Abstract
Objectives
Enhancer of zeste homolog 2 (EZH2) is a histone methyl transferase that mediates epigenetic silencing of tumor suppressor genes. It is commonly over-expressed in several solid tumors and has been shown to be a prognostic biomarker. We investigated patterns of EZH2 expression in endometrial cancer.
Methods
Evaluation of EZH2 expression was completed on both early and advanced stage endometrioid adenocarcinoma tissues and a subset of matched normal mullerian tissue samples, from participants enrolled in Gynecologic Oncology Group (GOG) protocol 210, using real time reverse transcription polymerase chain reaction (RT-PCR) and western blot (WB) analysis. Non-parametric methods were used to assess differences in mRNA and protein expression respectively with known clinical/pathologic prognostic factors. Survival analysis was performed using techniques including Cox proportional hazards (PH) model to evaluate differences in progression free survival (PFS) and overall survival (OS) based on EZH2 expression.
Results
Eighty-seven patient samples were analyzed that included 60 tumors and 27 matched-normal tissue specimens. EZH2 mRNA (p < .0001) and protein expression (p < .0001) in tumor specimens were significantly higher than in matched-normal tissue. In primary tumors, EZH2 protein expression was associated with lympho-vascular space invasion (LVSI, p = .044), and EZH2 mRNA expression was associated with age (p = .037). Differences in EZH2 expression between primary tumors and matched normal tissue were not associated with other known clinical and pathologic factors. However, there did appear to be a trend toward decreased progression-free survival among patients with high EZH2 expression levels.
Conclusions
Our results confirm the differential expression of EZH2 in uterine cancers compared to normal tissues. However, there were no statistically significant differences in survival associated with EZH2 expression in patients with endometrial cancer.
Keywords: EZH2, Endometrial carcinoma, Prognosis
1. Introduction
Endometrial cancer is the most common gynecologic malignancy [1 ]. Fortunately, many are diagnosed at an early stage. However, in patients with advanced stage or recurrent disease there is a substantial need for novel therapeutic options. In an effort to identify new targets, investigators have explored the relevance of epigenetic modifiers in cancer therapeutics. One such potential candidate is Enhancer of zeste homolog 2, (EZH2). EZH2 is a histone methyl transferase that mediates gene silencing by catalyzing trimethylation on lysine 27 of histone H3 (H3K27Me3) [2]. As a member of the polycomb group of genes (PcG) it has been implicated in nucleosome modification, chromatin remodeling, and interaction with various transcriptional regulators [3]. EZH2 has been targeted for inhibition because it is upregulated in multiple cancers and is critical for pathways that control cellular proliferation, angiogenesis, and survival [4–6]. This has led to the development of oral EZH2 inhibitors that have demonstrated activity in early clinical trials with a favorable safety profile in non-Hodgkin lymphoma and advanced solid tumors, including soft tissue sarcoma [7].
EZH2 is overexpressed in prostate, breast, ovarian, lung, liver, renal, gastric, esophageal, colorectal cancers, and melanoma [8–19]. In many of these, EZH2 expression is also correlated with higher proliferation and aggressive behavior of cancer cells, as well as poor prognosis. Indeed, studies have shown that overexpression of EZH2 in endometrial cancer cell lines promotes cellular proliferation, migration, and invasion in vivo [20]. Therefore, the current study has two specific aims: first, to test the hypothesis that there is differential expression of EZH2 in endometrial tumors compared to normal tissue; second, to examine the relationship between EZH2 expression and other known clinicopathologic factors that may correlate with prognosis.
2. Materials & methods
2.1. Research design and tissue specimens
Approval to conduct this study was obtained from the University of California, Irvine Institutional Review Board (HS#2011–8162). ln this study, the expression of EZH2 was investigated in both early and advanced stage endometrioid adenocarcinoma tissues, in addition to a subset of matched normal mullerian tissue samples. Specimens were provided by the Gynecologic Oncology Group (GOG) tissue bank from the GOG-210 clinical trial. Full details of the study population have been published previously [21]. This study was a part of GOG-8025 that had tumor tissue samples randomly selected within each stage group (stage I/II vs stage III/IV) in patients with endometrioid endometrial cancer based on a two-independent group design and availability of matched normal tissues [21]. Researchers conducting laboratory experiments were blinded to clinical data. The primary endpoints of interest in this study are differences in protein and mRNA expression of EZH2 as assessed by WB analysis and RT-PCR. Each experiment was carried out in triplicate and with positive and negative controls for EZH2 expression obtained from commercially available endometrial cell lines. Clinical and pathologic characteristics were prospectively and reliably collected by the GOG from multiple institutions.
2.2. Control cell lines and cell culture
The human endometrial cell lines utilized in this study ECC-1 (endometrial adenocarcinoma cell line) and T-HESC (endometrial stromal cell line) were purchased from American Type Culture Collection (ATCC, Manassas, VA). In accordance with ATCC guidelines, ECC-1 was grown in RPMI-1640 medium supplemented with 5% FBS; T-HESC was cultured in a phenol-free DMEM-F12 1:1 mixture supplemented with 1% ITS + premix, and 10% charcoal treated FBS. All cells were supplemented with penicillin (100 units/mL) and streptomycin (100 μg/mL) and maintained at 37 °C in a humidified atmosphere of 5% CO2.
2.3. Protein isolation and Western blot analysis
Cell extracts were prepared in RIPA lysis buffer containing protease inhibitors (Sigma, St. Louis, MO). Cell lysates were centrifuged, the supernatant was collected, and the BCA protein assay kit was used to determine protein concentration per package protocol (Thermo Fisher Scientific, Rockford, IL). Protein lysates containing equal amounts of protein were then separated on 12% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad, Hercules, CA), and electrophoretically transferred to a Hybond-ECL membrane (GE Healthcare, Piscataway, NJ). Blots were then blocked for 1 h in TBST (10 mM Tris-HCL, pH 8.0, 150 mM NaCl, and 0.05% Tween-20) containing 5% blocking grade non-fat dry milk (Bio-Rad, Hercules, CA), and then incubated with primary antibody at 4 °C overnight. Antibodies for EZH2 and GAPDH (loading control) used in WB were purchased from Cell Signaling Technology (Danvers, MA). Blots were then washed 3 times in TBST and incubated for 90 min at room temperature with HRP-conjugated goat anti-rabbit or anti-mouse IgG secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactive bands were visualized using the SuperSignal West Pico chemiluminescence detection system (Thermo Fisher Scientific, Rockford, IL).
Relative adjusted density was calculated using Image-J software and visual inspection of EZH2 bands with positive (ECC-1) and negative controls (T-HESC); these measurements were strongly correlated and the dichotomous outcome was used to simplify analysis. Therefore, results were reported as the presence or absence of immunoreactive bands.
2.4. Real-time reverse transcription-polymerase chain reaction (RT-PCR)
RNA was isolated from all tissue samples using the TRIzol reagent (Invitrogen, Carlsbad, CA) as previously reported by Eskander et al. [22]. A High Capacity cDNA Reverse Transcription kit was utilized to synthesize complementary DNA from 2 μg of total RNA per the manufacturer protocol (Applied Biosystems, Foster City, CA). Real time PCR amplification reactions for EZH2 were then carried out using the CFX Connect™ system (Bio-Rad) as per manufacturer’s guidelines. GAPDH gene expression was used for normalization. EZH2 and GAPDH primers were obtained from Qiagen (Valencia, CA). Data was then analyzed using the comparative Ct method as previously described in detail else-where [23]. Briefly, PCR results were analyzed using gene expression study software to calculate normalized expression ratios and determine the relative fold-change in EZH2 expression in samples compared to control cell lines. Each experiment was performed in triplicate.
2.5. Statistical analysis
Either exact or Monte-Carlo simulation-based non-parametric methods such as Spearman correlation coefficient tests, Kruskal-Wallis tests, Fisher exact tests, chi-squared tests, McNemar tests were used to explore the associations of EZH2 expression (either mRNA or protein) in primary tumor tissue and EZH2 expression difference between primary tumor tissue and the matched normal tissue with each of the prognostic factors and site of first disease recurrence, respectively. EZH2 mRNA expression difference was defined as EZH2 mRNA expression in primary tumor minus EZH2 mRNA expression in normal tissue. When exploring differences in EZH2 protein expression on WB, the EZH2 protein expression difference was defined as EZH2 protein expression in primary tumor minus EZH2 protein expression in normal tissue, where positive WB was coded as 1 and negative WB was coded as 0. Progression-free survival (PFS) was defined as the duration of time from study entry to date of evidence of disease recurrence or progression, death, or the date of last contact, whichever occurs first. PFS is censored in patients who are alive and have not experienced disease progression or recurrence. Overall survival (OS) was defined as the duration of time from study entry to the time of death due to any cause or the date of last contact. The site of first recurrence was classified as locoregional if within the vagina or pelvis and distant if otherwise.
Monte-Carlo permutation-based log-rank tests were utilized to investigate the relationships of EZH2 expression difference between primary tumor tissue and the matched normal tissue with PFS, and the relationships of EZH2 expression in primary tumor tissues with PFS, respectively. Univariate Cox proportional hazards (PH) model was used to estimate hazard ratio (HR) and corresponding profile likelihood (PL) confidence interval (Cl). In addition, the associations of PFS with each of the other interesting clinical and pathologic factors were explored by score tests from univariate Cox PH model, respectively. The relationships of OS with EZH2 expression were only characterized by Kaplan-Meier curves due to small number of events (i.e., 7 events out of 60 patients with primary tumor tissues, 3 events out of 27 patients with matched tumor and normal tissues).
An arbitrary significance level of 0.05 was used to classify individual statistical hypothesis test results as interesting and worthy of further investigation. No adjustment was made for multiple tests due to the exploratory nature of this study. Statistical analyses were performed using SAS software (version 9.4 SAS Institute Inc., Cary, NC, USA).
3. Results
Eighty-seven tissue specimens from sixty patients with endometrioid endometrial adenocarcinoma enrolled in GOG-210 were provided for this study, as well as matched normal mullerian tissues samples from twenty-seven subjects. The summary of patient and tumor pathologic characteristics for these 60 patients is shown in Table 1. Positive EZH2 protein expression by WB in the primary tumor was observed in 39 patients (65%, 90% CI 54%–75%). EZH2 protein expression in primary tumor tissue was associated with lympho-vascular space invasion (p = .044), but other clinical and pathologic factors including age, stage, grade, nodal involvement or disease status were not associated with EZH2 protein expression (see Table 2). The median EZH2 mRNA expression in primary tumor tissue was 8.29 with a mean of 10.26 (range: 0.79–52.6; SD 8.77). Older patients were found to have reduced EZH2 mRNA expression levels within the evaluated patient cohort (p = .037; r = −0.27).
Table 1.
Patient and Tumor Characteristics for All Participants.
| Characteristic | Total |
|
|---|---|---|
| N | % | |
| Age (years) | ||
| ≤54 | 12 | 20.0 |
| 55–69 | 39 | 65.0 |
| ≥70 | 9 | 15.0 |
| BMI (kg/m2) | ||
| <25 | 10 | 16.7 |
| 25–34 | 28 | 46.7 |
| ≥35 | 22 | 36.7 |
| Stage | ||
| I/II | 32 | 53.3 |
| III/IV | 28 | 46.7 |
| Tumor grade | ||
| 1/2 | 49 | 81.7 |
| 3 | 11 | 18.3 |
| Tumor penetration | ||
| Inner half | 29 | 48.3 |
| Outer half | 24 | 40.0 |
| Serosa | 3 | 5.0 |
| Unknown | 4 | 6.7 |
| Cytology | ||
| Negative | 46 | 76.7 |
| Positive | 9 | 15.0 |
| Suspicious | 3 | 5.0 |
| Unknown | 2 | 3.3 |
| LVSI | ||
| Absent | 38 | 63.3 |
| Present | 20 | 33.3 |
| Unknown | 2 | 3.3 |
| Nodal status | ||
| Negative | 40 | 66.7 |
| Positive | 16 | 26.7 |
| Unknown | 4 | 6.7 |
| Site of first recurrence | ||
| Distant | 5 | 8.3 |
| Loco-regional | 6 | 10.0 |
| NED | 49 | 81.7 |
| Matched tissue | ||
| No | 33 | 55.0 |
| Yes | 27 | 45.0 |
| Total | 60 | 100.0 |
BMI = body mass index; LVSI = lympho-vascular space invasion; NED = no evidence of disease.
Table 2.
EZH2 protein expression (WB) in primary tumor by known prognostic factors and recurrence.
| Characteristic | Positive WB |
Negative WB |
Total |
P-valuea | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Age (years)* | 0.1658 | ||||||
| ≤54 | 5 | 12.8% | 7 | 33.3% | 12 | 20.0% | |
| 55–69 | 28 | 71.8% | 11 | 52.4% | 39 | 65.0% | |
| ≥70 | 6 | 15.4% | 3 | 14.3% | 9 | 15.0% | |
| BMI (kg/m2)* | 0.7491 | ||||||
| <25 | 7 | 17.9% | 3 | 14.3% | 10 | 16.7% | |
| 25–34 | 18 | 46.2% | 10 | 47.6% | 28 | 46.7% | |
| ≥35 | 14 | 35.9% | 8 | 38.1% | 22 | 36.7% | |
| Stage | 1.0000 | ||||||
| I/II | 21 | 53.8% | 11 | 52.4% | 32 | 53.3% | |
| III/IV | 18 | 46.2% | 10 | 47.6% | 28 | 46.7% | |
| Tumor grade | 0.0779 | ||||||
| 1/2 | 29 | 74.4% | 20 | 95.2% | 49 | 81.7% | |
| 3 | 10 | 25.6% | 1 | 4.8% | 11 | 18.3% | |
| Tumor penetration | 0.7187 | ||||||
| Inner half | 18 | 46.2% | 11 | 52.4% | 29 | 48.3% | |
| Outer half | 18 | 46.2% | 6 | 28.6% | 24 | 40.0% | |
| Serosa | 2 | 5.1% | 1 | 4.8% | 3 | 5.0% | |
| Unknown | 1 | 2.6% | 3 | 14.3% | 4 | 6.7% | |
| Cytology | 0.7090 | ||||||
| Negative | 30 | 76.9% | 16 | 76.2% | 46 | 76.7% | |
| Positive | 5 | 12.8% | 4 | 19.0% | 9 | 15.0% | |
| Suspicious | 3 | 7.7% | 0 | 0.0% | 3 | 5.0% | |
| Unknown | 1 | 2.6% | 1 | 4.8% | 2 | 3.3% | |
| Vascular invasion | 0.0439 | ||||||
| Absent | 22 | 56.4% | 16 | 76.2% | 38 | 63.3% | |
| Present | 17 | 43.6% | 3 | 14.3% | 20 | 33.3% | |
| Unknown | 0 | 0.0% | 2 | 9.5% | 2 | 3.3% | |
| Nodal status | 0.7613 | ||||||
| Negative | 27 | 69.2% | 13 | 61.9% | 40 | 66.7% | |
| Positive | 10 | 25.6% | 6 | 28.6% | 16 | 26.7% | |
| Unknown | 2 | 5.1% | 2 | 9.5% | 4 | 6.7% | |
| Site of first recurrence | 0.1462 | ||||||
| Distant | 5 | 12.8% | 0 | 0.0% | 5 | 8.3% | |
| Loco-regional | 5 | 12.8% | 1 | 4.8% | 6 | 10.0% | |
| NED | 29 | 74.4% | 20 | 95.2% | 49 | 81.7% | |
| EZH2 mRNA* | 0.0944 | ||||||
| <3.6 | 3 | 7.7% | 6 | 28.6% | 9 | 15% | |
| 3.6–9 | 17 | 43.6% | 7 | 33.3% | 24 | 40% | |
| >9 | 19 | 48.7% | 8 | 38.1% | 27 | 45% | |
| Total | 39 | 65.0% | 21 | 35.0% | 60 | 100.0% | |
Monte-Carlo simulation-based tests excluding observations with values as unknown/suspicious: Spearman rank correlation coefficient tests for age, BMI and EZH2 mRNA, Pearson’s chi-square tests for tumor penetration and recurrence status, Fisher exact test for the rest of the prognostic factors.
3.1. EZH2 expression is higher in endometrial cancer than normal tissue
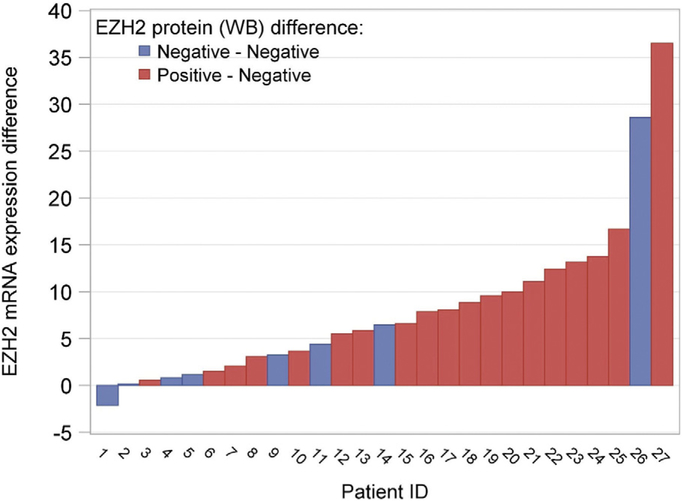
Primary tumor tissue and matched normal tissue samples were available for 27 patients and of these 24 patients had stage I disease, 2 had stage II, and 1 patient had stage III disease. EZH2 mRNA expression difference was defined as EZH2 mRNA expression in primary tumor minus EZH2 mRNA expression in matched normal tissue. In these patient samples, the average of EZH2 mRNA expression difference was 8.11, and the median was 6.44 (p < .0001). EZH2 mRNA expression was significantly higher in primary tumor tissue than matched normal tissue. The EZH2 mRNA expression difference among patients with both tumor and normal tissue data is displayed in Fig. 1. There were no statistically significant associations between EZH2 mRNA expression difference and the clinical or pathologic prognostic factors included age, BMI, stage, tumor grade, tumor penetration, LVSI, nodal status, or disease status (results not shown).
Fig. 1.
EZH2 mRNA expression difference and protein expression between tumor tissue and matched normal tissue by patient ID.
Negative EZH2 protein expression was observed in all of the matched normal tissues; however, 70% (p = .0001; 90% CI 53%–84%) of the matched primary tumor tissue had positive EZH2 protein expression. There were no statistically significant associations found between EZH2 protein expression differences and clinical or pathologic prognostic factors included age, BMI, stage, tumor grade, tumor penetration, LVSI, nodal status, or disease status, respectively (results not shown).
3.2. EZH2 & recurrence
EZH2 protein expression was observed in 39 of the 60 (65%) tumor specimens. There were 11 patients (18.3%) in the cohort who experienced disease recurrence. All those who experienced initial recurrence at a distant site expressed EZH2 on WB and of those with locoregional recurrence; only one did not express EZH2, although these differences were not statistically significant (see Table 2).
3.3. EZH2 & survival
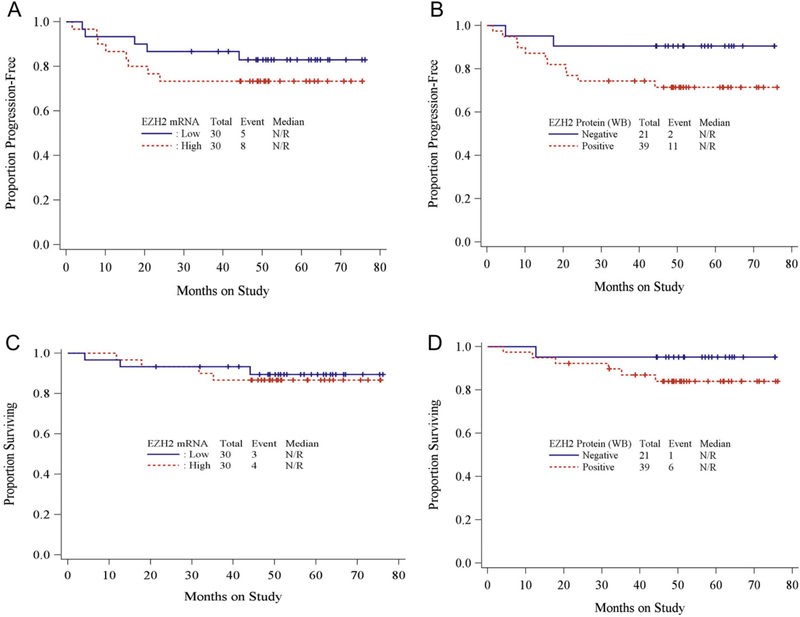
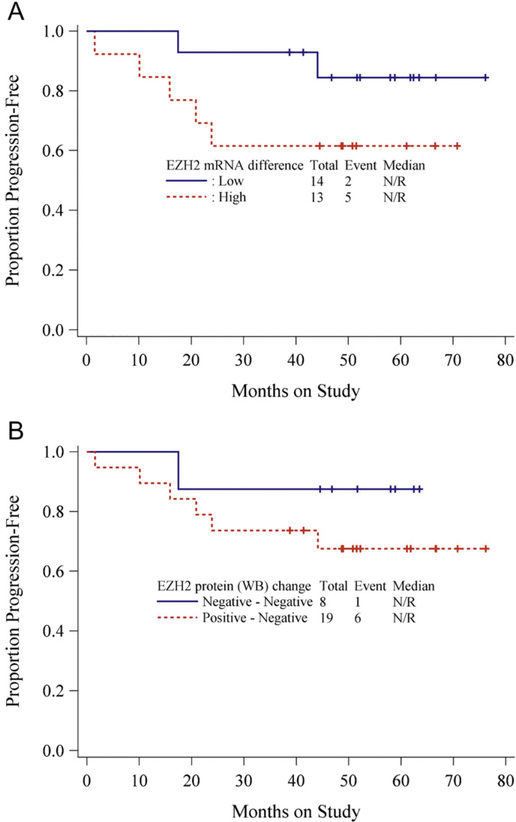
The survival distributions for PFS and OS by EZH2 expression in primary tumors were characterized by Kaplan-Meier curves [Fig. 2A–D]. While the curves appear to separate suggesting that patients with high EZH2 expression tended to have a worse progression-free survival and overall survival when compared to patients with lower EZH2 expression, the differences are not statistically significant. In patients with matched normal tissue specimens, due to the small sample size and small number of events, Monte-Carlo permutation-based log-rank tests were used to study the associations between the EZH2 expression difference (mRNAand protein, respectively) for progression-free survival (see Table 3). There was no statistically significant association between EZH2 expression difference and PFS. However, in patients with high EZH2 mRNA expression (primary tumor vs. matched normal tissue) there was a trend toward decreased PFS and lack of EZH2 expression on WB might be protective (Fig. 3A–B). The associations of PFS with age, BMI, stage, grade, depth of invasion, lympho-vascular invasion, cytology, nodal status and EZH2 expressions in primary tumor were explored by univariate Cox PH models as shown in Table 4. No statistically significant associations were found.
Fig. 2.
AKaplan-Meier curve forprogression-free survival (PFS) by EZH2 mRNAexpression level in tumor tissue (Hazard Ratio (HR) = 0.59,95% CI = 0.178–1.769; log-rank test p-value = .3611 ) (EZH2 mRNA: Low [≤median] vs High [>median]) B. Kaplan-Meier curve for PFS by EZH2 protein expression in tumor tissue (HR = 0.307,95% CI 0.047–1.143; log-ranktest p-value 0.117) (EZH2 protein: negative vs positive) C. Kaplan-Meier curve for overall survival (OS) by EZH2 mRNAexpression level in tumor tissue (HR = 1.292,95% CI 0.285–6.556; log-ranktest p-value 0.737) D. Kaplan-Meier curve for OS by EZH2 protein expression in tumor tissue (HR = 0.289,95% CI 0.015–1.692; log-rank test p-value 0.221 ).
Table 3.
PFS and EZH2 expression difference.
| Characteristic | Hazard ratio | 95% CI | P-valuea |
|---|---|---|---|
| EZH2 mRNAexpression difference: low(≤median) vs high (>median) | 0.311 | 0.044 to 1.444 | 0.1103 |
| EZH2 protein (WB) difference: negative - negative vs positive - negative | 0.352 | 0.019 to 2.062 | 0.2836 |
Monte-Carlo permutation-based log-rank tests.
Fig. 3.
A Kaplan-Meier curve for PFS by EZH2 mRNA expression difference between tumor tissue and matched normal tissue B. Kaplan-Meier curve for PFS by EZH2 protein expression (WB) difference between tumor tissue and matched normal tissue.
Table 4.
Associations of each prognostic factors including EZH2 expression in primary tumor tissue with PFS.
| Characteristic | Hazard ratio | 95% CI | P-valuea |
|---|---|---|---|
| Age: high(>median) vs low(≤median) | 2.422 | 0.789 to 8.942 | 0.1283 |
| bmi: high(>median) vs low(≤median) | 0.788 | 0.254 to 2.374 | 0.6683 |
| EZH2 mRNA: high(>median) vs low(≤median) | 0.59 | 0.178 to 1.769 | 0.3611 |
| EZH2 protein (WB): negative vs positive | 0.307 | 0.047 to 1.143 | 0.117 |
| Stage: I/II vs III/IV | 0.968 | 0.321 to 3.008 | 0.9534 |
| Tumor grade: 1/2 vs 3 | 0.674 | 0.206 to3.005 | 0.5458 |
| Tumor penetration: inner half vs outer half/serosa | 0.752 | 0.242 to 2.263 | 0.6066 |
| Cytology: negative vs positive/suspicious | 1.153 | 0.297 to 7.562 | 0.8556 |
| Nodal status: negative vs positive | 0.664 | 0.201 to 2.538 | 0.5118 |
| Vascular invasion: absent vs present | 0.4 | 0.129 to 1.207 | 0.089 |
Score test from univariate Cox PH model except p-values for EZH2 expressions resulting from Monte-Carlo permutation-based log-rank tests.
4. Discussion
4.1. The molecular mechanism by which EZH2 acts in endometrial cáncer remains unclear
However, it is known that the histone methyl transferase activity of EZH2 leads to epigenetic modification of tumor suppressor gene expression and given it is highly expressed in multiple tumor types— EZH2 is an attractive target for drug development in endometrial cancer [24,25]. This line of inquiry is further supported by the primary results of the current study, which confirm that there is differential expression of EZH2 in uterine cancers compared to normal tissues and EZH2 upregulation in many tumors. Regarding the secondary objectives to examine clinical characteristics and prognosis, it is important to note that the nature of this study was exploratory using available samples. Unfortunately, due to the small sample size, these results must be interpreted with caution and no conclusions can be inferred regarding the relationship between EZH2 and known prognosticators of outcome. Overall, there were too few events, and consequently a lack of power to detect differences in survival in the accessible sub-population of GOG 210 analyzed. In other larger studies, EZH2 expression has been shown to correlate with clinical characteristics in endometrial carcinomas that included endometrioid as well as serous and clear cell histology [26]. The lack of meaningful clinical correlations in the present work may be related to the limited sample size and potential selection bias, or may represent a true lack of association between EZH2 expression and prognosis in endometrial cancer.
The clinical relevance of the EZH2 mediated signaling is illustrated in the fact that several EZH2 specific inhibitors have been developed and have demonstrated anti-tumor effects in various malignancies as single agents [27,28]. NRG GY014 is currently investigating the utility of the EZH2 inhibitor, tazemetostat, in recurrent endometrioid endometrial cancer and recurrent endometrioid/clear cell carcinoma of the ovary.
Furthermore, new data regarding the therapeutic potential of combined epigenetic and immunotherapeutic agents suggests there will be further need for molecular epigenetic targets such as EZH2 in the future. Several studies have demonstrated that epigenetics plays an important role in tumor immunogenicity and immune evasion [29]. In animal models, combined treatment with EZH2 inhibitors improved the efficacy of programmed death-ligand 1 (PD-L1) checkpoint blockade [30]. Odunsi et al. published the first phase 1 clinical trial of combined epigenetic targeting agents and immunotherapy as an addition to second-line chemotherapy in ovarian cancer patients [31]. It demonstrated that the combination is safe and associated with favorable immunologic responses and a high rate of stable disease or partial responses in a challenging patient population. This represents a new and exciting avenue of exploration for other gynecologic malignancies with un-met clinical needs.
Ultimately, as our understanding of the contribution of epigenetic modifiers in cancer therapeutics evolves, novel single agent, or combinatorial approaches may be identified that are effective in treating aggressive malignancies.
HIGHLIGHTS.
EZH2 expression is higher in endometrial tumors than normal tissues.
The majority of patients who experienced disease recurrence had tumors with high EZH2 expression.
EZH2 expression was not associated with statistically significant differences in progression free or overall survival.
Acknowledgments
Financial support was provided by the Division of Gynecologic Oncology institutional NIH T-32 training grant (Ruth L. Kirschstein NRSA Institutional Training Research Grant, 2T32 CA-060396-11). Also supported in part by National Cancer Institute award number P30CA062203; the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517); the GOG Tissue Bank supported by NCI U24 CA114793, NRG Oncology (1 U10 CA 180822) and NRG Operations (U10CA180868).
The following NRG Oncology/Gynecologic Oncology Group member institutions participated in this study: Ohio State University Comprehensive Cancer Center, University of Oklahoma Health Sciences Center, Penn State Milton S. Hershey Medical Center, Washington University School of Medicine, University of Massachusetts Memorial Health Care, Duke University Medical Center, University of Minnesota Medical Center-Fairview, University of Iowa Hospitals and Clinics, Indiana University Hospital/Melvin and Bren Simon Cancer Center, University of New Mexico, Women and Infants Hospital, Roswell Park Comprehensive Cancer Center, Northwestern University, University of Colorado Cancer Center - Anschutz Cancer Pavilion, University of North Carolina at Chapel Hill, University of Texas Southwestern Medical Center, Wake Forest University Health Sciences, Cooper Hospital University Medical Center, University of Virginia, Tacoma General Hospital, Mayo Clinic, Case Western Reserve University, North Shore University Hospital, Yale University, University of Arkansas Medical Center, Michigan Cancer Research Consortium Community Clinical Oncology Program, Stony Brook University Medical Center, Women’s Cancer Center of Nevada, University of Pittsburgh Cancer Institute (UPCI), University of California at Los Angeles Health System, Gynecologic Oncology Network/Brody School of Medicine, University of California Medical Center at Irvine-Orange Campus, University of Illinois, Gynecologic Oncology of West Michigan PLLC, University of Wisconsin Hospital and Clinics, New York University Medical Center, Water Reed National Military Medical Center, Fox Chase Cancer Center, University of Chicago, University of Cincinnati, The Hospital of Central Connecticut, Abington Memorial Hospital, Aurora Women’s Pavilion of Aurora West Allis Medical Center, Evanston CCOP-North Short University Health System, Wayne State University/Karmanos Cancer Institute, University of Mississippi Medical Center, Cleveland Clinic Foundation, Fred Hutchinson Cancer Research Center, University of Alabama at Birmingham, Tufts-New England Medical Center, Moffit Cancer Center and Research Institute, Delaware/Christiana Care CCOP, Saint Vincent Hospital, Abramson Cancer Center of the University of Pennsylvania, Wisconsin NCI Community Oncology Research Program, Memorial Sloan-Kettering Cancer Center, Fletcher Allen Health Care, Mount Sinai School of Medicine, William Beaumont Hospital, Cancer Research for the Ozarks NCORP, UCSF-Mount Zion and Rush University Medical Center.
Footnotes
Declaration of competing interest
The authors have no competing interests to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2017, CA Cancer J. Clin 67 (1 ) (2017) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. , Role of histone H3 lysine 27 methylation in Polycomb-group silencing, Science 298 (5595) (2002) 1039–1043. [DOI] [PubMed] [Google Scholar]
- [3].Simon JA, Tamkun JW, Programming off and on states in chromatin: mechanisms of Polycomb and trithorax group complexes, Curr. Opin. Genet. Dev 12 (2) (2002) 210–218. [DOI] [PubMed] [Google Scholar]
- [4].Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K, EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer, EMBOJ. 22 (20) (2003) 5323–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, et al. , Regulation of tumor angiogenesis by EZH2, Cancer Cell 18 (2) (2010) 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tang X, Milyavsky M, Shats I, Erez N, Goldfinger N, Rotter V, Activated p53 suppresses the histone methyltransferase EZH2 gene, Oncogene 23 (34) (2004) 5759–5769. [DOI] [PubMed] [Google Scholar]
- [7].Italiano A, Soria JC, Toulmonde M, Michot JM, Lucchesi C, Varga A, et al. , Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study, LancetOncol 19 (5) (2018) 649–659. [DOI] [PubMed] [Google Scholar]
- [8].Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, et al. , EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast,J. Clin. Oncol 24 (2) (2006) 268–273. [DOI] [PubMed] [Google Scholar]
- [9].Choi JH, Song YS, Yoon JS, Song KW, Lee YY, Enhancer of zeste homolog 2 expression is associated with tumor cell proliferation and metastasis in gastric cancer, APMIS 118 (3) (2010) 196–202. [DOI] [PubMed] [Google Scholar]
- [10].Huqunv, Ishikawa R, Zhang J, Miyazawa H, Goto Y, Shimizu Y, et al. , Enhancer of zeste homolog 2 is a novel prognostic biomarker in nonsmall cell lung cancer, Cancer 118 (6) (2012) 1599–1606. [DOI] [PubMed] [Google Scholar]
- [11].Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, et al. , EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells, Proc. Nati. Acad. Sci. U. S. A 100 (20) (2003) 11606–11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee HW, Choe M, Expression of EZH2 in renal cell carcinoma as a novel prognostic marker, Pathol. Int 62 (11) (2012) 735–741. [DOI] [PubMed] [Google Scholar]
- [13].Li H, Cai Q, Godwin AK, Zhang R, Enhancer of zeste homolog 2 promotes the proliferation and invasion of epithelial ovarian cancer cells, Mol. Cancer Res. 8 (12) (2010) 1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Matsukawa Y, Semba S, Kato H, Ito A, Yanagihara K, Yokozaki H, Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer, Cancer Sci. 97 (6) (2006) 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rao ZY, Cai MY, Yang GF, He LR, Mai SJ, Hua WF, et al. , EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-beta1 and is a predictor of outcome in ovarian carcinoma patients, Carcinogenesis 31 (9) (2010) 1576–1583. [DOI] [PubMed] [Google Scholar]
- [16].Sudo T, Utsunomiya T, Mimori K, Nagahara H, Ogawa K, Inoue H, et al. , Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma, Br.J. Cancer 92 (9) (2005) 1754–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. , The polycomb group protein EZH2 is involved in progression of prostate cancer, Nature 419 (6907) (2002) 624–629. [DOI] [PubMed] [Google Scholar]
- [18].Wang CG, Ye YJ, Yuan J, Liu FF, Zhang H, Wang S, EZH2 and STAT6 expression profiles are correlated with colorectal cancer stage and prognosis, World J. Gastroenterol 16 (19) (2010) 2421–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang W, Wang F, Zong G, Liu R, Zhang Y, Luan Y, et al. , Prognostic significance of EZH2 expression in patients with digestive cancers: a meta-analysis, Int. J. Clin. Exp. Med 8 (9) (2015) 16043–16049. [PMC free article] [PubMed] [Google Scholar]
- [20].Eskander RN, Ji T, Huynh B, Wardeh R, Randall LM, Hoang B, Inhibition of enhancer of zeste homolog 2 (EZH2) expression is associated with decreased tumor cell proliferation, migration, and invasion in endometrial cancer cell lines, Int. J. Gynecol. Cancer 23 (6) (2013) 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, et al. , Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial, Gynecol. Oncol 129 (2) (2013) 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eskander RN, Ali S, Dellinger T, Lankes HA, Randall LM, Ramirez NC, et al. , Expression patterns of the Wnt pathway inhibitors Dickkopf3 and secreted frizzled-related proteins 1 and 4 in endometrial endometrioid adenocarcinoma: an NRG oncology/gynecologic oncology group study, Int. J. Gynecol. Cancer 26 (1 ) (2016) 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tang Y, Simoneau AR, Xie J, Shahandeh B, Zi X, Effects of the kava chalcone flavokawain a differ in bladder cancer cells with wild-type versus mutant p53, Cancer Prev. Res. (Phila.) 1 (6) (2008) 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ihira K, Dong P, Xiong Y, Watari H, Konno Y, Hanley SJ, et al. , EZH2 inhibition suppresses endometrial cancer progression via miR-361/Twist axis, Oncotarget 8 (8) (2017) 13509–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Alldredge JK, Eskander RN, EZH2 inhibition in ARID1A mutated clear cell and endometrioid ovarian and endometrioid endometrial cancers, Gynecol Oncol Res Pract 4 (2017) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gu Y, Zhang J, Guan H, Expression of EZH2 in endometrial carcinoma and its effects on proliferation and invasion of endometrial carcinoma cells, Oncol. Lett 14 (6) (2017) 7191–7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Geng J, Li X, Zhou Z, Wu CL, Dai M, Bai X, EZH2 promotes tumor progression via regulating VEGF-A/AKT signaling in non-small cell lung cancer, Cancer Lett. 359 (2) (2015) 275–287. [DOI] [PubMed] [Google Scholar]
- [28].Knutson SK, Kawano S, Minoshima Y, Warholic NM, Huang KC, Xiao Y, et al. , Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma, Mol. Cancer Ther 13 (4) (2014) 842–854. [DOI] [PubMed] [Google Scholar]
- [29].Dunn J, Rao S, Epigenetics and immunotherapy: the current state of play, Mol. Immunol 87 (2017) 227–239. [DOI] [PubMed] [Google Scholar]
- [30].Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. , Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy, Nature 527 (7577) (2015) 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Odunsi K, Matsuzaki J, James SR, Mhawech-Fauceglia P, Tsuji T, Miller A, et al. , Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer, Cancer Immunol Res 2 (1) (2014) 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]