Abstract
Background
We aimed to assess the efficacy of ultrasound-guided bilateral erector spinae plane block (ESPB) compared to intrathecal morphine (ITM) for analgesia after elective cesarean delivery under spinal anesthesia.
Methods
In total, 140 parturients scheduled for elective cesarean section under spinal anesthesia were randomly allocated into two equal groups. The ESPB-group received 10 mg hyperbaric bupivacaine intrathecally through spinal anesthesia, followed by an ESPB at the ninth thoracic transverse process with 20 mL of 0.5% bupivacaine immediately after the operation. The ITM-group received 10 mg hyperbaric bupivacaine with 100 mcg morphine intrathecally through spinal anesthesia, followed by a sham block at the end of the surgery. The visual analogue scale (VAS) score for pain at several postoperative time points, total opioid consumption, and time to the first analgesic request were evaluated. Statistical analysis was performed with the independent t-test and linear mixed-effects models. The Kaplan–Meier estimator and the log-rank test were used to compare the primary and secondary outcomes of the groups.
Results
No significant differences were observed between the groups regarding patient characteristics; in the post-operative period (0–24 hrs), VAS scores (at rest) were, on average, 0.25 units higher in the ITM group. The total tramadol consumption in the first 24 hrs was significantly higher in the ITM group than in the ESPB group (101.71 ± 25.67 mg vs 44 ± 16.71 mg, respectively). The time to the first analgesic request was 4.93±0.82 hrs in the ITM group and 12±2.81 hrs in the ESPB group. Patient satisfaction did not differ significantly.
Conclusion
ESPB has a successful postoperative analgesic effect and may limit opioid consumption in parturients undergoing elective caesarean delivery.
Keywords: erector spinae plane block, intrathecal morphine, cesarean, analgesia
Introduction
Cesarean section is usually accompanied by moderate to severe pain.1 The pain has both somatic and visceral components, and parturients usually describe it as arising from the abdominal wall incision.2 Insufficient postoperative analgesia is one of the most common factors leading to poor patient satisfaction following caesarean section.3 Effective postoperative analgesia facilitates early mobilization of the mother and infant care, prevents postoperative morbidity, increases patient satisfaction, and decreases the duration of hospital stay.4
The ideal method for postoperative pain management after caesarean section under spinal anesthesia remains unknown. There are many techniques, such as those involving spinal and/or systemic opioids, being used as part of a multimodal postoperative analgesic protocol.5 However, opioids administered using both techniques are frequently associated with adverse effects such as nausea, vomiting, lethargy, itching, risk of delayed maternal respiratory depression, delayed initiation of breastfeeding, and impaired mother-infant bonding.5,6
Multimodal analgesic techniques including abdominal nerve blocks and truncal blocks—such as transversus abdominis plane (TAP) blocks—with parenteral analgesics are becoming popular for post-cesarean pain relief.7 Ultrasound (US)-guided bilateral erector spinae plane block (ESPB) is a new technique being used for analgesia after cesarean section.8
ESPB was initially described by Forero et al for thoracic analgesia at the T5 transverse process.9 ESPB delivers widespread, potent analgesia unilaterally. The block is achieved by injecting an anesthetic into the plane between the erector spinae muscle and the transverse process; the anesthetic diffuses into the paravertebral space through spaces among nearby vertebrae. The anesthetic then blocks the dorsal and ventral rami of the spinal nerves.9,10
We hypothesized that the bilateral ESPB would effectively reduce postoperative pain after elective cesarean section and that it may be used, instead of intrathecal morphine (ITM), as part of a multimodal opioid-sparing analgesic procedure.
The aim of our study was to evaluate the efficacy of ESPB compared to ITM for analgesia after elective cesarean delivery under spinal anesthesia.
Methods
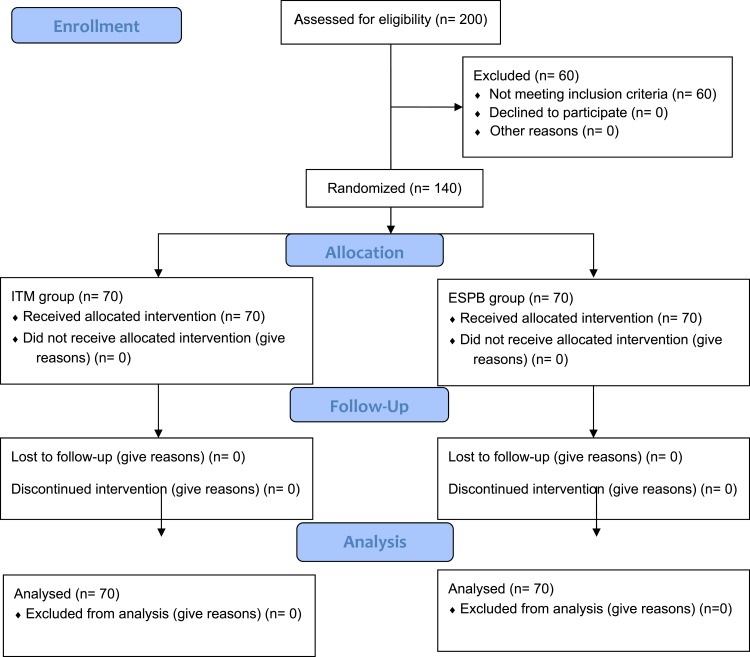
This randomized, prospective, double-blind study was conducted in accordance with the tenets of the Declaration of Helsinki. The study design was approved by the ethical review board of Fayoum University Hospital, and written informed consent was acquired from all participants. The study was conducted after registration on ClinicalTrials.gov (NCT03935412; principal investigator: Mohamed Ahmed Hamed; date of registration: March 1, 2019, no plan to share individual participant data (IPD)). Prospective participants were scheduled for elective cesarean section under spinal anesthesia between March 5, 2019 and July 5, 2019. This manuscript adheres to the applicable CONSORT guidelines [Figure 1].
Figure 1.
Patient flowchart diagram.
Abbreviations: n, number; ITM, intrathecal morphine; ESPB, erector spinae plane block.
Parturients were suitable for enrollment if they met the following inclusion criteria: (1) age 18–40 years; (2) American Society of Anesthesiologists physical status classification ΙΙ; and (3) scheduled for elective cesarean section using a low transverse Pfannenstiel incision with spinal anesthesia. The exclusion criteria were as follows: (1) major hepatic, renal, or cardiovascular disease; (2) local infection; (3) bleeding disorder; (4) any contraindication for spinal anesthesia; or (5) known allergy to any drug used in the study.
Participants were randomly allocated into two equal groups (the ITM group or the ESPB group) using computer-generated random numbers placed in separate opaque envelopes that were opened by the study investigators just before performing the intrathecal block. The participants and data collectors were unaware of group allocation until study completion.
Preoperative investigations, including an electrocardiogram (ECG), complete blood count, renal function tests, liver function tests, and a coagulation profile, were performed. All parturients fasted for 8 hrs prior to the operation. Upon arrival to the operating room, intravenous (IV) access was obtained (one 18G peripheral venous cannula), and the routine monitoring procedures (pulse oximetry, ECG, and noninvasive blood pressure monitoring) were initiated. All parturients received 1 mg granisetron and 50 mg ranitidine intravenously as premedications, and 10 mL.kg−1 of Ringer’s lactate solution was infused over a period of 15 mins as a preload. With the parturient in a sitting position, the skin on the back was sterilized.3 mL of 2% lidocaine was then administered as a subcutaneous infiltration. Spinal anesthesia was performed using a midline method into the L4-5 interspaces with a 25G Quincke spinal needle. After confirming free flow of the cerebrospinal fluid through the needle, 10 mg of 0.5% hyperbaric bupivacaine was slowly introduced for those in the ESPB group; those in the ITM group received a spinal injection of 10 mg of hyperbaric 0.5% bupivacaine plus 100 mcg of preservative-free morphine. The patient was then immediately moved to the supine position with a 15° left tilt and fitted with an oxygen mask. After confirming an adequate anesthesia level, the surgical procedure was performed with continuous hemodynamic monitoring and recording. If the systolic blood pressure reduced to 20% below the baseline or less than 90 mmHg, 5 mg ephedrine was administered intravenously. Moreover, if the heart rate slowed to 50 beats/min or less, 0.5 mg atropine was administered intravenously. Upon delivery of the fetus, 10 units of oxytocin were given by IV infusion.
At the end of the cesarean section surgery, parturients in the ESPB group underwent bilateral ESPB at the level of the ninth thoracic transverse process using a linear US transducer (Phillips; Saronno, Italy). Parturients turned to a lateral position, and the transducer was positioned vertically 3 cm to the side of the midline to visualize the muscles of the back, the transverse process, and the pleura among the two transverse processes. Then, after subcutaneous infiltration of 3 mL of 2% lidocaine, a 22G blunt needle (Spinocan, B. Braun Melsungen AG, Germany) was introduced in the cranial-caudal direction toward the transverse process (T9) using the in-plane method till the needle tip crosses all the muscles. After ensuring negative aspiration, interfascial injection of 20 mL of 0.5% bupivacaine (200 mg) was performed. The procedure was repeated on the opposite side of the back.
Sham blocks were used for participants in the ITM group; the sham blocks involved a non-invasive ultrasound scan, and a short bevel needle was gently pressed on both sides. We instructed the participants to report any signs of local anesthetic toxicity throughout the injection procedure (eg, change in mental status, anxiety, oral numbness, and ringing in the ears). For all participants, spinal level was assessed and recorded before the block. At the end of the cesarean section, parturients were transported to a postoperative anesthesia care unit (PACU), and the routine monitoring procedures were followed. Any intraoperative or postoperative nausea or vomiting was managed with 10 mg metoclopramide. All participants received 30 mg ketorolac intravenously at the time of the ESPB or sham blocks. Participants were transferred to the obstetrics ward when they attained a modified Aldrete score ≥9. Throughout the first 24 hrs, patients received IV paracetamol 1 g every 8 hrs for postoperative analgesia according to the obstetric department protocol. They also received intravenous tramadol through a patient-controlled analgesia (PCA) system (concentration of 4 mg/mL); with; a 20 mg dose, a 10 mins lockout interval and a 50 mg 1 hr limit as supplementary analgesia.
Postoperative mean arterial blood pressure, postoperative pulse rate, and postoperative visual analogue scale (VAS) pain score (ranging from 0 to 10, where 0 indicated no pain and 10 indicated maximum pain) were measured at rest and with cough. All of these parameters were assessed upon arrival in the PACU and at 4, 8, 12, 16, and 24 hrs. The total tramadol intake in 24 hrs and the time to the first analgesic request using the PCA system were obtained from electronic memory of the PCA device. Participant satisfaction was assessed on a 4-point scale (1: excellent, 2: good 3: fair, 4: poor). Adverse effects included nausea and vomiting (0 = no symptoms, 1 = only nausea, 2 = nausea and vomiting), respiratory depression (respiratory rate less than 10), sedation (0 = awake and alert, 1 = quietly awake, 2 = asleep but easily arousable, 3 = deep sleep, responding to painful stimulus), and pruritus (1 = no pruritus, 2 = mild pruritus, 3 = moderate pruritus, 4 = severe pruritus). The data collector was blinded to group distribution.
The primary outcome of our study was the severity of pain, measured using the VAS score 8 hrs after the surgery during rest. Secondary outcomes were the total tramadol intake, VAS score at other postoperative time points (upon arrival to the PACU and 4, 12, 16, and 24 hrs after surgery), the time to the first analgesic request, participant satisfaction, and any side effects or complications.
Statistical Analysis
Sample size was calculated using G*Power version 3.1.9.4. A minimal sample size of 64 patients in each group was needed for a power level of 0.80, alpha level of 0.05 (two tailed), and a medium effect size of 0.50 for the VAS score at 8 hrs (it is equal to a mean difference of 1 point, with an SD of 2 points). To overcome the loss to follow up, the calculated sample size was increased by 10% to reach 70 participants in each group.
The study data were prospectively entered into a computer database for further analysis using SPSS Statistics for Windows, Version 20.0 (SPSS Inc., Chicago, IL, USA). Numerical variables such as age and body mass index were normally distributed and described as mean ± standard deviation (SD). An independent t-test was used to compare the mean values of the two groups. The VAS scores at different time points were not normally distributed and were presented as median and interquartile range (IQR); linear mixed models were used to account for repeated measures of VAS scores. A fixed effect model was used for the intervention group, and a random effect model was used to adjust for repeated measures over time. The time-to-event variables were evaluated using the Kaplan–Meier method, and the log-rank test was used to compare the groups. Qualitative data were presented as numbers and percentages, and the chi-squared test was used to determine significance. A two-sided P-value of <0.05 was considered statistically significant.
Results
This study involved 140 parturients scheduled for elective cesarean section (70 parturients in each group); no parturients were excluded from the study. No significant differences were observed between the groups regarding patient characteristics (age, body mass index, parity, spinal level, or duration of surgery) [Table 1].
Table 1.
Comparison Between Intrathecal Morphine and Erector Spinae Groups According to Demographic and Operative Data
| ITM Group (n=70) | ESPB Group (n=70) | p-value | |
|---|---|---|---|
| Age (years) | 27.57 ± 6.11 | 27.97 ± 6.03 | 0.69 |
| BMI | 25.54 ± 4.74 | 25.71 ± 4.68 | 0.83 |
| Parity | |||
| Nulliparous | 28 (40%) | 25 (35.7%) | 0.72 |
| Multiparous | 42 (60%) | 45 (64.3%) | |
| Spinal level | |||
| T4/T5 | 23 (36.5%) | 17 (24.3%) | 0.45 |
| T6/T9 | 18 (28.6%) | 21 (30%) | |
| T10 | 14 (22.2%) | 20 (28.6%) | |
| Lower thanT10 | 8 (12.7%) | 12 (17.1%) | |
| Duration of surgery (minutes) | 39.83 ± 11.97 | 39.69 ± 11.81 | 0.94 |
Note: Variables are reported as mean ± SD or number and percent.
Abbreviations: ITM, intrathecal morphine; ESPB, erector spinae plane block; BMI, body mass index; T, thoracic; N, number.
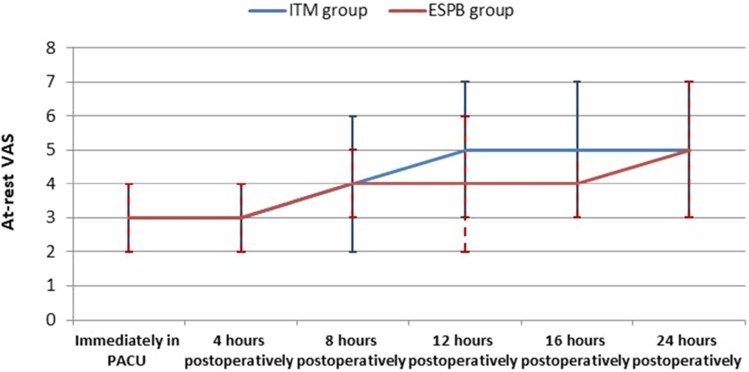
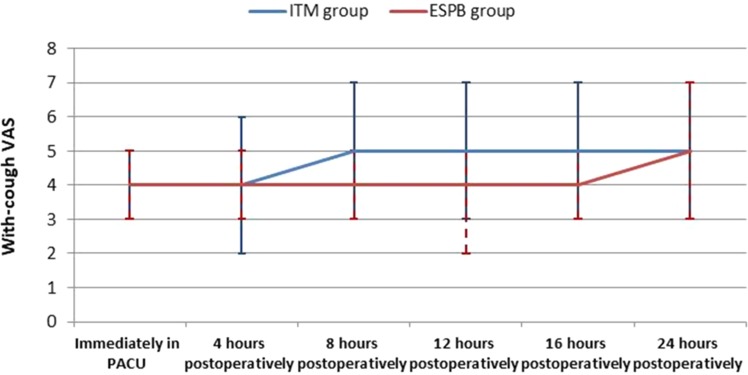
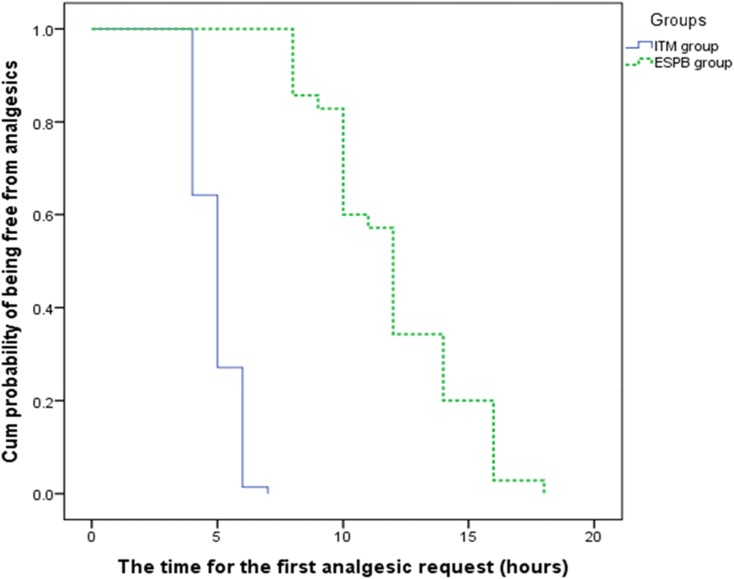
Table 2 shows the differences in VAS pain scores (at rest and while coughing) between the groups over time. Figure 2 show during the post-operative period (0–24 hrs), at-rest VAS scores were, on average, 0.25 units higher in the ITM group (estimate = 0.25, 95% CI = 0.07–0.43, t = 2.678, p = 0.008). During the first 8 hrs, at-rest VAS scores were, on average, 0.31 units higher in the ITM group (estimate = 0.31, 95% CI = 0.08–0.54, t = 2.712, p = 0.008). Figure 3 during the post-operative period (0–24 hrs), with-cough VAS scores were, on average, 0.34 units higher in the ITM group (estimate = 0.34, 95% CI = 0.17–0.52, t = 3.971, p = <0.0001). During the first 8 hrs, with-cough VAS scores were, on average, 0.33 units higher in the ITM group (estimate = 0.33, 95% CI = 0.12–0.53, t = 3.168, p = 0.002).The total tramadol consumption in the first 24 hrs was significantly higher in the ITM group than in the ESPB group (101.71 ± 25.67 mg vs 44 ± 16.71 mg, respectively) [Table 3]. The time to the first analgesic request differed significantly between the groups (4.93 ±0.82 hrs in ITM group, 12 ± 2.81 hrs in the ESPB group). The time to the first analgesic request was significantly shorter in the ITM group, as described using the Kaplan–Meier method (p <0.0001) [Table 3, Figure 4]. No significant differences were observed between the groups regarding participant satisfaction [Table 4]. No adverse effects or complications were documented in either group.
Table 2.
Comparison Between Intrathecal Morphine and Erector Spinae Groups According to Visual Analog Scale (VAS) at Rest and with Cough
| VAS | ITM Group (n=70) | ESPB Group (n=70) | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Immediately in PACU | At rest | 3 | (3–4) | 3 | (3–4) |
| With cough | 4 | (3–4) | 4 | (3–4) | |
| 4 hrs postoperatively | At rest | 3 | (3–4) | 3 | (3–4) |
| With cough | 4 | (3–5) | 4 | (3–4) | |
| 8 hrs postoperatively | At rest | 4 | (4–6) | 4 | (4–5) |
| With cough | 5 | (4–6) | 4 | (4–5) | |
| 12 hrs postoperatively | At rest | 5 | (4–6) | 4 | (3–5) |
| With cough | 5 | (4–6) | 4 | (4–5) | |
| 16 hrs postoperatively | At rest | 5 | (4–6) | 4 | (4–5) |
| With cough | 5 | (4–6) | 4 | (4–5) | |
| 24 hrs postoperatively | At rest | 5 | (4–6) | 5 | (4–6) |
| With cough | 5 | (4–6) | 5 | (4–6) | |
Abbreviations: VAS, visual analog scale; ITM, intrathecal morphine; ESPB, erector spinae plane block; N, number; PACU, post anesthetic care unit.
Figure 2.
At-rest VAS.
Abbreviations: VAS, visual analog scale; ITM, intrathecal morphine; ESPB, erector spinae plane block; PACU, post anesthetic care unit.
Figure 3.
With-cough VAS.
Abbreviations: VAS, visual analog scale; ITM, intrathecal morphine; ESPB, erector spinae plane block; PACU, post anesthetic care unit.
Table 3.
Comparison Between Intrathecal Morphine and Erector Spinae Groups According to Total Tramadol Analgesic Consumption in mg Within 24 hrs and First Analgesic Requirement in Hours
| ITM Group (n=70) | ESPB Group (n=70) | p-value | |
|---|---|---|---|
| Total tramadol consumption (mg) | 101.71 ± 25.67 | 44 ± 16.71 | 0.00* |
| First analgesic requirement (hours) | 4.93 ±0.82 | 12 ± 2.81 | 0.00* |
Notes: All variables are reported as mean ± SD. *Statistically significant.
Abbreviations: ITM, intrathecal morphine; ESPB, erector spinae plane block; N, number.
Figure 4.
The time for the first analgesic request.
Abbreviations: ITM, intrathecal morphine; ESPB, erector spinae plane block.
Table 4.
Patient Satisfaction
| Satisfaction | ITM Group (n=70) | ESPB Group (n=70) | p-value |
|---|---|---|---|
| Excellent | 30 (42.9%) | 37 (52.9%) | 0.3 |
| Good | 40 (57.1%) | 33 (47.1%) |
Note: All variables are reported as number and percent.
Abbreviations: ITM, intrathecal morphine; ESPB, erector spinae plane block; N, number.
Discussion
Pain after cesarean section complicates the postoperative recovery. Eisenach et al11 demonstrated that acute pain following delivery represents a significant risk for persistent pain and depression, and it remains a challenge to provide postoperative pain management that is adequate and safe for both mother and baby.12
Intrathecal morphine has been described as highly effective and is the mainstay method for pain control after cesarean section.13 Unfortunately, its use is accompanied by undesirable drawbacks such as nausea and vomiting, urine retention, pruritus, and the risk of delayed maternal respiratory depression.14 Currently, multimodal opioid-sparing analgesia has become a popular alternative for postoperative pain management.15
Many publications have validated the ESPB as an effective component of a multimodal regimen for postoperative analgesia for different types of surgery. Ultrasound-guided ESPB was recently described by Forero et al at the level of the 5th thoracic vertebra with a successful unilateral complete sensory blockade, effectively spreading local anesthetic from C7-T1 to T8.9
ESPB was first described for thoracic pain management,9 thoracoscopic lobectomy,16 breast surgeries,17 and costal fractures.18 Further publications described its use for abdominal surgeries such as ventral hernia repair,19 abdominoplasty,20 bariatric surgeries,21 laparoscopic abdominal surgeries,22 cesarean section8,23 and total abdominal hysterectomy.24
To assess the efficacy of ESPB in patients undergoing ventral hernia repair, Chin et al19 performed the block at T7 TP in a cadaveric model and evaluated the degree of injectate distribution using a computed tomography scan. They reported that the injectate was distributed cranially to the upper thoracic levels and caudally to the L2-L3 transverse processes. As such, we decided to perform the block at the T9 TP level.
The aim of the present study was to evaluate the analgesic effect of ESPB in comparison with ITM after elective cesarean section under spinal anesthesia. We observed a prolonged duration of analgesia among parturients in the ESPB group. The VAS scores were higher in the ITM group during the first 8 hrs both at-rest and with cough. Moreover, we observed higher VAS scores in the ITM group during the post-operative period (0–24 hrs) both at-rest and with cough.
Previous studies have shown ESPB to be a successful technique for postoperative analgesia with variable duration. In a study by Tulgar et al,22 the analgesic effect of ESPB extended for 17, 13, and 16 hrs in three patients undergoing multiple abdominal procedures. In a recent study by Hamed et al,24 the analgesic effect of ESPB extended for 12 hrs in patients undergoing total abdominal hysterectomy.
In a recently published case report by Altinpulluk et al,8 reported long-term analgesia in a patient undergoing lower abdominal cesarean section after bilateral ESPB using single injection; they reported numeric rating scale scores of 1 to 3 in the first 24 hrs.
The current study demonstrated that, compared to ITM, ESPB significantly delayed the time to the first analgesic request and significantly lowered tramadol consumption.
In their randomized controlled trial, Kanazi et al13 demonstrated that ITM provides better analgesia than does transverse abdominis plane block, with lower VAS pain scores, delayed request for supplemental analgesic, and less tramadol consumption after cesarean section. They explained their findings by the effectiveness of ITM to treat somatic and visceral pain arising from the wound and the uterus, respectively. Conversely, transverse abdominis plane block deals only with somatic pain.
ESPB also provides both somatic and visceral abdominal analgesia when administered at the level of T7-9 TP.19,21 As such, ESPB at the level of T9 can provide effective analgesia after caesarean section and reduce the consumption of opioids and their adverse effects. This indicates ESPB can successfully be used for analgesia after caesarean delivery.
The current study showed no difference in participant satisfaction despite better pain control in the ESPB group. This can be attributed to the fact that participant satisfaction is a complex phenomenon and does not depend on pain control alone.
We observed no side effects in this study. Despite the drawbacks of ITM, no nausea or vomiting were noted. This may be due to the premedication of 1 mg granisetron administered to all participants. No urine retention was recorded, as all participants were catheterized using a Foley catheter. We also did not observe any pruritus or respiratory depression. The incidence of pruritus related to ITM varies widely from 0% to 100%; this wide range of variation may explain why we did not observe any cases of pruritus among our participants. We observed no complications in this study, the only complication after ESPB was a pneumothorax as described by Ueshima.25
Limitations of the current study include the inability to assess the success rate and distribution of ESPB due to the residual block from intrathecal anesthesia, which continues in the early postoperative period. Additionally, limited data were available regarding the effectiveness of ESPB for postoperative analgesia after caesarean delivery; this led to inadequate result comparisons. Additionally, all patients had a Foley catheter, it would not be properly assessed postoperative urinary retention.
Even with these limitations, the data of the current study may be used to validate ESPB as a successful technique for both decreasing pain scores and opioid consumption so that it can be used in multimodal analgesia and opioid-sparing protocols.
In conclusion, ESPB has a successful postoperative analgesic effect and limits opioid consumption in parturients undergoing caesarean delivery.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Leung AY. Postoperative pain management in obstetric anesthesia—new challenges and solutions. J Clin Anesth. 2004;16:57–65. doi: 10.1016/j.jclinane.2003.02.012 [DOI] [PubMed] [Google Scholar]
- 2.Urbanczae L. Transverse abdominis plane block. Anesth Intensive Ther. 2009;35:137–141. [Google Scholar]
- 3.Mishriky BM, George RB, Habib AS. Transversus abdominis plane block for analgesia after Cesarean delivery: a systematic review and meta-analysis. Can J Anaesth. 2012;59(8):766–778. doi: 10.1007/s12630-012-9729-1 [DOI] [PubMed] [Google Scholar]
- 4.Buhagiar L, Cassar OA, Brincat MP, et al. Predictors of post-caesarean section pain and analgesic consumption. J Anaesthesiol Clin Pharmacol. 2011;27(2):185. doi: 10.4103/0970-9185.81822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fusco P, Scimia P, Paladini G, et al. Transversus abdominis plane block for analgesia after Cesarean delivery: a systematic review. Minerva Anestesiol. 2015;81(2):195–204. [PubMed] [Google Scholar]
- 6.White PF. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg. 2005;101:S5–S22. doi: 10.1213/01.ANE.0000177099.28914.A7 [DOI] [PubMed] [Google Scholar]
- 7.Baeriswyl M, Kirkham KR, Kern C, Albrecht E. The analgesic efficacy of ultrasound-guided transversus abdominis plane block in adult patients: a meta-analysis. Anesth Analg. 2015;121:1640–1654. doi: 10.1213/ANE.0000000000000967 [DOI] [PubMed] [Google Scholar]
- 8.Altinpulluk EY, Simón DG, Fajardo-Pérez M. Erector spinae plane block for analgesia after lower segment caesarean section: case report. Rev Esp Anestesiol Reanim. 2018;65(5):284–286. doi: 10.1016/j.redar.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 9.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–627. doi: 10.1097/AAP.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 10.Ueshima H, Otake H. Similarities between the retrolaminar and erector spinae plane blocks. Reg Anesth Pain Med. 2017;42:123–124. doi: 10.1097/AAP.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 11.Eisenach JC, Pan PH, Smiley R, Lavand’homme P, Landau R, Houle TT. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87–94. doi: 10.1016/j.pain.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava U, Verma S, Singh TK, et al. Efficacy of trans abdominis plane block for post cesarean delivery analgesia: a double-blind, randomized trial. Saudi J Anaesth. 2015;9(3):298–302. doi: 10.4103/1658-354X.154732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanazi GE, Aouad MT, Abdallah FW, et al. The analgesic efficacy of subarachnoid morphine in comparison with ultrasound-guided transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg. 2010;111(2):475–481. doi: 10.1213/ANE.0b013e3181e30b9f [DOI] [PubMed] [Google Scholar]
- 14.Dahl JB, Jeppesen IS, Jorgensen H, Wetterslev J, Moiniche S. Intraoperative and postoperative analgesic efficacy and adverse effects of intrathecal opioids in patients undergoing cesarean section with spinal anesthesia: a qualitative and quantitative systematic review of randomized controlled trials. Anesthesiology. 1999;91:1919–1927. doi: 10.1097/00000542-199912000-00045 [DOI] [PubMed] [Google Scholar]
- 15.Rafiq S, Steinbruchel DA, Wanscher MJ, et al. Multimodal analgesia versus traditional opioid based analgesia after cardiac surgery, a randomized controlled trial. J Cardiothoracic Surg. 2014;9(52):1–8. doi: 10.1186/1749-8090-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scimia P, Basso Ricci E, Droghetti A, Fusco P. The ultrasound-guided continuous erector spinae plane block for postoperative analgesia in video-assisted thoracoscopic lobectomy. RegAnesthPainMed. 2017;42:537. doi:doi: 10.1097/AAP.0000000000000615 [DOI] [PubMed] [Google Scholar]
- 17.Bonvicini D, Giacomazzi A, Pizzirani E. Use of the ultrasound-guided erector spinae plane block in breast surgery. Minerva Anestesiol. 2017;83:1111–1112. doi:doi: 10.23736/S0375-9393.17.12141-3 [DOI] [PubMed] [Google Scholar]
- 18.Hamilton DL, Manickam B. Erector spinae plane block for pain relief in rib fractures. Br J Anaesth. 2017;118:474–475. doi:doi: 10.1093/bja/aew387 [DOI] [PubMed] [Google Scholar]
- 19.Chin KJ, Adhikary S, Sarwani N, Forero M. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia. 2017;72:452–460. doi: 10.1111/anae.13814 [DOI] [PubMed] [Google Scholar]
- 20.Ashok J, Priyanka J, Neelam S. The erector spinae block for postoperative analgesia in abdominoplasty—a case report. BAOJ Anesth. 2017;1:1. [Google Scholar]
- 21.Chin KJ, Malhas L, Perlas A. The erector spinae plane block provides visceral abdominal analgesia in bariatric surgery: a report of 3 cases. Reg Anesth Pain Med. 2017;42:372–376. doi: 10.1097/AAP.0000000000000615 [DOI] [PubMed] [Google Scholar]
- 22.Tulgar S, Selvi O, Kapakli MS. Erector spinae plane block for different laparoscopic abdominal surgeries: case series. Anesthesiology. 2018;2018:3947281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santonastaso DP, Addis DC, Mastronardi C, Pini R, Agnoletti V. Ultrasound guided erector spinae plane block for post-operative pain control after caesarean section. J Clin Anesth. 2019;58:45–46. doi: 10.1016/j.jclinane.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Hamed MA, Goda AS, Basiony MM, Fargaly OS, Ahmed Abdelhady M. Erector spinae plane block for postoperative analgesia in patients undergoing total abdominal hysterectomy: a randomized controlled study original study. J Pain Res. 2019;12:1393–1398. doi: 10.2147/JPR.S196501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueshima H. Pneumothorax after the erector spinae plane block. J Clin Anesth. 2018;48:12. doi: 10.1016/j.jclinane.2018.04.009 [DOI] [PubMed] [Google Scholar]