: 10.1128/jcm.33.12.3264-3269.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majhdi F., Minocha H.C., Kapil S. Isolation and characterization of a coronavirus from elk calves with diarrhea. J Clin Microbiol. 1997;35:2937–2942. doi: 10.1128/jcm.35.11.2937-2942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kin N., Miszczak F., Diancourt L., Caro V., Moutou F., Vabret A., Ar Gouilh M. Comparative molecular epidemiology of two closely related coronaviruses, bovine coronavirus (BCoV) and human coronavirus OC43 (HCoV-OC43), reveals a different evolutionary pattern. Infect, Genet Evol. 2016;40:186–191. doi: 10.1016/j.meegid.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Y., Zhang Y., Liang X., Lou F., Oglesbee M., Krakowka S., Li J. Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. mBio. 2015;6 doi: 10.1128/mBio.00064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu D.K., Leung C.Y., Gilbert M., Joyner P.H., Ng E.M., Tse T.M., Guan Y., Peiris J.S., Poon L.L. Avian coronavirus in wild aquatic birds. J Virol. 2011;85:12815–12820. doi: 10.1128/JVI.05838-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lau S.K.P., Wong E.Y.M., Tsang C.C., Ahmed S.S., Au-Yeung R.K.H., Yuen K.Y., Wernery U., Woo P.C.Y. Discovery and sequence analysis of four deltacoronaviruses from birds in the middle east reveal interspecies jumping with recombination as a potential mechanism for avian-to-avian and avian-to-mammalian transmission. J Virol. 2018;92 doi: 10.1128/JVI.00265-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Huang Y.W., Dickerman A.W., Pineyro P., Li L., Fang L., Kiehne R., Opriessnig T., Meng X.J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. mBio. 2013;4:e00737–00713. doi: 10.1128/mBio.00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; A nice study on the origin of emerging PEDV in the USA.
- 48.Liu C., Tang J., Ma Y., Liang X., Yang Y., Peng G., Qi Q., Jiang S., Li J., Du L. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J Virol. 2015;89:6121–6125. doi: 10.1128/JVI.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teeravechyan S., Frantz P.N., Wongthida P., Chailangkarn T., Jaru-Ampornpan P., Koonpaew S., Jongkaewwattana A. Deciphering the biology of porcine epidemic diarrhea virus in the era of reverse genetics. Virus Res. 2016;226:152–171. doi: 10.1016/j.virusres.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bevins S.N., Lutman M., Pedersen K., Barrett N., Gidlewski T., Deliberto T.J., Franklin A.B. Spillover of swine coronaviruses, United States. Emerg Infect Dis. 2018;24:1390–1392. doi: 10.3201/eid2407.172077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Lee D.U., Kwon T., Je S.H., Yoo S.J., Seo S.W., Sunwoo S.Y., Lyoo Y.S. Wild boars harboring porcine epidemic diarrhea virus (PEDV) may play an important role as a PEDV reservoir. Vet Microbiol. 2016;192:90–94. doi: 10.1016/j.vetmic.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study reporting PEDV infection of wild pigs, which can be an important PEDV reservoir.
- 52.Lau S.K., Woo P.C., Li K.S., Huang Y., Wang M., Lam C.S., Xu H., Guo R., Chan K.H., Zheng B.J. Complete genome sequence of bat coronavirus HKU2 from Chinese horseshoe bats revealed a much smaller spike gene with a different evolutionary lineage from the rest of the genome. Virology. 2007;367:428–439. doi: 10.1016/j.virol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Li W., Hulswit R.J.G., Kenney S.P., Widjaja I., Jung K., Alhamo M.A., van Dieren B., van Kuppeveld F.J.M., Saif L.J., Bosch B.J. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. Proc Natl Acad Sci U S A. 2018;115:E5135–E5143. doi: 10.1073/pnas.1802879115. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported that PDCoV uses APN as an entry receptor via binding to the phylogenetically conserved catalytic domain of APN. It partially explains the fact that PDCoV efficiently infects cells of a broad species range and the mechanism of interspecies transmission of the virus from birds to pigs.
- 54.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Hulswit R.J., de Haan C.A., Bosch B.J. Coronavirus spike protein and tropism changes. Adv Virus Res. 2016;96:29–57. doi: 10.1016/bs.aivir.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nice and comprehensive review of the coronavirus spike protein and inter- and intraspecies tropism changes.
- 56.Walls A.C., Tortorici M.A., Bosch B.J., Frenz B., Rottier P.J.M., DiMaio F., Rey F.A., Veesler D. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531:114–117. doi: 10.1038/nature16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W., van Kuppeveld F.J.M., He Q., Rottier P.J.M., Bosch B.J. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016;226:117–127. doi: 10.1016/j.virusres.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Shirato K., Maejima M., Islam M.T., Miyazaki A., Kawase M., Matsuyama S., Taguchi F. Porcine aminopeptidase N is not a cellular receptor of porcine epidemic diarrhea virus, but promotes its infectivity via aminopeptidase activity. J Gen Virol. 2016;97:2528–2539. doi: 10.1099/jgv.0.000563. [DOI] [PubMed] [Google Scholar]; It is the first report showing experimental evidence that the previously claimed PEDV receptor APN is not the cellular receptor for PEDV.
- 59.Li W., Luo R., He Q., van Kuppeveld F.J.M., Rottier P.J.M., Bosch B.J. Aminopeptidase N is not required for porcine epidemic diarrhea virus cell entry. Virus Res. 2017;235:6–13. doi: 10.1016/j.virusres.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji C.M., Wang B., Zhou J., Huang Y.W. Aminopeptidase-N-independent entry of porcine epidemic diarrhea virus into Vero or porcine small intestine epithelial cells. Virology. 2018;517:16–23. doi: 10.1016/j.virol.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reguera J., Santiago C., Mudgal G., Ordono D., Enjuanes L., Casasnovas J.M. Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schultze B., Krempl C., Ballesteros M.L., Shaw L., Schauer R., Enjuanes L., Herrler G. Transmissible gastroenteritis coronavirus, but not the related porcine respiratory coronavirus, has a sialic acid (N-glycolylneuraminic acid) binding activity. J Virol. 1996;70:5634–5637. doi: 10.1128/jvi.70.8.5634-5637.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Krempl C., Schultze B., Laude H., Herrler G. Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J Virol. 1997;71:3285–3287. doi: 10.1128/jvi.71.4.3285-3287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]; A nice study on the connection between sialic acid binding activity and the enteropathogenicity of TGEV.
- 64.Schwegmann-Wessels C., Herrler G. Sialic acids as receptor determinants for coronaviruses. Glycoconjugate J. 2006;23:51–58. doi: 10.1007/s10719-006-5437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu X., Liu S., Wang X., Luo Z., Shi Y., Wang D., Peng G., Chen H., Fang L., Xiao S. Contribution of porcine aminopeptidase N to porcine deltacoronavirus infection. Emerg Microbes Infect. 2018;7:65. doi: 10.1038/s41426-018-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vlasova A.N., Zhang X., Hasoksuz M., Nagesha H.S., Haynes L.M., Fang Y., Lu S., Saif L.J. Two-way antigenic cross-reactivity between severe acute respiratory syndrome coronavirus (SARS-CoV) and group 1 animal CoVs is mediated through an antigenic site in the N-terminal region of the SARS-CoV nucleoprotein. J Virol. 2007;81:13365–13377. doi: 10.1128/JVI.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin C.M., Gao X., Oka T., Vlasova A.N., Esseili M.A., Wang Q., Saif L.J. Antigenic relationships among porcine epidemic diarrhea virus and transmissible gastroenteritis virus strains. J Virol. 2015;89:3332–3342. doi: 10.1128/JVI.03196-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Z.F., Meng X.J. Antigenic cross-reactivity between the nucleocapsid protein of severe acute respiratory syndrome (SARS) coronavirus and polyclonal antisera of antigenic group I animal coronaviruses: implication for SARS diagnosis. J Clin Microbiol. 2004;42:2351–2352. doi: 10.1128/JCM.42.5.2351-2352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma Y., Zhang Y., Liang X., Oglesbee M., Krakowka S., Niehaus A., Wang G., Jia A., Song H., Li J. Two-way antigenic cross-reactivity between porcine epidemic diarrhea virus and porcine deltacoronavirus. Vet Microbiol. 2016;186:90–96. doi: 10.1016/j.vetmic.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marthaler D., Raymond L., Jiang Y., Collins J., Rossow K., Rovira A. Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerg Infect Dis. 2014;20:1347–1350. doi: 10.3201/eid2008.140526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niederwerder M.C., Hesse R.A. Swine enteric coronavirus disease: a review of 4 years with porcine epidemic diarrhoea virus and porcine deltacoronavirus in the United States and Canada. Transboundary and Emerg Dis. 2018;65:660–675. doi: 10.1111/tbed.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu Z., Zhang Y., Gong L., Huang L., Lin Y., Qin J., Du Y., Zhou Q., Xue C., Cao Y. Isolation and characterization of a highly pathogenic strain of Porcine enteric alphacoronavirus causing watery diarrhoea and high mortality in newborn piglets. Transboundary Emerg Dis. 2018 doi: 10.1111/tbed.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y., Wu Q., Huang L., Yuan C., Wang J., Yang Q. An alternative pathway of enteric PEDV dissemination from nasal cavity to intestinal mucosa in swine. Nat Commun. 2018;9:3811. doi: 10.1038/s41467-018-06056-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X., Lin C.M., Annamalai T., Gao X., Lu Z., Esseili M.A., Jung K., El-Tholoth M., Saif L.J., Wang Q. Determination of the infectious titer and virulence of an original US porcine epidemic diarrhea virus PC22A strain. Vet Res. 2015;46:109. doi: 10.1186/s13567-015-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas J.T., Chen Q., Gauger P.C., Gimenez-Lirola L.G., Sinha A., Harmon K.M., Madson D.M., Burrough E.R., Magstadt D.R., Salzbrenner H.M. Effect of porcine epidemic diarrhea virus infectious doses on infection outcomes in naive conventional neonatal and weaned pigs. PloS One. 2015;10 doi: 10.1371/journal.pone.0139266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J. Porcine deltacoronavirus: overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res. 2016;226:71–84. doi: 10.1016/j.virusres.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Q., Wang L., Zheng Y., Zhang J., Guo B., Yoon K.J., Gauger P.C., Harmon K.M., Main R.G., Li G. Metagenomic analysis of the RNA fraction of the fecal virome indicates high diversity in pigs infected by porcine endemic diarrhea virus in the United States. Virol J. 2018;15:95. doi: 10.1186/s12985-018-1001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Q., Hu R., Tang X., Wu C., He Q., Zhao Z., Chen H., Wu B. Occurrence and investigation of enteric viral infections in pigs with diarrhea in China. Arch Virol. 2013;158:1631–1636. doi: 10.1007/s00705-013-1659-x. [DOI] [PubMed] [Google Scholar]
- 79.Lin C.M., Saif L.J., Marthaler D., Wang Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016;226:20–39. doi: 10.1016/j.virusres.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet J. 2015;204:134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81•.Moon H.W., Kemeny L.J., Lambert G., Stark S.L., Booth G.D. Age-dependent resistance to transmissible gastroenteritis of swine. III. Effects of epithelial cell kinetics on coronavirus production and on atrophy of intestinal villi. Vet Pathol. 1975;12:434–445. doi: 10.1177/0300985875012005-00610. [DOI] [PubMed] [Google Scholar]; A nice study on innate age-dependent resistance to transmissible gastroenteritis.
- 82.Annamalai T., Saif L.J., Lu Z., Jung K. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet Immunol Immunopathol. 2015;168:193–202. doi: 10.1016/j.vetimm.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jung K., Annamalai T., Lu Z., Saif L.J. Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs. Vet Microbiol. 2015;178:31–40. doi: 10.1016/j.vetmic.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hammerberg C., Schurig G.G., Ochs D.L. Immunodeficiency in young pigs. Am J Vet Res. 1989;50:868–874. [PubMed] [Google Scholar]
- 85.Jung K., Miyazaki A., Saif L.J. Immunohistochemical detection of the vomiting-inducing monoamine neurotransmitter serotonin and enterochromaffin cells in the intestines of conventional or gnotobiotic (Gn) pigs infected with porcine epidemic diarrhea virus (PEDV) and serum cytokine responses of Gn pigs to acute PEDV infection. Res Vet Sci. 2018;119:99–108. doi: 10.1016/j.rvsc.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Langel S.N., Paim F.C., Lager K.M., Vlasova A.N., Saif L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts. Virus Res. 2016;226:93–107. doi: 10.1016/j.virusres.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song Q., Stone S., Drebes D., Greiner L.L., Dvorak C.M., Murtaugh M.P. Characterization of anti-porcine epidemic diarrhea virus neutralizing activity in mammary secretions. Virus Res. 2016;226:85–92. doi: 10.1016/j.virusres.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diel D.G., Lawson S., Okda F., Singrey A., Clement T., Fernandes M.H.V., Christopher-Hennings J., Nelson E.A. Porcine epidemic diarrhea virus: an overview of current virological and serological diagnostic methods. Virus Res. 2016;226:60–70. doi: 10.1016/j.virusres.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crawford K., Lager K., Miller L., Opriessnig T., Gerber P., Hesse R. Evaluation of porcine epidemic diarrhea virus transmission and the immune response in growing pigs. Vet Res. 2015;46:49. doi: 10.1186/s13567-015-0180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90•.Gimenez-Lirola L.G., Zhang J., Carrillo-Avila J.A., Chen Q., Magtoto R., Poonsuk K., Baum D.H., Pineyro P., Zimmerman J. Reactivity of porcine epidemic diarrhea virus structural proteins to antibodies against porcine enteric coronaviruses: diagnostic implications. J Clin Microbiol. 2017;55:1426–1436. doi: 10.1128/JCM.02507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; A nice study on the immunogenicity and reactivity among porcine enteric CoV structural proteins.
- 91.de Arriba M.L., Carvajal A., Pozo J., Rubio P. Mucosal and systemic isotype-specific antibody responses and protection in conventional pigs exposed to virulent or attenuated porcine epidemic diarrhoea virus. Vet Immunol Immunopathol. 2002;85:85–97. doi: 10.1016/s0165-2427(01)00417-2. [DOI] [PubMed] [Google Scholar]
- 92.de Arriba M.L., Carvajal A., Pozo J., Rubio P. Isotype-specific antibody-secreting cells in systemic and mucosal associated lymphoid tissues and antibody responses in serum of conventional pigs inoculated with PEDV. Vet Immunol Immunopathol. 2002;84:1–16. doi: 10.1016/S0165-2427(01)00386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ouyang K., Shyu D.L., Dhakal S., Hiremath J., Binjawadagi B., Lakshmanappa Y.S., Guo R., Ransburgh R., Bondra K.M., Gauger P. Evaluation of humoral immune status in porcine epidemic diarrhea virus (PEDV) infected sows under field conditions. Vet Res. 2015;46:140. doi: 10.1186/s13567-015-0285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin C.M., Ghimire S., Hou Y., Langel S.N., Vlasova A.N., Saif L.J., Wang Q. Pathogenicity and immunogenicity of attenuated porcine epidemic diarrhea virus PC22A strain in conventional weaned pigs. BMC Vet Res. 2019 doi: 10.1186/s12917-018-1756-x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Derbyshire J.B., Jessett D.M., Newman G. An experimental epidemiological study of porcine transmissible gastroenteritis. J Comp Pathol. 1969;79:445–452. doi: 10.1016/0021-9975(69)90064-4. [DOI] [PubMed] [Google Scholar]
- 96.Cao L., Ge X., Gao Y., Ren Y., Ren X., Li G. Porcine epidemic diarrhea virus infection induces NF-kappaB activation through the TLR2, TLR3 and TLR9 pathways in porcine intestinal epithelial cells. J Gen Virol. 2015;96:1757–1767. doi: 10.1099/vir.0.000133. [DOI] [PubMed] [Google Scholar]
- 97.Xu X., Zhang H., Zhang Q., Huang Y., Dong J., Liang Y., Liu H.J., Tong D. Porcine epidemic diarrhea virus N protein prolongs S-phase cell cycle, induces endoplasmic reticulum stress, and up-regulates interleukin-8 expression. Vet Microbiol. 2013;164:212–221. doi: 10.1016/j.vetmic.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu X., Zhang H., Zhang Q., Dong J., Liang Y., Huang Y., Liu H.J., Tong D. Porcine epidemic diarrhea virus E protein causes endoplasmic reticulum stress and up-regulates interleukin-8 expression. Virol J. 2013;10:26. doi: 10.1186/1743-422X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang D., Fang L., Shi Y., Zhang H., Gao L., Peng G., Chen H., Li K., Xiao S. Porcine epidemic diarrhea virus 3C-like protease regulates its interferon antagonism by cleaving NEMO. J Virol. 2015;90:2090–2101. doi: 10.1128/JVI.02514-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ding Z., Fang L., Jing H., Zeng S., Wang D., Liu L., Zhang H., Luo R., Chen H., Xiao S. Porcine epidemic diarrhea virus nucleocapsid protein antagonizes beta interferon production by sequestering the interaction between IRF3 and TBK1. J Virol. 2014;88:8936–8945. doi: 10.1128/JVI.00700-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Q., Shi K., Yoo D. Suppression of type I interferon production by porcine epidemic diarrhea virus and degradation of CREB-binding protein by nsp1. Virology. 2016;489:252–268. doi: 10.1016/j.virol.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Q., Ke H., Blikslager A., Fujita T., Yoo D. Type III interferon restriction by porcine epidemic diarrhea virus and the role of viral protein nsp1 in IRF1 signaling. J Virol. 2018;92 doi: 10.1128/JVI.01677-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin C.M., Annamalai T., Liu X., Gao X., Lu Z., El-Tholoth M., Hu H., Saif L.J., Wang Q. Experimental infection of a US spike-insertion deletion porcine epidemic diarrhea virus in conventional nursing piglets and cross-protection to the original US PEDV infection. Vet Res. 2015;46:134. doi: 10.1186/s13567-015-0278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crawford K., Lager K.M., Kulshreshtha V., Miller L.C., Faaberg K.S. Status of vaccines for porcine epidemic diarrhea virus in the United States and Canada. Virus Res. 2016;226:108–116. doi: 10.1016/j.virusres.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 105.Song D., Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44:167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okda F.A., Lawson S., Singrey A., Nelson J., Hain K.S., Joshi L.R., Christopher-Hennings J., Nelson E.A., Diel D.G. The S2 glycoprotein subunit of porcine epidemic diarrhea virus contains immunodominant neutralizing epitopes. Virology. 2017;509:185–194. doi: 10.1016/j.virol.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li C., Li W., Lucio de Esesarte E., Guo H., van den Elzen P., Aarts E., van den Born E., Rottier P.J.M., Bosch B.J. Cell attachment domains of the porcine epidemic diarrhea virus spike protein are key targets of neutralizing antibodies. J Virol. 2017;91 doi: 10.1128/JVI.00273-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chattha K.S., Roth J.A., Saif L.J. Strategies for design and application of enteric viral vaccines. Annu Rev Anim Biosci. 2015;3:375–395. doi: 10.1146/annurev-animal-022114-111038. [DOI] [PubMed] [Google Scholar]
- 109.Bjustrom-Kraft J., Woodard K., Gimenez-Lirola L., Setness B., Ji J., Lasley P., Nelson E., Zhang J.Q., Baum D., Gauger P. Serum and mammary secretion antibody responses in porcine epidemic diarrhea-immune gilts following porcine epidemic diarrhea vaccination. JSHAP. 2018;26:34–40. [Google Scholar]
- 110.Song D., Moon H., Kang B. Porcine epidemic diarrhea: a review of current epidemiology and available vaccines. Clin Exp Vac Res. 2015;4:166–176. doi: 10.7774/cevr.2015.4.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jung K., Hu H., Saif L.J. Calves are susceptible to the newly emerged porcine deltacoronavirus, but not to the swine enteric alphacoronavirus, porcine epidemic diarrhea virus. Arch Virol. 2017 doi: 10.1007/s00705-017-3351-z. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tamura K., Nei M., Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu H., Jung K., Vlasova A.N., Chepngeno J., Lu Z., Wang Q., Saif L.J. Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States. J Clin Microbiol. 2015;53:1537–1548. doi: 10.1128/JCM.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jung K., Miyazaki A., Hu H., Saif L.J. Susceptibility of porcine IPEC-J2 intestinal epithelial cells to infection with porcine deltacoronavirus (PDCoV) and serum cytokine responses of gnotobiotic pigs to acute infection with IPEC-J2 cell culture-passaged PDCoV. Vet Microbiol. 2018;221:49–58. doi: 10.1016/j.vetmic.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]