Abstract
Background
Binge-eating disorder (BED) is associated with impaired quality of life and has a number of untoward public health associations. There are few established pharmacological treatments for BED, and available options are not suitable for all individuals. Vortioxetine is a recently developed pharmacological agent with effects on the serotonergic but also other neurochemical systems, which has yet to be evaluated in this context.
Methods
Eighty adults with BED were recruited for a double-blind, placebo-controlled study. Participants received 12-week treatment with vortioxetine (10mg/day for one week, then increasing to 20mg/day) or placebo in a parallel design. The primary efficacy outcome measures were binge eating frequency and weight. Safety data were collected. Effects of active versus placebo treatment were characterized using linear repeated measures models.
Results
Both vortioxetine and placebo treatment were associated with significant reductions in binge eating frequency. Vortioxetine did not differentiate significantly from placebo on any efficacy measure. Frequency of adverse events did not differ between groups.
Discussion
Vortioxetine was not more effective than placebo in the treatment of BED. The ability to detect pharmacological treatment benefit may have been hindered by the relatively high placebo response and drop out. Future work should seek to better understand and predict placebo response in BED, with a view to more targeted treatment interventions and, potentially, sample enrichment.
Keywords: binge eating, obesity, pharmacology, treatment, antidepressant
Introduction
Binge-eating disorder (BED) is characterized by recurrent episodes of excessive food consumption accompanied by a sense of loss of control and psychological distress, but without the inappropriate compensatory weight-loss behaviors of bulimia nervosa (American Psychiatric Association, APA, 2013). Binge eating disorder has been recognized as a serious public health problem as it is often associated with obesity, metabolic syndrome, and psychiatric comorbidities, including depression (Kessler et al., 2013; Mathes et al., 2009). Many patients are undertreated despite BED’s association with functional impairments and personal and social difficulties leading to a poor quality of life (Hudson et al., 2007; Kornstein et al., 2016; Hay et al., 2017).
Current treatments for BED are often inadequate and difficult to access for patients. Cognitive behavioral therapy has demonstrated benefit for BED in a recent meta-analysis (Hilbert et al., 2019) but finding trained psychologists can be challenging. Lisdexamfetamine was recently approved by the FDA for BED (McElroy et al., 2015; McElroy et al., 2016) but, as with other psychostimulant medications, it carries risk of diversion (Cassidy et al., 2015), and potential for misuse especially in individuals vulnerable to addiction (Clemow & Walker, 2014). Other currently available medications, used off-label for BED, include antidepressants and anticonvulsants, both of which may reduce binge eating behaviors but anticonvulsants are often poorly tolerated (McElroy et al., 2012; Goracci et al., 2015). Therefore, additional clinical trials are needed to identify effective treatments.
The pathophysiology of BED is likely quite complex (Kessler et al., 2016) as eating itself, and moreover a pathological form of eating, involves multiple brain regions. Therefore, finding a specific target for pharmacological interventions for BED has proven challenging. The hypothalamic nuclei appear to regulate appetite/hunger, emotional triggers to eating may implicate the amygdala, the rewarding aspects of food may be largely centered on the nucleus accumbens, and disrupted self-regulation may suggest lack of top-down control involving the prefrontal cortices (for a review of neuroimaging studies in BED see Donnelly et al., 2018). Given the varied neural circuitry arguably involved in the pathophysiology of BED, and prior treatment data, the neurochemical dysfunction of BED may include serotonergic, opioidergic, dopaminergic, glutamatergic, and noradrenergic systems (David et al., 2009; Johnson and Kenny, 2010; Latagliata et al., 2010). Thus, a medication to target BED would ideally be multi-modal in terms of its pharmacology.
Vortioxetine has distinctive properties that make it a promising option for people with BED. Animal and human in vitro studies indicate that several neurotransmitter systems may be impacted by vortioxetine, with the drug enhancing levels of serotonin (via multiple serotonin targets), noradrenaline, dopamine, and acetylcholine in certain areas of the brain, as well as modulating γ-aminobutyric acid and glutamate neurotransmission (Gibb and Deeks, 2014; Leiser et al., 2015). In addition, vortioxetine appears to have cognitive enhancing potential, which may be of value in disorders characterized by executive dyscontrol (Reiter et al., 2017; Mahableshwarkar et al., 2015; Al-Sukhni et al., 2015). This suggests that vortioxetine could improve the symptoms of BED by complementary pathways. Given the serious public health problems associated with BED, and the likelihood of success of vortioxetine in treating the disorder, the aim of the present study was to examine the efficacy and safety of vortioxetine compared to placebo in adults with moderate to severe BED. We hypothesized that vortioxetine would be more effective than placebo in reducing the number of binge eating days per week after 12 weeks of treatment when compared to baseline.
Methods
Participants
Eighty adults with primary BED were recruited for a double-blind, placebo-controlled pilot study in which vortioxetine or placebo was administered in a 1:1 fashion. Participants were recruited using media advertisements. All participants were required to have had BED per DSM-5 criteria for at least a year.
The institutional review board for the University of Chicago approved the study and the informed consent process. Prior to obtaining informed consent, the primary investigator and/or trained study personnel discussed potential risks of the study and alternative treatments with participants. After receiving a complete description of the study, participants provided written informed consent. This study was carried out in accordance with the Declaration of Helsinki. Data were collected between September 1, 2016 and May 17, 2018. The study was registered at ClinicalTrials.gov (identifier: NCT02528409).
The following were the inclusion criteria for the study: Men and women aged 18-65 years with a primary DSM-5 diagnosis of BED for at least one year; at least 3 binge eating days per week for the 2 weeks before the baseline visit; and an ability to understand and sign the consent form.
Exclusion criteria included: 1) unstable medical illness based on history or clinically significant abnormalities on baseline physical examination (currently stable medical illness was allowed, for example diabetes, treated hypothyroidism, hypertension); 2) current pregnancy or lactation, or inadequate contraception in women of childbearing potential; 3) participants considered an immediate suicide risk based on the Columbia Suicide Severity rating Scale (C-SSRS) (www.cssrs.columbia.edu/docs); 4) past 12-month psychotic disorder, bipolar disorder, or major depressive disorder (the rationale for exclusion of depression was to avoid the confounding variable in a small sample of depression possibly worsening the binge behavior); 5) past 6-month alcohol or substance use disorder; 6) illegal substance use based on urine toxicology screening; 7) initiation of psychological or weight-loss interventions within 3 months of screening; 8) use of any other prescription psychotropic medication (except a PRN hypnotic or PRN benzodiazepine); 9) previous treatment with vortioxetine; 10) currently taking OTC weight loss medications; and 11) cognitive impairment that interferes with the capacity to understand and self-administer medication or provide written informed consent. Concomitant medications that were deemed to have no psychotropic qualities were allowed.
Procedures
Participants who met inclusion/exclusion criteria were randomized at the conclusion of their baseline visit. All 80 subjects were randomized to vortioxetine or placebo (1:1 randomization) and received 10mg/day or placebo during the first week of the study (the first dose was started the day immediately after the baseline visit). All participants received 10mg or placebo for 7 days and then were evaluated with all measures at week 1 (7 days after baseline). At the week 1 visit, participants were started on 20mg/day (or remained on placebo) for the remainder of the study. They returned at week 2 (corresponding to having been on 20mg/day for one week) and were evaluated with all measures. After week 2 visit, participants were seen every two weeks for the remainder of the 12-week study period (this corresponds to visits at week 4, 6, 8, 10, and 12). Week 12 evaluation was the study endpoint for purposes of efficacy. After study conclusion (at week 12), the dose was tapered off during a 1-week follow-up period. All participants returned at week 13 for final safety evaluation only.
Randomization and Dosing
Participants were randomized (1:1) to receive placebo or vortioxetine by the investigational pharmacy at the University of Chicago in block sizes of eight, using computer-generated randomization with no clinical information, independent of the study team. The study blind was maintained by over-encapsulation, and by making placebo and active treatments appear identical in size, weight, shape, and color.
Dosage changes/reductions outside the above-defined protocol were not permitted. Because nausea, and possibly headache, were the most likely side effects anticipated at the 20mg dose, participants were allowed to use over-the-counter options as needed for headaches or nausea. The use of over-the-counter options was documented at each visit. If side effects continued and were intolerable, the participant was discontinued from the study.
Assessments
Assessment of BED at baseline and at follow-up visits was performed with the following: Binge eating diary (we were aware that this itself could be therapeutic but felt it would be so in both groups), Clinical Global Impressions–Improvement and Severity Scale (Guy, 1976), the Three-Factor Eating Questionnaire (Stunkard and Messick, 1985), and the Yale-Brown Obsessive Compulsive Scale modified for Binge Eating (BE-YBOCS) (Deal et al., 2015). Weight was recorded using a calibrated scale with the participants not wearing shoes, rounded to the nearest 0.5 pounds, and converted to kilograms (to convert, multiply by 0.45) for data reporting purposes.
Baseline psychiatric evaluation was conducted using the MINI International Neuropsychiatric Interview (Sheehan et al., 1998). The baseline and follow-up visits included the following assessments: Urine pregnancy test (for women of childbearing years) and urine drug screen (all subjects), and a psychiatric evaluation (using the following measures: Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960); Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959); patient-rated version of the Sheehan Disability Scale (SDS) (Sheehan, 1983); and the Columbia Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2011). A finger stick blood sample was collected to measure blood glucose levels.
Safety Assessments
Safety and tolerability were assessed using spontaneously reported adverse events data, Columbia–Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2011), vital signs, and by evaluating premature termination. Safety assessments (C-SSRS, sitting blood pressure, heart rate, adverse effects, and concomitant medications) were documented at each visit. Assessment of side effects was done at each visit. Adverse events were coded by system organ class and preferred term using the Medical Dictionary for Regulatory Activities (Med- DRA) Version 11.1.
Data Analysis
The study sample size calculation indicated 80 patients (40/group) were needed to achieve 80% with an effect size of 0.65 when using a 2-group t-test with a significance level of 0.05. Under the achieved sample size of 30 patients (15/group), however, the actual power to detect this change is 40%; there is 56% power to detect an effect size of 0.7.
Descriptive summaries were generated overall and by randomization group. Outcomes were also summarized at all study time points by group. Changes within each group from baseline to endpoint were statistically evaluated using Wilcoxon signed rank tests. Changes from baseline to week 12 between groups were compared using linear repeated measures models with a random intercept for subject. The dependent variable for each model was one outcome variable as defined in the protocol. Independent variables included age at onset, study visit, treatment group, and a visit by treatment group interaction term. For the primary outcome of binge eating frequency, log(binge eating days +1) was modeled and log(binge eating at baseline +1) by study visit interaction was included. Model fit was assessed. The coefficient of interest was the study visit by treatment group interaction. All analyses were conducted in SAS v9.4 and R. There was no imputation for missing data. Statistical analyses were conducted by a team of professional statisticians independent of the research with no knowledge as to which group constituted placebo or medication. Missing data were not imputed. For descriptive summaries, all available data were used for each summary. For the regression models, complete case analysis was used within each time period. For example, if someone missed week 2-3 visit, their data for the other visits were still used in the model. However, if they were missing a baseline predictor (i.e. baseline value or age of onset), then all of their data was omitted.
Given the number of comparisons we adjusted the threshold for what we considered significant using a Bonferroni correction. Specifically, for the primary outcome measures of binge eating frequency and weight, the significance threshold for Table S2 would be modified by 2, so p-values need to be < 0.025 instead of p<0.05 in order to be significant; and the significance threshold for the secondary outcomes should be adjusted by 10 (the number of secondary measures), so p should be < 0.005 (0.05/10) to be significant.
Results
Participant Characteristics
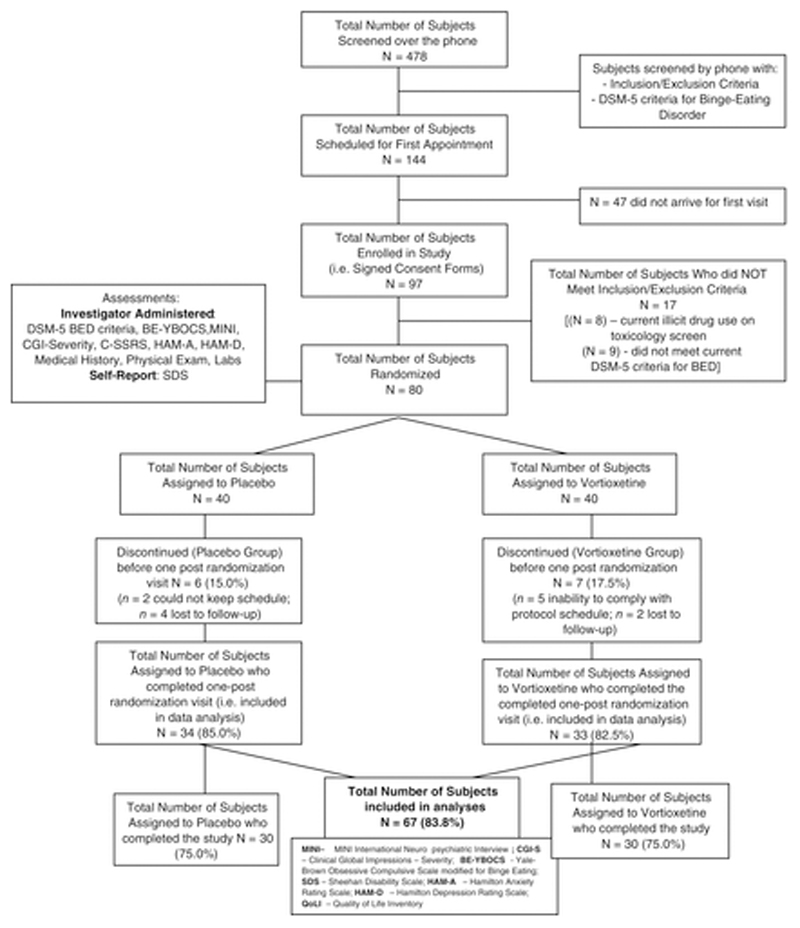
A total of 80 participants were enrolled in the study (see Figure 1). Of these, 67 were included in both the efficacy and safety analysis for this study (13 failed to return for at least one post-randomization visit). Of the 67, only 30 (45%) had primary efficacy data at baseline and week 12. Both groups experienced similar drop-out levels. The rate of study completion did not differ significantly between treatment groups, and there were no statistically significant pretreatment differences between completers and non-completers on any measure. The average number of days of study participation (standard deviation) were 62.3 days (35.2) for placebo and 74.2 days (29.5) for vortioxetine, though both groups had median participation of 91 days (p-value for group difference = 0.1006).
Figure 1.
Consort diagram. Subject flow diagram for vortioxetine versus placebo in the treatment of binge-eating disorder.
Demographic summaries are presented in Table 1 for all participants and by study group. The groups had similar characteristics except that the placebo group had significantly earlier onset of symptoms (age of onset was included as a covariate in outcome analyses). Regarding gender diversity, the proportion of females within each treatment group was the same. Regarding racial diversity, most of the study participants were African American (53%) or Hispanic/Latino (17%) with only 22% identifying as white Caucasian.
Table 1. Demographic Summaries.
| All Participants (n=67) | Randomization Group |
|||
|---|---|---|---|---|
| Placebo (n = 34) | Vortioxetine (n=33) | p-value | ||
| Age (Years) - Mean (SD) | 40.0 (13.1) | 39.8 (13.2) | 40.3 (13.2) | 0.89 |
| Age at Onset (Years) – Mean (SD), missing n=6 | 24.1 (13.7) | 18.7 (9.8) | 29.4 (14.9) | 0.002 |
| Body Mass Index (kg/m2) – Mean (SD) | 37.9 (8.8) | 36.5 (7.9) | 39.3 (9.6) | 0.20 |
| Days of Binge Eating per Week at Baseline – Mean (SD) | 4.3 (1.5) | 4.3 (1.6) | 4.4 (1.5) | 0.74 |
| Sex – n (%) | 0.86 | |||
| Female | 45 (67.2%) | 22 (64.7%) | 23 (69.7%) | |
| Male | 22 (32.8%) | 12 (35.3%) | 10 (30.3%) | |
| Race – n (%) | 0.18 | |||
| African American | 36 (53.7%) | 16 (47.1%) | 20 (60.6%) | |
| Caucasian | 15 (22.4%) | 6 (17.7%) | 9 (27.3%) | |
| Hispanic/Latino | 12 (17.9%) | 8 (23.5%) | 4 (12.1%) | |
| Asian | 2 (3.0%) | 2 (5.9%) | 0 (0.0%) | |
| Multiple Races | 2 (3.0%) | 2 (5.9%) | 0 (0.0%) | |
| Education – n (%) | 0.40 | |||
| Less than high school | 3 (4.5%) | 1 (2.9%) | 2 (6.1%) | |
| Graduated high school/GED | 11 (16.4%) | 3 (8.8%) | 8 (24.2%) | |
| Some college | 25 (37.3%) | 13 (38.2%) | 12 (36.4%) | |
| College graduate | 17 (25.4%) | 11 (32.4%) | 6 (18.2%) | |
| Post graduate (College +) | 11 (16.4%) | 6 (17.7%) | 5 (15.2%) | |
| Marital Status – n (%) | 0.23 | |||
| Single | 47 (70.2%) | 26 (76.5%) | 21 (63.6%) | |
| Married | 9 (13.4%) | 2 (2.9%) | 7 (21.2%) | |
| Divorced/separated | 9 (13.4%) | 5 (14.7%) | 4 (12.1%) | |
| Living together/engaged | 1 (1.5%) | 1 (2.9%) | 0 (0.0%) | |
| Widowed | 1 (1.5%) | 0 (0.0%) | 1 (3.0%) | |
| Employment Status – n (%) | 0.38 | |||
| Employed full time | 21 (31.3%) | 12 (35.3%) | 9 (27.3%) | |
| Employed part time | 15 (22.4%) | 8 (23.5%) | 7 (21.2%) | |
| Student | 5 (7.5%) | 4 (11.8%) | 1 (3.0%) | |
| Retired | 2 (3.0%) | 0 (0.0%) | 2 (6.1%) | |
| Unemployed | 23 (34.3%) | 10 (29.4%) | 13 (39.4%) | |
| Other | 1 (1.5%) | 0 (0.0%) | 1 (3.0%) | |
| Current health conditions (yes) – n (%) | ||||
| High blood pressure | 16 (25.0%) | 9 (29.0%) | 7 (21.2%) | 0.67 |
| High cholesterol | 5 (7.8%) | 1 (3.2%) | 4 (12.1%) | 0.36 |
| Diabetes | 6 (9.4%) | 3 (9.7%) | 3 (9.1%) | 1.00 |
Efficacy Results
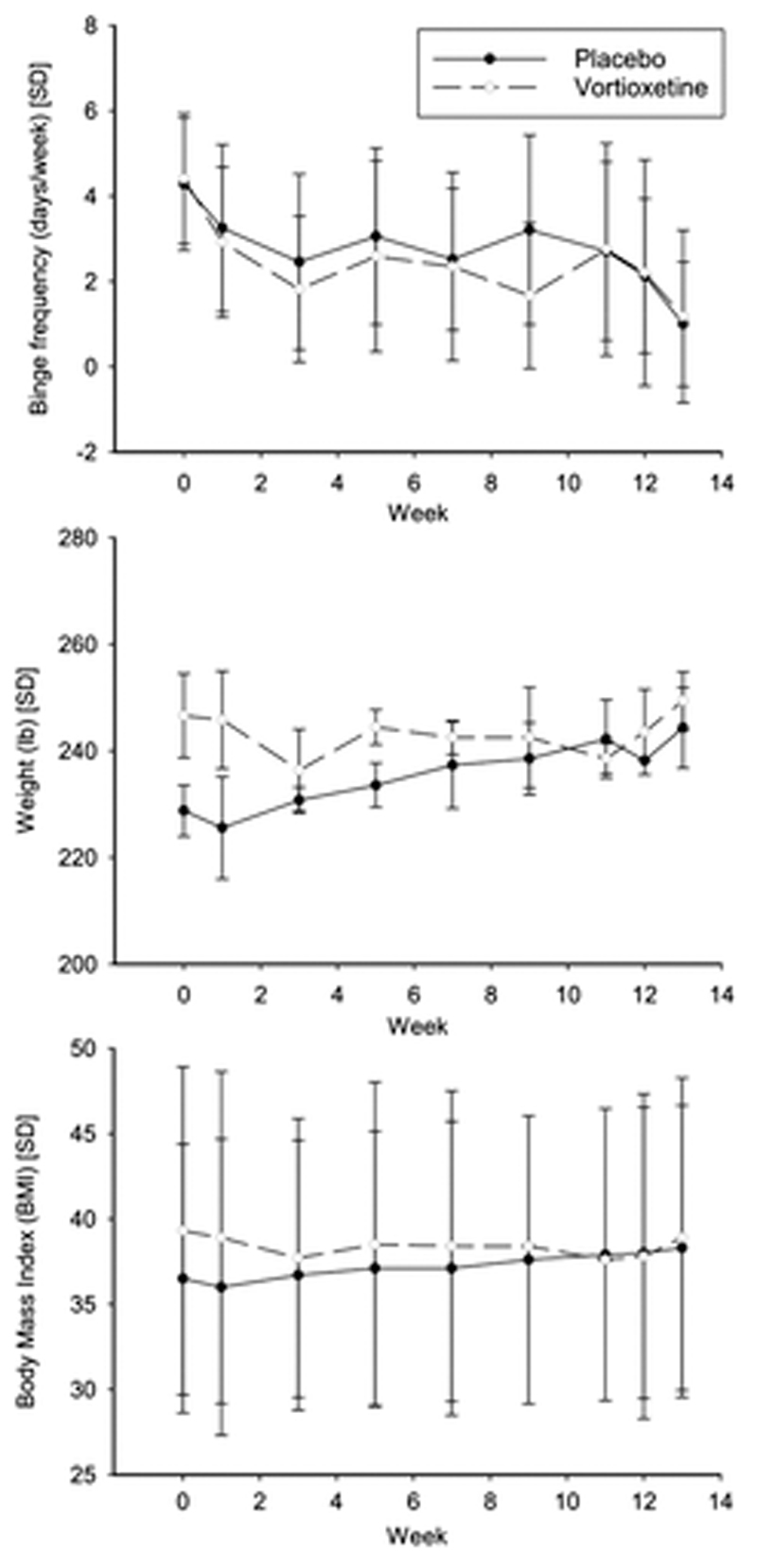
The primary efficacy endpoint for this study was the number of binge eating episodes per week. Efficacy results, also for weight and BMI, at each time point are summarized in Figure 2. Table S1 contains all participants in the efficacy analysis and shows descriptive summaries of binge frequency, weight and BMI by group at each time point. Binge eating frequency significantly reduced over time in both groups. Weight and BMI did not change significantly over time in either group.
Figure 2.
Graphs showing efficacy measures over time in each treatment arm. Top: Binge episodes per week; middle: Weight (lb); bottom: Body mass indexes (BMI). Regression modeling indicated that binge frequency significantly increased over time in each treatment arm (p = .008 for placebo; p = .006 for active treatment). Weight and BMI did not change significantly over time in either group (all p > .10). There was no significant treatment arm by time interactions (all p > 0.10)
In the regression analysis (Table S2), the main outcome was log(binge eating frequency +1). Significant variables included log(binge eating frequency +1) at baseline and, just barely, the interaction of binge eating and visit number. Neither group nor the interaction of group and visit was significant. No variables were significant in the model with weight as the outcome. As age of onset was significant between groups, a term for that was included in the models.
Secondary Measures
In addition to the regression analyses (presented in Table S2), we assessed the percentage of participants in each group who improved in binge frequency from baseline to week 12 using the CGI as defined by: Complete improvement = 100% decrease (no binge eating); Marked improvement = 75% - <100% decrease; Moderate improvement = 50% - <75% decrease; and No improvement = <50% decrease. Just under half of all participants showed no improvement and just over half showed some improvement. It should be noted that the cell sizes here are small and that some people increased in binge frequency from baseline to week 12. The differences between groups are not statistically significant (Fisher’s exact p=0.62).
Other secondary measures are shown in Tables S3 & S4. For eating-related questionnaire outcomes, all regression models show a significant time trend; the binge eating scale model also shows a significant relationship with age of binge eating onset (Table S3). For other secondary measures (Table S4), there was significant change in all BE-YBOCS scores from baseline to week 12 shown in the regression model (significant visit predictor), as did SDS. The HAM-D and HAM-A measures did not show significant change, nor did their regression models have significant predictors.
Safety and Tolerability
All 67 participants were included in the safety analysis (i.e. had at least 1 post-baseline visit that included a safety assessment) (Table 2). Overall, 37 (55.2%) participants experienced at least one adverse event (AE). On average, participants reported AEs during 2 visits (range 1 – 6 visits). The most common AE was nausea, occurring 9 times in placebo and 19 times in the vortioxetine group. The majority of the AEs were rated as mild or moderate.
Table 2. Adverse Event Information by Group.
| All Participants (n=67) | Randomization Group |
|||
|---|---|---|---|---|
| Placebo (n = 34) | Vortioxetine (n=33) | p-value | ||
| Any adverse event reported – n (%) | 37 (55.2%) | 17 (50.0%) | 20 (60.6%) | 0.53 |
| Number of visits per participant with AEs reported – n (%) | 0.43 | |||
| 0 | 30 (44.8%) | 17 (50.0%) | 13 (39.4%) | |
| 1 | 15 (22.4%) | 9 (26.5%) | 6 (18.2%) | |
| 2 | 12 (17.9%) | 5 (14.7%) | 7 (21.2%) | |
| 3 | 2 (3.0%) | 0 (0.0%) | 2 (6.1%) | |
| 4 | 5 (7.5%) | 3 (8.8%) | 2 (6.1%) | |
| 5 | 1 (1.5%) | 0 (0.0%) | 1 (3.0%) | |
| 6 | 2 (3.0%) | 0 (0.0%) | 2 (6.1%) | |
| Number of AEs reported (total across participants and visits) | 124 | 49 | 75 | N/A |
| Average number of AEs per participant (across all visits) | 3.4 | 2.9 | 3.8 | N/A |
| Description of most common AEs (> 3 reports) – n | N/A | |||
| Nausea | 28 | 9 | 19 | |
| Dry Mouth | 12 | 5 | 7 | |
| Headaches | 11 | 7 | 4 | |
| Dizziness | 10 | 5 | 5 | |
| Insomnia | 7 | 2 | 7 | |
| Vomiting | 5 | 2 | 3 | |
| Anxiety | 4 | 0 | 4 | |
| Gas/bloating | 4 | 3 | 1 | |
| Diarrhea | 4 | 0 | 4 | |
| Constipation | 4 | 3 | 1 | |
Discussion
This randomized, double-blind, 13-week clinical trial failed to find that vortioxetine was more effective than placebo for BED based on our primary and secondary outcome measures of binge frequency, binge eating response or remission, eating pathology, and body weight. Vortioxetine was not, however, associated with a significantly higher rate of adverse events or a higher discontinuation rate due to adverse events than placebo.
The lack of efficacy found for vortioxetine for BED may be due to several non-mutually exclusive reasons. One possible explanation is that vortioxetine is simply not effective for BED. A second possible explanation for these negative results is that the drug is effective, but the failure to detect a drug-placebo difference was due to chance (a type II error). This is more likely to occur in relatively small-scale studies (as this one is). Third, vortioxetine may in fact be genuinely effective, but study itself may have been incapable of detecting an effect. This third point could be due to what has been seen as a relatively high placebo response in studies of BED (Blom et al., 2014; Ziauddeen et al., 2013). Future studies that could possibly control for the large placebo response, or recruit samples enriched for low likelihood of placebo response (such as through a placebo run-in) could better determine if vortioxetine might be an effective treatment intervention.
This study had several limitations that should be considered. First, because of the small sample size, the study was inadequately powered to detect clinically important treatment effects of moderate size. Therefore, the study had a relatively high chance of a type II error (i.e., namely failing to reject the null hypothesis when the null hypothesis is in fact false), which would result in the study being a failed trial rather than a negative trial. Next, it is possible that the large placebo effect observed for reduction in binge episode frequency may have obscured the study's ability to detect a significant treatment effect. Another limitation is a high overall attrition rate – high attrition is common in studies of BED. Furthermore, vortioxetine may not have been given for an adequate period of time as used in this trial, though the duration was reasonable in light of extant positive binge eating trials and established clinical trial norms. Finally, persons with major depressive disorder were excluded and it is possible that vortioxetine might be efficacious in BED when it co-occurs with major depressive disorder.
In summary, in a 13 week trial in outpatients with BED, vortioxetine was not superior to placebo in reducing binge episode or binge day frequency, in improving eating disorder psychopathology related to binge eating, or in reducing body weight. It would be potentially informative in future work to examine whether the high placebo response rate in BED can be mitigated, such as through placebo run-ins, or sample enrichment if predictors of placebo response can be identified. It may also be possible that vortioxetine can help some people with BED lose significant amounts of weight.
Supplementary Material
Acknowledgements
PDAStats (Minneapolis, MN) performed statistical analyses, blinded to group assignment.
Footnotes
Conflicts of Interest and Disclosures
This study was funded by an investigator-initiated research grant from Takeda Pharmaceuticals to Dr. Grant. Dr. Grant receives yearly compensation from Springer Publishing for acting as Editor-in-Chief of the Journal of Gambling Studies and has received royalties from Oxford University Press, American Psychiatric Publishing, Inc., Norton Press, and McGraw Hill. Dr. Chamberlain consults for Cambridge Cognition, Shire and Promentis. Ms. Redden report no financial relationships with commercial interests. Dr. Chamberlain’s involvement in this study was supported by a Wellcome Trust Clinical Fellowship (110049/Z/15/Z).
Trial Registration: ClinicalTrials.gov identifier: NCT02528409
References
- Al-Sukhni M, Maruschak NA, McIntyre RS. Vortioxetine : a review of efficacy, safety and tolerability with a focus on cognitive symptoms in major depressive disorder. Expert Opinion on Drug Safety. 2015;14(8):1291–1304. doi: 10.1517/14740338. [DOI] [PubMed] [Google Scholar]
- Blom TJ, Mingione CJ, Guerdjikova AI, Keck PE, Jr, Welge JA, McElroy SL. Placebo response in binge eating disorder: a pooled analysis of 10 clinical trials from one research group. European Eating Disorders Review. 2014;22(2):140–146. doi: 10.1002/erv.2277. [DOI] [PubMed] [Google Scholar]
- Cassidy TA, Varughese S, Russo L, Budman SH, Eaton TA, Butler SF. Nonmedical Use and Diversion of ADHD Stimulants Among U.S. Adults Ages 18-49: A National Internet Survey. Journal of Attention Disorders. 2015;19(7):630–640. doi: 10.1177/1087054712468486. [DOI] [PubMed] [Google Scholar]
- Clemow DB, Walker D. The potential for misuse and abuse of medications in ADHD: a review. Postgraduate Medicine. 2014;126(5):64–81. doi: 10.3810/pgm.2014.09.2801. [DOI] [PubMed] [Google Scholar]
- Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, King N, Curtis C, Kennedy JL. Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 2009;17(6):1220–1225. doi: 10.1038/oby.2009.52. [DOI] [PubMed] [Google Scholar]
- Deal LS, Wirth RJ, Gasior M, Herman BK, McElroy SL. Validation of the Yale-Brown obsessive compulsive scale modified for binge eating. International Journal of Eating Disorders. 2015;48(7):994–1004. doi: 10.1002/eat.22407. [DOI] [PubMed] [Google Scholar]
- Donnelly B, Touyz S, Hay P, Burton A, Russell J, Caterson I. Neuroimaging in bulimia nervosa and binge eating disorder: a systematic review. Journal of Eating Disorders. 2018;6:3. doi: 10.1186/s40337-018-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb A, Deeks ED. Vortioxetine: first global approval. Drugs. 2014;74(1):135–145. doi: 10.1007/s40265-013-0161-9. [DOI] [PubMed] [Google Scholar]
- Goracci A, di Volo S, Casamassima F, Bolognesi S, Benbow J, Fagiolini A. Pharmacotherapy of binge-eating disorder: a review. Journal of Addiction Medicine. 2015;9(1):1–19. doi: 10.1097/ADM.0000000000000089. [DOI] [PubMed] [Google Scholar]
- Guerdjikova AI, Mori N, Blom TJ, Keck PE, Jr, Williams SL, Welge JA, McElroy SL. Lisdexamfetamine dimesylate in binge eating disorder: a placebo controlled trial. Human Psychopharmacology. 2016;31(5):382–391. doi: 10.1002/hup.2547. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976. pp. 76–338. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay P, Mitchison D, Collado AEL, González-Chica DA, Stocks N, Touyz S. Burden and health-related quality of life of eating disorders, including Avoidant/Restrictive Food Intake Disorder (ARFID), in the Australian population. Journal of Eating Disorders. 2017;5:21. doi: 10.1186/s40337-017-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert A, Petroff D, Herpertz S, Pietrowsky R, Tuschen-Caffier B, Vocks S, Schmidt R. Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. Journal of Consulting and Clinical Psychology. 2019;87(1):91–105. doi: 10.1037/ccp0000358. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Benjet C, Bruffaerts R, et al. The prevalence and correlates of binge eating disorder in the World Health OrganizationWorld Mental Health Surveys. Biological Psychiatry. 2013;73(9):904–914. doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RM, Hutson PH, Herman BK, Potenza MN. The neurobiological basis of binge-eating disorder. Neuroscienece & Biobehavioral Reviews. 2016;63:223–238. doi: 10.1016/j.neubiorev.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Kunovac JL, Herman BK, Culpepper L. Recognizing Binge-Eating Disorder in the Clinical Setting: A Review of the Literature. Primary Care Companion CNS Disorders. 2016;18(3) doi: 10.4088/PCC.15r01905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latagliata EC, Patrono E, Puglisi-Allegrac S, Ventura R. Food seeking in spite of harmful consequences is under prefrontal cortical noradrenergic control. BMC Neuroscience. 2010;11:15. doi: 10.1186/1471-2202-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SC, Li Y, Pehrson AL, Dale E, Smagin G, Sanchez C. Serotonergic Regulation of Prefrontal Cortical Circuitries Involved in Cognitive Processing: A Review of Individual 5-HT Receptor Mechanisms and Concerted Effects of 5-HT Receptors Exemplified by the Multimodal Antidepressant Vortioxetine. ACS Chemical Neuroscience. 2015;6(7):970–986. doi: 10.1021/cn500340j. [DOI] [PubMed] [Google Scholar]
- Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RS. A Randomized, Placebo-Controlled, Active-Reference, Double-Blind, Flexible-Dose Study of the Efficacy of Vortioxetine on Cognitive Function in Major Depressive Disorder. Neuropsychopharmacology. 2015;40(8):2025–2037. doi: 10.1038/npp.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes WF, Brownley KA, Mo X, Bulik CM. The biology of binge eating. Appetite. 2009;52(3):545–553. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Hudson JI, Capece JA, Beyers K, Fisher AC, Rosenthal NR. Topiramate Binge Eating Disorder Research Group. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biological Psychiatry. 2007;61(9):1039–1048. doi: 10.1016/j.biopsych.2006.08.008. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova AI, Mori N, O’Melia AM. Pharmacological management of binge eating disorder: current and emerging treatment options. Therapeutics and Clinical Risk Management. 2012;8:219–241. doi: 10.2147/TCRM.S25574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Hudson JI, Mitchell JE, Wilfley D, Ferreira-Cornwell MC, Gao J, Wang J, Whitaker T, Jonas J, Gasior M. Efficacy and safety of lisdexamfetamine for treatment of adults with moderate to severe binge-eating disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(3):235–246. doi: 10.1001/jamapsychiatry.2014.2162. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Hudson J, Ferreira-Cornwell MC, Radewonuk J, Whitaker T, Gasior M. Lisdexamfetamine Dimesylate for Adults with Moderate to Severe Binge Eating Disorder: Results of Two Pivotal Phase 3 Randomized Controlled Trials. Neuropsychopharmacology. 2016;41(5):1251–1260. doi: 10.1038/npp.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter AM, Heinze HJ, Schlagenhauf F, Deserno L. Impaired Flexible Reward-Based Decision-Making in Binge Eating Disorder: Evidence from Computational Modeling and Functional Neuroimaging. Neuropsychopharmacology. 2017;42(3):628–637. doi: 10.1038/npp.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV. The Anxiety Disease. New York: Scribner's; 1983. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Chamberlain SR, Nathan PJ, Koch A, Maltby K, Bush M, Tao WX, Napolitano A, Skeggs AL, Brooke AC, Cheke L, et al. Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Molecular Psychiatry. 2013;18(12):1287–1293. doi: 10.1038/mp.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.