Abstract
Background
Vegetatively propagated crops are globally significant in terms of current agricultural production, as well as for understanding the long-term history of early agriculture and plant domestication. Today, significant field crops include sugarcane (Saccharum officinarum), potato (Solanum tuberosum), manioc (Manihot esculenta), bananas and plantains (Musa cvs), sweet potato (Ipomoea batatas), yams (Dioscorea spp.) and taro (Colocasia esculenta). In comparison with sexually reproduced crops, especially cereals and legumes, the domestication syndrome in vegetatively propagated field crops is poorly defined.
Aims and Scope
Here, a range of phenotypic traits potentially comprising a syndrome associated with early domestication of vegetatively propagated field crops is proposed, including: mode of reproduction, yield of edible portion, ease of harvesting, defensive adaptations, timing of production and plant architecture. The archaeobotanical visibility of these syndrome traits is considered with a view to the reconstruction of the geographical and historical pathways of domestication for vegetatively propagated field crops in the past.
Conclusions
Although convergent phenotypic traits are identified, none of them are ubiquitous and some are divergent. In contrast to cereals and legumes, several traits seem to represent varying degrees of plastic response to growth environment and practices of cultivation, as opposed to solely morphogenetic ‘fixation’.
Keywords: Asexual (clonal) reproduction, vegetative propagation, phenotype, early agriculture, developmental plasticity, archaeobotany
THE SIGNIFICANCE OF VEGETATIVELY PROPAGATED PLANTS
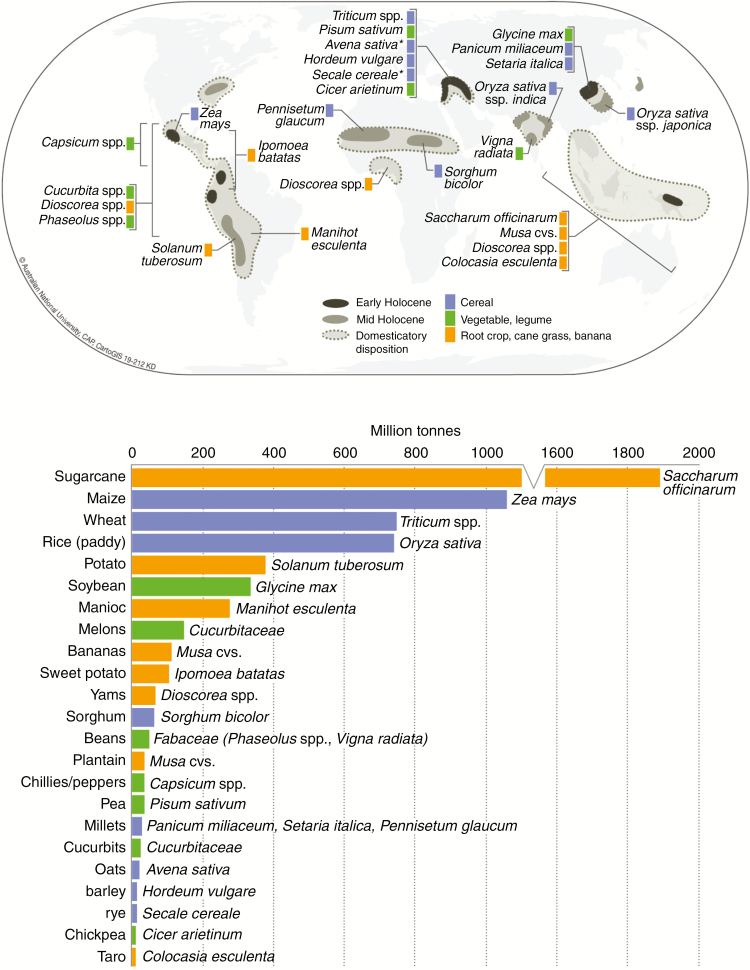
Vegetatively propagated plants are among the world’s most important subsistence and commercial crops, especially in the wet tropics and sub-tropics. Globally significant foods that are vegetatively propagated include bananas and plantain (Musa cvs), manioc (cassava, Manihot esculenta), potato (Solanum tuberosum), sugarcane (Saccharum officinarum), sweet potato (Ipomoea batatas), taro (Colocasia esculenta) and yams (Dioscorea spp.) (Fig. 1). Other important vegetatively grown crops include arrowroot (Maranta arundinacea), old cocoyam (Xanthosoma sagittifolium), ginger (Zingiber officinale) and turmeric (Curcuma longa).
Fig. 1.
Loci of domestication for globally significant food crops (upper; after Fuller et al., 2014: fig. 1) and annual global production (lower; FAO, 2016) for major agricultural crops grown in fields (monoculture) and plots (polyculture). Arboricultural/silvicultural crops, such as trees, palms and pandanus, and fodder crops are excluded. Groups of crops are colour-coded according to: sexually reproduced cereals (blue); sexually reproduced legumes and vegetables (green); and vegetatively propagated bananas, root crops and sugarcane (orange). Notes: 1. In the map (upper), an asterisk connotes that plants probably moved as a weed from region of origin and domesticated in another locale; oats (Avena sativa) and rye (Secale cereale) originated in South-west Asia and were probably domesticated in eastern-central Europe during the late Holocene. 2. In the graph (lower), yield of sugarcane may represent total crop biomass, while other crops are usually given as primary product only.
In this paper, we propose a domestication syndrome of convergent evolutionary traits for vegetatively propagated crops, namely bananas, cane grasses and root crops ordinarily grown in cultivated plots or fields. Definitions of domestication syndrome vary considerably; some are general and refer to a suite of traits that mark a crop’s divergence from its wild ancestor(s). The suite includes traits that are desirable to humans, yet are not necessarily beneficial to the plant, and need not be uniform from species to species (Meyer et al., 2012). Other definitions are more restrictive and link the collection of phenotypic traits associated with domestication to genetic changes in the domesticated crop relative to its wild progenitor (Gepts, 2004; Allaby, 2014). Although domestication syndromes are sometimes considered fixed by genetic changes (Zohary, 1984; Ladizinsky, 1985, 1998; Lenser and Theißen, 2013; Martinez-Ainsworth and Tenaillon, 2016; Kistler et al., 2018; Pickersgill, 2018), this may not be an absolute requirement because the genetic correlates for phenotypically expressed traits are not known for most crops (Smýkal et al., 2018).
The domestication histories and status of several vegetatively propagated plants are confounded because no known wild ancestor exists, for example greater yam (Dioscorea alata) and sweet potato (I. batatas; Muñoz-Rodríguez et al., 2018). In other cases, little is known about the ecology, genetics and cultivation history of plants that were probably once important staples and now widely spread geographically, such as Alocasia macrorrhizos and Xanthosoma spp. (Brown, 2000). For these crops, inferences regarding domestication history can only be drawn from present-day plants.
For domesticates descended from a wild conspecific ancestor, genetic analyses of modern and historic populations can assist in the interpretation of origins, yet most studies are limited by sampling coverage, a bias towards major cultivar groups, and genetic reshuffling through time (Roullier et al., 2013). Other domesticates are true cultigens; they are products of introgression, namely interspecific or intrasub-specific hybridization reflecting sexual reproduction, such as AA diploid banana cultivars (Musa), sugarcane (members of the Saccharum complex) and potatoes (members of the Solanum brevicaule complex). Diploid hybridization presumably preceded the generation of sterile, vegetatively propagated cultivars, including several major triploid banana cultivar groups (Perrier et al., 2011) and sugarcane polyploids (Grivet et al., 2004).
For context, we initially provide an overview of the significance of different modes of reproduction for the emergence of agriculture across the globe. As a means of bridging the gaps in knowledge for the domestication of sexually and asexually reproducing crop plants, we characterize asexual reproduction in plants and different types of vegetative propagation practice. We then present several domestication syndrome traits for vegetative crops, namely the behavioural, physical and chemical traits that emerged as a result of human selection under early forms of cultivation and are common to derived cultivars. We then consider the archaeobotanical visibility of these phenotypic traits for reconstructing the domestication of vegetatively propagated plants in the past.
EARLY AGRICULTURE AND MODES OF REPRODUCTION
Early and later forms of agriculture vary in their reliance on sexual and asexual modes of reproduction (Fig. 1; Sauer, 1952; Harlan, 1971; Harris, 1977; Ladizinsky, 1998; Piperno and Pearsall, 1998; Zohary and Hopf, 2000; Denham et al., 2007).
Cultivation based on sexual reproduction through the planting of fertilized seed is commonly associated with annuals, especially cereals and legumes, as well as a broad range of oil seeds, soft-stemmed fruits and vegetables. Several globally significant cereals, in terms of modern production, contributed to early forms of regional agriculture (Fuller et al., 2014): maize (Zea mays) in Mesoamerica; rice (Oryza sativa) in southern China and South-east Asia; wheat (Triticum spp.) and barley (Hordeum vulgare) in South-west Asia; sorghum (Sorghum bicolor) in East Africa; pearl millet (Pennisetum glaucum) in West Africa; and broomcorn millet (Panicum miliaceum) and foxtail millet (Setaria italica) in northern China. Other cereals and pseudocereals were incorporated into regional farming practices: Panicum sumatrense, Brachiaria ramosa and Paspalum scrobiculatum in India (Murphy and Fuller, 2017); buckwheat (Fagopyrum esculentum) on the Tibetan plateau (Hunt et al., 2018); quinoa (Chenopodium quinoa) in the Andes (Bruno, 2009); and pitseed goosefoot (Chenopodium berlandieri) in the Mississippi Basin (Smith and Yarnell, 2009). Legumes were also domesticated as part of these early cultivation practices, including: peas (Pisum sativum; Trněný et al., 2018), chickpea (Cicer arietinum; van Oss et al., 2015) and lentil (Lens culinaris; Sonnante et al., 2009) in South-west Asia; beans (Phaseolus spp.) in the Americas (Rendón-Anaya et al., 2017); soybean (Glycine max) in China (Lee et al., 2011; Zong et al., 2017); cowpea (Vigna unguiculata) in Africa (D’Andrea et al., 2007); and multiple pulses including mungbean (Vigna radiata), horsegram (Macrotyloma uniflorum) and pigeon pea (Cajanus cajan) in India (Fuller and Harvey, 2006; Fuller and Murphy, 2018; Fuller et al., 2019).
A domestication syndrome of convergent evolutionary traits has been proposed for many of these sexually reproduced crops (Harlan et al., 1973; Hammer, 1984; Vaughan et al., 2007; Meyer et al., 2012; Abbo et al., 2014; Fuller et al., 2014). For some researchers, a single key trait, such as loss of wild-type seed dispersal, has been singled out as the only marker of domestication (e.g. Zohary, 1984; Murgia et al., 2017), with other changes considered to be more loosely related to plant evolution under cultivation. However, such an approach pre-supposes the nature of past human–plant interactions rather than inferring those interactions from empirical evidence. Given that any crop population will be undergoing selection for multiple traits at any one time, including the potential for previously unrecognized targets of selection (Vaughan et al., 2007), a broader conception of a domestication syndrome is useful as it offers multiple proxies for documenting the process of coevolution between crops and humans.
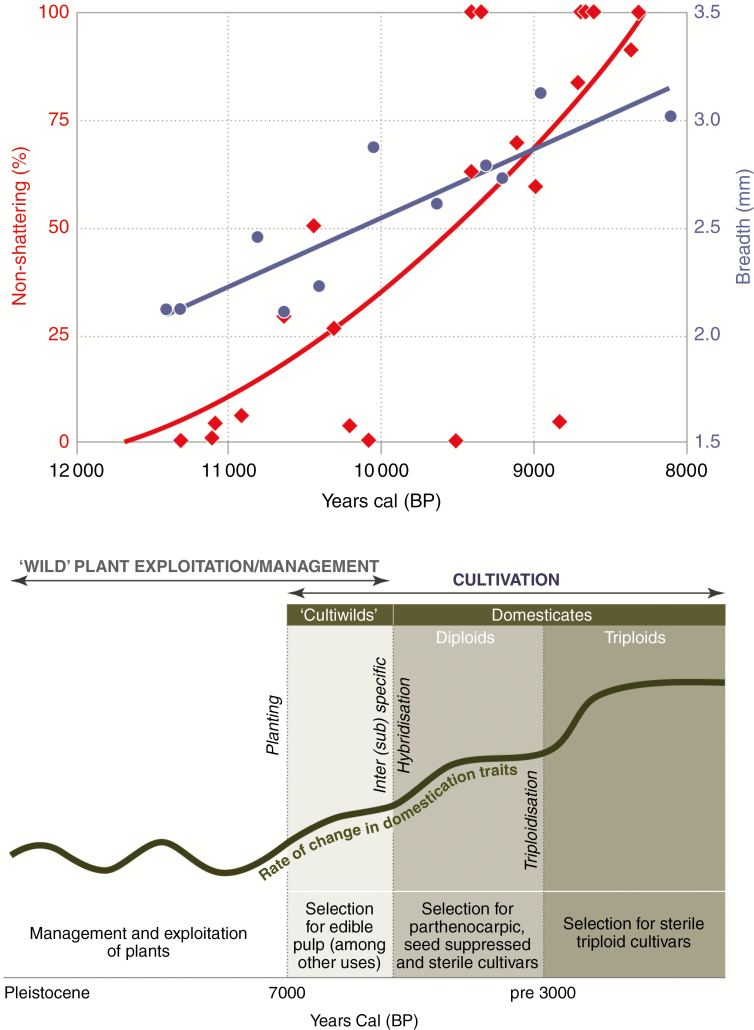
The major mechanisms of domestication inferred from archaeological remains have been determined from detailed studies of the phenotypes (macrobotanical and microbotanical) and, more recently, genotypes (ancient DNA) of a sub-set of sexually reproducing crops, primarily cereals (Allaby et al., 2018; Kistler et al., 2018; Schreiber et al., 2018; Stitzer and Ross-Ibarra, 2018; Scott et al., 2019) and legumes (Smýkal et al., 2015; Bitocchi et al., 2017). However, most ancient DNA has been obtained from derived cultivars rather than from the oldest domesticated plant remains. Clear physical changes in morphological architectures associated with reproduction and propagation, namely non-shattering rachis of barley (Fig. 2), wheat (Tanno and Wilcox, 2012), rice (Fuller et al., 2009; Barron et al., 2017) and sorghum (Winchell et al., 2017), as well as the rapid evolution of the cob in maize (Piperno and Pearsall 1998; Stitzer and Ross-Ibarra,, 2018), have been documented.
Fig. 2.
Comparison of the unilinear domestication episode for barley (upper; Hordeum vulgare) with the multistaged and stepwise domestication trajectory for bananas (lower; Musa cvs). Upper: the domestication episode for barley (Hordeum vulgare) potentially extends from approx. 11 500 cal BP to approx. 8500 cal BP and is reconstructed from archaeobotanical evidence at multiple sites for percentages of non-shattering cultivars (red) and increasing grain breadth (blue; Fuller et al., 2014: tables S2 and S3). Lower: rates of change in domesticatory traits are inferred to increase across three thresholds: early planting of diploid cultiwilds; hybridization to generate diploid cultivars; and triploidization with subsequent widespread dispersal (Perrier et al., 2011; De Langhe et al., 2015).
In comparison, there is limited archaeobotanical, ecological and genetic information regarding the phenotypic trajectories of domestication for vegetatively propagated crops. Yet, an examination of 203 crop species, including 115 vegetatively propagated crops, found between five and seven domestication syndrome traits, with an average of 2.8 traits per species (Meyer et al., 2012). Vegetatively propagated root crops did not exhibit significantly fewer traits than annual seed crops.
Early farming emerged in several regions based on the vegetative propagation of staple crops that today are globally significant, most notably the Americas – manioc (M. esculenta), potato (S. tuberosum) and sweet potato (I. batatas) (Ugent and Peterson, 1988; Piperno and Pearsall, 1998) – and the Indo-Pacific region extending from eastern India to New Guinea – aroids (Araceae), bananas (Musa cvs), sugarcane (Saccharum officinarum) and yams (Dioscorea spp.) (Burkill, 1935; Li, 1970; Yen, 1973). Thus, despite sexually reproduced plants being at the forefront of most archaeobotanical research on plant domestication, understanding vegetative propagation is equally significant for a more complete and balanced perspective on human selection, early domestication and global agriculture.
Vegetative propagation is especially important for unravelling the history of early cultivation and domesticatory practices in the wet tropics and sub-tropics, mountainous regions, wetland habitats and deserts (Harris, 1972). For instance, several regionally important crop plant assemblages are based on vegetative principles: potato (S. tuberosum), oca (Oxalis tuberosa), ulluco (Ullucus tuberosus) and mashua (Tropaeolum tuberosum) in the Central Andes (National Research Council, 1989); enset (Ensete ventricosum) and yam (Dioscorea cayenensis) in Ethiopia (Hildebrand, 2007; Borrell et al., 2019); plantain (Musa cvs), Plectranthus spp., taro (C. esculenta) and yams (D. rotundata-cayenensis complex) in western Africa (Fuller and Hildebrand, 2013); aroids (Alocasia macrorrhizos, Amorphophallus paeonifolius, C. esculenta and Cyrtosperma merkusii) and swamp sago (Metroxylon sagu) in the Indo-Pacific (Ruddle et al., 1978; Brown, 2000; Santosa et al., 2017); and bananas (Musa cvs), taro (C. esculenta) and yams (Dioscorea spp.), together with edible cane grasses (Saccharum officinarum, Saccharum edule and Setaria palmifolia) in the New Guinea region (Barrau, 1955; Yen, 1973; Denham, 2018).
Vegetatively propagated crops are often characterized as being of local or regional significance, as well as lacking expansive capacity (Harris, 2002). Yet at the time of European exploration of the globe from the 15th century onwards, vegetatively propagated crops had the widest longitudinal ranges of any food crops in the world: bananas (Musa cvs) were distributed across the ‘Old World’, from West Africa (Mbida et al., 2001) and Iberia (Dozy, 1961) to eastern Polynesia (Yen, 1973); and taro (C. esculenta) spread from uncertain homelands in South-east Asia, eastwards into Polynesia and westwards to the eastern Mediterranean where it was known by the ancient Greeks and Romans (Grimaldi et al., 2018). As yet, the complex histories of domestication and pre-historic dispersal for many vegetatively propagated food crops are poorly understood, largely as a result of low archaeobotanical visibility and poor preservation, as well as the limited phenotypic and genetic characterization of ancient and modern plants from wild and domesticated sources. Most interpretations rely heavily on genetic inferences from modern populations with only limited archaeobotanical support, such as for manioc (Wang et al., 2014), potato (Hardigan et al., 2017), taro (Chaïr et al., 2016) and some yams (Scarcelli et al., 2019); an exception is the banana which is present in many archaeological phytolith records (Fig. 2; Perrier et al., 2011; Castillo and Fuller, 2016).
ASEXUAL REPRODUCTION IN PLANTS
Many plants have two modes of reproduction: sexual reproduction from fertilized seed; and asexual reproduction, i.e. clonal growth through regeneration from plant structures (Stebbins, 1950; Harper, 1977; Abrahamson, 1980). Asexual reproduction by-passes pollination and production of fertilized seed; instead, offspring genetically identical to the parent plant, namely clones, are produced by processes that are more akin to growth than to reproduction (Abrahamson, 1980: p. 89). In trying to characterize the non-equivalence of asexual and sexual reproduction, genetically distinct individuals in a population can be considered as genets, while the genetically identical individuals arising from asexual reproduction of a genet are ramets (Harper, 1977; Abrahamson, 1980).
Asexual reproduction in plants occurs in two principal forms: agamospermy and vegetative reproduction (Abrahamson, 1980). Agamospermy is parthenogenic seed production, also referred to as apomixis (Silverton, 2008: p. 157). Apomictic seeds are clones of the mother plant that are packaged and dispersed in the trappings of sexually produced progeny (Silverton, 2008: pp. 457–458). They differ from ramets in that they still go through the same developmental programme (seedling, juvenile and reproductive adult stages) as any other seed propagation cycle. Manioc is capable of apomixis (Ellstrand, 2003: p. 80), but it is not a major reproductive strategy for any major crop plant discussed here.
Advantages of vegetatively reproduced offspring include loss of juvenility, rapid development and higher growth rates compared with seedlings because propagules are better provisioned initially with a larger food supply (Table 1; Abrahamson, 1980: p. 96) and may even start as miniature versions of the parent plant with developed root systems (Silverton, 2008: p. 157). Amongst flowering plants, vegetative reproduction is a low-risk adaptation in certain environments for proliferating the genet through the production of independent and hardy ramets (Abrahamson, 1980: p. 96). Through vegetative reproduction, individual genetic lineages may be extremely long lived; the aspen (Populus termuloides) colony in southern Utah, known colloquially as ‘Pando’, is approx. 8000–10 000 years old (Mock et al., 2008: p. 4828).
Table 1.
Expected differences between asexually and sexually produced offspring (adapted from Williams, 1975 and Abrahamson, 1980: table 5.2)
| Asexual offspring | Sexual offspring |
|---|---|
| Mitotically standardized | Meiotically diversified |
| Produced more continuously | Seasonally limited |
| Develop close to parent | More widely dispersed |
| Develop more immediately | Often more dormant |
| Develop more directly to reproductive stage | Develop more slowly through a non-reproductive stage |
| Phenotype and optimum genotype predictable from those of parent | Phenotype and genotype less predictable |
| Low mortality rate | High mortality rate |
The frequency of plants capable of vegetative reproduction is highly variable in different flora. Ecosystems where vegetative reproduction is noted include: high latitudes, such as boreal forests; high altitudes, where some species may lose the ability to sexually reproduce; aquatic habitats containing species that vegetatively reproduce via bulbs, corms and rhizomes, and where the fragmentation of stems and stolons can take over the dispersal function of seeds; and habitats prone to fire in which strong vegetative reproducers employ a ‘sit and wait’ strategy with fast growth to recolonize newly burned habitat (Abrahamson, 1980: p. 93; Eckert, 2002). In the wet tropics, sexual reproduction is more common than asexual reproduction (Abrahamson 1980: 92), though asexual reproduction is a significant strategy for many species important to people (Hather, 1996).
PATHWAYS TO DOMESTICATION
The domestication of vegetative crops, like many sexually reproduced crops, is unlikely to have been a single capture event (McKey et al., 2010). In considering the pathways to domestication of clonally propagated plants, numerous anthropic selective processes would have operated on exploited plants within a landscape, including direct selection of favoured phenotypes (which may have included translocation of whole plants or plant parts capable of reproduction, e.g. yam heads), as well as indirect processes of selection: modification of local environments through clearing and burning; modification of the immediate growth environment, such as disturbance of soils to increase looseness and friability; and the creation of anthropic habitats favouring particular phenotypes (Yen, 1989; Hather, 1996; Terrell et al., 2003; Harris, 2007; Barton and Denham, 2018). In all of these pathways there exists potential for phenotypic change to occur and accumulate over short and prolonged time periods either through genetic changes, involving some degree of sexual recombination or mutation, or through more immediate genetic expression within changed ecological conditions that influence the ‘plasticity’ of growth form (de Kroon et al., 1994: pp. 125–126).
As vegetative propagators, humans have acted as important dispersal agents of desired genets, moving ramets that would often only disperse locally through spreading roots, stolons and suckers adjacent to the parent. Globally, people have introduced genets into new ecological zones and regions, such as the dispersal of major cultivars, while regionally people moved plants into places of lower plant density with reduced competition, such as the translocation of yams from the Ethiopian lowlands where they occur wild to higher elevations where they do not (Hildebrand, 2007). An important advantage of vegetative propagation for both humans and plants is independence from external pollination, such that plants are able to colonize new habitats outside of the natural range in which flowering occurs and where pollinators are absent (Abrahamson, 1980: p. 963). It also has the potential to remove plants away from natural pests (Chen et al., 2018), thereby increasing survival rates and vigour. In dispersing plants and plant parts, whether deliberately or inadvertently, such events were probably important mechanisms in plant domestication through the generation of asexually reproducing phenotypes (outside of their natural range), new phenotypes of individual species and through the spontaneous creation of new hybrids of related species, i.e. sympatric hybridization (Clement et al., 2010).
Arguably, vegetative propagation can be characterized as a form of instant domestication (Stetter et al., 2017) that enables more controlled selection of favoured phenotypic characteristics than under sexual reproduction. In theory, vegetative propagation enables instantaneous genetic isolation of preferred phenotypes; in practice, though, spontaneous sexually reproduced progeny may also be incorporated into clonally reproduced crop assemblages thereby enabling gene flow and potentially prolonging the period of domestication. In general terms, species or specific phenotypes of a species have been selected based on: ease of growth, hardiness and resistance to stress (whether disease, pest or environmentally induced); productivity (including caloric, protein and oil yield, synchronicity of yield and interannual reliability of production); ease of processing (such as a hard seed coat or nut casing, extraction of the edible portion or spininess); ease of cooking (pounding, soaking, heating, roasting, etc.); and selection for secondary characteristics such as toxicity, acridity, colour, palatability and texture (McKey et al., 2012; Meyer et al., 2012; Smýkal et al., 2018).
THE DOMESTICATION SYNDROMES OF VEGETATIVELY PROPAGATED CROPS
Despite the complicated and poorly documented domestication histories of most vegetatively propagated plants, some common phenotypic characteristics can be proposed for cultivars of diverse crop types, including grasses, herbs and tuberous plants. Even though archaeobotanical, biological, ecological and genetic information is often incomplete, these phenotypic commonalities can be tentatively compared with the domestication syndrome in sexually reproduced crops (Table 2). Syndrome traits comprise those associated with early domestication common to all derived varieties rather than improvement/diversification traits that have arisen in only some regional varieties (Purugganan and Fuller, 2009; Meyer and Purugganan, 2013). None of these traits is ubiquitous, with convergence and divergence exhibited for several traits among vegetatively propagated crops (McKey et al., 2010; Meyer et al., 2012).
Table 2.
Domestication traits in sexually vs. asexually reproduced plants
| Trait category | Domestication in sexually propagated plants | Domestication in asexually propagated plants |
|---|---|---|
| Mode of reproduction | 1. Partial or complete loss of asexual reproduction ability | 1. Partial or complete loss of sexual reproduction ability |
| 2. Increased uniformity in seed germination traits; loss of dormancy | 2. Increased uniformity in clonal reproduction traits | |
| Plant life cycle | Shift towards annual life cycle based on sexual reproduction from seed | Shift towards perennial life cycle based on vegetative production of suckers, shoots, underground storage organs (USOs) and other viable plant parts |
| Yield of edible portion | 1. Increased size in seeds of cereals, legumes, nuts and stone fruits | 1. Increased size of edible vegetative storage organs (often the organ used for clonal propagation) |
| 2. Increased number of fruits and seeds | 2. Increased number of edible organs | |
| 3. Increased ratio of edible to non-edible plant parts in a whole plant | 3. Increased ratio of edible to non-edible plant parts in a whole plant | |
| Ease of harvesting | Development of non-shattering seed heads/pods | Development of bunched or fused vegetative storage organs |
| Development of easily separated USOs/bud separation | ||
| Timing of production | Synchronous production of harvested parts within a plant and between plants | Asynchronous and more continuous production of harvested parts, with in-ground storage for some USOs |
| Plant architecture | Changes in: | Changes in: |
| Apical dominance | Apical dominance | |
| Branch arrangements | Branch arrangements | |
| Leaf arrangements | Leaf arrangements | |
| Defensive adaptations | Loss of defensive adaptations (spines, hard seed casings, toxicity, acridity) to enhance harvesting, processing and consumption | Loss of defensive adaptations (spines, hard seed casings, toxicity, acridity) to enhance harvesting, processing and consumption |
| Pre-domestication traits | ||
| Ease of storage | 1. Traits that favour survival of seeds used for propagation | 1. Traits that favour survival of USOs used for propagation |
| 2. Traits that favour preservation of seeds used for consumption | 2. Traits that favour preservation of USOs used for consumption | |
| Post-domestication traits | Improvement/diversification/dispersal | |
| Photoperiod sensitivity | Changes in photoperiod sensitivity according to latitude, reproductive cycle of the wild type and latitudinal origin of the wild type | Changes in photoperiod sensitivity according to latitude and reproductive cycle of the wild type and latitudinal origin of the wild type |
| Environmental tolerance | Traits that enable cultivation in wider environmental range (altitudinal, latitudinal, water conditions, wind conditions and soil type) | Traits that enable cultivation in wider environmental range (altitudinal, latitudinal, water conditions, wind conditions and soil type) |
| Disease resistance | Reduced resistance to disease and pests due to human selection following continued sexual reproduction of sub-population | Dramatic reduction in resistance to disease and pests due to low genetic variability in clonally reproduced cultivars (despite somatic mutation) |
| Palatability | Selection for various desired traits, often involving a loss of defensive chemical adaptations | Selection for various desired traits, often involving a loss of defensive chemical adaptations |
| Processing | Selection for reduction or ease of removal of inedible portions (free-threshing cereals, seed integument, nutshells and pod shells) | Selection for reduction or ease of removal of inedible portions (skin and fibre) |
Sexually reproduced plants include cereals, legumes, leafy vegetables, and many fruit and nut trees; asexually reproduced plants include root crops, grasses and vegetables, as well as palms, pandanus and trees. While propagation may be predominantly sexual or asexual for a given crop, many crop taxa reproduce naturally using both modes of reproduction. Note that in many clonally propagated fruit trees, fertilization is still essential for crop production. When one form or the other of propagation is favoured for a crop that has both modes of reproduction, the dominance of one form of reproduction is the focus of selection and constitutes a domestication trait.
Mode of reproduction
Asexual reproduction can develop in plants to become dominant in cultivars in numerous ways. Natural processes such as spontaneous mutations, polyploidization and hybridization combined with preferential anthropic selection of parthenocarpic, seed-suppressed and seedless forms have led to hybrid dysfunction, reproductive dysfunction and potential loss of capacity for sexual reproduction. Although the reduced ability for sexual reproduction is convergent in many vegetatively propagated crops, it is achieved through different phenotypic changes and different types of human–plant domesticatory practice. The loss of sexual reproductive capacity has been accompanied by a shift towards perennial life cycles.
As people have preferentially utilized the vegetative mode of reproduction, some cultivated plants partially or completely lost their capacity to reproduce sexually through the accumulation of genetic characteristics (e.g. asynchronous flowering, somatic mutations, seed suppression and polyploidy) that would naturally be deleterious to the plant. Similar loss of sex has been noted for plants in environments marginal for viable sexual reproduction (Eckert, 2002; Barrett, 2015). Prolonged clonal reproduction potentially led to the loss of sexual reproductive capacity for greater yam (D. alata; Alexander and Coursey, 1969) and Ethiopian domesticated enset (E. ventricosum; Hildebrand, 2003; Borrell et al., 2019). In contrast, many other crops have maintained sexual reproductive capacity despite prolonged asexual cultivation, such as manioc and sweet potato.
Parthenocarpy is a spontaneous mutation that enables plants to produce mature fruits without fertilization (Gustafson, 1942). The resultant fruits can contain embryonic, immature or impartially formed seeds that are often more digestible. Parthenocarpy enables plants to be moved beyond the natural range to new environments in which they are unable to reproduce sexually, perhaps due to unfavourable climate, an absence of pollinators or an absence of sexual partners. Although plants can then be subject to asexual reproduction, some may still reproduce sexually if fertilized, as occurs in many fig varieties (Ficus carica; Condit, 1947). Anthropic selection of parthenocarpic forms of diploid banana (Musa cvs) in the South-east Asia–New Guinea region was fundamental to the domestication of major cultivar groups during the mid-Holocene (Perrier et al., 2011). Subsequent human selection during cultivation led to the creation of seed-suppressed and eventually seedless forms in parthenocarpic plants, e.g. vestigial seeds in most banana cultivars (Musa cvs) today, or the seed reduction in enset cultivars (Hildebrand, 2003).
Hybrid dysfunction leading to sterility is a possible factor underlying vegetative domestication. Introgression, or hybridization, can lead to sterility and necessitate asexual modes of reproduction in a plant. Sterile hybrid cultivars with odd sets of chromosomes were also generated through polyploidization and subsequently propagated by people, including triploid bananas (Musa cvs; Perrier et al., 2011), polyploid cane grasses (Saccharum spp.; Premachandran et al., 2011) and, arguably, some yams (Dioscorea spp.; Lebot, 2009). Cultivated polyploids probably developed spontaneously, such as when cultivated banana diploids were brought together or into contact with other cultivated or wild diploids (Perrier et al., 2011).
Polyploidy, though, should not be considered a domestication trait. Although 78 % of perennial crop plants, of which 90 % were primarily vegetatively propagated, were claimed to exhibit ploidy changes as a domestication trait (Ramsey and Schemske, 2002), the proportion of polyploids among crops is not statistically different from that among wild species of the same families (Meyer et al., 2012). Rather than being a product of domestication, polyploidy is a natural phenomenon that drives speciation in plants, conferring greater flexibility with the appearance of novel traits (Alix et al., 2017; Smýkal et al., 2018). Humans have benefited from this phenomenon and selected polyploid variants due to useful agronomic traits; triploids are associated with greater disease resistance and wider environmental tolerance than diploids.
The domestication histories for some crop plants that are predominantly vegetatively propagated today include episodes of sexual reproduction in the past, including cultigens and some derived from a wild conspecific ancestor, such as the greater yam (D. alata; Lebot et al., 1998). Such overlaps between sexual and asexual modes of reproduction continue to the present; several vegetatively propagated species undergo spontaneous sexual reproduction with wild (where present) or cultivated populations. The resultant progeny are then incorporated into a cultivator’s vegetatively propagated stock to increase cultivar diversity, as documented for manioc (M. esculenta; Rival and McKey, 2008; Clement et al., 2010), sweet potato (I. batatas; Yen, 1974) and yams (Dioscorea spp.; Dumont and Vernier, 2000), as well as for diploid banana (Musa) and taro (C. esculenta) cultivars (Kennedy and Clarke, 2004). As McKey et al. (2010) observed, cultivated stock of many vegetative crops reflects clonal and sometimes spontaneous sexual reproduction; the different reproductive strategies have differing selective pressures that produce complex domestication pathways.
On the whole, vegetatively propagated cultivars tend to lose sexual reproductive capacity with a concomitant increase in phenotypic characteristics associated with asexual reproduction. Cultivars tend towards parthenocarpy, seed suppression, triploidy/polyploidy and sterility. However, these are tendencies rather than inevitable transformations.
Yield of the edible portion
As in sexually reproduced plants, the yield of the edible portion – often the size, but also the availability of useful nutrients – has increased in vegetatively propagated field crops. The increase can be observed in many underground storage organs (USOs), the fruits of bananas and plantains, and the sugar-enriched stems of some cane grasses. For instance, starch content and storage root yield have been selected for in manioc (Wang et al., 2014), with similar claimed genetic selection in potatoes (Hardigan et al., 2017) and some yams (Scarcelli et al., 2019). Often, the increased size of the edible plant part derives from structures used for vegetative propagation, such as in most USOs (Table 3) including yam tubers (Dioscorea spp.; Zannou et al., 2006) and taro corms (C. esculenta; Matthews et al., 2012), as well as stems of cane grasses (S. officinarum; James, 2004). In other plants, these characteristics do not align, such as increased fruit size in banana cultivars cultivated from suckers and increased tuber size in sweet potatoes reproduced from vine slips.
Table 3.
Parenchymatous storage organs in non-woody plants exploited by people and ordinarily propagated vegetatively, primarily underground storage organs (USOs) (after Abrahamson, 1980; Hather, 1998, 1994a, 2000)
| Plant structure | Description | Examples |
|---|---|---|
| Bulb | Rounded underground storage organ comprised of a short stem surrounded by fleshy scale leaves or leaf bases | Garlic (Allium sativum); lily (Lilium spp.); onion (Allium cepa) |
| Bulbil | Tuber produced in the axil of a leaf capable of adventitious root growth. Propagation by fragmentation and adventitious growth | Bitter/cheeky yam (Dioscorea bulbifera) |
| Caudex | Vertical multimodal swelling of a stem base. Sometimes referred to as a pachycaul stem. This may or may not constitute the reproductive structure of the plant | Baobab (Adansonia spp.); cycads (Cycas spp., Zamia spp.); giant taro (Alocasia macrorrhiza); tree ferns (Alsophila spp.) |
| Corm | Vertical multimodal tuber of 1 year or more duration, producing ephemeral shoots. Each node on a corm has the capacity to produce daughter corms. Propagation by axillary replacement, fragmentation and adventitious growth | Canna (Canna edulis); cocoyam (Xanthosoma saggitifolium); eddoe (Colocasia antiquorum); elephant foot yam (Amorphophallus spp.); enset (Ensete ventricosum); fern (e.g. Pteridium esculentum); swamp taro (Cyrtosperma merkusii); taro (Colocasia esculenta); water chestnut (Eleocharis dulcis) |
| Rhizome | Perennial horizontal axis more or less homogenously swollen or unswollen supporting ephemeral leaves and flowering axes arising vertically at nodes. Propagation by fragmentation and adventitious growth | Arrowroot (Maranta arundinacea); galangal (Alpina sp.); ginger (Zingiber officinale); oca (Oxalis tuberosa); tumeric (Curcuma sp.); typha (Typha spp.) |
| Rhizome tuber | Multiple swollen regions along the length of, or terminally attached to, a rhizomatous axis. Propagation by fragmentation and adventitious growth | Cyperus (Cyperus spp.); Scirpus spp. |
| Root tuber | Swollen regions along the length of an otherwise unswollen root system. Occasional vegetative propagative capability by adventitious growth | Cassava/manioc (Manihot esculenta); leren (Calathea allouia); murnong (Microseris scapigera); pencil yam (Vigna lanceolata); sweet potato (Ipomoea batatas) |
| Stolon tuber | Swollen regions along the length of, and terminally attached to, a stolon. In the yams, swelling may be massive forming large, long or thick tubers. Propagation by fragmentation and adventitious growth. | Arrow head (Sagittaria sagittifolia); Plectranthus spp.; lotus root (Nelumbo nucifera); potato (Solanum tuberosum); yams (Dioscorea spp.) |
Classifications are not necessarily exclusive for a particular plant, given changes in plant structures during life cycles, such as rhizome–caudex in Typha spp.: Hather, 2000: p. 16). Many USOs are exploited by people for food and used for propagation; however, this is not always the case. For example, bananas (Musa cvs) are exploited for fruit and reproduced from suckers growing from a corm at the base of the pseudostem, while sweet potato (Ipomoea batatas) is exploited for root tubers and can be reproduced from root tubers and vine slips (stem cuttings).
Tap roots are excluded here, as although a swollen secondary root of a biennial or perennial herbaceous plant, the crops are ordinarily reproduced from seed, as a tap root has no vegetative propagative capability.
Domestication has also favoured plants with greater in-ground storage capacity. Yams (Dioscorea spp.) are an important resource across the tropics due to lengthy tuber dormancy (2–4 months at ambient temperature) facilitating storage for 4–6 months without significant deterioration of nutritional properties (Lebot, 2009). Similarly, piecemeal harvesting of USOs can occur over extended periods, such as up to 9 months in sweet potato (Lebot, 2009).
Increased yield is also facilitated by increasing the number of edible parts within a plant and increasing the ratio of edible to non-edible parts. These can be affected in numerous ways: from increasing the quantity of extractable sugar within cane grass stems, to increasing the number and size of fruits and USOs on a plant. Some of these changes are likely to be the result, at least initially, of phenotypic plasticity, as has been identified for traditional cultivation practices of African wild yams (Dioscorea spp.), where putative ‘fixation’ of newly desired traits may take between 3 and 5 years (Zannou et al., 2006), while relaxation of cultivation practices results in yams returning to the wild phenotype (Dumont and Vernier, 2000).
In some vegetatively propagated plants, a reduction in seed size (seed suppression) occurs – such as bananas (Musa cvs) – with a concomitant increase in size of the edible fleshy part of the fruit. Seed suppression has also been noted in some sexually reproduced crops, such as some varieties of cultivated citrus fruits (Citrus spp.; Roose et al., 1995) and chempedak (Artocarpus integer; Primack, 1985). In other edible fruits, increased seed size occurred with domestication (Fuller, 2018), even if later suppression of seeds became possible with vegetative reproduction, for example in date palms (Phoenix dactylifera) and grapes (Vitis vinifera). In a few sexually reproduced crops that are grown for roots and not seeds, such as carrot (Daucus carota) and burdock (Arctium lappa), there is also a noted increase in seed size (Kluyver et al., 2017). Hence, the correlation between seed size and domestication is not ubiquitous; rather it appears correlated with sexual proclivity.
Despite selection under cultivation for millennia, the degrees to which tuber size and starch contents reflect genetic control or conditions of growth are unclear. Morphological changes in plant and tuber morphology have been noted for numerous root crops, such as size and shape in yams (Dioscorea spp.; Lea, 1966; Hather, 2000). They depend upon growth environment and cultivation practices, including degree of soil preparation in garden plots, staking of vines, spacing between plants and weeding. For instance, failure to adequately prepare and maintain cultivated yam plots, especially to enable sufficient leaf area, leads to reduced yields and cultivars rapidly deteriorate, losing beneficial traits and becoming ‘feral’ (Vernier et al., 2003). These morphological changes can be attributed in part to plastic responses on the part of plants to reduced soil nutrient levels, when increased root surface to volume ratios, i.e. longer thinner roots, allow a plant to take up more minerals from its rhizosphere; this has been characterized as ‘plant nutrient-foraging plasticity’ (Sultan, 2015: p. 81; see also Hodge, 2014). Phenotypic variability, which may be linked to subtle environmental differences, as well as likely gene flow between wild and cultivated yams, often makes definition of domesticated, feral and wild populations extremely difficult (Scarcelli et al., 2006; cf. Scarcelli et al., 2019).
Ease of harvesting
The development of fused, multiple or aggregate syncarps, as well as bunching, may have evolved in response to human-mediated domestication, as well as plausibly to enhance seed dispersal by other animals. These morphological changes are demonstrated by a range of fruit- and nut-bearing species, including bananas (Musa cvs), berries (Rubus spp.), breadfruit (A. altilis), figs (F. carica) and pandanus (Pandanus spp.). In contrast, there is a tendency for greater separation amongst USOs – such as in potatoes (S. tuberosum), sweet potatoes (I. batatas) and yams (Dioscorea spp.) – especially in more friable, cultivated soils, which is plausibly a plastic response to growth environment as much as the product of genetic change. The contrasting fusion of fruit/nuts vs. separation in USOs probably results from practices of human harvesting, selection and cultivation, as well as responses to growth environment.
Timing of production
Asynchronous production in vegetatively propagated crops is a function of two factors: climate and human selection. Today vegetative forms of cultivation predominate as forms of subsistence agriculture in wet tropical and sub-tropical regions, principally where climates are perhumid and less seasonal. On the whole, vegetative forms of agriculture, or vegeculture, are anticipated to be less seasonal and to enable cultivation of crops at different times of the year. There are notable exceptions: some vegetative crops are major staples in highly seasonal climates, such as potatoes (S. tuberosum) in northern Europe and sweet potato (I. batatas) on the North Island of New Zealand, although neither plant originates in those regions. For some plants, aseasonal climates lead to less predictable fruit production, in terms of interplant synchronicity of production and periodicity of fruit production by individual plants (Bourke et al., 2004). Overall, vegetative crop plants display considerable variation: cultivated yams (Dioscorea spp.) are persistently photoperiod sensitive despite extensive breeding programmes (Lebot, 2009). While domesticated seed crops are characterized by more even ripening and narrowing of the harvest window (Ladizinsky, 1998; Fuller, 2007), human selection seems to have pushed for a broadening harvest window for many vegetative domesticates.
Plant architecture
Apical dominance is manifest in several vegetatively propagated crops, including potato (S. tuberosum), sugarcane (S. officinarum), taro (C. esculenta) and yams (Dioscorea spp.). Apical dominance is well known in seed crops, often involving selection for taller, erect plants and fewer side branches, or more compact plants, as it allows more plants to fit into each unit of cultivated soil (Doust, 2007; Fuller et al., 2010). In vegetatively propagated crops, there is much variation: yams have been characterized as exhibiting apical dominance (Coursey, 1967; Passam, 1977), while others propose basal dominance (Mozie, 1984); in manioc (M. esculenta), apical dominance becomes more marked with reduced spacing between plants (Streck et al., 2017); and triploid AAB plantains (Musa cvs) exhibit more marked apical dominance than diploid and AAA triploid cultivar groups of banana (Ortiz and Vuylsteke, 1994). However, some crops exhibit considerable morphological plasticity, reflecting the growth environment; for instance, wild and cultivated manioc (M. esculenta) grow as a liana in forest and dense vegetation, yet as a shrub in open savanna and gardens (Ménard et al. 2013). Apical dominance can be reduced through removal of the shoot tip or sucker in most crops, leading to plural lateral bud development.
Apical dominance is also expressed in terminal flower and seed head/pod character. Although ordinarily associated with sexually reproduced crops, such as soybean (Glycine max) and cereal panicles that become larger and concentrated on fewer stalks (Doust, 2007; Fuller, 2007), comparable morphological transformations may have occurred in some vegetatively propagated crops. For example, lowland pitpit (S. edule) is a cane grass that is cultivated from cuttings for its unopened flower heads that are cooked as a vegetable in lowland New Guinea (French, 2006).
Defensive adaptations
The loss of defensive adaptations, such as spines and armatures, in some cultivars may be indications of domestication, as exhibited by several cultivated aroids (Alocasia macrorrhizos, Amorphophallus paeoniifolius, and Cyrtosperma merkusii; Brown 2000) and many yams (Mignouna and Dansi, 2003; Vernier et al., 2003). For example, giant swamp taro (C. merkusii) is cultivated from Peninsular Malaysia across the Micronesian atolls to far eastern Polynesia (Hay, 1988: p. 433). Normally the plant is heavily armatured, but under cultivation is usually without armatures and larger in size. Cultivated varieties of elephant foot yam (A. paeoniifolius) also have smoother stalks, as well as fewer raphides (calcium oxalate crystals) and lower to no alkaloid content (Brown, 2000).
Many crops have been selected for reduced acridity, bitterness, irritability and toxicity to thereby decrease processing requirements and increase palatability, such as lower glycoalkaloids in potatoes (S. tuberosum), lower calcium oxalate crystals in taro (C. esculenta) and lower dioscorine in yams (Dioscorea spp.). A secondary metabolite that may impact negatively on human health are cyanogenic glucosides that are hydrolysed by β-glycosidases into hydrocyanic acid (HCN). A single dose of 1–3 mg kg–1 of body weight is lethal to most vertebrates (Oke, 1969). The presence of cyanogenic glucosides is a heritable trait that may be present in all individuals of some plant species, while others are heterogenous and may contain acyanogenic individuals (Gleadow and Møller, 2014: p. 163). Cyanogenic glucosides occur in higher concentrations in young plants, and production appears to be influenced partially by genetic control (Wang et al., 2014) and partially by local environmental factors such as herbivory, the presence of toxins in soil, reduced soil nutrients, drought and shade (Gleadow and Møller, 2014: p. 170). Depending upon the degree of plasticity inherent in the plant, the act of bringing young plants into a cultivated plot with better soil, sunshine and water may be enough to significantly reduce the production of cyanogenic glucosides. Under drought stress, manioc (M. esculenta) tubers increase in toxicity, sometimes to hazardous concentrations, but this can be reversed by watering (Gleadow and Møller, 2014: p. 171). Pathways to domestication may involve selection of plants with appropriate phenotypic properties, including concentrations of phytochemicals; they may also involve harnessing the plasticity of plants under cultivation through changes in local environmental conditions and the removal of conditions that stress young plants in their early growth phase.
A focus on less acrid, bitter and toxic varieties is not ubiquitous. In manioc (M. esculenta), cultivars are grouped into two main types, sweet and bitter, based on their respective higher and lower cyanogenic glucoside contents. Bitter manioc requires leaching, mashing and heating to remove toxins, whereas sweet manioc requires only standard cooking, and some varieties can be eaten raw (Rival and McKey, 2008). Early cultivation may have selected for reduced toxicity in manioc, with highly toxic forms selected later for higher productivity on poor soils and greater storability, even though they require advanced detoxification methods (Arroyo-Kalin, 2012). Similarly, some yams have retained bitterness or toxicity and are still highly poisonous to people, such as the Asiatic bitter yam (D. hispida), despite prolonged exploitation for approx. 20 000 years (Barton and Paz, 2007).
While selection for removal of phytochemicals, usually secondary metabolites, is desirable in plants targeted for general consumption, it seems equally plausible that there has been selection towards greater levels of toxicity in some species, as with bitter manioc (M. esculenta). The persistence of bitterness and toxicity in some crops may have reduced competition from mammals, such as tapir, pigs and deer, as well as loss to pests, such as beetles and other insects during cultivation and storage. Thus definitions of domestication traits in plants are more complicated than assuming the direction of selection is solely towards a reduction in phytochemicals through time; rather they require consideration of the various stages of food supply – including propagation, cultivation, harvesting, storage, distribution, processing and cooking – and the total range of plant uses – including medicines, toxins, mastics and fibres, as well as food.
Pre- and post-domestication traits
Some characteristics often assumed to derive from domestication are associated with pre-domestication and post-domestication processes. For instance, ease of storage is potentially a factor that led foragers to initially target a given species, together with its culinary and nutritional benefits. Several other characteristics are best considered as secondary domestication traits associated with varietal diversification (Ladizinsky, 1998; Purugganan and Fuller, 2009; Meyer and Purugganan, 2013). Foremost, the vast phenotypic variation exhibited in most root crops, comprising several hundred cultivars in manioc, potato, taro and sweet potato, among many others, results from centuries and millennia of cultivation. Environmental tolerance, photoperiod sensitivity and disease resistance are also likely to result from later cultivation practices. Selection of tolerant and resistant phenotypes would presumably be based on cultivator experience, namely seeing which varieties grow best in specific environments. As varieties were moved into new environments, different phenotypes with different characteristics were preferred. Triploids generally have greater environmental tolerance compared with diploids of the same crop because of the broader genetic inheritance of the former. However, clonally reproduced, sterile triploids can be highly susceptible to pathogens given the narrow genetic base of cultivated stock, as witnessed with the decimation of global Gros Michel banana plantations by a strain of Panama disease (Fusarium oxysporum) in the early 1950s. Further, several traits associated with the consumption, processing and cooking of a crop have been refined as secondary domestication traits, including shape, colour, texture and taste.
A domestication syndrome?
While subject to prolonged and continued cultivation, vegetatively propagated field crops exhibit several domestication traits that are broadly convergent across a range of different groups of plants, including grasses, root crops and vegetables. None of these domestication traits is ubiquitous and there is considerable divergence among crops for some traits. The domestication syndrome of convergent traits proposed here for vegetatively propagated crops is, therefore, only preliminary.
Significant focus on the genetic aspects of domestication has contributed to our understanding of the mechanisms and origins of many domesticates and key domestication traits. However, phenotypes of clonal plants are not just genetically controlled; they may display various degrees of ‘plasticity’ resulting from environmental influences (de Kroon et al., 1994: pp. 125–126). Plasticity is defined as phenotypic change that is environmentally induced, though the direction and the magnitude of that change are genetically determined. There appears to be considerable variation among clonal plants in the degree to which observed phenotypic change may be considered plastic or non-plastic (i.e. that which is under direct genetic control) in different ecological conditions (Ding and Chen, 2018; Liu et al., 2019). More ‘fixed’ morphogenetic changes include a shift towards asexual modes of reproduction and an increased edible portion in some plants. Other traits seem to be more plastic, such as yield, ease of harvesting, timing of fruit production, some aspects of plant architecture and some defensive adaptations. Consequently, phenotypes readily revert to ‘wild type’ when left to grow feral.
THE ARCHAEOBOTANY OF DOMESTICATION UNDER VEGETATIVE PROPAGATION
Three advances in archaeobotany have aided the investigation of early agriculture and plant domestication based on vegetative reproduction: phytoliths (Piperno 2006); starch granules (Torrence and Barton 2006), sometimes supplemented with raphide identification (Loy 2006); and archaeological parenchyma (Hather 2000) (Figs 3, 4). Ordinarily, these microfossil techniques are only able to reliably discriminate to genus or family level; higher resolution inferences of species, subspecies and domestication status are often problematic (e.g. Mercader et al., 2018). Despite these limitations, the application of this suite of techniques has raised the visibility of early plant exploitation and cultivation practices based on vegetative propagation in the lowland neotropics (Piperno and Pearsall, 1998), New Guinea region (Denham et al., 2003; Golson et al., 2017) and West African rain forest (Mbida et al., 2001). For instance, at Kuk Swamp in the highlands of Papua New Guinea, microfossils from stratigraphic contexts and as residues on stone artefacts provide evidence for the presence and use of various plants, respectively (Golson et al., 2017). However, interpretations of early cultivation, as a surrogate measure for the intensity of domesticatory relationships, have relied upon the association of archaeobotanical remains with multiple lines of contextual evidence, including: archaeological features associated with cultivation, such as field systems, mounds and ditches; palaeosols and feature fills consistent with plot preparation, tillage and cultivation; and, palaeoecological records of forest clearance, weedy and fallow floral assemblages, and burning (Denham, 2018). Carpological and anthracological remains can further inform on the taxonomic composition of agricultural forests typically integrating vegetatively propagated bananas, cane grasses and root crops, helping to identify indirect processes of domestication via anthropogenic manipulation of the growth environment.
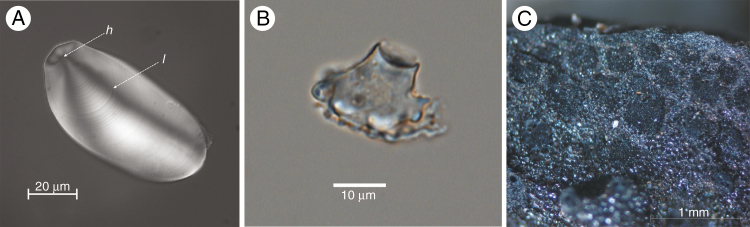
Fig. 3.
Microfossil techniques for the investigation of vegetatively propagated crops. (A) Photomicrograph of starch granule of Disocorea hispida indicating diagnostic elements: h = hilum, l = lamellae (modern reference sample). (B) Photomicrograph of volcaniform phytolith of an AAw banana (Musa sp.; modern reference sample from Ngezi Forest, Pemba). (C) Photomicrograph of a transverse section through a sugarcane (Saccharum officinarum) stem fragment from a 200- to 300-year-old domestic context at Kuk Swamp (Lewis et al., 2016: fig. 3d).
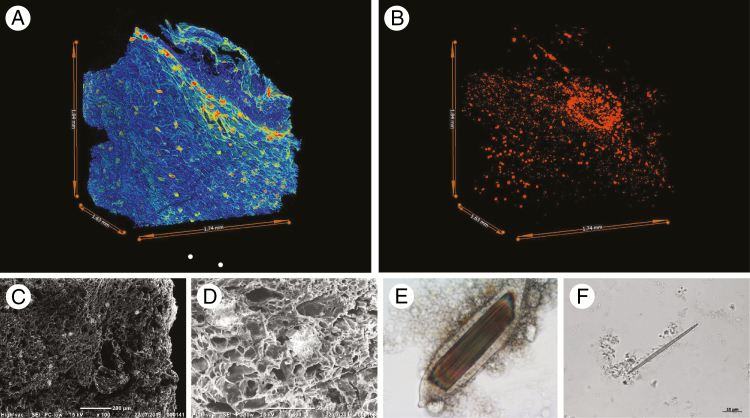
Fig. 4.
Archaeobotanical techniques for the investigation of calcium oxalate in taro (C. esculenta). (A) MicroCT visualization of a parenchyma fragment with low density areas in blue (cell walls) and high density areas in red (druses and raphide bundles comprising calcium oxalate crystals). (B) MicroCT visualization showing the distribution of high density areas in (A). (C and D) Scanning electron microscopy images of taro parenchyma with druses visible as lighter concentrations. (E) Photomicrograph of a cell packed with raphides. (F) Photomicrograph of a raphide showing needle-like morphology and an asymmetric proximal tip (lower right). All images are from modern reference samples.
The initial step in the archaeobotany of vegetative plant domestication is to obtain a species identification, with subsequent discrimination of wild/domesticated morphotypes where possible. Although species-level identification is often achievable for most fruit- and nut-bearing species from seeds and nut fragments, respectively, it has proven problematic for a number of cane grasses, root crops and vegetables (Pearsall, 2000). For instance, although many species of aroids have large numbers of calcium oxalate crystals present as druses and raphides in cellular tissues (Brown, 2000: pp. 276–277), which may be identifiable to genus level (Crowther, 2009a), they are not ordinarily well preserved in archaeological contexts nor are they ordinarily identified during archaeobotanical investigations (though see Loy et al., 1992). As a result, the identification of taro in the past has been heavily reliant on charred parenchyma and starch granule morphometrics (Fullagar et al., 2006). Nonetheless, the exploitation of several vegetatively propagated plants has been identified in archaeobotanical contexts using phytoliths and starch granules. Even though microfossils do not ordinarily, or reliably, discriminate between wild and domesticated types, these remains are often inferred to represent cultivation because they were found outside the natural range (e.g. Piperno and Pearsall, 1998; Vrydaghs et al., 2003; Chandler-Ezell et al., 2006).
Macro-remains of preserved fruits, tubers and stem fragments can preserve in desiccated, waterlogged or charred form. Macrobotanical tuber fragments of potato (S. tuberosum) have been documented from 10 000-year-old archaeological contexts in the Chilca Canyon, central coast of Peru (Engel, 1970). The desiccated macro-remains of achira (Canna edulis), manioc, potato and sweet potato were found at multiple sites dating from approx. 4250 to 3500 years ago in the Casma Valley, Peru (Ugent et al., 1981, 1982, 1984, 1986; Ugent and Peterson, 1988). Banana (Musa sp.) skin peelings, taro (C. esculenta) corms and sugarcane (S. officinarum) stem sections at the Red Sea port of Quseir al-Qadim in Egypt indicate westward trade in vegetative cultivars to Africa by 1040–1160 AD (Van der Veen and Morales, 2011).
Charred parenchyma fragments are encountered on archaeological sites across the globe, but there have been recurrent problems with obtaining reliable taxonomic identifications (Hather, 1988, 1991, 1994a, b, 1996, 2000; Paz, 2001; Oliveira, 2008; Barton et al., 2016). Fragments of charred sweet potato (I. batatas) have been identified from several sites in Hawai’i dating from 1300 AD (Ladefoged et al., 2005) and in eastern Polynesia from 1000 to 1200 AD (Hather and Kirch, 1991). Charred sugarcane (S. officinarum) stem and sweet potato (I. batatas) tuber fragments from recent domestic contexts at Kuk Swamp have been identified using optical microscopy and micro-computed tomography (microCT) and are suggestive of continuities with ethnographic lifestyles in the highlands of New Guinea (Lewis et al., 2016; Pritchard et al., 2018). However, such robust identifications are rare.
Even if a species-level identification is possible, the discrimination of domesticates from wild types is problematic. Problems result from: a limited understanding of plant ecology, phenology and genetics; a lack of clarity in terms of domestication traits; and uncertainties in the archaeobotanical identification of domestication traits in plant macro- and microfossils. Further, any domestication traits in vegetatively propagated plants may be difficult to identify in the archaeobotanical record because the specific traits preserved need not be ‘fixed’ in the same way as early domestication traits in some sexually reproduced crops, such as non-shattering spikelet bases. Rather, phenotypic traits in many vegetatively propagated plants still seem to exhibit considerable developmental plasticity, which makes interpretations of domestication status for archaeobotanical remains from the distant past problematic. The implications of phenotypic plasticity for using archaeobotany to reconstruct the fixation of traits during a domestication episode in the past are unclear. Some aspects of USO macromorphology and plant parenchyma, such as cell wall thickness and size, as well as starch granule morphology and size, may be plastic to varying (and largely unknown) degrees, thereby confounding the charting of domestication in the archaeobotanical record using these macrofossil and microfossil techniques.
The implications of morphological plasticity at the microscale, namely in terms of starch granule and phytolith morphometrics, parenchyma cell wall thicknesses and phytochemistry, require systematic study for most species, whether in terms of domestication status (Ugent et al., 1982; Perry, 2002; Barton et al., 2016; Herzog et al., 2018) or of growth environment (Field, 2006). Increases in parenchyma cell size and cell wall thickness have been identified between wild and domesticated varieties of some taro (C. esculenta) and some yams (Dioscorea spp.; Barton et al., 2016), but the field overall lacks systematic study. In contrast, volcaniform phytoliths in bananas (Musa cvs) show an approx. 20 % increase in crater size from AA diploids to cultivated AAA triploids (Ball et al., 2006; Vrydaghs et al., 2009), although this size change is not consistent across all diploid and triploid cultivar groups (De Langhe et al., 2019). Similarly, elevated or reduced levels of acridity, bitterness, toxicity and other irritants in some tuberous plants are associated with domestication, although contents often vary with life cycle stage (Sunell and Healey, 1979, 1985) and growing conditions, e.g. soil nutrients, water stress, shade and herbivore behaviour (Metlen et al., 2009). Potentially, phytochemical contents could be measured in desiccated or charred parenchymatous tissues if suitably preserved, such as calcium oxalate raphides and druses in taro (Fig. 4; C. esculenta; Crowther, 2009b).
Taken together, archaeobotanical evidence for the early domestication of vegetative plants is relatively sparse and often ambiguous. Although phenotypic differences between domesticates and wild precursor(s) are known for many vegetatively propagated crop plants, the timing for the emergence of domestic traits and the duration of the domestication episode have not been tracked in the archaeobotanical record. In part, archaeobotanical techniques may not always be suitable – as noted above for starch granules, phytoliths and archaeological parenchyma – for differentiating between wild and domestic plants. More significantly, these archaeobotanical techniques have not been systematically applied and comprehensive modern reference collections of wild and domesticated plants have not been developed for most vegetative crops. More fundamentally, the effects of developmental plasticity are poorly understood in terms of how plant macrofossils and microfossils of vegetatively propagated crops present in the archaeobotanical record.
CONCLUSION
A domestication syndrome of convergent evolutionary traits has been proposed for sexually reproduced crops that can be tracked in the archaeobotanical record through the emergence of non-shattering cultivars and, to a lesser extent, through increased seed size (Fuller et al., 2014, 2018). Several domestication syndrome traits in these crops are fixed and have known genetic markers, namely there is some correspondence between phenotype and genotype (Fuller and Allaby, 2009; Meyer and Purugganan, 2013). Increasingly, ancient DNA can be used to track directly the emergence of genetic markers of domestication for sexually reproduced plants in the past (Jaenicke-Despres et al., 2013; Castillo et al., 2016; Allaby et al., 2018; Kistler et al., 2018; Scott et al., 2019).
Equivalent syndrome traits associated with the early domestication of vegetatively propagated crops are not so clear. There are convergent tendencies to lose sexual reproductive capacity and increase the size of the edible portion, although other traits are divergent, and none is ubiquitous. Whereas in sexually reproduced plants phenotypic and genotypic transformations associated with early domestication are portrayed as occurring in lockstep, considerable variation exhibited by vegetatively propagated plants probably represents phenotypic plasticity rather than genotypic variation. Currently, the application of DNA to the investigation of clonal domestication is limited, partly due to poor biomolecular preservation in charred plant tissues and partly due to the lack of application, especially to desiccated plant remains.
Although the domestication syndrome in sexually reproduced plants may be overstated (Meyer et al., 2012; Abbo et al., 2014), phenotypic traits are still characterized as correlated with genotypes resulting from human-directed selection and genetic isolation (Fuller and Allaby, 2009; Larson et al., 2014). In this sense, sexual domestication processes represent a Darwinian ‘best fit’ to human selection and anthropic environments. In vegetatively propagated plants, plastic adaptation to growth environments fulfils a similar function in terms of driving phenotypic variation. Root growth, in particular, responds dynamically to soil conditions (Hodge, 2014; Sultan, 2015: pp. 80–81). Whilst the effects of plasticity are arguably more evident in the phenotypes of vegetatively propagated plants, there is still an underlying element of genetic alteration through natural mutation, introgression and other phenomena affecting the genetic code and how it is expressed phenotypically through time. Hence, although the implications of phenotypic plasticity for charting the early domestication of vegetatively propagated crops are poorly characterized, these do not negate the genetic underpinnings of subsequent crop improvement resulting from millennia of cultivation.
Many clonal domestication traits in vegetatively propagated crops result from active and recurrent practical management of the plant and its growth environment by people. Although genetic markers provide the biomolecular scaffolding for any domestication traits, recurrent human practices that manage plants and growth environments influence phenotypic expression. We wonder if the role of cultivation practices in determining the expression of domestication traits has been underestimated in vegetatively propagated field crops, as well as in some sexually reproduced crops.
Vegetatively propagated crops are globally significant to understanding the emergence of agriculture, as well as to planning for more sustainable agricultural futures. Yet the domestication histories for most vegetatively propagated crops are poorly known. Domestication syndrome traits in vegetative crops represent tendencies in human-mediated plant evolution that reflect a combination of permanent genetic changes and impermanent plastic responses to practices of cultivation, including plant propagation and managing the conditions of growth, such as vegetation clearance and plot preparation. The respective roles of genetic regulation and phenotypic plasticity in the development and expression of domestication traits are uncertain. Co-ordinated botanical and archaeobotanical research is urgently needed in different parts of the world to further our understanding of how people domesticated plants through various practices of vegetative propagation in the past.
FUNDING
This work was supported by an Australian Research Council Future Fellowship grant (FT FT150100420) to T.D.
ACKNOWLEDGEMENTS
We thank Kay Dancey and Karina Pelling (ANU Cartography) for assistance with drafting of figures. We also thank many colleagues for discussions over the years on related themes, including: Robin Allaby, Mike Bourke, Edmond de Langhe, Andrew S. Fairbairn, Ilaria Grimaldi, Peter Matthews, Chris Stevens and Luc Vrydaghs. We also thank Pat Heslop-Harrison, Simcha Lev-Yadun and an anonymous reviewer for constructive comments on earlier drafts.
LITERATURE CITED
- Abbo S, van-Oss RP, Gopher A, Saranga Y, Ofner I, Peleg Z. 2014. Plant domestication versus crop evolution: a conceptual framework for cereals and grain legumes. Trends in Plant Science 19: 351–360. [DOI] [PubMed] [Google Scholar]
- Abrahamson WG. 1980. Demography and vegetative reproduction. In: Solberg OT, ed. Demography and evolution in plant populations. Oxford: Blackwell Scientific Publications, 89–106. [Google Scholar]
- Alexander J., Coursey DG. 1969. The origins of yam cultivation. In: Ucko PJ, Dimbleby GW, eds. The domestication and exploitation of plants and animals. London: Gerald Duckworth, 405–425. [Google Scholar]
- Alix K, Gerard PR, Schwarzacher T, Heslop-Harrison JS. 2017. Polyploidy and interspecific hybridisation: partners for adaptation, speciation and evolution in plants. Annals of Botany 120: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaby RG. 2014. Domestication syndrome in plants. In: Smith C, ed. Encyclopedia of global archaeology. New York: Springer, 2182–2184. [Google Scholar]
- Allaby RG, Ware RL, Kistler L. 2018. A re-evaluation of the domestication bottleneck from archaeogenomic evidence. Evolutionary Applications 12: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo-Kalin M. 2012. Slash–burn–churn: landscape history and crop cultivation in pre-Columbian Amazonia. Quarternary International 249: 4–18. [Google Scholar]
- Ball TB, Vrydaghs L, Van Den Hauwe I, Manwaring J, De Langhe E. 2006. Differentiating banana phytoliths: wild and edible Musa acuminata and Musa balbisiana. Journal of Archaeological Science 33: 1228–1236. [Google Scholar]
- Barrau J. 1955. Subsistence agriculture in Melanesia. Noumea: New Caledonia. [Google Scholar]
- Barrett SC. 2015. Influences of clonality on plant sexual reproduction. Proceedings of the National Academy of Sciences, USA 112: 8859–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A, Turner M, Beeching L, et al. 2017. MicroCT reveals domesticated rice (Oryza sativa) within pottery sherds from early Neolithic sites (4150–3265 cal BP) in Southeast Asia. Scientific Reports 7: 7410. doi: 10.1038/s41598-017-04338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton H, Denham TP. 2018. Vegecultures and the social–biological transformations of plants and people. Quaternary International 489: 17–25. [Google Scholar]
- Barton H, Paz V. 2007. Subterranean diets in the tropical rainforests of Sarawak, Malaysia. In: Denham TP, Iriarte J, Vrydaghs L, eds. Rethinking agriculture: archaeological and ethnoarchaeological perspectives. Walnut Creek, CA: Left Coast Press, 50–77. [Google Scholar]
- Barton H, Paz V, Carlos AJ. 2016. Plant food remains from the Niah Caves: macroscopic and microscopic approaches. In: Barker G, Farr L, eds. Archaeological investigations in the Niah Caves, Sarawak, Vol. 2 Cambridge: McDonald Institute for Archaeological Research, 455–468. [Google Scholar]
- Bitocchi E, Rau D, Bellucci E, et al. 2017. Beans (Phaseolus ssp.) as a model for understanding crop evolution. Frontiers in Plant Science 8: 722. doi: 10.3389/fpls.2017.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell JS, Biswas MK, Goodwin M, et al. 2019. Enset of Ethiopia: a poorly characterized but resilient starch staple. Annals of Botany 127: 747–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke RM, Camarotto C, D’Souza EJ, Nema K, Tarepe TN, Woodhouse S. 2004. Production patterns of 180 economic crops in Papua New Guinea. Canberra: Coombs Academic Publishing. [Google Scholar]
- Brown D. 2000. Aroids: plants of the arum family, 2nd edn Portland, OR: Timber Press. [Google Scholar]
- Bruno M. 2009. Practice and history in the transition to food production. Current Anthropology 50: 703–706. [Google Scholar]
- Burkill IH. 1935. A dictionary of the economic products of the Malay Peninsula. London: Crown Agents. [Google Scholar]
- Castillo CC, Fuller DQ. 2016. Bananas: the spread of a tropical forest fruit as an agricultural staple. In: Lee-Thorp J, Katzenberg MA, eds. The Oxford handbook of the archaeology of diet. Oxford: Oxford University Press. doi: 10.1093/oxfordhb/9780199694013.013.7. [Google Scholar]
- Castillo CC, Tanaka K, Sato YI, et al. 2016. Archaeogenetic study of prehistoric rice remains from Thailand and India: evidence of early japonica in South and Southeast Asia. Archaeological and Anthropological Sciences 8: 523–543. [Google Scholar]
- Chaïr H, Traore RE, Duval MF, et al. 2016. Genetic diversification and dispersal of taro (Colocasia esculenta (L.) Schott). PLoS One 11: e0157712. doi: 10.1371/journal.pone.0157712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler-Ezell K, Pearsall DM, Zeidler JA. 2006. Root and tuber phytoliths and starch grains document manioc (Manihot esculenta), arrowroot (Maranta arundinacea) and llerén (Calathea sp.) at the Real Alto site, Ecuador. Economic Botany 60: 103–120. [Google Scholar]
- Chen YH, Ruiz-Arocho J, von Wettberg EJB. 2018. Crop domestication: anthropogenic effects on insect–plant interactions in agroecosystems. Current Opinion in Insect Science 29: 56–63. [DOI] [PubMed] [Google Scholar]
- Clement CR, de Cristo-Araújo M, d’Eeckenbrugge GC, Pereira AA, Picanço-Rodridgues D. 2010. Origins and domestication of Native American crops. Diversity 2: 72–106. [Google Scholar]
- Condit IJ. 1947. The fig. Waltham, MA: Chronica Botanica. [Google Scholar]
- Coursey DG. 1967. Yams. An account of the nature, origins, cultivation and utilisation of the useful members of the Dioscoreaceaa. London: Longmans. [Google Scholar]
- Crowther A. 2009a Morphometric analysis of calcium oxalate raphides and assessment of their taxonomic value for archaeological microfossil studies. In: Haslam M, Robertson G, Crowther A, Nugent S, Kirkwood L, eds. Archaeology under a microscope: studies in residue and ancient DNA analysis in honour of Thomas H. Loy. Canberra: Australian National University Press, 102–128. [Google Scholar]
- Crowther A. 2009b Re-viewing raphides: issues with the identification and interpretation of calcium oxalate crystals in microfossil assemblages. In: Fairbairn AS, O’Connor S, Marwick B, eds. New directions in archaeological science. Canberra: Australian National University Press, 105–118. [Google Scholar]
- D’Andrea A, Kahlheber S, Logan A, Watson D. 2007. Early domesticated cowpea (Vigna unguiculata) from Central Ghana. Antiquity 81: 686–698. [Google Scholar]
- De Langhe E, Perrier X, Donohue M, Denham TP. 2015. The original banana split: multidisciplinary implications of the generation of African and Pacific Plantains in Island Southeast Asia. Ethnobotany Research and Applications 14: 299–312. [Google Scholar]
- De Langhe E, Vrydaghs L, Perrier X, Denham TP. 2019. Fahien reconsidered: Pleistocene exploitation of wild bananas and Holocene introduction of Musa cultivars to Sri Lanka. Journal of Quaternary Science 34: 405–409. [Google Scholar]
- Denham TP. 2018. Tracing early agriculture in the highlands of New Guinea: plot, mound and ditch. Oxford: Routledge. [Google Scholar]
- Denham TP, Iriarte J, Vrydaghs L, eds. 2007. Rethinking agriculture: archaeological and ethnoarchaeological perspectives. Walnut Creek, CA: Left Coast Press. [Google Scholar]
- Denham TP, Haberle SG, Lentfer C, et al. 2003. Origins of agriculture at Kuk Swamp in the Highlands of New Guinea. Science 301: 189–193. [DOI] [PubMed] [Google Scholar]
- Ding M, Chen ZJ. 2018. Epigenetic perspectives on the evolution and domestication of polyploid plants and crops. Current Opinion in Plant Biology 42: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doust AN. 2007. Architectural evolution and its implications for domestication in grasses. Annals of Botany 100: 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozy RPA. 1961. Le calendrier de cordoue de L’année 961: texte Arabe et ancienne traduction Latine accompagnée d’une traduction française annotée par Ch. Pellat. Leiden: E. J. Brill, 1873. [Google Scholar]
- Dumont R, Vernier P. 2000. Domestication of yams (Dioscorea cayenensis-rotundata) within the Bariba ethnic group in Benin. Outlook on Agriculture 29: 137–142. [Google Scholar]
- Eckert CG. 2002. The loss of sex in clonal plants. Evolutionary Ecology 15: 501–520. [Google Scholar]
- Ellstrand NC. 2003. Dangerous liaisons? When cultivated plants mate with their wild relatives. Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- Engel FA. 1970. Explorations of the Chilca Canyon, Peru. Current Anthropology 11: 55–58. [Google Scholar]
- FAO 2016. FAOSTAT. Rome: Food and Agriculture Organisation of the United Nations; http://www.fao.org/faostat/en. [Google Scholar]
- Field J. 2006. Reference collections. In: Torrence R, Barton H, eds. Ancient starch research. Walnut Creek, CA: Left Coast Press, 95–114. [Google Scholar]
- French B. 2006. Food plants of Papua New Guinea: a compendium, revised edn. Burnie, Tasmania: Food Plants International, Inc. [Google Scholar]
- Fullagar R, Field J, Denham TP, Lentfer C. 2006. Early and mid Holocene processing of taro (Colocasia esculenta) and yam (Dioscorea sp.) at Kuk Swamp in the Highlands of Papua New Guinea. Journal of Archaeological Science 33: 595–614. [Google Scholar]
- Fuller DQ. 2007. Contrasting patterns in crop domestication and domestication rates: recent archaeobotanical insights from the Old World. Annals of Botany 100: 903–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DQ. 2018. Long and attenuated: comparative trends in the domestication of tree fruits. Vegetation History and Archaeobotany 27:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DQ, Allaby R. 2009. Seed dispersal and crop domestication: shattering, germination and seasonality in evolution under cultivation. In: Østergaard L, ed. Annual Plant Reviews 38: fruit development and seed dispersal. Oxford: Wiley-Blackwell, 238–295. [Google Scholar]
- Fuller DQ, Harvey E. 2006. The archaeobotany of Indian pulses: identification, processing and evidence for cultivation. Environmental Archaeology 11: 219–246. [Google Scholar]
- Fuller DQ, Hildebrand EA. 2013. Domesticating plants in Africa. In: Mitchell P, Lane P, eds. The Oxford handbook of African archaeology. Oxford: Oxford University Press, 507–525. [Google Scholar]
- Fuller DQ, Murphy C. 2018. The origins and early dispersal of horsegram (Macrotyloma uniflorum), a major crop of ancient India. Genetic Resources and Crop Evolution 65: 285–305. [Google Scholar]
- Fuller DQ, Qin L, Zheng Y, Zhao Z, Chen X, Hosoya LA, Sun GP. 2009. The domestication process and domestication rate in rice: spikelet bases from the Lower Yangtze. Science 323: 1607–1610. [DOI] [PubMed] [Google Scholar]
- Fuller DQ, Allaby RG, Stevens C. 2010. Domestication as innovation: the entanglement of techniques, technology and chance in the domestication of cereal crops. World Archaeology 42:13–28. [Google Scholar]
- Fuller DQ, Denham TP, Arroyo-Kalin M, et al. 2014. Convergent evolution and parallelism in plant domestication revealed by an expanding archaeological record. Proceedings of the National Academy of Sciences, USA 111: 6147–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DQ, Lucas L, Gonzalez Carretero L, Stevens C. 2018. From intermediate economies to agriculture: trends in wild food use, domestication and cultivation among early villages in Southwest Asia. Paléorient 44: 61–76. [Google Scholar]
- Fuller DQ, Murphy C, Kingwell-Banham E, Castillo CC, Naik S. 2019. Cajanus cajan origins and domestication: the South and Southeast Asian archaeobotanical evidence. Genetic Resources and Crop Evolution 66: 1175–1188. [Google Scholar]
- Gepts P. 2004. Crop domestication as a long-term selection experiment. Plant Breeding Reviews 24: 1–44. [Google Scholar]
- Gleadow RM, Møller BL. 2014. Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annual Review of Plant Biology 65: 155–185. [DOI] [PubMed] [Google Scholar]
- Golson J, Denham TP, Hughes PJ, Swadling P, Muke J, eds. 2017. Ten thousand years of cultivation at Kuk Swamp in the highlands of Papua New Guinea. Terra Australis 46 Canberra: Australian National University Press. [Google Scholar]
- Grimaldi I, Muthukumuran S, Tozzi G, et al. 2018. Literary evidence for taro in the ancient Mediterranean: a chronology of names and uses in a multilingual world. PLoS One 13: e0198333. doi: 10.1371/journal.pone.0198333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivet L, Daniels C, Glaszman JC, D’Hont A. 2004. A review of recent molecular genetics evidence for sugarcane evolution and domestication. Ethnobotany Research and Applications 2: 9–17. [Google Scholar]
- Gustafson FG. 1942. Parthenocarpy: natural and artificial. The Botanical Review 8: 599–654. [Google Scholar]
- Hammer K. 1984. Das Domestikationssyndrom. Kulturpflanze 32: 11–34. [Google Scholar]
- Hardigan MA, Laimbeer FPE, Newton L, et al. 2017. Genome diversity of tuber-bearing Solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato. Proceedings of the National Academy of Sciences, USA 114: E9999–E10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JR. 1971. Agricultural origins: centers and non-centers. Science 174: 468–474. [DOI] [PubMed] [Google Scholar]
- Harlan JR, De Wet JMJ, Price EG. 1973. Comparative evolution of cereals. Evolution 27: 311–325. [DOI] [PubMed] [Google Scholar]
- Harris DR. 1972. The origins of agriculture in the tropics. American Scientist 60: 180–193. [Google Scholar]
- Harris DR. 1977. Alternative pathways toward agriculture. In: Reed C, ed. Origins of agriculture. The Hague: Mouton, 179–243. [Google Scholar]
- Harris DR. 2002. The expansion capacity of early agricultural systems: a comparative perspective on the spread of agriculture. In: Bellwood P, Renfrew C, eds. Examining the farming/language dispersal hypothesis. Cambridge: McDonald Institute for Archaeological Research, 31–40. [Google Scholar]
- Harris DR. 2007. Agriculture, cultivation and domestication: exploring the conceptual framework of early food production. In: Denham TP, Iriarte J, Vrydaghs L, eds. Rethinking agriculture: archaeological and ethnoarchaeological perspectives. Walnut Creek, CA: Left Coast Press, pp. 16–35. [Google Scholar]
- Harper JL. 1977. Population biology of plants. London: Academic Press. [Google Scholar]
- Hather JG. 1988. The morphological and anatomical interpretation and identification of charred vegetative parenchymatous plant remains. Unpublished PhD thesis, University College London. [Google Scholar]
- Hather JG. 1991. The identification of charred archaeological remains of vegetative parenchymatous tissue. Journal of Archaeological Science 18: 661–675. [Google Scholar]
- Hather JG. 1994a A morphological classification of roots and tubers and its bearing on the origins of agriculture in Southwest Asia and Europe. Journal of Archaeological Science 21: 719–724. [Google Scholar]
- Hather JG. 1994b The identification of charred root and tuber crops from archaeological sites in the Pacific. In: Hather JG, ed. Tropical archaeobotany: applications and new developments. London: Routledge, 51–64. [Google Scholar]
- Hather JG. 1996. The origins of tropical vegeculture: Zingiberaceae, Araceae and Dioscoreaceae in Southeast Asia. In: Harris DR, ed. The origins and spread of agriculture and pastoralism in Eurasia. London: University College London Press, 538–550. [Google Scholar]
- Hather JG. 2000. Archaeological parenchyma. London: Archaeotype Publications. [Google Scholar]
- Hather JG, Kirch PV. 1991. Prehistoric sweet potato (Ipomoea batatas) from Mangaia Island, Central Polynesia. Antiquity 65: 887–893. [Google Scholar]
- Hay A. 1988. Cyrtosperma (Araceae) and its Old World allies. Blumea 33:427–469. [Google Scholar]
- Herzog NM, Louderback LA, Pavlik BM. 2018. Effects of cultivation on tuber and starch granule morphometrics of Solanum jamesii and implications for interpretation of the archaeological record. Journal of Archaeological Science 98: 1–6. [Google Scholar]
- Hildebrand EA. 2003. Enset, yams and honey: ethnoarchaeological approaches to the origins of horticulture in southwest Ethiopia. Unpublished PhD thesis, Washington University in St Louis. [Google Scholar]
- Hildebrand EA. 2007. A tale of two tuber crops: how attributes of enset and yams may have shaped prehistoric human–plant interactions in southwest Ethiopia. In: Denham TP, Iriarte J, Vrydaghs L, eds. Rethinking agriculture: archaeological and ethnoarchaeological perspectives. Walnut Creek, CA: Left Coast Press, 273–298. [Google Scholar]
- Hodge A. 2014. The plastic plant: root responses to heteroneneous supplies of nutrients. New Phytologist 162: 9–24. [Google Scholar]
- Hunt HV, Shang X, Jones MK. 2018. Buckwheat: a crop from outside the major Chinese domestication centres? A review of the archaeobotanical, palynological and genetic evidence. Vegetation History and Archaeobotany 27: 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke-Despres V, Buckler ES, Smith BD, et al. 2003. Early allelic selection in maize as revealed by ancient DNA. Science 302: 1206–1208. [DOI] [PubMed] [Google Scholar]
- James G. 2004. An introduction to sugarcane. In: James G, ed. Sugarcane, 2nd edn Oxford: Blackwell. [Google Scholar]
- Kennedy J, Clarke WC. 2004. Cultivated landscapes of the Southwest Pacific. Resource Management in the Asia-Pacific (RMAP) Working Paper 50. Canberra: RMAP, Australian National University. [Google Scholar]
- Kistler L, Maezumi SY, Gregorio de Souza J, et al. 2018. Multiproxy evidence highlights a complex evolutionary legacy of maize in South America. Science 362: 1309–1313. [DOI] [PubMed] [Google Scholar]
- Kluyver TA, Jones G, Pujol B, et al. 2017. Unconscious selection drove seed enlargement in vegetable crops. Evolution Letters 1: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kroon H, Stuefer JF, Dong N, During HJ. 1994. Plant morphology and its ecological significance. Folia Geobotanica & Phytotaxonomica 29: 123–138. [Google Scholar]
- Ladefoged TN, Graves MW, Coil JH. 2005. The introduction of sweet potato in Polynesia: early farmers in Hawai’i. Journal of the Polynesian Society 114: 359–373. [Google Scholar]
- Ladizinsky G. 1985. Founder effect in crop-plant evolution. Economic Botany 39: 191–199. [Google Scholar]
- Ladizinsky G. 1998. Plant evolution under domestication. London: Kluwer Academic. [Google Scholar]
- Larson G, Piperno DR, Allaby RG, et al. 2014. Current perspectives and the future of domestication studies. Proceedings of the National Academy of Sciences, USA 111: 6139–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea DAM. 1966. Yam growing in the Maprik area. Papua New Guinea Agricultural Journal 18: 5–16. [Google Scholar]
- Lebot V. 2009. Root and tuber crops: cassava, sweet potato, yams and aroids. Crop Production Science in Horticulture, Volume 17 Wallingford, UK: CABI Publishing. [Google Scholar]
- Lebot V, Trilles B, Noyer JL, Modesto J. 1998. Genetic relationships between Dioscorea alata L. cultivars. Genetic Resources and Crop Evolution 45: 499–509. [Google Scholar]
- Lee GA, Crawford G, Liu L, Sasaki Y, Chen X. 2011. Archaeological soybean (Glycine max) in East Asia: does size matter? PLoS One 6: e26720. doi: 10.1371/journal.pone.0026720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenser C, Theiβen G. 2013. Molecular mechanisms involved in convergent crop domestication. Trends in Plant Science 18: 704–714. [DOI] [PubMed] [Google Scholar]
- Lewis T, Denham TP, Golson J. 2016. A renewed archaeological and archaeobotanical assessment of house sites at Kuk Swamp in the highlands of Papua New Guinea. Archaeology in Oceania 51: 91–103. [Google Scholar]
- Li H-L. 1970. The origin of cultivated plants in Southeast Asia. Economic Botany 24: 3–19. [Google Scholar]
- Liu W, Chen L, Zhang S, et al. 2019. Decrease of gene expression diversity during domestication of animals and plants. BMC Evolutionary Biology 19: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy TH. 2006. Raphides. In: Torrence R, Barton H, eds. Ancient starch research. Walnut Creek, CA: Left Coast Press, 136. [Google Scholar]
- Loy TH, Spriggs M, Wickler S. 1992. Direct evidence for human use of plants 28,000 years ago: starch residues on stone artefacts from the northern Solomon Islands. Antiquity 66: 898–912. [Google Scholar]
- Martinez-Ainsworth NE, Tenaillon ML. 2016. Superheroes and masterminds of plant domestication. Comptes Rendus Biologies 339: 268–273. [DOI] [PubMed] [Google Scholar]
- Matthews PJ, Agoo EMG, Tandang DN, Madulid DA. 2012. Ethnobotany and ecology of wild taro (Colocasia esculenta) in the Philippines: implications for domestication and dispersal. In: Spriggs M, Addison D, Matthews PJ, eds. Irrigated taro (Colocasia esculenta) in the Indo-Pacific: biological, social and historical perspectives. Osaka: National Museum of Osaka, Senri Ethnological Studies; 78, 307–340. [Google Scholar]
- Mbida Mindzie C, Doutrelepont H, Vrydaghs L, et al. 2001. First archaeological evidence of banana cultivation in central Africa during the third millennium before present. Vegetation History and Archaeobotany 10: 1–6. [Google Scholar]
- McKey D, Elias M, Pujol B, Duputié A. 2010. The evolutionary ecology of clonally propagated domesticated plants. New Phytologist 186: 318–332. [DOI] [PubMed] [Google Scholar]
- McKey D, Elias M, Pujol B, Duputié A. 2012. Ecological approaches to crop domestication. In: Gepts P, Famula TR, Bettinger RL, et al. , eds. Biodiversity in agriculture: domestication, evolution and sustainability. New York: Cambridge University Press, 377–406. [Google Scholar]
- Ménard L, McKey D, Muhlen GS, Vlair B, Rowe NP. 2013. The evolutionary fate of phenotypic plasticity and functional traits under domestication in manioc: changes in stem biomechanics and the appearance of stem brittleness. PLoS One 8: e74727. doi: 10.1371/journal.pone.0074727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader J, Abtosway M, Bird R, et al. 2018. Morphometrics of starch granules from sub-Saharan plants and the taxonomic identification of ancient starch. Frontiers of Earth Science 6: 169–265. [Google Scholar]
- Metlen KL, Aschehoug ET, Callaway RM. 2009. Plant behavioural ecology: dynamic plasticity in secondary metabolites. Plant, Cell & Environment 32: 641–653. [DOI] [PubMed] [Google Scholar]
- Meyer RS, Purugganan MD. 2013. Evolution of crop species: genetics of domestication and diversification. Nature Reviews Genetics 14: 840–852. [DOI] [PubMed] [Google Scholar]
- Meyer RS, DuVal AE, Jensen HR. 2012. Patterns and processes in crop domestication: an historical review and quantitative analysis of 203 global food crops. New Phytologist 196: 29–48. [DOI] [PubMed] [Google Scholar]
- Mignouna HD, Dansi A. 2003. Yam (Dioscorea spp.) domestication by the Nago and Fon ethnic groups in Benin. Genetic Resources and Crop Evolution 50: 519–528. [Google Scholar]
- Mock KE, Rowe CA, Hooten MB, Dewoody J, Hipkins VD. 2008. Clonal dynamics in western North American aspen (Populus termuloides). Molecular Ecology 17: 4827–4844. [DOI] [PubMed] [Google Scholar]
- Mozie O. 1984. The nature of shoot dominance in white yam tubers Dioscorea rotundata. Journal of Agriculture of the University of Puerto Rico 68: 335–340. [Google Scholar]
- Muñoz-Rodríguez P, Carruthers T, Wood JRI, et al. 2018. Reconciling conflicting phylogenies in the origin of sweet potato and dispersal to Polynesia. Current Biology 28: 1246–1256. [DOI] [PubMed] [Google Scholar]
- Murgia ML, Attene G, Rodriguez M, et al. 2017. Comprehensive phenotypic investigation of the ‘pod-shattering syndrome’ in common bean. Frontiers in Plant Science 8: 251. doi: 10.3389/fpls.2017.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CA, Fuller DQ. 2017. The agriculture of early India. In: Shugart H, ed. Oxford research encyclopedia of environmental science. Oxford: Oxford University Press. doi: 10.1093/acrefore/9780199389414.013.169. [Google Scholar]
- National Research Council. 1989. Lost crops of the Incas. Washington, DC: National Academy Press. [Google Scholar]
- Oke OL. 1969. The role of hydrocyanic acid in nutrition. World Review of Nutrition and Dietetics 11: 170–198. [DOI] [PubMed] [Google Scholar]
- Oliveira N. 2008. Subsistence archaeobotany: food production and the agricultural transition in East Timor. Unpublished PhD thesis, Australian National University. [Google Scholar]
- Ortiz R, Vuylsteke D. 1994. Genetic analysis of apical dominance and improvement of suckering behavior in plantain. Journal of the American Society of Horticultural Science 119: 1050–1053. [Google Scholar]
- van Oss R, Abbo S, Eshed R, et al. 2015. Genetic relationships in Cicer sp. expose evidence for geneflow between the cultigen and its wild progenitor. PLoS One 10: e0139789. doi: 10.1371/journal.pone.0139789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passam HC. 1977. Sprouting and apical dominance of yam tubers. Tropical Science 19: 29–39. [Google Scholar]
- Paz V. 2001. Archaeobotany and cultural transformation: patterns of early plant utilisation in northern Wallacea. Unpublished PhD thesis, University of Cambridge. [Google Scholar]
- Pearsall DM. 2000. Paleoethnobotany: a handbook of procedures, 2nd edn London: Academic Press. [Google Scholar]
- Perrier X, De Langhe E, Donohue M, et al. 2011. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proceedings of the National Academy of Sciences, USA 108: 11311–11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. 2002. Starch granule size and the domestication of manioc (Manihot esculenta) and sweet potato (Ipomoea batatas). Economic Botany 56: 335–349. [Google Scholar]
- Pickersgill B. 2018. Parallel vs. convergent evolution in domestication and diversification of crops in the Americas. Frontiers in Ecology and Evolution 6: 56. doi: 10.3389/fevo.2018.00056 [Google Scholar]
- Piperno DR. 2006. Phytoliths: a comprehensive guide for archaeologists and paleoecologists. Lanham: AltaMira. [Google Scholar]
- Piperno DR, Pearsall DM. 1998. The origins of agriculture in the lowland neotropics. San Diego: Academic Press. [Google Scholar]
- Premachandran MN, Prathima PT, Lekshmi M. 2011. Sugarcane and polyploidy. Journal of Sugarcane Research 1: 1–15. [Google Scholar]
- Primack RB. 1985. Comparative studies of fruits in wild and cultivated trees of chempedak (Artocarpus integer) and terap (Artocarpus odoratissimus) in Sarawak, East Malaysia with additional information on the reproductive biology of the Moraceae in Southeast Asia. Malayan Nature Journal 39: 1–39. [Google Scholar]
- Pritchard J, Lewis T, Beeching L, Denham TP. 2018. An assessment of microCT technology for the investigation of charred archaeological parenchyma from house sites at Kuk Swamp, Papua New Guinea. Journal of Anthropological and Archaeological Sciences 11: 1927–1938. [Google Scholar]
- Purugganan MD, Fuller DQ. 2009. The nature of selection during plant domestication. Nature 457: 843–848. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Schemske DW. 2002. Neopolyploidy in flowering plants. Annual Review of Ecology and Systematics 33: 589–639. [Google Scholar]
- Rendón-Anaya M, Montero-Vargas JM, Saburido-Álvarez S, et al. 2017. Genomic history of the origin and domestication of common bean unveils its closest sister species. Genome Biology 18: 60. doi: 10.1186/s13059-017-1190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival L, McKey D. 2008. Domestication and diversity in manioc (Manihot esculenta Crantz ssp. esculenta, Euphorbiaceae). Current Anthropology 49: 1119–1128. [Google Scholar]
- Roose ML, Soost RK, Cameron JW. 1995. Citrus. In: Smartt J, Simmonds NW, eds. Evolution of crop plants, 2nd edn Harlow: Longman, 443–448. [Google Scholar]
- Roullier C, Benoit L, McKey DB, Lebot V. 2013. Historical collections reveal patterns of diffusion of sweet potato in Oceania obscured by modern plant movements and recombination. Proceedings of the National Academy of Sciences, USA 110: 2205–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle K, Johnson D, Townsend P, Rees J. 1978. Palm sago: a tropical starch from marginal lands. Canberra: Australian National University Press. [Google Scholar]
- Santosa E, Lian CL, Sugiyama N, Misra RS, Boonkorkaew P, Thanomchit K. 2017. Population structure of elephant foot yams (Amorphophallus paeoniifolius (Dennst.) Nicolson) in Asia. PLoS One 12: e0180000. doi: 10.1371/journal.pone.0180000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer CO. 1952. Agricultural origins and their dispersals. New York: American Geographical Society. [Google Scholar]
- Scarcelli N, Tostain S, Mariac C, et al. 2006. Genetic nature of yams (Dioscorea sp.) domesticated by farmers in Benin (West Africa). Genetic Resources and Crop Evolution 53: 121–130. [Google Scholar]
- Scarcelli N, Cubry P, Akakpo R, et al. 2019. Yam genomics supports West Africa as a major cradle of crop domestication. Science Advances 5: eaaw1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrieber M, Stein N, Mascher M. 2018. Genomic approaches for studying crop evolution. Genome Biology 19: 140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M, Botigué L, Brace S, et al. 2019. A 3,000-year-old Egyptian emmer wheat genome reveals dispersal and domestication history. Nature Plants 5: 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvertown J. 2008. The evolutionary maintenance of sexual reproduction: evidence from the ecological distribution of asexual reproduction in clonal plants. International Journal of Plant Science 169: 157–168. [Google Scholar]
- Sonnante G, Hammer K, Pignone D. 2009. From the cradle of agriculture a handful of lentils: history of domestication. Rendiconti Lincei 20: 21–37. [Google Scholar]
- Smith BD, Yarnell RA. 2009. Initial formation of an indigenous crop complex in eastern North America at 3800 BP. Proceedings of the National Academy of Sciences, USA 106: 6561–6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smýkal P, Coyne CJ, Ambrose MJ, et al. 2015. Legume crops phylogeny and genetic diversity for science and breeding. Critical Reviews in Plant Sciences 34: 43–104. [Google Scholar]
- Smýkal P, Nelson M, Berger JD, Wettberg E. 2018. The impact of genetic changes during crop domestication on healthy food development. Agronomy 8: 26. [Google Scholar]
- Stebbins GL. 1950. Variation and evolution in plants. New York: Colombia University Press. [Google Scholar]
- Stetter MG, Gates DJ, Mei W, et al. 2017. How to make a domesticate. Current Biology 27: R896–R900. [DOI] [PubMed] [Google Scholar]
- Stitzer MC, Ross-Ibarra J. 2018. Maize domestication and gene interaction. New Phytologist 220: 395–408. [DOI] [PubMed] [Google Scholar]
- Streck NA, Pinheiro DG, Zanon AJ, et al. 2017. Effect of plant spacing on growth, development and yield of cassava in a subtropical environment. Crop Production and Management 75: 407–415. [Google Scholar]
- Sultan SE. 2015. Organism and environment. ecological development, niche construction and adaptation. Oxford: Oxford University Press. [Google Scholar]
- Sunell LA, Healey PL. 1979. Distribution of calcium oxalate crystal idioblasts in corms of taro (Colocasia esculenta). American Journal of Botany 66: 1029–32. [Google Scholar]
- Sunell LA, Healey PL. 1985. Distribution of calcium oxalate crystal idioblasts in leaves of taro (Colocasia esculenta). American Journal of Botany 72: 1854–1860. [Google Scholar]
- Tanno K, Willcox G. 2012. Distinguishing wild and domestic wheat and barley spikelets from early Holocene sites in the Near East. Vegetation History and Archaeobotany 21: 107–115. [Google Scholar]
- Terrell JE, Hart JP, Barut S, et al. 2003. Domesticated landscapes: the subsistence ecology of plant and animal domestication. Journal of Archaeological Method and Theory 10: 323–368. [Google Scholar]
- Torrence R, Barton H, eds. 2006. Ancient starch research. Walnut Creek, CA: Left Coast Press. [Google Scholar]
- Trněný O, Brus J, Hradilová I, et al. 2018. Molecular evidence for two domestication events in the pea crop. Genes 9: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugent D, Peterson LW. 1988. Archaeological remains of potato and sweet potato in Peru. Circular (International Potato Center) 16: 1–10. [Google Scholar]
- Ugent D, Pozorski S, Pozorski T. 1981. Prehistoric remains of the sweet potato from the Casma Valley of Peru. Phytologia 49: 401–415. [Google Scholar]
- Ugent D, Pozorski S, Pozorski T. 1982. Archaeological potato tuber remains from the Casma Valley of Peru. Economic Botany 36: 182–192. [Google Scholar]
- Ugent D, Pozorski S, Pozorski T. 1984. New evidence for ancient cultivation of Canna edulis in Peru. Economic Botany 38: 417–432. [Google Scholar]
- Ugent D, Pozorski S, Pozorski T. 1986. Archaeological manioc (Manihot) from coastal Peru. Economic Botany 40: 78–102. [Google Scholar]
- Van der Veen M, Morales J. 2011. Summer crops – from trade to innovation. In: Van der Veen M, ed. Consumption, trade and innovation. Exploring the botanical remains from the Roman and Islamic ports at Quseir al-Qadim, Egypt. Frankfurt: Africa Magna Verlag, 75–119. [Google Scholar]
- Vaughan DA, Balazs E, Heslop-Harrison JS. 2007. From crop domestication to super-domestication. Annals of Botany 100: 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernier P, Orkwor GC, Dossou AR. 2003. Studies of yam domestication and farmers’ practices in Benin and Nigeria. Outlook on Agriculture 32: 35–41. [Google Scholar]
- Vrydaghs L, Swennen RR, Mbida C, Doutrelepont H, De Langhe E, de Maret P. 2003. The banana phytolith as a direct marker of early agriculture: a review of the evidence. In: Hart DM, Willis LA, eds. Phytolith and starch research in the Australia - Pacific - Asian regions: the state of the art. Terra Australis 19. Canberra: Pandanus Books, 175–185. [Google Scholar]
- Vrydaghs L, Ball TB, Volkaert H, Van Den Houwe I, Manwaring J, De Langhe E. 2009. Differentiating the volcaniform phytoliths of bananas: Musa acuminata. Ethnobotany Research & Applications 7: 239–246. [Google Scholar]
- Wang W, Feng B, Xiao J, et al. 2014. Cassava genome from a wild ancestor of cultivate varieties. Nature Communications 5: 5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. 1975. Sex and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- Winchell F, Stevens CJ, Murphy C, Champion L, Fuller DQ. 2017. Evidence for sorghum domestication in Fourth Millennium BC eastern Sudan: spikelet morphology from ceramic impressions of the Butana Group. Current Anthropology 58: 673–683. [Google Scholar]
- Yen DE. 1973. The origins of Oceanic agriculture. Archaeology and Physical Anthropology in Oceania 8: 68–85. [Google Scholar]
- Yen DE. 1974. The sweet potato and Oceania: an essay in ethnobotany. Honolulu: Bishop Museum Press. [Google Scholar]
- Yen DE. 1989. The domestication of environment. In: Harris DR, Hillman GC, eds. Foraging and farming: the evolution of plant exploitation. London: Unwin Hyman, 55–75. [Google Scholar]
- Zannou A, Richards P, Struik PC. 2006. Knowledge of yam variety development: insights from farmers’ and researchers’ practices. Knowledge Management for Development Journal 2: 30–39. [Google Scholar]
- Zohary D. 1984. Modes of evolution in plants under domestication. In: Grant WF, ed. Plant biosystematics. New York: Academic Press, 579–586. [Google Scholar]
- Zohary D, Hopf M. 2000. Domestication of plants in the Old World. Oxford: Oxford University Press. [Google Scholar]
- Zong Y, Yao S, Crawford GW, et al. 2017. Selection for oil content during soybean domestication revealed by X-ray tomography of ancient beans. Scientific Reports 7: 43595. [DOI] [PMC free article] [PubMed] [Google Scholar]