Abstract
Background and Aims
The success of invasive plants can be attributed to many traits including the ability to adapt to variable environmental conditions. Whether by adaptation, acclimation or phenotypic plasticity, these plants often increase their resource-use efficiency and, consequently, their fitness. The goal of this study was to examine the hydraulic and eco-physiological attributes of sun and shade populations of Pteridium aquilinum, a weedy fern, to determine whether the presence of vessels and other hydraulic attributes affects its success under a variety of light conditions.
Methods
Hydraulic traits such as cavitation resistance, hydraulic conductivity, photosynthesis and water potential at turgor loss point were measured on fronds from sun and shade populations. Anatomical and structural traits such as conduit diameter and length, stomatal density and vein density were also recorded. Diurnal measures of leaf water potential and stomatal conductance complement these data.
Key Results
Gas exchange was nearly double in the sun plants, as was water-use efficiency, leaf-specific conductivity, and stomatal and vein density. This was largely achieved by a decrease in leaf area, coupled with higher xylem content. There was no significant difference in petiole cavitation resistance between the sun and shade leaves, nor in xylem-specific conductivity. Hydraulic conduit diameters were nearly equivalent in the two leaf types.
Conclusions
Shifts in leaf area and xylem content allow P. aquilinum to occupy habitats with full sun, and to adjust its physiology accordingly. High rates of photosynthesis explain in part the success of this fern in disturbed habitats, although no change was observed in intrinsic xylem qualities such as cavitation resistance or conduit length. This suggests that P. aquilinum is constrained by its fundamental body plan, in contrast to seed plants, which show greater capacity for hydraulic adjustment.
Keywords: Plasticity, xylem, embolism, turgor loss point, stomatal density, stomatal conductance, photosynthesis, modulus of elasticity
Introduction
The success of invasive or weedy species may be due to a combination of factors, including adaptive physiology. One theory suggests that ‘phenotypic divergence’ confers a competitive edge because invaders may have traits that fall outside of the norm in a given community (Hutchinson, 1959; MacArthur and Levins, 1967; Ordonez et al., 2010). Alternatively, ‘phenotypic convergence’ may allow an invader to better adapt to local conditions because its traits are more closely aligned with the native plant community (Smith and Knapp, 2001; Curtis, 2003; Ordonez et al., 2010). Key studies have shown that some invasive species use resources such as light, water and nutrients more efficiently than natives, supporting the former hypothesis (Funk and Vitousek, 2007). Physiological acclimation or phenotypic plasticity may play an important role in all these scenarios allowing invaders to maximize fitness in a novel environment (Davidson et al., 2011). For example, a few co-ordinated changes allow leaves to alter their morphology and physiology to variable levels of light. Sun leaves exhibit higher rates of leaf water transport (KLEAF) and thus higher rates of gas exchange (Boardman, 1977; Bassow and Bazzaz, 1998; Scoffoni et al., 2018), and these functional differences are linked to structural changes such as shifts in vein density and stomatal density, which support increased KLEAF (Brodribb et al., 2007; Murphy et al., 2014; Scoffoni et al., 2015). Hydraulic attributes are rarely examined in studies of invasive species’ physiology, so our goal here was to capture the structural, hydraulic and photosynthetic adjustments of sun and shade populations of bracken fern, Pteridium aquilinum, a weedy cosmopolitan fern.
The vast majority of ferns are found in shady understorey habitats where evapo-transpirative demand is typically low. With underground rhizomes that give rise to leaves, terrestrial ferns have an ancestral body plan that relies solely on tracheid-based primary xylem, imposing a hard limit on leaf water transport (Brodribb, 2007; Pittermann et al., 2011, 2013, 2015). However, P. aquilinum (Dennstaedtiaceae) is one of a limited number of ferns to tolerate full sun, and one of the very few known ferns to have evolved true xylem vessels (Carlquist and Schneider, 2007; Pittermann et al., 2011). Xylem vessels appear later in the fossil record than tracheids and are more morphologically flexible with respect to lumen diameter and conduit length (Aloni, 2015; Wilson, 2016). Hence, the evolution of vessels has generally led to greater hydraulic efficiency and faster growth rates in angiosperms compared to conifers and ferns, allowing angiosperms to explore a broader morphospace of vascular structure and function (Spicer and Groover, 2010; Aloni, 2015; Gensel, 2018). There is little doubt that hydraulic attributes contributed to the ecological success of angiosperms and so we wondered whether this success may be shared by P. aquilinum, one of the most widespread vascular plants that occupies both low light and full sun habitats.
Pteridium aquilinum is a weedy and invasive fern that grows globally throughout temperate and subtropical regions (Page, 1982, 2002). It is a nuisance species, difficult to eradicate, toxic to both humans and livestock if consumed in large quantities, and it often pollutes local water sources with its particular blend of chemical constituents (Marrs and Watt, 2006; Vetter, 2009; Robinson et al., 2010). Ecological disturbances, principally from human activity, have expanded the range of P. aquilinum, where it responds to environmental heterogeneity with what appears to be considerable phenotypic plasticity (Page, 1982; Marrs and Watt, 2006; Der et al., 2009). Pteridium aquilinum can grow in low-nutrient habitats (Watt, 1940; Cary and Pittermann, 2018) and it can tolerate a range of light levels, from full sunlight to near complete shade (Page, 1976; den Ouden, 2000; Marrs and Watt, 2006). Fronds from either of these sites have a strikingly different appearance. Fronds in full sun are short, with a vertical orientation and thick cuticles (Boodle, 1904; den Ouden, 2000; Fig. 1). By contrast, fronds in the understorey can reach over 1.5 m in length and have a larger leaf area. The ability of P. aquilinum to flourish so well in such diverse habitats may due to its adaptive vascular traits (Marrs and Watt, 2006).
Fig. 1.
Examples of sun and shade populations of P. aquilinum on the University of California Santa Cruz campus. Sun populations (top panels) are vertically oriented, and have thicker leaves with more pronounced venation than the shade-grown fronds (bottom panels). Sun fronds have a greater petiolar fraction of vascular tissue (top right) than shade plants (bottom left).
Given the functional importance of vessels and the success of P. aquilinum across different habitats, we hypothesized that sun-adapted fronds of P. aquilinum are likely to have more efficient water transport, as evidenced by higher rates of gas exchange, leaf-specific hydraulic conductivity (KLEAF), xylem specific conductivity (KS), a tendency toward long, wide conduits, higher leaf vein and stomatal density, as well as greater resistance to drought-induced embolism in the frond petioles. By contrast, shade-adapted fronds may exhibit lower rates of photosynthesis, reduced phloem content, higher turgor loss point and thinner leaves. We focused primarily on water relations and hydraulic attributes because of their crucial link to plant survival and their value for comparison to vessel-bearing angiosperms.
MATERIAL AND METHODS
Plant material
Fronds of P. aquilinum (L.) Kuhn were sampled from wild populations within 1 km of one another on the University of California, Santa Cruz campus reserve during mid-summer (2014–2017). Page (1976) classified the regional subspecies as Pteridium aquilinum ssp. lanuginosum-pubescens. The campus reserve is located on California’s coastal range, which experiences a Mediterranean climate modulated by summer fog (Dawson, 1998). Shaded populations were along road cuts, trails or under moderate canopy cover of redwood (Sequoia sempervirens) forest, growing in permeable, black silty loam soils topped with a dense layer of redwood leaf duff (Haff et al., 2008; Fig. 1). The sun populations grew in open south-sloping meadows of invasive grasses on a sandy, light brown loam with a thin cover of organic matter (Haff et al., 2008; Fig. 1). Sample size is six to eight fronds per ‘treatment’ unless otherwise indicated.
Several attempts at cultivating P. aquilinum from rhizome cuttings and spore germination in a common garden setting failed, and this difficulty was confirmed by others in correspondence. Apparently, long segments of the rhizome with a terminal bud must be removed for successful cultivation, but we were not granted permission to disrupt soils on campus. Based on conversations with experts (J. Der, California State University, Fullerton, CA) and the close proximity of the campus populations, we strongly suspect that the sun and shade plants comprise the single regional subspecies. However, in the absence of common garden experiments, we refrain from attributing any phenotypic response to plasticity.
Diurnal measures
Habitat conditions and water relations of sun and shade P. aquilinum plants were monitored hourly from 0700 to 1900 h in early June 2015 (n = 6 shade plants, n = 6 sun plants). These parameters consisted of temperature, wind speed and relative humidity (Kestrel 3000 Wind Meter, Kestrelmeters.com, Minneapolis, MN, USA). Vapour pressure deficit (kPa) was calculated from air temperature and relative humidity. Photosynthetically active radiation (PAR; µmol m−2 s−1) was determined with a PAR sensor (LI-250A Light Meter, LI-COR, Lincoln, NB, USA) and volumetric water content (%) was measured in the morning using a soil moisture probe (Hydrosense II, Campbell Scientific, Logan, UT, USA). An SCI-1 porometer was used to measure stomatal conductance (mmol m−2 s−1) and leaf temperature (Decagon Devices, Pullman, WA, USA), with stomatal conductance for each individual plant reported as the average of three measures from each frond. Water potential (MPa) was measured on apical pinnae using a Scholander pressure chamber (1505D-EXP, PMS Instrument Co., St. Albany, OR, USA).
Hydraulic conductivity and vulnerability curves
Plants are variably adapted to the drought-induced entry and expansion of air into xylem conduits known as embolism, which may severely disrupt water transport. To examine this in P. aquilinum, we measured the general susceptibility of petiole xylem to embolism in fronds from both sun and shade populations, as well as their native levels of embolism.
Vulnerability curves were constructed on eight fronds from each population. On the day of collection, healthy fronds were bagged for 15 min before 0900 h. Fronds were then cut under water at the petiole base and placed in two plastic bags with wet paper towels for transport to the lab. Once there, the leaf petioles were excised and re-trimmed under water, then degassed at −95 kPa to remove any emboli from the xylem conduits. The segments were then fitted to a hydraulic apparatus filled with 20 mm KCl solution to drive flow. Maximum hydraulic conductivity (KMAX, kg m MPa−1 s−1) was computed as the mass flow rate standardized for segment path length (0.142 m), with a correction for any background flow at 0 kPa taken before and after pressure flow at ~6 kPa (Hacke et al., 2000). KMAX was divided by the xylem and leaf area to determine xylem-specific hydraulic conductivity (KS) and leaf specific hydraulic conductivity (KLEAF), respectively.
Following the KMAX measures, vulnerability curves were constructed by spinning the petioles in a Sorvall RC5C centrifuge at speeds known to induce a range of xylem pressures (Thermo Fisher, Scientific, Waltham, MA, USA). The segments were spun for 5 min at each speed in a custom rotor designed for 0.142 m segments; this was done at incrementally higher speeds until samples were 90 % embolized (Alder et al., 1997). Petiole hydraulic conductivity was measured after each spin (KSPIN). These data were used to construct a vulnerability curve, in which the segment percentage loss of conductivity due to embolism (PLC) is plotted as a function of the xylem pressures attained during the spin (Pockman et al., 1995). PLC was computed as:
| (1) |
A standard function was used to find the water potential at which the petioles reached 12, 50 and 88 PLC (P12, P50, P88) (Pammenter and Van der Willigen, 1998). Less than 1 % of the conduits in the petiole were longer than 0.118 m (see below).
Native PLC values were determined on petioles at midday, and this was done in early September, at the end of the growing season. Eight fronds from each population were covered in a black plastic bag for at least 15 min, after which the water potential was measured on pinnae located at mid-rachis. Fronds were cut under water at the petiole base, transported to the lab and processed for hydraulic measures as described above. Native conductivity was measured first (KNATIVE), then the segment was degassed at −95 kPa and KMAX was measured. PLC was computed as described above.
Frond morphology and anatomy
Total frond leaf area was measured using a leaf area meter (LI-3100, LI-COR). Specific leaf area (SLA) was obtained by first measuring leaf area of a frond sub-sample, drying it at 65 °C for 2 d and computing SLA as leaf area divided by leaf dry mass.
Measures of stomatal and vein density were made on one pinnule at mid-rachis for each frond. These samples were cleared in 1 m KOH for 2–4 d, and washed in bleach for 1 min to remove trace pigments. The samples were then stained in toluidine blue and mounted onto slides. The leaf abaxial side was photographed at 20× magnification, the images were stitched together with Fiji software (Schindelin et al., 2012) and vein density was measured (vein length divided by lamina area; mm mm−2) using Fiji. To capture stomatal density, the pinnule was then photographed at four random locations at 200× magnification. At each location the number of stomata was counted in regions with an area of 0.725 mm2; we also measured the length (µm) and width (µm) of 20 randomly selected stoma. Stomatal density is presented as the number of stomata observed per unit area (no. mm−2). These photos were also used for measures of epidermal cell area (μm2) made on ten cells per individual.
Xylem traits were captured on the petioles used to construct the vulnerability curves by making hand-sections with a razor blade. Sections were stained in phloroglucinol and photographed using a light microscope mounted with a digital camera (instruments cited above). For each section, conduit lumen area, xylem area, phloem area and sectional area were measured using ImageJ. Xylem conduit lumen areas were converted to diameters (D) by treating them as circles: where A is the lumen area. Hydraulic diameters were computed according to Kolb and Sperry (1999) as Dh = ΣD5/ΣD4. Xylem and phloem areas were divided by total area of the cross-section to obtain the xylem and phloem fractional area (FX and FP). Leaf area was divided by xylem area for estimates of Huber values.
Mean conduit lengths were also compared between the sun and shade plants. The stipe and rachis of each frond were trimmed underwater to about 45 cm in length and flushed with purified water for 1 h at 100 kPa. A silicone fluid (Rhodosil RTV-14, Bluestar Silicones, Rock Hill, SC, USA) was mixed with a UV-stain (Uvitex OB, Ciba Specialty Chemicals, Basel, Switzerland), dissolved in 1 % chloroform, and then degassed for 2 h as previously described (Venturas et al., 2016). The basal end of the stipe was shaved with a razor blade, mounted into a closed tubing apparatus and injected with the UV silicone mixture for 24 h under 50 kPa of pressure. During injection, fronds were wrapped in a plastic bag with wet paper towels to prevent desiccation. Fronds were cured at room temperature for 48 h in humidified bags following the injection treatment.
Cured samples were sectioned in a transverse plane with a razor blade at 0, 0.7, 1.4, 2.9, 5.8, 11.8 and 24 cm from the injection point. Micrographs of each section were captured using a fluorescence microscope equipped with digital camera (Zeiss Stereo Discover V.12 with Axiocam HRc digital camera, Carl Zeiss Microscopy, LLC, Thornwood, NY, USA). Silicone-filled and non-filled vessels were counted from each section using ImageJ. The proportion of filled to non-filled vessels per section was related to an exponential decay function that was used to calculate mean conduit length (Sperry et al., 2005).
Leaf gas exchange
Leaf CO2 assimilation rate was measured in response to changing CO2 concentrations using a portable gas exchange analyser (LI-6400XT, LI-COR; n = 7 per treatment). CO2 levels were set to 400, 0, 400, 600, 800, 1000 and 1200 µmol CO2 mol−1, PAR was set to 1600 and 1000 µmol m−2 s−1 for the sun and shade fronds, respectively, and VPD was kept at 1 kPa. Cuvette temperature varied between the sun and shade site at 21.8 ± 0.4 and 24.1 ± 0.4 °C respectively. CO2 assimilation curves were analysed using the R software package plantecophys (Duursma, 2015; R Core Team, 2019), which fits a curve using the Farquhar–von Caemmerer model to obtain values of maximum carboxylation (VCMAX), maximum electron transport rate responsible for RuP2 regeneration (JMAX) and the mitochondrial respiration in light (RD; Farquhar et al. 1980).
Leaf pressure–volume curves
Pressure–volume curves determined differences in osmotica (ΨΠMAX, MPa) and the turgor loss point (Ψ TLP; MPa) between P. aquilinum sun and shade leaves. Healthy fronds from wild populations were cut at mid-stipe and transported back to the laboratory in humidified bags. Frond samples were trimmed to mid-rachis underwater and hydrated for ~2 h. Most samples reached water potentials in the range of values measured between predawn and midday.
Hydrated samples were then further trimmed underwater down to apical pinnae of 10–15 cm length, and these were alternatively weighed on an analytical balance and measured for water potential as they dried. Between measures, samples were placed in sealed bags in darkness for about 15 min to equilibrate water content and slow drying rate. Each sample was measured 10–15 times during dry-down, with at least five measures past the inflection point to capture the linear portion of the pressure–volume curve. After dry-down measures, samples were oven dried at 65 °C for ≥72 h and then weighed on an analytical balance to determine the dry mass.
Samples were inspected for signs of over-saturation by plotting the mass of water loss in relation to leaf water potential. Points with an initial steep decline in water potential were removed to avoid the analysis of excessively hydrated leaves (Meinzer et al., 2014). Relative water content (RWC) was computed according to:
| (2) |
where Wf is fresh weight (g), Wd is dry weight (g) and Ws is saturated weight (g) of the leaf sample. Saturated leaf weight was taken as the intercept of the initial slope – above the inflection point – where leaf water mass corresponded with a water potential of 0 MPa.
Analyses of turgor loss point and osmotic potential were performed by plotting the relative water deficit (RWD; 1 – RWC) and inverse water potential. The last three points beyond the inflection point were fitted with a linear regression, and points were then added towards the inflection point until the goodness of fit was maximized. The water potential at which the linear slope intercepts the inverse water potential axis was the maximum leaf osmotic potential (Ψ ΠMAX). To find the water potential at the turgor loss point (Ψ TLP), we found the point that best occupied the transition of the linear portion of the data to the curve, using the linear regression. The slope of turgor pressure in relation to RWC represented the leaf modulus of elasticity (ε).
Biomechanics and tissue density
Pteridium aquilinum fronds were tested for differences in biomechanical properties. Measured biomechanical properties were modulus of elasticity (MOE), a metric of stiffness, and modulus of rupture (MOR), a metric of strength. Fronds from each habitat (n = 16) were cut at the stipe base and transported to California State University Bakersfield. Stipe samples were trimmed down to 20 cm and the end diameters were measured with digital calipers (Marathon Watch Company Ltd, Richmond Hill, Ontario, Canada). Samples were placed into a material properties testing machine (Instron, Norwood, MA, USA) that performed a four-point bending test. We used the approach of Jacobsen et al. (2005) to calculate MOE and MOR.
The tissue density (mass per unit volume) of stipes was determined on the same samples used to test for MOE and MOR. After performing the four-point bending test, undamaged portions of samples of each frond type were trimmed down to 2 cm in length and saturated with water using vacuum infiltration for >12 h. The displacement method was used to measure sample volume using a four-digit precision balance (Sartorius, Göttingen, Germany). Samples were then dried in an oven at 60 °C for ≥72 h and weighed to determine the dry mass. Stipe tissue density was calculated as wet volume divided by dry mass.
Statistical analysis
The data were analysed using a two-sample Student’s t-test, or, if lacking equal variance, using a Welch’s t-test (R Core Team, 2019). The data were manually inspected for normal distribution and equal variance in R, following methods outlined by Mangiafico (2016). For each test, α = 0.05. For ease of reference, all averages, errors (1 s.d.) and test outputs are reported in Table 1 and ranked in order of significance.
Table 1.
Means, standard deviation (s.d.), and Student’s or Welch’s t-test outputs comparing sun and shade P. aquilinum frond traits. Traits are listed by P-value from smallest to largest.
| Trait | Abbreviation | Mean ± 1 s.d. | t | d.f. | P | |
|---|---|---|---|---|---|---|
| Sun | Shade | |||||
| Stipe density (kg m−3) | 330.38 ± 33.21 | 257.61 ± 21.66 | 7.340 | 30 | 3.55E-08 | |
| Specific leaf area (cm2 g−1) | SLA | 65.32 ± 5.17 | 271.54 ± 39.05 | −12.825 | 10 | 1.56E-07 |
| Stomatal density (mm−2) | SD | 100.97 ± 19.50 | 35.86 ± 4.92 | 9.158 | 14 | 2.75E-07 |
| Maximum electron transport rate (µmol m−2 s−1) | J MAX | 167.49 ± 23.97 | 72.17 ± 10.97 | 9.566 | 12 | 5.77E-07 |
| Maximum carboxylation rate (µmol m−2 s−1) | V CMAX | 82.40 ± 12.14 | 39.25 ± 5.49 | 8.568 | 12 | 1.85E-06 |
| Minimum water potential (MPa) | ψ MIN | −1.83 ± 0.22 | −0.74 ± 0.29 | −7.48 | 10 | 2.11E-05 |
| Vein density (mm mm−2) | VD | 2.93 ± 0.35 | 2.15 ± 0.14 | 5.885 | 14 | 3.97E-05 |
| Xylem fraction (%) | F X | 8.81 ± 0.83 | 6.20 ± 0.56 | 6.385 | 10 | 7.99E-05 |
| Phloem fraction (%) | F P | 17.15 ± 0.75 | 13.89 ± 1.25 | 5.483 | 10 | 0.0003 |
| Leaf epidermal cell area (µm2) | 3660 ± 888 | 6695 ± 1077 | −5.325 | 10 | 0.0003 | |
| Maximum stomatal conductance (mmol m−2 s−1) | g SMAX | 462 ± 76 | 282 ± 36 | 5.229 | 10 | 0.0003 |
| Leaf to xylem area ratio (m2 cm−2) | A L : AX | 3.94 ± 2.05 | 17.82 ± 5.21 | −6.072 | 6.51 | 0.0007 |
| Conduit diameter (µm) | D | 24.65 ± 0.78 | 21.67 ± 0.54 | 6.280 | 6 | 0.0008 |
| Leaf specific hydraulic conductivity 105 × (kg m−1 MPa−1 s−1) | K LEAF | 26.78 ± 18.95 | 5.39 ± 3.49 | 5.206 | 7.92 | 0.0008 |
| Stipe modulus of elasticity (N mm−2) | MOE | 6821 ± 2009 | 9091 ± 1658 | −3.484 | 30 | 0.0015 |
| Water potential at 88 % loss of hydraulic conductivity (MPa) | P88 | −2.59 ± 0.34 | −2.06 ± 0.20 | −3.741 | 14 | 0.0021 |
| Water potential at turgor loss point in pinnae (MPa) | Ψ TLP | −2.20 ± 0.16 | −1.72 ± 0.21 | −3.801 | 8 | 0.0052 |
| Intrinsic water use efficiency (µmol CO2 mol H2O−1) | WUE | 61.67 ± 6.59 | 34.13 ± 38.94 | 3.208 | 12 | 0.0075 |
| Maximum osmotic water potential in pinnae (MPa) | Ψ ΠMAX | −1.83 ± 0.21 | −1.48 ± 0.12 | −3.068 | 8 | 0.0154 |
| Pinnae modulus of elasticity (MPa) | ε | 18.84 ± 3.62 | 14.44 ± 3.79 | 1.965 | 8.78 | 0.0818 |
| Water potential at 50 % loss of hydraulic conductivity (MPa) | P50 | −1.76 ± 0.26 | −1.51 ± 0.28 | −1.857 | 14 | 0.0845 |
| Stipe modulus of rupture (N mm−2) | MOR | 80.29 ± 14.31 | 73.13 ± 13.37 | 1.464 | 30 | 0.1536 |
| Percentage loss of hydraulic conductivity at midday (%) | PLCmidday | 44.22 ± 25.30 | 28.18 ± 20.05 | 1.4214 | 18 | 0.1723 |
| Guard cell length (µm) | 48.58 ± 2.33 | 49.95 ± 1.87 | −1.300 | 13.36 | 0.2160 | |
| Proportion of xylem conduits filled at 0.058 m from silicone injection point ( %) | 2.38 ± 0.77 | 3.31 ± 1.59 | −1.292 | 10 | 0.2256 | |
| Hydraulic conduit diameter (µm) | D H | 68.68 ± 2.68 | 70.38 ± 1.38 | −1.123 | 6 | 0.3044 |
| Guard cell width (µm) | 14.66 ± 0.63 | 14.08 ± 1.20 | 0.785 | 14 | 0.4450 | |
| Xylem-specific hydraulic conductivity (kg m−1 kPa−1 s−1) | K S | 21.01 ± 6.57 | 23.35 ± 5.07 | −0.690 | 10 | 0.5059 |
| Day respiration (µmol m−2 s−1) | R D | 1.22 ± 0.23 | 1.38 ± 1.09 | −0.375 | 12 | 0.7141 |
| Mean conduit length (m) | L C | 0.014 ± 0.004 | 0.015 ± 0.005 | −0.308 | 10 | 0.7645 |
| Proportion of xylem conduits filled at 0.118 m from silicone injection point (%) | 0.52 ± 0.25 | 0.58 ± 0.61 | −0.180 | 10 | 0.8604 | |
| Water potential at 12 % loss of hydraulic conductivity (MPa) | P12 | −0.89 ± 0.55 | −0.93 ± 0.59 | 0.148 | 14 | 0.8843 |
RESULTS
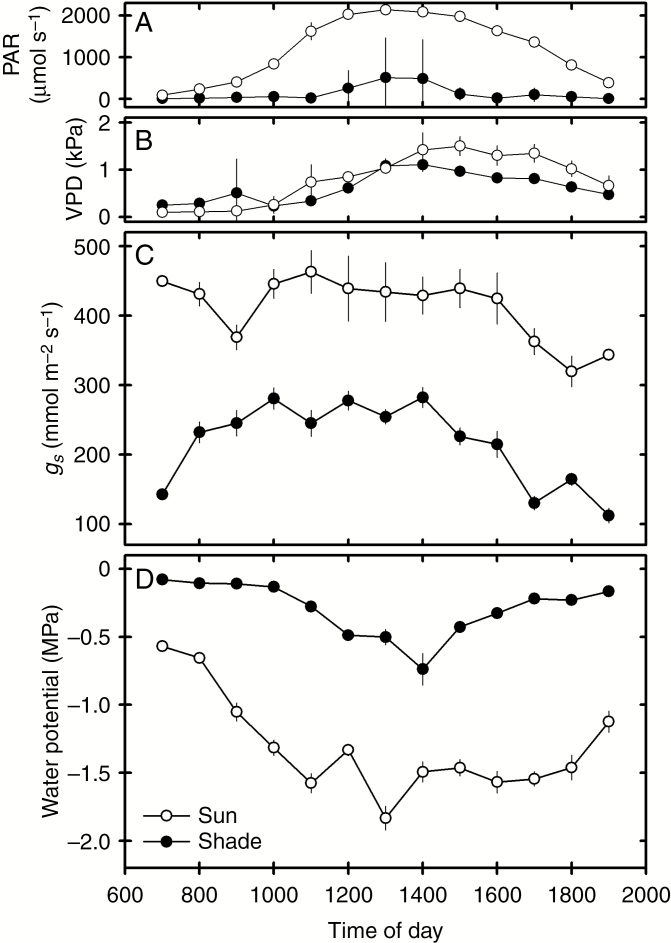
The habitat of the sun- and shade-adapted P. aquilinum fronds differed considerably. Whereas the sun plants experienced full sun at midday, the sun flecks in the shade habitat occasionally reached over 1400 μmol m−2 s−1 (Fig. 2). The air vapour pressure deficit (VPD) peaked at 1.45 kPa in the sun but there was considerable overlap between the two sites (Fig. 2) Soils from the sun sites had a volumetric water content of 4.33 ± 0.46 %, compared to the 16.45 ± 2.20 % at shade sites. The air was almost still in the shade but wind speeds varied from 0.4 to 1.7 m s−1 in full sun. Consequently, stomatal conductance was close to double in sun plants than in shade plants, peaking midday at 463 ± 76 and 282 ± 5 mmol m−2 s−1 respectively (Fig. 2). In line with these differences in habitat and leaf water loss, minimum midday frond water potential (ψ ΜΙΝ) was consistently lower in the sun populations than in the shade: sun populations reached their nadir at −1.9 MPa, while the shade plants rarely dipped below −0.5 MPa (Fig. 2).
Fig. 2.
Diurnal measures of (A) photosynthetically active radiation (PAR), (B) vapour pressure deficit (VPD), (C) stomatal conductance (gS) and (D) leaf water potential (ψ) in sun and shade populations of P. aquilinum (open and closed circles, respectively).
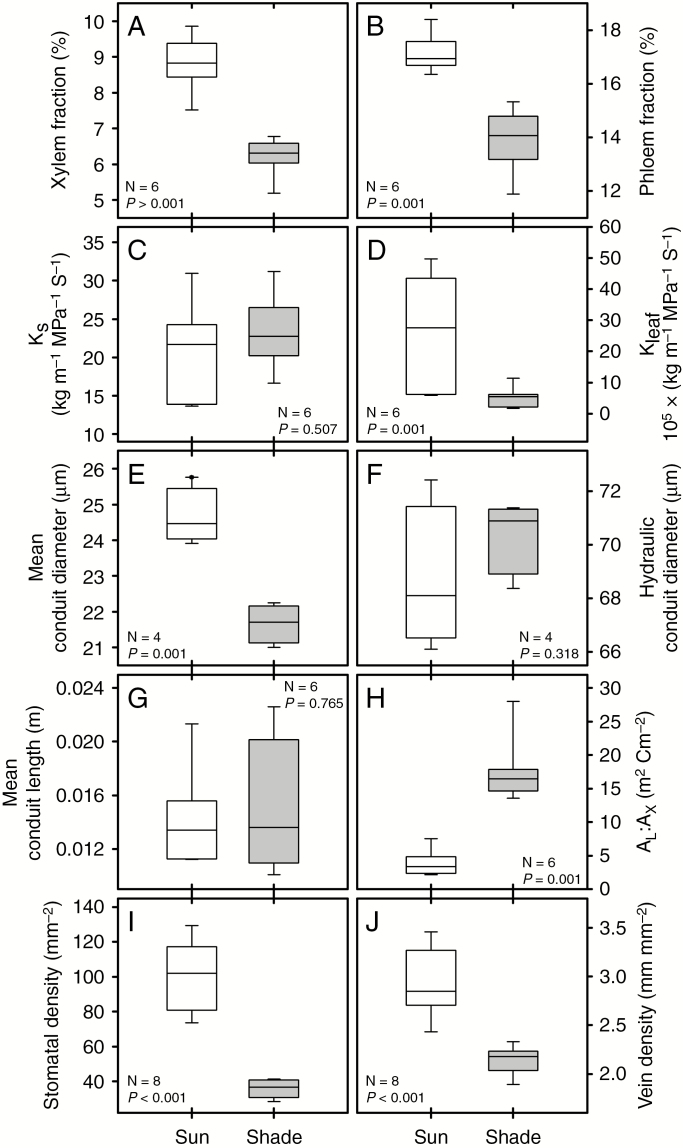
The fronds were also morphologically distinct. Sun fronds were shorter with an average length of 0.68 ± 0.021 m compared to shade fronds (0.96 ± 0.05 m), they had 30 % lower leaf area (898 ± 149 cm2) than shade fronds (1259 ± 205 cm2) and thicker, tougher leaves as manifest by their significantly higher specific leaf area (SLA; Table 1). Importantly, the sun fronds had more xylem and phloem on a petiole cross-sectional basis (the xylem and phloem fraction respectively, FX and FP; Fig. 3), and when combined with their smaller leaves, their ratio of leaf area to xylem area was 78 % lower than in shade-grown plants (AL : AX; Fig. 3). Taken together, the sun ferns cope with the greater heat load by reducing leaf area and investing into more substantial vascular infrastructure in comparison with the shade plants.
Fig. 3.
Hydraulic and anatomical trait comparisons in sun and shade fronds of P. aquilinum (white and grey boxplots, respectively): (A, B) cross-petiolar xylem and phloem fraction, (C, D) xylem-specific (KS) and leaf-specific conductivity (KLEAF), (E, F) mean conduit diameter (D) and hydraulic conduit diameter (DH), (G) mean conduit length (LC), (H) the ratio of leaf area to xylem area (AL: AX), and (I, J) stomatal density (SD) and vein density (VD).
Both the low-light and the high-light plants showed a surprisingly similar hydraulic response. The specific xylem conductivity of the petiole (KS) was no different in the sun and shade plants, although KLEAF was significantly higher in the sun-exposed fronds (Fig. 3). This is because the sun plants have smaller leaves and thus a more favourable leaf area to petiole xylem area ratio (Fig. 3). The similarity in KS in the two treatments can probably be explained by the fronds’ similar hydraulic conduit diameters (DH), which averaged 70.38 ± 1.38 and 68.68 ± 2.68 µm for the sun and shade leaves respectively (Fig. 3). DH better reflects xylem hydraulic capacity than mean conduit diameters (DMEAN) because conductance is highly sensitive to even small changes in conduit diameter (Kolb and Sperry,, 1999). Nevertheless, at an average diameter of 24.65 ± 0.78 µm, DMEAN was significantly higher in the sun than in the shade plants but the absolute difference between the two groups was only 3 µm (Fig. 3). Mean tracheid/vessel length was equivalent for the two leaf types (1.4 and 1.5 cm for sun and shade leaves, respectively) with less than 1 % of the frond conduits reaching lengths over 11.8 cm (Fig. 3).
Stomatal density (SD) and vein density (VD) were significantly higher in sun leaves than in fronds from the understorey. SD averaged 100.97 ± 19.5 stomata mm−2 in the sun leaves, whereas only 35.86 ± 4.92 stomata mm−2 were counted in the shade leaves, a result that is consistent with the gSMAX of these plants in situ (Figs 2 and 3). The response of leaf vein density was functionally co-ordinated with stomatal density; the sun leaves had a VD of 2.93 ± 0.35 mm mm−2 whereas the VD of shade leaves averaged 2.15 ± 0.14 mm mm−2 (Fig. 3). Stomatal size, as indicated by guard cell length and width, was no different despite the pronounced difference in stomatal density, although epidermal cells were 45 % smaller in the sun leaves than in the shade leaves (Table 1).
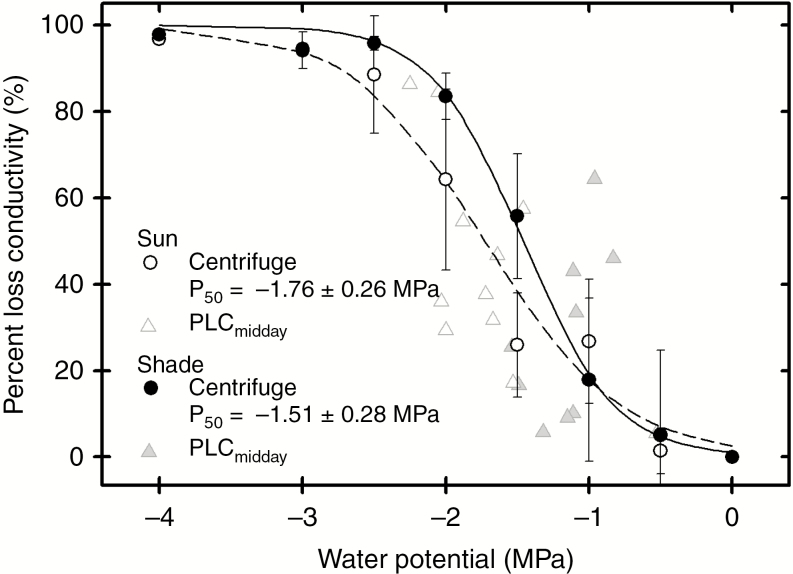
Petiole xylem vulnerability to air-induced embolism was equivalent in the sun and shade plants (Fig. 4). P50 ranged from −1.13 to −2.31 MPa across all samples, which is consistent with the water potentials these plants experienced in the field (Fig. 2). All petioles were 100 % embolized between −3 and −4 MPa. Midday petiole PLC values were statistically indistinguishable between fronds from both habitats, but they generally overlapped with their respective vulnerability curves (Fig. 4).
Fig. 4.
Percentage loss of petiole conductivity in response to xylem pressure in sun and shade fronds of P. aquilinum.
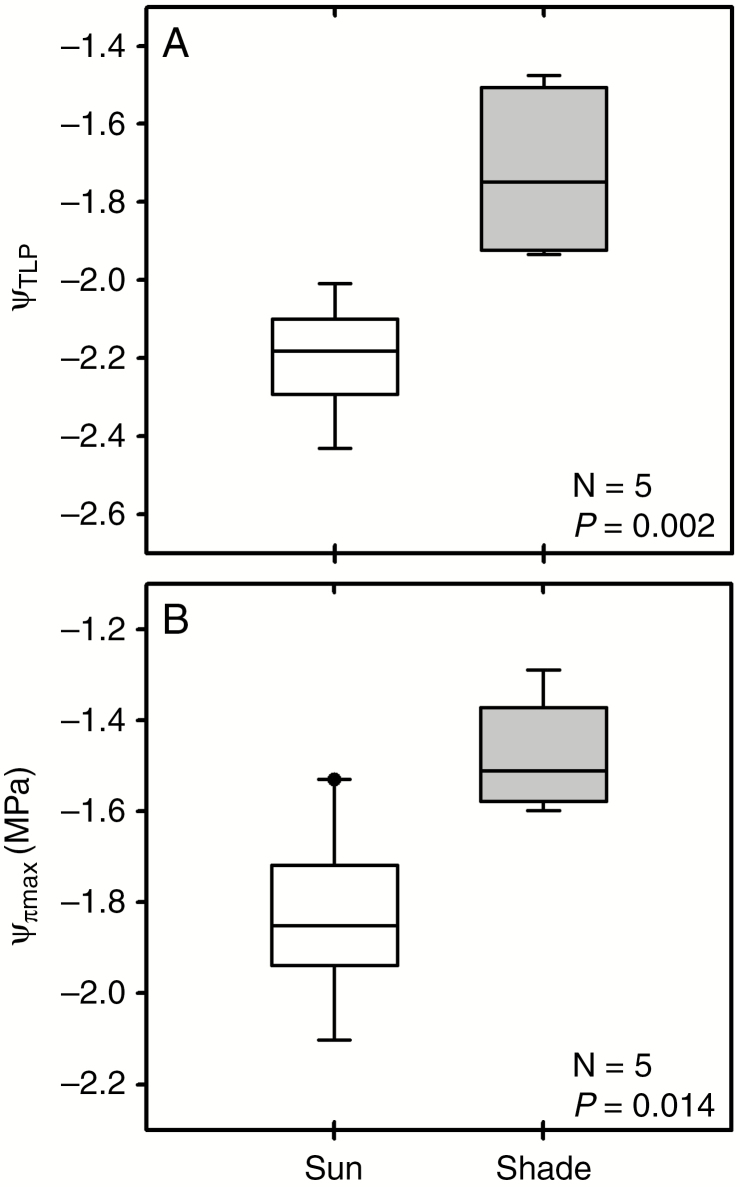
Both the water potential at turgor loss point (Ψ TLP) and the maximum osmotic potential (Ψ Π) at full hydration were significantly lower in the sun plants than in those from the shade (Fig. 5), indicating greater resilience to wilting as well as a greater role for osmotic regulation of water potential in sun-exposed plants. The leaf modulus of elasticity was similar in both leaf types (Table 1). Representative pressure–volume curves are available in the Supplementary Data, Information S1.
Fig. 5.
(A) Water potential at turgor loss point (Ψ TLP) and (B) maximum osmotic potential (Ψ ΠMAX) in sun and shade fronds of P. aquilinum.
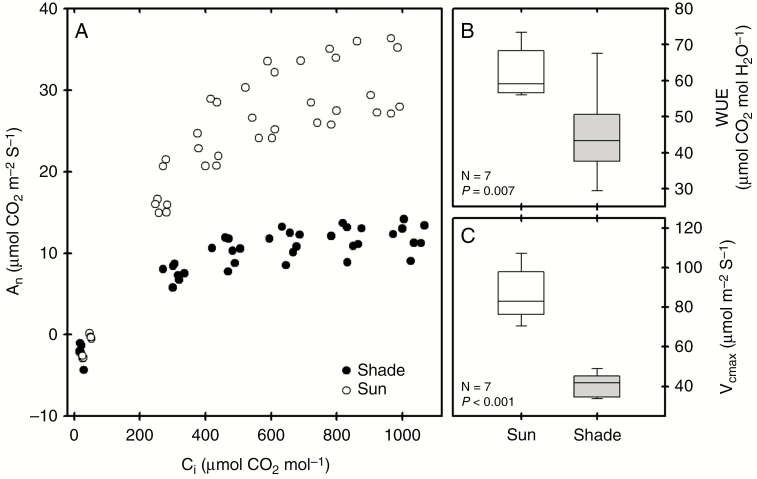
Consistent with adaptation to higher light levels, the sun-exposed fronds had higher rates of photosynthesis than the shade leaves, peaking at 32 µmol m−2 s−1 at a leaf CO2 (Ci) of 1000 µmol m−2 s−1. By contrast, photosynthesis in the shade leaves saturated at a Ci of approximately 500 µmol m−2 s−1 reached a maximum assimilation rate of 14 µmol m−2 s−1 at the maximum Ci (Fig. 6). Sun fronds had more than double the rates of maximum electron transport (JMAX) and carboxylation (CMAX) than the shade leaves, indicating an up-regulation of both the Calvin cycle and light reactions of photosynthesis (Fig. 6). Similarly, intrinsic water use efficiency (WUE) in sun plants was almost double that of the shaded fronds (Fig. 6). No differences were observed in dark respiration rates in both plant types (Table 1).
Fig. 6.
Gas-exchange response of sun and shade fronds of P. aquilinum (open and shaded boxplots, respectively). (A) The response of assimilation (An) to increasing leaf CO2 levels (Ci). (B, C) The intrinsic leaf water-use efficiency (WUE) and maximum carboxylation rate (Vcmax).
The biomechanical properties of the petioles differed between the sun and shade plants. The sun plants had significantly more flexible petioles, with a modulus of elasticity (MOE) of 6821 ± 2009 N mm−2 as compared to shade-grown fronds, in which MOE averaged 9091 ± 1659 N mm−2 (Table 1). While the modulus of rupture was no different in the two treatments, the sun-grown leaves had significantly denser petioles than the shade plants (Table 1), probably due to the high number of starch grains in the parenchyma tissue (Supplementary Data, Information S2).
Discussion
Vascular adjustment contributed to the success of P. aquilinum in its range of habitats but the means by which this was achieved differed from our predictions. Given the evapo-transpirative demand encountered by P. aquilinum at high light we expected these plants to have a greater fraction of long and wide conduits, as well as higher resistance to cavitation but instead our results show that the intrinsic xylem transport (KS) and embolism resistance of both sun and shade fronds were, for the most part, equivalent. Large differences in mean conduit diameter were swamped by the contributions of the larger, hydraulically significant conduits (DH) and an abundance of long vessels in both sun and shade fronds. Similarly, the nearly equivalent P50 values in petioles from both populations suggest that neither pit membrane structure nor vascular integration adjusted to the contrasting demand for water as might be expected based on earlier reports (Schoonmaker et al., 2010; Brodersen et al., 2012, 2014). Instead, we observed that the sun leaves had more xylem and phloem on a cross-sectional basis, and that when coupled with a lower leaf area, this led to a near doubling of gSMAX and WUE. Taken together, vascular acclimation of P. aquilinum to light is accomplished primarily by reductions in leaf area and a concurrent increase in xylem content.
Smaller, thicker, vertically oriented leaves are characteristic of high light environments (Niinemets, 2010; Leigh et al., 2012; Sessa and Givnish,, 2014; Scoffoni et al., 2015), and this was also a key attribute of sun-adapted P. aquilinum fronds. Reduced leaf area promotes water conservation while a vertical disposition reduces photoinhibition and heat loading (Givnish,, 1988). Thicker leaves have the additional advantage of buffering thermal fluctuations caused by variation in light intensity and air speed (Leigh et al., 2012), which too is adaptive for the sun populations of P. aquilinum. Indeed, P. aquilinum sun leaves had greater specific leaf area than those growing in the shade probably due to increased lignification, as observed in P. aquilinum elsewhere (Jones, 1983). Exposed fronds in high sun environments are subject to persistent agitation by wind, so along with tougher leaves, the petioles of the sun fronds were more flexible, as evidenced by their lower MOE compared to the shade plants (Table 1). A concurrent explanation is that the petioles of the shade plants were stiffer to support their higher leaf areas.
The high rates of photosynthesis in P. aquilinum are exceptional among ferns. Most ferns occupy low-light environments such as the forest understorey, so their assimilation rates rarely exceed 10 µmol m−2 s−1 (Brodribb et al., 2007; Watkins et al., 2010; Pittermann et al., 2011; Sessa and Givnish, 2014). However, the sun plants of P. aquilinum had leaf to xylem area ratios that were over four times lower than those of the understorey populations, thereby significantly increasing their KLEAF (Figure 3; Table 1). Leaf-specific conductance must be functionally co-ordinated with appropriate stomatal and vein densities, both of which were observed to be greater in the sun fronds than in shade-grown plants (Scoffoni et al., 2015). That being said, we suspect that it was the reduction in leaf area that led to increased stomatal and vein density in sun leaves, rather than developmental up-regulation in these traits per se because the epidermal cells shrank in size while stomatal size remained the same (Table 1). The consequence of this hydraulic and anatomical co-ordination was that the sun plants had much higher rates of gas exchange than the low-light population (Figs 3 and 6; Table 1; see also Brodribb et al., 2007; Sessa and Givnish, 2014; Scoffoni et al., 2015). Previous studies of P. aquilinum physiology report assimilation values up to 15 µmol m−2 s−1 at a Ci of 400 µmol mol−1 (Caporn et al., 1999) but several sun leaves from the Santa Cruz sun populations achieved rates beyond 20 µmol m−2 s−1 (Fig. 6). High rates of carbon uptake may allow P. aquilinum to grow quickly, and thus spread and outcompete existing vegetation, especially following frost, fire or other disturbance (Marrs and Watt, 2006).
Pteridium aquilinum plants from both sun and understorey populations operate perilously close to wilting. The ψ MIN of the sun plants reached −1.83 ± 0.22 MPa, only 0.37 MPa away from the water potential at turgor loss point, and practically equivalent to their average P50 (Table 1). By contrast, the safety margins are slightly higher in the shade-grown fronds at 0.74 MPa from the turgor loss point and 0.77 MPa from the P50. Studies on Dryopteris arguta, a perennial, similarly sized fern that is widely distributed on North America’s west coast, revealed that southern California populations are capable of significant osmotic adjustment, reaching an osmotic potential at turgor loss point below −6 MPa at the end of the dry season (Holmlund et al., 2016). Pteridium aquilinum is drought-deciduous and thus unlikely to adjust to the same extent. The native PLC values are consistent with the vulnerability curves, as well as the expectation that shade-growing individuals should experience milder conditions and thus less embolism. It is also possible that these ferns experience some degree of transport failure during the growing season, but given their high hydraulic capacity and redundancy (Brodersen et al., 2012), this may not significantly impact leaf function.
Pteridium aquilinum is a noxious, cosmopolitan and highly adaptive weed. Large fronds may have xylem with vessels in excess of 15 cm in length, its conduit diameters are on par with those of some ring-porous species, and its vein and stomatal densities are on the high end of the range relative to other ferns (Boyce et al., 2009; Franks and Beerling, 2009; Pittermann et al., 2011). Yet despite its physiological capacity, this outlier fern remains, to a large extent, hydraulically canalized in that its acclimation to variable light levels consisted of simple adjustments in leaf area, epidermal cell size and xylem content. In contrast to angiosperms and conifers which may alter conduit size and pit membrane attributes (Plavcová and Hacke, 2012; Bryukhanova and Fonti, 2013; Petit et al., 2016), P. aquilinum’s vulnerability to cavitation remained largely unchanged, as did the fundamental attributes of its xylem tissue. Neither did the presence of vessels confer any particular hydraulic advantage to the sun-exposed fronds. Pteridium aquilinum’s apparent adjustments are functionally adept but they are also relatively straightforward given that they entail the addition of existing tissue types rather than their fine-tuning. This may be part of a broader suite of limitations among ferns that may be related in part to their ancestral physiology and the absence of a vascular cambium (Pittermann et al., 2011; Brodribb et al., 2017). Alternatively, this response could, quite simply, be specific to our target taxon. Four species comprise the genus Pteridium, with P. aquilinum, P. caudatum, P. esculentum, P. semihastatum and their numerous subspecies located around the world (Marrs and Watt, 2006). Further research into Pteridium’s adaptive physiology may reveal other functional strategies that confer this genus with such exceptional competitive ability.
Supplementary data
Supplementary data are available online at https://academic.oup.com/aob and consist of the following.
Information S1: Examples of pressure–volume curves generated from sun and shade fronds of P. aquilinum.
Information S2: The parenchyma tissue from petioles in sun fronds of P. aquilinum were packed with starch grains, relative to the shade fronds.
ACKNOWLEDGEMENTS
We are grateful to Anna Jacobsen for assistance with the Instron instrument and the conduit length measures. Feedback from the handling Editor and two reviewers improved the manuscript.
Funding
This study was supported by NSF grants IOS-1258186 and 1656876.
LITERATURE CITED
- Alder NN, Pockman WT, Sperry JS, Nuismer S. 1997. Use of centrifugal force in the study of xylem cavitation. Journal of Experimental Botany 48: 665–674. [Google Scholar]
- Aloni R. 2015. Ecophysiological implications of vascular differentiation and plant evolution. Trees 29: 1–16. [Google Scholar]
- Bassow SL, Bazzaz FA. 1998. How environmental conditions affect canopy leaf-level photosynthesis in four deciduous tree species. Ecology 79: 2660–2675. [Google Scholar]
- Boardman NK. 1977. Comparative photosynthesis of sun and shade plants. Annual Review of Plant Physiology 28: 355–377. [Google Scholar]
- Boodle LA. 1904. The structure of the leaves of the Bracken (Pteris aquilina, Linn.) in relation to environment. The Botanical Journal of the Linnean Society 35: 659–669. [Google Scholar]
- Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA. 2009. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proceedings of the Royal Society of London B: Biological Sciences 276: 1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen C, Jansen S, Choat B, Rico C, Pittermann J. 2014. Cavitation resistance in seedless vascular plants: the structure and function of interconduit pit membranes. Plant Physiology 165: 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen CR, Roark LC, Pittermann J. 2012. The physiological implications of primary xylem organization in two ferns: fern xylem and physiology. Plant, Cell & Environment 35: 1898–1911. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ. 2007. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144: 1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SA, Murphy MRC. 2017. Xylem and stomata, coordinated through time and space. Plant, Cell & Environment 40: 872–880. [DOI] [PubMed] [Google Scholar]
- Bryukhanova M, Fonti P. 2013. Xylem plasticity allows rapid hydraulic adjustment to annual climatic variability. Trees 27: 485–496. [Google Scholar]
- Curtis DC. 2003. Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annual Reviews of Ecology and Systematics 34: 183–211. [Google Scholar]
- Caporn SJM, Brooks AL, Press MC, Lee JA. 1999. Effects of long-term exposure to elevated CO2 and increased nutrient supply on bracken (Pteridium aquilinum). Functional Ecology 13: 107–115. [Google Scholar]
- Carlquist S, Schneider EL. 2007. Tracheary elements in ferns: new techniques, observations, and concepts. American Fern Journal 97: 199–211. [Google Scholar]
- Cary KL, Pittermann J. 2018. Small trees, big problems: comparative leaf function under extreme edaphic stress. American Journal of Botany 105: 50–59. [DOI] [PubMed] [Google Scholar]
- Davidson AM, Jennions M, Nicotra AB. 2011. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecology Letters 14: 419–431. [DOI] [PubMed] [Google Scholar]
- Dawson TE. 1998. Fog in the California redwood forest: ecosystem inputs and use by plants. Oecologia 117: 476–485. [DOI] [PubMed] [Google Scholar]
- Der JP, Thomson JA, Stratford JK, Wolf PG. 2009. Global chloroplast phylogeny and biogeography of bracken (Pteridium; Dennstaedtiaceae). American Journal of Botany 96: 1041–1049. [DOI] [PubMed] [Google Scholar]
- Duursma RA. 2015. Plantecophys - an r package for analysing and modelling leaf gas exchange data. PLoS ONE 10: e0143346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer SV, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Beerling DJ. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences 106: 10343–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk JL, Vitousek PM. 2007. Resource-use efficiency and plant invasion in low-resource systems. Nature 446: 1079–1081. [DOI] [PubMed] [Google Scholar]
- Gensel PG. 2018. Early Devonian woody plants and implications for the early evolution of vascular cambia. In: Krings M, Harper CJ, Cuneo NR, Rothwell GW, eds. Transformative paleobotany: papers to commemorate the life and legacy of Thomas N. Taylor. Amsterdam: Elsevier, 21–33. [Google Scholar]
- Givnish TJ. 1988. Adaptation to sun and shade: a whole plant perspective. Australian Journal of Plant Physiology 15: 63–92. [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J. 2000. Drought experience and cavitation resistance in six shrubs from the Great Basin, Utah. Basic and Applied Ecology 1: 31–41. [Google Scholar]
- Haff TM, Brown MT, Tyler WB. 2008. The natural history of the UC Santa Cruz campus, 2nd edn Santa Cruz: Bay Tree Bookstore, University of California. [Google Scholar]
- Holmlund HI, Lekson VM, Gillespie BM, et al. 2016. Seasonal changes in tissue-water relations for eight species of ferns during historic drought in California. American Journal of Botany 103: 1607–1617. [DOI] [PubMed] [Google Scholar]
- Hutchinson GE. 1959. Homage to Santa Rosalia or why are there so many kinds of animals? The American Naturalist 93: 145–159. [Google Scholar]
- Jacobsen AL, Ewers FW, Pratt RB, Paddock WA, Davis SD. 2005. Do xylem fibers affect vessel cavitation resistance? Plant Physiology 139: 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CG. 1983. Phytochemical variation, colonization, and insect communities: the case of bracken fern. In: Denno RF,, McClure MS, eds. Variable plants and herbivores in natural and managed systems. New York:Academic Press, 513–549. [Google Scholar]
- Kolb KJ, Sperry JS. 1999. Differences in drought adaptation between subspecies of sagebrush (Artemisia tridentata). Ecology 80: 2373–2384. [Google Scholar]
- Leigh A, Sevanto S, Ball MC, et al. 2012. Do thick leaves avoid thermal damage in critically low wind speeds? New Phytologist 194: 477–487. [DOI] [PubMed] [Google Scholar]
- Macarthur R, Levins R. 1967. The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist 101: 377–385. [Google Scholar]
- Mangiafico SS. 2016. Summary and analysis of extension program evaluation in R. Version 1.18.1. rcompanion.org/documents/RHandbookProgramEvaluation.pdf.
- Marrs RH, Watt AS. 2006. Biological Flora of the British Isles: Pteridium aquilinum (L.) Kuhn. Journal of Ecology 94: 1272–1321. [Google Scholar]
- Meinzer FC, Woodruff DR, Marias DE, McCulloh KA, Sevanto S. 2014. Dynamics of leaf water relations components in co-occurring iso- and anisohydric conifer species. Plant, Cell & Environment 37: 2577–2586. [DOI] [PubMed] [Google Scholar]
- Murphy MRC, Jordan GJ, Brodribb TJ. 2014. Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant, Cell & Environment 37: 124–131. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. 2010. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecological Research 25: 693–714. [Google Scholar]
- Ordonez A, Wright IJ, Olff H. 2010. Functional differences between native and alien species: a global-scale comparison. Functional Ecology 24: 1353–1361. [Google Scholar]
- den Ouden JHB.The role of bracken (Pteridium aquilinum) in forest dynamics. Thesis, University of Wageningen. 2000.
- Page CN. 1976. The taxonomy and phytogeography of bracken – a review. Botanical Journal of the Linnean Society 73: 1–34. [Google Scholar]
- Page CN. 1982. The history and spread of bracken in Britain. Proceedings of the Royal Society of Edinburgh. Section B. Biological Sciences 81: 3–10. [Google Scholar]
- Page CN. 2002. Ecological strategies in fern evolution: a neopteridological overview. Review of Palaeobotany and Palynology 119: 1–33. [Google Scholar]
- Pammenter NW, Van der Willigen C. 1998. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiology 18: 589–593. [DOI] [PubMed] [Google Scholar]
- Petit G, Savi T, Consolini M, Anfodillo T, Nardini A. 2016. Interplay of growth rate and xylem plasticity for optimal coordination of carbon and hydraulic economies in Fraxinus ornus trees. Tree Physiology 36: 1310–1319. [DOI] [PubMed] [Google Scholar]
- Pittermann J, Brodersen CB, Watkins JJ. 2013. The physiological resilience of fern sporophytes and gametophytes: advances in water relations offer new insights into an old lineage. Frontiers in Plant Science 4: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittermann J, Limm E, Rico C, Christman MA. 2011. Structure–function constraints of tracheid-based xylem: a comparison of conifers and ferns. New Phytologist 192: 449–461. [DOI] [PubMed] [Google Scholar]
- Pittermann J, Watkins JE, Cary KL, et al. 2015. The structure and function of xylem in seed-free vascular plants: an evolutionary perspective. In: Hacke U, ed. Functional and ecological xylem anatomy. Cham: Springer International Publishing, 1–37. [Google Scholar]
- Plavcová L, Hacke UG. 2012. Phenotypic and developmental plasticity of xylem in hybrid poplar saplings subjected to experimental drought, nitrogen fertilization, and shading. Journal of Experimental Botany 63: 6481–6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockman WT, Sperry JS, O’Leary JW. 1995. Sustained and significant negative water pressure in xylem. Nature 378: 715. [Google Scholar]
- R Core Team 2019. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.r-project.org/index.html [Google Scholar]
- Robinson R, Sheffield E., Sharpe J. 2010. Problem ferns: their impact and management. In: Mehltreter K, Walker LR, Sharpe JM, eds. Fern ecology. Cambridge: Cambridge University Press, 255–322 [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonmaker AL, Hacke UG, Landhäusser SM, Lieffers VJ, Tyree MT. 2010. Hydraulic acclimation to shading in boreal conifers of varying shade tolerance. Plant, Cell & Environment 33: 382–393. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Kunkle J, Pasquet-Kok J, et al. 2015. Light-induced plasticity in leaf hydraulics, venation, anatomy, and gas exchange in ecologically diverse Hawaiian lobeliads. New Phytologist 207: 43–58. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Albuquerque C, Cochard H, et al. 2018. The causes of leaf hydraulic vulnerability and its influence on gas exchange in Arabidopsis thaliana. Plant Physiology 178: 1584–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa EB, Givnish TJ. 2014. Leaf form and physiology of Dryopteris species distributed along light gradients in Eastern North America. Functional Ecology 28: 108–123. [Google Scholar]
- Smith MD, Knapp AK. 2001. Physiological and morphological traits of exotic, invasive exotic, and native plant species in tallgrass prairie. International Journal of Plant Sciences 162: 785–792. [Google Scholar]
- Sperry JS, Hacke UG, Wheeler JK. 2005. Comparative analysis of end wall resistivity in xylem conduits. Plant, Cell & Environment 28: 456–465. [Google Scholar]
- Spicer R, Groover A. 2010. Evolution of development of vascular cambia and secondary growth. New Phytologist 186: 577–592. [DOI] [PubMed] [Google Scholar]
- Venturas MD, Rodriguez-Zaccaro FD, Percolla MI, Crous CJ, Jacobsen AL, Pratt RB. 2016. Single vessel air injection estimates of xylem resistance to cavitation are affected by vessel network characteristics and sample length. Tree Physiology 36: 1247–1259. [DOI] [PubMed] [Google Scholar]
- Vetter J. 2009. A biological hazard of our age: Bracken fern [Pteridium aquilinum (L.) Kuhn] — a review. Acta Veterinaria Hungarica 57: 183–196. [DOI] [PubMed] [Google Scholar]
- Watkins JE, Holbrook MN, Zwieniecki MA. 2010. Hydraulic properties of fern sporophytes: consequences for ecological and evolutionary diversification. American Journal of Botany 97: 2007–2019. [DOI] [PubMed] [Google Scholar]
- Watt AS. 1940. Contributions to the ecology of bracken (Pteridium aquilinum): I. The rhizome. New Phytologist 4: 401–422. [Google Scholar]
- Wilson JP. 2016. Hydraulics of Psilophyton and evolutionary trends in plant water transport after terrestrialization. Review of Palaeobotany and Palynology 227: 65–76. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.